Abstract
The clitoris is a leading player in female sexual arousal, if not the main protagonist. Despite this role, studies performed on this structure with specific neuroanatomical techniques are few. This study focuses on glans clitoris innervation, with special emphasis on sensory corpuscles and the presence of the mechanotransducer protein PIEZO2 in these structures. Six glans clitoris samples were obtained at autopsy covering an age spectrum between 52 and 83 years old. Several types of nerve terminations including free nerve endings, genital endbulbs as well as Meissner‐like corpuscles and Pacinian corpuscles, but not Ruffini corpuscles, were found. Although corpuscular morphology in the glans clitoris was subtly different from the cutaneous digital counterparts, their basic composition was comparable for both Pacinian and Meissner‐like corpuscles. Genital endbulbs showed heterogeneous morphology, and the axons usually exhibited a typical “wool ball” or “yarn ball” aspect. Some of them were lobulated and variably encapsulated by endoneurial elements (65%); from the capsule originate septa that divides the genital endbulbs, suggesting that they are found in clusters rather than as single corpuscles. In addition, most corpuscles in the glans clitoris showed axonal PIEZO2 immunoreactivity, thus, suggesting a mechanical role and molecular mechanisms of mechanosensibility similar to those of digital Meissner's corpuscles. Our results demonstrate that sensory corpuscles of the glans clitoris are similar to those of other glabrous skin zones, as most genital organs are characterized by clusters of corpuscles and the occurrence of the mechanoprotein PIEZO2 in the axons. These findings strongly suggest that PIEZO2 participates in erotic and sexual mechanical sensing.
Keywords: clitoral innervation, clitoris, glabrous skin, PIEZO2, sensory corpuscles
Clitoris is a leading player in female sexuality. Women glans clitoris contains free nerve endings, genital endbulbs, Meissner‐like corpuscles and Pacinian corpuscles, but not Ruffini corpuscles. The basic composition and immunohistochemical profile of glans clitoris corpuscles is superimposable with counterpart digital sensory corpuscles, including the occurrence of an endoneurium‐related capsule y about 65%. Most corpuscles in the glans clitoris showed axonal Piezo2 immunoreactivity, thus, suggesting this mechanoprotein participates in the sexual mechanosensing.
![]()
1. INTRODUCTION
Vertebrate skin contains specialized sensory organs collectively referred to as sensory corpuscles, which are supplied by peripheral processes of primary sensory neurons that encode non‐painful mechanical stimuli (low‐threshold mechanoreceptors, LTMRs; Abraira & Ginty, 2013; Fleming & Luo, 2013; Zimmerman et al., 2014). A group of these structures localized in the erogenous zones are involved in sexual pleasure.
In women, the glans clitoris is generally considered the structure most involved in sexual pleasure, and it is a key element required to reach orgasm. It is a fibrovascular, non‐erectile, densely innervated structure located in the midline that is the only external manifestation of the clitoris (O'Connell et al., 2005; Pauls, 2015; Puppo & Puppo, 2015; Jackson et al., 2019). It is covered by specialized glabrous skin, fine‐tuned for sexual pleasure sensations and reproductive reflexes. This particular skin specialization is associated with different combinations of LTMRs, rendering it neurophysiologically and functionally distinct from the clitoris as a whole as the centre for triggering the orgasmic response (Pauls, 2015). However, it has other reproductive functions. In fact, stimulation of the clitoris activates the brain to instigate changes in the female genital tract which are of major importance in facilitating reproductive success (Levin, 2020).
The macroscopic innervation of the glans clitoris occurs primarily via the dorsal nerve of the clitoris, a branch of the pudendal nerve, with contributions from the cutaneous branch of the ilioinguinal nerve, the genital branch of the genitofemoral nerve and the perineal branch of the posterior femoral cutaneous nerve (Pauls, 2015; Yeung & Pauls, 2016). Microscopic innervation of the glans clitoris was analysed by classical neuroanatomists for more than 100 years using silver impregnation methods, and different morphotypes of sensory corpuscles were described. All these studies were compiled by Seto (1963) in his classical book “Studies on the sensory innervation (human sensibility).” He affirms that the adult human clitoris contains abundant “genital nerve bodies” divided into three types, as well as Pacinian corpuscles, adjacent to branched nerve endings. More recently, immunohistochemical techniques identifying specific markers for axonal and Schwann‐related cells were used (Vega et al., 2009). Shih et al. (2013), using immunohistochemistry for the axon and glial cells, identified some Pacinian corpuscles and abundance of corpuscular receptors (genital endbulbs) within the glans clitoris, variably arranged in the subepithelial tissues. In the monograph “Anatomic study of the clitoris and the bulbo‐clitoral organ,” Di Marino and Lepidi (2014) report that the human glans clitoris contains standard tactile corpuscles including Meissner's, Ruffini's and Pacinian corpuscles, as well as corpuscles specialized to the clitoris such as Krause corpuscles. Meissner‐like corpuscles and genital endbulbs have been also described in the labia minora (Feito et al., 2018).
Since the glans clitoris is covered by thin glabrous skin, it may be assumed that the sensory nerve endings share the structure and immunohistochemical properties of digital ones. The periaxonic cells that form sensory corpuscles are continuous with the cells of nerve trunks, except for the perineurium which can be either present (Pacinian corpuscles) or absent (Meissner's corpuscles; Vega et al., 2009; Feito et al., 2016; García‐Piqueras et al., 2017, 2020). Thus, the first goal of this study was to analyse whether the sensory corpuscles present in the glans clitoris share basic immunohistochemical characteristics with the digital ones, including a capsule of endoneurial and/or perineurial filiation.
In sensory corpuscles, conversion of mechanical stimuli into electrical signals involves mechanogated ion channels. Recent studies have shown that PIEZO2 is required for mechanotransduction in mammalian cells (Coste et al., 2010) and is expressed in LTMRs of mammalian dorsal root ganglia (Ranade et al., 2014; Honoré et al., 2015). PIEZO2 is also present in Merkel discs and isolated Merkel cells, as well as in Meissner's corpuscles (Ikeda et al., 2014; Maksimovic et al., 2014; Ranade et al., 2014; Woo et al., 2014; García‐Mesa et al., 2017; García‐Piqueras et al., 2019). As far as we know, PIEZO2 expression in genital sensory corpuscles has not been reported, despite the fact that genital tactile stimulation is a critical component of sexual arousal. Furthermore, there is general agreement that it is possible to have an orgasm through direct simulation of the glans clitoris (Jannini et al., 2012). Therefore, the second aim of this study was to analyse whether PIEZO2 is the mechanotransducer present in these sensory structures.
2. METHODS
2.1. Tissue processing
Six glans clitoris from un‐embalmed female cadavers were obtained from our laboratory collection (Registro Nacional de Biobancos, Sección colecciones, Ref. C‐0001627), and the study was approved by the Ethical Committee for Biomedical Research of the Principality of Asturias, Spain (Cod. CElm, PAst: Proyecto 266/18). All these materials were obtained in compliance with Spanish law (RD 1301/2006; Ley 14/2007; DR 1716/2011; Orden ECC 1414/2013). The age range was 52–83 years. These materials were routinely embedded in paraffin and cut into serial sections 10 µm thick.
2.2. Single immunohistochemistry
Deparaffinized and rehydrated sections were processed for indirect immunohistochemistry using Leica Bond™ Polymer Refine Detection Kit (Leica Biosystems™, Newcastle, UK) following the manufacturer's instructions. Because of the continuity between the axon and periaxonic cells of the sensory corpuscles and the cells of the nerve trunks, we used immunohistochemistry to examine the expression of axonal (neuron‐specific enolase: NSE), Schwann‐related cell (S100 protein: S100P), endoneurial (CD34 antigen) or perineurial (Glucose transporter 1: Glut1) markers in the glans clitoris. Furthermore, we used an antibody against PIEZO2 to detect this mechanoprotein. Table 1 summarizes the primary antibodies used in the study to examine the various corpuscular constituents. Indirect immunohistochemistry included several negative and positive controls as well as internal positive and negative controls.
Table 1.
Primary antibodies used in this study
| Antigen | Origin | Dilution | Supplier |
|---|---|---|---|
| CD34 (clone QB‐END/10) | Mouse | Prediluted | Master Diagnostica a |
| Glut1 | Rabbit | 0.5 µg/ml | Cell Marque b |
| NSE (clone BBS/NC/VI‐H14) | Mouse | 1:1000 | Dako c |
| NFP (clone 2F11) | Mouse | 1:100 | Dako c |
| PIEZO2 f | Rabbit | 1:200 | Sigma‐Aldrich d |
| S100 protein (clone 4C4.9) | Mouse | 1:1000 | ThermoFisher Scientific e |
| S100 protein | Rabbit | 1:1000 | Dako c |
Abbreviations: Glut1, glucose transporter 1; NFP, neurofilament protein; NSE, neuron‐specific enolase.
Granada, Spain
Seattle, WA, USA
Glostrup, Denmark
Saint Louis, MS, USA
Freemont, CA, USA
Amino acid sequence recognized: FEDENKAAVRIMAGDNVEICMNLDAASFSQHNP.
2.3. Double immunofluorescence
In deparaffinized and rehydrated sections, non‐specific binding was reduced (incubation for 30 minutes with a solution of 5% bovine serum albumin in Tris‐buffered saline (TBS), pH 7.4). Sections were then incubated overnight at 4°C in a humid chamber with a 1:1 (v/v) mixture of anti‐S100P and anti‐NSE; anti‐S100P and anti‐CD34; anti‐S100P and anti‐Glut1; anti‐S100P and anti‐PIEZO2; or anti‐NSE and anti‐PIEZO2. The dilutions of the antibodies for double immunofluorescence were as in Table 1. After rinsing, sections were incubated for 1 hour with Alexa Fluor 488‐conjugated goat anti‐rabbit IgG (Serotec™, Oxford, UK, diluted 1:1000), rinsed again and incubated for another hour with a Cy3‐conjugated donkey anti‐mouse antibody (Jackson‐ImmunoResearch™, Baltimore, MD, USA, diluted 1:50). Both steps were performed at 20°C room temperature in a dark, humid chamber. Thereafter, sections were washed and mounted with Fluoromount Gold (ThermoFisher, Runcoen, UK), and finally, sections were counterstained with DAPI (4′,6‐diamidino‐2‐phenylindole; 10 ng/ml) to label nuclei. Triple staining was detected using a Leica DMR‐XA automatic fluorescence microscope coupled with Leica Confocal Software, version 2.5 (Leica Microsystems, Heidelberg GmbH, Germany), and captured images were processed using the software ImageJ, version 1.43 g at the Master Biophotonics Facility, McMaster University, Ontario, Canada (www.macbiophotonics.ca). As controls, representative sections were processed in the same way as described above, using non‐immune rabbit or mouse sera instead of primary antibodies or while omitting primary antibodies during incubation.
2.4. Quantitative analyses
Ten sections of glans clitoris samples, 10 µm thick, 100 µm apart, were processed for S100P + CD34 immunohistochemistry and used to identify sensory corpuscles. The sections were scanned by an SCN400F scanner (Leica Biosystems), and the scans were computerized using SlidePath Gateway LAN software (Leica Biosystems™). Then, in five randomly selected fields of 400 µm2 each per section (Figure S1a,b), the number of sensory corpuscles were counted by two independent observers (YG‐M and JAV). Values are expressed as the mean of sensory corpuscles by mm2. Due to the low number of sampled corpuscles, no statistical analysis was carried out.
3. RESULTS
The glans clitoris is richly innervated by nerve fibres of different diameters that form a network apparently denser at the periphery than in the central part of the organ. These fibres form perivascular plexuses, terminate as free nerve endings (especially in dermal papillae) and form different morphotypes of sensory corpuscles (Figure 1).
Figure 1.
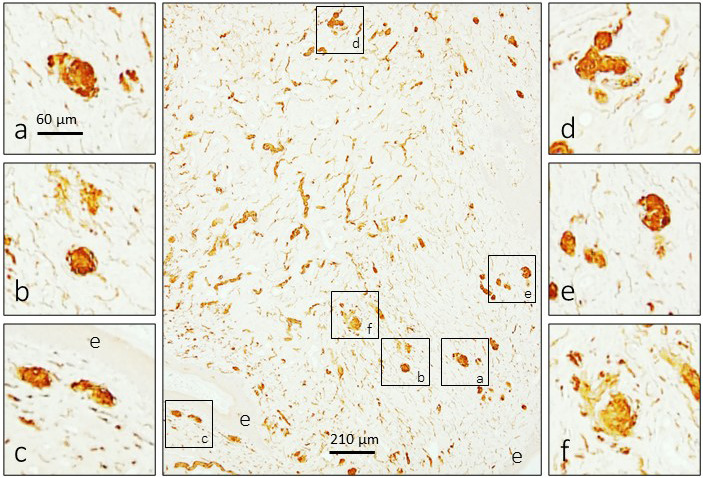
Immunohistochemical detection of S100 protein in the human glans clitoris. The glans clitoris contains a dense network of nerve fibres, the ends of which can form different morphotypes of sensory corpuscles localized subepithelially but also in the central part of the organ. e: epithelium
3.1. Morphotypes of sensory corpuscles
Sensory corpuscles in the glans clitoris were abundant and of variable morphology and size. They consisted of a very tightly coiled axon (with a “wool ball” or “yarn ball” appearance), with remarkable differences in the diameter of axonal branches, embedded in densely packed Schwann‐like cells.
Sensory corpuscles localized immediately beneath the epithelium were smaller than deeper ones and showed an irregular and fragmented aspect (Figure 2a–c). Despite their localization, axon morphology (Figure 3a,b) and arrangement of Schwann‐related cells differed from those of typical cutaneous Meissner corpuscles. Sensory corpuscles localized in deeper subepithelial tissues (Figures 2d–i and 3c–f), as well as those located in the inner part of the glans clitoris, showed the typical morphology of the so‐called genital endbulbs. The size of these bodies was very variable, but there was a tendency to increase in size from surface to deeper regions of the skin (see Figure 2).
Figure 2.
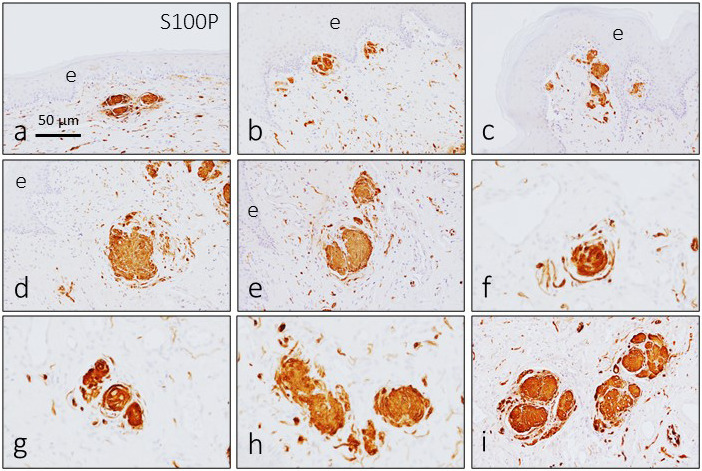
Immunohistochemical detection of S100 protein in the sensory corpuscles of the human glans clitoris. The Schwann‐related cells of glans clitoris sensory corpuscles display a strong immunoreactivity for S100 protein. Sensory corpuscle morphology was variable, and the size increased from the subepithelial zone (a–c) to the intermediate zone (d, e) and deep zone (f–i). Some of the sensory corpuscles were lobulated. e, epithelium
Figure 3.
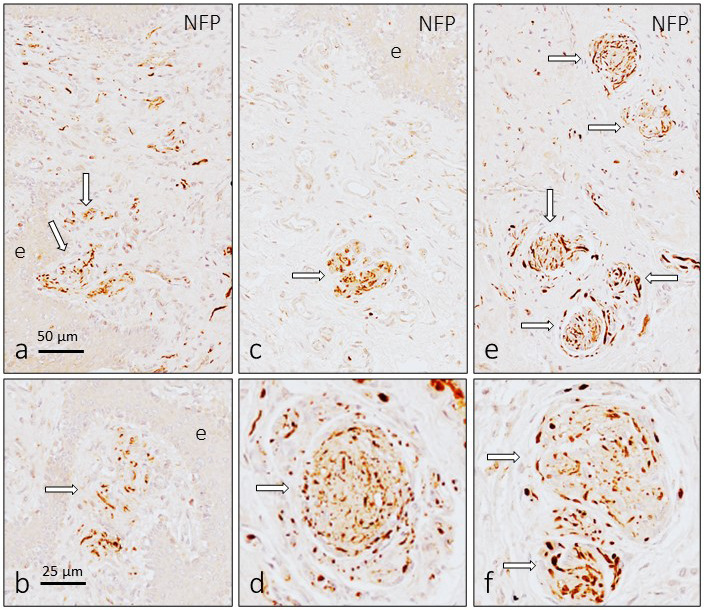
Immunohistochemical detection of neurofilament protein and neuron‐specific enolase in the sensory corpuscles of the human glans clitoris. The arrangement of the axons in the dermal papillae was similar to that of cutaneous Meissner corpuscles (a and b), whereas in those placed in deep subepithelial tissues and in the central part of the organ (c–f), the axon was very tightly coiled and look like a “wool ball” or “yarn ball” with remarkable differences in the diameter of the axonal profiles
The intricate relationship between axons and Schwann‐related cells, independently of corpuscular location and size and leading to a complex arrangement of both structures, is depicted in Figures 4 and 5.
Figure 4.
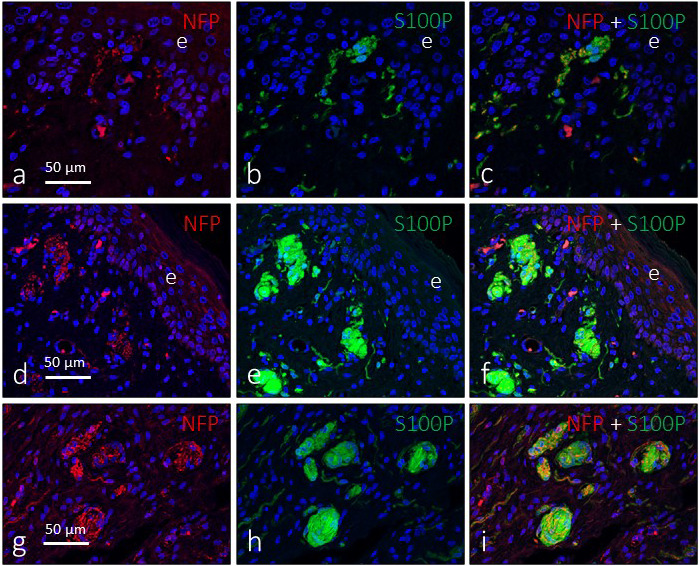
Double immunofluorescence for S100 protein (green fluorescence) and neurofilament protein (red fluorescence) in subepithelial (a–f) and intermediate (g–i) sensory corpuscles in the glans clitoris. Sections were counterstained with DAPI to ascertain structural details. Objective 63×/1.40 oil; pinhole 1.37; XY resolution 139.4 nm and Z resolution 235.8 nm. e, epithelium
Figure 5.
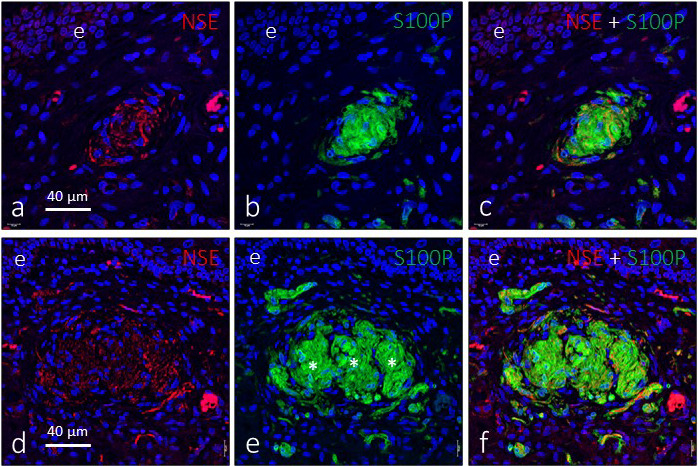
Double immunofluorescence for S100 protein (green fluorescence) and neuron‐specific enolase (red fluorescence) in genital endbulbs of the deep zone of the glans clitoris. Some corpuscles seem to be lobulated (asterisks in e), and typically the axons are arranged in a “wool ball” or “yarn ball” shape with remarkable differences in the diameter of the axonal profiles. Sections were counterstained with DAPI to ascertain structural details. Objective 63×/1.40 oil; pinhole 1.37; XY resolution 139.4 nm and Z resolution 235.8 nm
One characteristic aspect of genital endbulbs was the apparent lobulation observed in about 60% of them. We performed immunohistochemistry for CD34 (an endoneurial marker) and Glut1 (a perineurial marker) to investigate whether this lobulation is real or not. Approximately 65% of the lobulated genital endbulbs presented endoneurial CD34‐positive septa dividing the corpuscles (Figure 6a–c); the remaining (approximately 35%) lacked an endoneurial capsule, and CD34 positivity was restricted to the endothelial cells of the capillaries that encircled the corpuscle (Figure 6d–f). In the dermis immediately below the epidermis, both capsulated and non‐capsulated genital endbulbs were observed (Figure 6g–i). Immunoreactivity for Glut1 was generally absent covering genital endbulbs, although it was positive in the perineurium of nerve trunks and the capsule of Pacinian corpuscles (data not shown).
Figure 6.
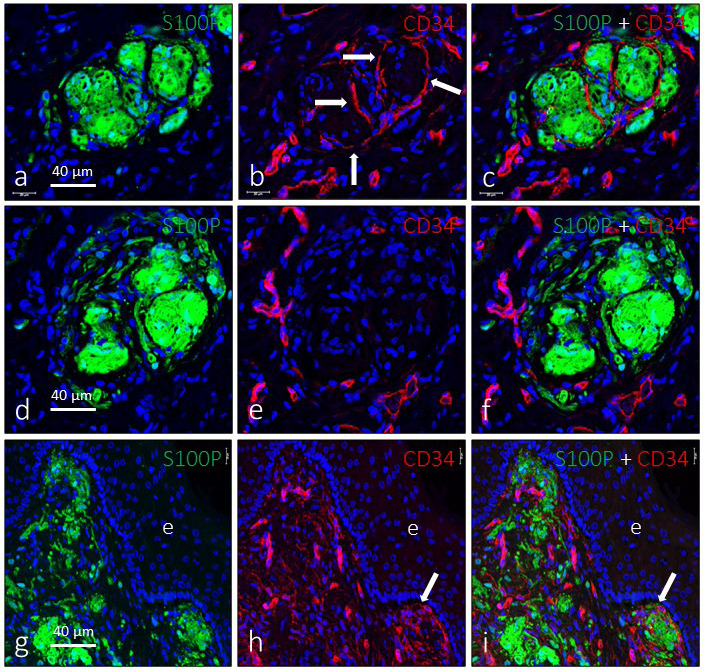
Evidence of lobulation of the genital endbulbs. Dual immunofluorescence for S100 protein (green fluorescence) and CD34 (red fluorescence) in deep (a–f) and subepithelial (g–i) sensory corpuscles. In some corpuscles (a–c), a well‐defined lobulation indicated by the presence of CD34‐positive septa was observed (arrows in b), whereas in others no evidence of capsulation or internal lobulation was observed (d–f). Subepithelial corpuscles were seen with either capsulation or no capsulation (g–i). Sections were counterstained with DAPI to ascertain structural details. Objective 63×/1.40 oil; pinhole 1.37; XY resolution 139.4 nm and Z resolution 235.8 nm
In addition to Meissner‐like corpuscles and genital endbulbs, well‐differentiated Pacinian corpuscles were observed in the central part of the glans clitoris (Figure 7). They showed an irregular morphology, but all their components were present (axon, inner‐core Schwann‐related cells, CD34‐positive intermediate layer and well‐developed capsule) with their typical immunohistochemical profile (Figure 7), including Glut1 immunoreactivity in the capsule.
Figure 7.
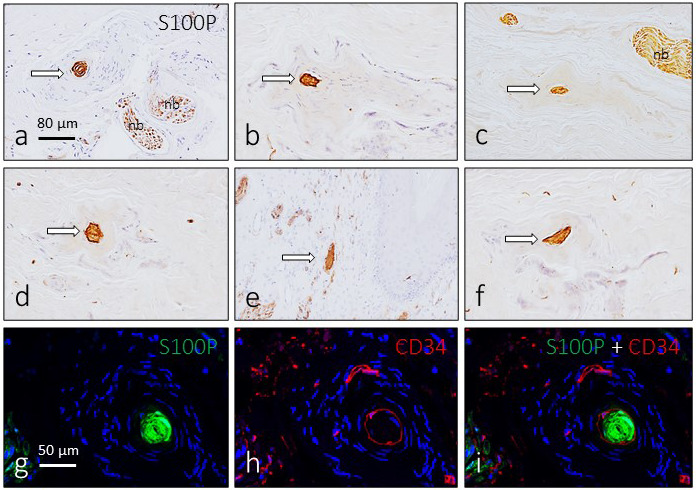
Pacinian corpuscles in the glans clitoris. In the central part of the organ, Pacinian corpuscles were regularly found. They showed an irregular morphology, but the axon, inner‐core cells and capsule had a typical arrangement. Arrows in a–f indicate the inner core which was intensely immunoreactive for S100 protein. These corpuscles also displayed CD34 immunofluorescence in the intermediate layer (g–i). Sections g–i were counterstained with DAPI to ascertain structural details. Objective 63×/1.40 oil; pinhole 1.37; XY resolution 139.4 nm and Z resolution 235.8 nm. nb: nerve bundle
3.2. Density of sensory corpuscles
The estimated density of sensory corpuscles in sections immunostained for S100P was 4.37 ± 0.7 mm2, and there was a trend in the density of sensory corpuscles that suggested an age‐dependent decline. In fact, in sections from individuals aged 52–60 years, the mean values were 5.22 ± 0.4 mm2, whereas in the sections from subjects aged 61–83 the mean values were 4.16 ± 0.8 mm2 (Figure S1a,b). Nevertheless, these values must be taken with caution since numbers were obtained from sections immunostained for S100P. Furthermore, when a lobulated corpuscular structure is seen in sections processed for simultaneous detection of S100P and CD34, if each lobule is considered to be an independent corpuscle, the mean values reach 13.9 ± 1.3 mm2 (Figure S1c–e).
3.3. Sensory corpuscles of the glans clitoris contain PIEZO2
It is generally accepted that activation of mechanically gated ion channels, especially PIEZO2, is at the origin of the detection of low‐ or high‐threshold mechanical stimuli (Ranade et al., 2014). The presence of PIEZO2 in the glans clitoris was investigated using simple and double immunohistochemistry. PIEZO2 was detected in scattered cells localized in cells of the basal layer of the superficial epithelium, which were identified as Merkel cells based on their localization and morphology (Figure 8a,b). Furthermore, PIEZO2 immunoreactivity was observed in all subepithelial sensory corpuscles, but especially in genital endbulbs (Figure 8). The pattern of distribution of PIEZO2 within these structures resembles “wool balls” or “yarn balls” (Figure 8c–e) and, as mentioned previously for conventional neuronal markers, the axonal profiles show different calibres. To confirm the axonal localization of PIEZO2, we performed double immunofluorescence with Schwann‐like and axonal markers. PIEZO2 never co‐localized with S100P (Figure 8f–h) and partially co‐localized with neuron‐specific enolase, especially in the larger axonal profiles (Figure 8i–k).
Figure 8.
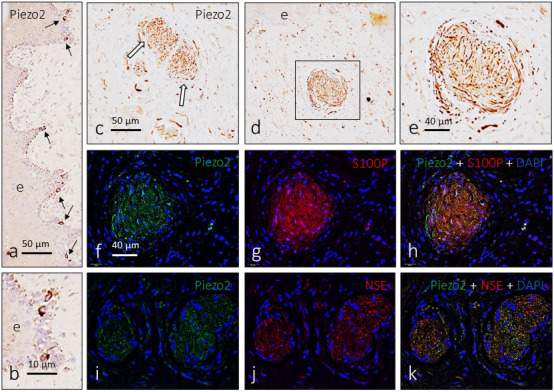
Immunodetection of PIEZO2 in the glans clitoris. PIEZO2 immunoreactivity is detected in cells of the epithelium basal layer (a and b) that were identified as Merkel cells (arrows in a). In the genital endbulbs, PIEZO2 shows a typical “wool ball” or “yarn ball” axonal pattern of distribution (c–e; arrows in c). Immunofluorescence confirmed that PIEZO2 is localized in the axon (i–k) and is absent from the Schwann‐related cells (f–h)
4. DISCUSSION
The skin is a multisensory organ that receives profuse sensory innervation from primary sensory neurons placed in the peripheral sensory ganglia (McGlone & Reilly, 2010; McGlone et al., 2014; Owens & Lumpkin, 2014). Cutaneous afferents can be distinguished anatomically based on their sensory terminals, or sensory corpuscles, and can be functionally classified based on the conduction speed of their action potentials (Rice & Albrecht, 2008; Gardner & Johnson, 2013).
The glans clitoris is covered by glabrous skin, which according to our results contains three main morphotypes of sensory corpuscles: Meissner‐like corpuscles, genital endbulbs and Pacinian corpuscles. These data are in good agreement with previous reports (Seto, 1963; Shih et al., 2013; Di Marino & Lepidi, 2014), especially with those form Shih et al. (2013). Conversely, we did not find Ruffini's or Krause corpuscles as reported by Di Marino and Lepidi (2014). Presumably, Meissner's and Pacinian corpuscles of the glans clitoris could be compared to their cutaneous counterparts and meet similar functions. Pauls (2015) affirms that the specialized glabrous skin of the glans clitoris contains combinations of LTMRs that make it functionally distinct, which ultimately determine the pleasure sensibility of the orgasm. In the words of Zimmerman et al. (2014) “Like individual instruments in an orchestra, each LTMR subtype conveys a specific feature of the forces acting on the skin, collectively culminating in a musical symphony of neural impulses that the brain translates as a touch.” However, the sexual response to sexually arousing stimuli is not only mechanical but also motivates an incentive‐based cycle comprising subjective experience and physiologic change (Basson, 2015).
Interestingly, although additional cases should be analysed to confirm our findings, we have found a trend in the density of sensory corpuscles that suggest a reduction with ageing, as occurs in the digital glabrous skin (García‐Piqueras et al., 2019). Nevertheless, we cannot affirm if all morphotypes are affected equally. These preliminary data seem oppose with studies that suggest that the clitoral sexual response and the female orgasm are not affected by ageing (Puppo, 2013); difficulty with orgasm in older women is often associated with a partner's erectile dysfunction (Granville and Pregler, 2018) rather than with age‐related decline in orgasm. Additional studies enabling more precise clinical‐histological correlation would likely be required to resolve this inconsistency.
The pattern of distribution of investigated antigens in the glans clitoris sensory corpuscles was identical to that in cutaneous ones. Axons were intensely immunoreactive for both NSE and neurofilament, and Schwann‐related cells displayed strong S100P immunoreactivity. The arrangement of the axon in a “wool ball” or “yarn ball” pattern is consistent with the pictures reported in classical studies using silver impregnation or immunohistochemical techniques (Seto, 1963; Shih et al., 2013; Di Marino and Lepidi, 2014). However, we added a new data regarding the structure of clitoral corpuscles: the occurrence of a capsule on endoneurial origin encircling the axon and the glial cells in 65% of genital endbulbs. Recently, we demonstrated that cutaneous Pacinian corpuscles (García‐Piqueras et al., 2017) as well as most Meissner's corpuscles (García‐Piqueras et al., 2020) have a CD34‐positive intermediate layer and a capsule of endoneurial origin; corpuscular perineurial Glut1‐positive derivatives are only present in Pacinian corpuscles (Feito et al., 2016). Endoneurial capsulation in glans clitoris sensory corpuscles was very heterogeneous: there were both capsulated and non‐capsulated structures, and, interestingly, in up to 65% of lobulated genital endbulbs there were internal septa dividing clusters of Schwann‐related cells and balls of axons. Thus, this raised the question of whether the lobulated genital endbulbs are single sensory corpuscles or clusters of sensory corpuscles. Although one can assume that a single‐axon branches and forms different “wool balls” based on these results alone, this concept should be revisited using 3D reconstructions from laser confocal records.
In LTMRs and the associated sensory corpuscles, mechanotransduction (i.e. conversion of mechanical stimuli into electrical signals) involves some potential mechanogated ion channels, which have been detected in both axon and Schwann‐related cells (Calavia et al., 2010; Cabo et al., 2015; Alonso‐González et al., 2017), and PIEZO2 has emerged as the most likely candidate responsible for sensory mechanotransduction (Ranade et al., 2014; García‐Mesa et al., 2017; García‐Piqueras et al., 2019). We have observed that the axon of glans clitoris sensory corpuscles displays PIEZO2 immunoreactivity similar to Meissner's corpuscles in human digital skin (García‐Mesa et al., 2017; García‐Piqueras et al., 2019). To our knowledge, the presence of mechanoproteins, and in particular PIEZO2, in the sensory corpuscles of genital organs is reported here for the first time. These findings are of interest since mechanical stimulation of the clitoris is essential for sexual arousal and orgasmic response (Jannini et al., 2012; Pauls, 2015; Levin, 2020), and PIEZO2 is critical for mechanotransduction (Wu et al., 2017). Nevertheless, it is important to keep in mind that women may experience other kind of orgasms in addition to the clitoral external orgasm (Jannini et al., 2012). Beside sexual pleasure, clitoral mechanical stimulation may also represent a treatment method for female sexual dysfunction (Billups, 2002) or female stress urinary incontinence (Sønksen et al., 2007).
CONFLICTS OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
YG‐M, LC and JM‐C performed the experiments. JF and OG‐S collected the material in compliance with ethical guidelines and performed part of the experiments. RC, and JG‐P quantified the data. JF and JAV designed the study, analysed the data and wrote the manuscript.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported in part by a grant from Gerencia Regional de Salud de Castilla y León to JF and JAV (GRS 1882/A/18). Y G‐M was supported by a Severo Ochoa grant from the Govern of the Principality of Asturias (Ref. BP17‐044). The authors thank Dr. Marta Guervos (Servicios Comunes de Investigación, Microscopia Confocal, Universidad de Oviedo) and Marta Sánchez‐Pitiot (Grupo de Histopatología Molecular, Instituto Universitario de Oncología del Principado de Asturias) for technical assistance.
García‐Mesa Y, Cárcaba L, Coronado C, et al. Glans clitoris innervation: PIEZO2 and sexual mechanosensitivity. J. Anat.2021;238:446–454. 10.1111/joa.13317
García‐Mesa and Cárcaba contributed equally to this study.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Abraira, V.E. & Ginty, D.D. (2013) The sensory neurons of touch. Neuron, 79, 618–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso‐González, P. , Cabo, R. , San José, I. , Gago, A. , Suazo, I.C. , García‐Suárez, O. et al. (2017) Human digital meissner corpuscles display immunoreactivity for the multifunctional ion channels Trpc6 and Trpv4. Anatomical Record (Hoboken), 300, 1022–1031. [DOI] [PubMed] [Google Scholar]
- Basson, R. (2015) Human sexual response. Handbook of Clinical Neurology, 130, 11–18. [DOI] [PubMed] [Google Scholar]
- Billups, K.L. (2002) The role of mechanical devices in treating female sexual dysfunction and enhancing the female sexual response. World Journal of Urology, 20, 137–141. [DOI] [PubMed] [Google Scholar]
- Cabo, R. , Alonso, P. , Viña, E. , Vázquez, G. , Gago, A. , Feito, J. et al. (2015) ASIC2 is present in human mechanosensory neurons of the dorsal root ganglia and in mechanoreceptors of the glabrous skin. Histochemistry and Cell Biology, 143, 267–276. [DOI] [PubMed] [Google Scholar]
- Calavia, M.G. , Montaño, J.A. , García‐Suárez, O. , Feito, J. , Guervós, M.A. , Germanà, A. et al. (2010) Differential localization of Acid‐sensing ion channels 1 and 2 in human cutaneous Pacinian corpuscles. Cellular and Molecular Neurobiology, 30, 841–848. [DOI] [PubMed] [Google Scholar]
- Coste, B. , Mathur, J. , Schmidt, M. , Earley, T.J. , Ranade, S. , Petrus, M.J. et al. (2010) Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science, 330, 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marino, V. & Lepidi, H. (2014) Sensory corpuscles Anatomic study of the clitoris and the bulbo‐clitoral organ. Switzerland: Springer International Publishing. [Google Scholar]
- Feito, J. , Cebrián‐Muiños, C. , Alonso‐Morrondo, E.J. , García‐Mesa, Y. , García‐Piqueras, J.. , Cobo, R.. et al. (2018) Hyperplastic sensory corpuscles in nevus sebaceus of labia minora pudendi. A case report. Journal of Cutaneous Pathology, 45, 777–781. [DOI] [PubMed] [Google Scholar]
- Feito, J. , Ramos‐García, J.L. , Gago, Á. , Cobo, J.L. , García‐Suárez, O. , Junquera, L.M. et al. (2016) Pacinian corpuscles in a cervical chondrocutaneous remnant: A case report and update of Pacinian corpuscles. American Journal of Dermatopathology, 38, 231–235. [DOI] [PubMed] [Google Scholar]
- Fleming, M.S. & Luo, W. (2013) The anatomy, function, and development of mammalian Aβ low‐threshold mechanoreceptors. Frontiers in Biology (Beijing), 8, 408–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García‐Mesa, Y. , García‐Piqueras, J. , García, B. , Feito, J. , Cabo, R. , Cobo, J. et al. (2017) Merkel cells and Meissner's corpuscles in human digital skin display Piezo2 immunoreactivity. Journal of Anatomy, 231, 978–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García‐Piqueras, J. , Cobo, R. , Cárcaba, L. , García‐Mesa, Y. , Feito, J. , Cobo, J. et al. (2020) The capsule of human Meissner corpuscles: Immunohistochemical evidence. Journal of Anatomy, 236, 854–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García‐Piqueras, J. , García‐Mesa, Y. , Cárcaba, L. , Feito, J. , Torres‐Parejo, I. , Martín‐Biedma, B. et al. (2019) Ageing of the somatosensory system at the periphery: Age‐related changes in cutaneous mechanoreceptors. Journal of Anatomy, 234, 839–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García‐Piqueras, J. , García‐Suárez, O. , Rodríguez‐González, M.C. , Cobo, J.L. , Cabo, R. , Vega, J.A. et al. (2017) Endoneurial‐CD34 positive cells define an intermediate layer in human digital Pacinian corpuscles. Annals of Anatomy ‐ Anatomischer Anzeiger, 211, 55–60. [DOI] [PubMed] [Google Scholar]
- Gardner, E.P. & Johnson, K.O. (2013) Touch In: Kandel E.R., Schwartz J.H., Jessell T.M., Siegelbaum S.A. and Hudspeth A.J. (Eds.) Principles of neural science. New York, USA: McGraw‐Hill, pp. 498–529. [Google Scholar]
- Granville, L. & Pregler, J. (2018) Women's sexual health and aging. Journal of the American Geriatrics Society, 66, 595–601. [DOI] [PubMed] [Google Scholar]
- Honoré, E. , Martins, J.R. , Penton, D. , Patel, A. , Demolombe, S. et al. (2015) The piezo mechanosensitive ion channels: May the force be with you! Reviews of Physiology Biochemistry and Pharmacology, 169, 25–41. [DOI] [PubMed] [Google Scholar]
- Ikeda, R. , Cha, M. , Ling, J. , Jia, Z. , Coyle, D. , Gu, J.G. et al. (2014) Merkel cells transduce and encode tactile stimuli to drive Aβ‐afferent impulses. Cell, 157, 664–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson, L.A. , Hare, A.M. , Carrick, K.S. , Ramirez, D.M.O. , Hamner, J.J. , Corton, M.M. et al. (2019) Anatomy, histology, and nerve density of clitoris and associated structures: Clinical applications to vulvar surgery. American Journal of Obstetrics and Gynecology, 221, 519.e1–519.e9. [DOI] [PubMed] [Google Scholar]
- Jannini, E.A. , Rubio‐Casillas, A. , Whipple, B. , Buisson, O. , Komisaruk, B.R. , Brody, S. et al. (2012) Female orgasm(s): One, two, several. The Journal of Sexual Medicine, 9, 956–965. [DOI] [PubMed] [Google Scholar]
- Levin, R.J. (2020) The clitoris – An appraisal of its reproductive function during the fertile years: Why was it, and still is, overlooked in accounts of female sexual arousal. Clinical Anatomy, 33, 136–145. [DOI] [PubMed] [Google Scholar]
- Maksimovic, S. , Nakatani, M. , Baba, Y. , Nelson, A.M. , Marshall, K.L. , Wellnitz, S.A. et al. (2014) Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature, 509, 617–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlone, F. & Reilly, D. (2010) The cutaneous sensory system. Neuroscience and Biobehavioral Reviews, 34, 148–159. [DOI] [PubMed] [Google Scholar]
- McGlone, F. , Wessberg, J. & Olausson, H. (2014) Discriminative and affective touch: Sensing and feeling. Neuron, 82, 737–755. [DOI] [PubMed] [Google Scholar]
- O'Connell, H.E. , Sanjeevan, K.V. & Hutson, J.M. (2005) Anatomy of the clitoris. Journal of Urology, 174, 1189–1195. [DOI] [PubMed] [Google Scholar]
- Owens, D.M. & Lumpkin, E.A. (2014) Diversification and specialization of touch receptors in skin. Cold Spring Harbor Perspectives in Medicine, 4, a013656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauls, R.N. (2015) Anatomy of the clitoris and the female sexual response. Clinical Anatomy, 28, 376–384. [DOI] [PubMed] [Google Scholar]
- Puppo, V. (2013) Anatomy and physiology of the clitoris, vestibular bulbs, and labia minora with a review of the female orgasm and the prevention of female sexual dysfunction. Clinical Anatomy, 26, 134–152. [DOI] [PubMed] [Google Scholar]
- Puppo, V. & Puppo, G. (2015) Anatomy of sex: Revision of the new anatomical terms used for the clitoris and the female orgasm by sexologists. Clinical Anatomy, 28, 293–304. [DOI] [PubMed] [Google Scholar]
- Ranade, S.S. , Woo, S.H. , Dubin, A.E. , Moshourab, R.A. , Wetzel, C. , Petrus, M. et al. (2014) Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature, 516, 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice, F.L. & Albrecht, P.J. (2008) Cutaneous mechanisms of tactile perception: Morphological and chemical organization of the innervation to the skin In: Smith D., Firestein S. and Beauchamp G. (Eds.) The senses: A comprehensive reference. Volume 6: Somatostatin. San Diego, CA: Academic. [Google Scholar]
- Seto, H. (1963) Studies on the sensory innervation (human sensibility), 2nd edition. Tokio, Japan: Igaku Shoin Ltd. [Google Scholar]
- Shih, C. , Cold, C.J. & Yang, C.C. (2013) Cutaneous corpuscular receptors of the human glans clitoris: descriptive characteristics and comparison with the glans penis. The Journal of Sexual Medicine, 10, 1783–1789. [DOI] [PubMed] [Google Scholar]
- Sønksen, J. , Ohl, D.A. , Bonde, B. , Laessøe, L. , McGuire, E.J. et al. (2007) Transcutaneous mechanical nerve stimulation using perineal vibration: A novel method for the treatment of female stress urinary incontinence. Journal of Urology, 178, 2025–2028. [DOI] [PubMed] [Google Scholar]
- Vega, J.A. , García‐Suárez, O. , Montaño, J.A. , Pardo, B. , Cobo, J.M. et al. (2009) The Meissner and Pacinian sensory corpuscles revisited new data from the last decade. Microscopy Research and Technique, 72, 299–309. [DOI] [PubMed] [Google Scholar]
- Woo, S.H. , Ranade, S. , Weyer, A.D. , Dubin, A.E. , Baba, Y. , Qiu, Z. et al. (2014) Piezo2 is required for Merkel‐cell mechanotransduction. Nature, 509, 622–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, J. , Lewis, A.H. & Grandl, J. (2017) Touch, tension, and transduction – The function and regulation of piezo ion channels. Trends in Biochemical Sciences, 42, 57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung, J. & Pauls, R.N. (2016) Anatomy of the vulva and the female sexual response. Obstetrics and Gynecology Clinics of North America, 43, 27–44. [DOI] [PubMed] [Google Scholar]
- Zimmerman, A. , Bai, L. & Ginty, D.D. (2014) The gentle touch receptors of mammalian skin. Science, 346, 950–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


