Abstract
The cartilaginous endplate (CEP) is a thin layer of hyaline cartilage, and plays an important role in the diffusion of nutrients into the intervertebral discs. Its damage may seriously affect the disc degeneration, and result in low back pain (LBP). However, the structural features of damaged CEPs have not been well characterized, and this hinders our understanding of the etiology of disc degeneration and pain. To present the structural features of micro‐damaged CEPs in patients with disc degeneration and LBP that might even be regarded as an initial factor for disc degeneration, we performed a histological study of micro‐damaged CEPs harvested from human lumbar intervertebral discs and analyzed its clinical implications. Human lumbar CEPs were excised from 35 patients (mean age 60.91 years) who had disc degeneration and LBP. Control tissue was obtained from 15 patients (mean age 54.67 years) with lumbar vertebral burst fractures. LBP and disability were assessed clinically, and all patients underwent anterior vertebral body fusion surgery. CEPs together with some adjacent nucleus pulposus (NP) were sectioned at 4 µm, and stained using H&E, Safranin O/Fast Green, and Alcian Blue. Immunostaining and PCR were used to identify various markers of degeneration, innervation, and inflammation. Histology demonstrated physical micro‐damage in 14/35 CEPs from the disc degeneration group. Six major types of damage could be distinguished: fissure, traumatic nodes, vascular mimicry, incorporation of NP tissue within the CEP, incorporation of bone within the CEP, and incorporation of NP and bone within the CEP. Pain and disability scores (ODI: p = 0.0190; JOA: p = 0.0205; JOABPEQ: p = 0.0034) were significantly higher in those with micro‐damaged CEPs (N = 14) than in those with non‐damaged CEPs (N = 21). CEP damage was significantly associated with elevated MMP3 (p = 0.043), MMP13 (p = 0.0191), ADAMTS5 (p = 0.0253), TNF‐α (p = 0.0011), and Substance P (p = 0.0028), and with reduced Sox9 (p = 0.0212), aggrecan (p = 0.0127), and type II collagen (p = 0.0139). In conclusion, we presented a new classification of human lumbar micro‐damaged CEPs. Furthermore, we verify disc degeneration, innervation, and discogenic pain in micro‐damaged CEPs.
Keywords: cartilaginous endplate, classification, innervation, low back pain, micro‐damage
1. INTRODUCTION
Severe and chronic low back pain (LBP) is an increasing clinical problem (Chou and Shekelle, 2010), and is commonly caused by intervertebral disc (IVD) degeneration (Deyo and Weinstein, 2001; Zhao et al., 2005). Despite the great socioeconomic impact of disc degeneration, the mechanisms of initiation and progression are still not well understood, and an appropriate disease‐based model is lacking (Vergroesen et al., 2015). A destructive cause and effect circle has been proposed which includes mechanical overloading, catabolic response of disc cells and reduced water binding by the extracellular matrix (ECM). These factors are variously interrelated, and eventually lead to a common degeneration pathway (Adams et al., 2000; Fields et al., 2010; Vergroesen et al., 2015; Wade et al., 2014).
Schemes of disc degeneration classification have included sub‐scores for major sub‐tissues: nucleus pulposus (NP), annulus fibrosus (AF) and cartilaginous endplate (CEP) (Boos et al., 2002; Zehra et al., 2019). The endplate may be most relevant clinically, considering that endplate defects are associated with pathologic disc innervation (Fields et al., 2014), and a history of severe back pain (Lama et al., 2018). Cadaver and histology studies show that tidemark avulsions, CEP ruptures, and bony endplate lesions are the predominant form of endplate irregularity (Berg‐Johansen et al., 2018b; Berg‐Johansen et al., 2018a; Fields et al., 2014; Kakitsubata et al., 2002; Tanaka et al., 1993; Wang et al., 2012), and that fragmentation of the CEP is associated with back pain and sciatica (Lama et al., 2014). However, the structural features of damaged CEPs have not been well characterized, and this hinders our understanding of the etiology of disc degeneration and pain.
In human adults, IVDs are the largest nonvascular organs, so nutrition of the NP and inner AF depends on diffusion of nutrients through the CEP (Giers et al., 2017). However, diffusion into degenerated discs can be reduced by ossification or calcification of the CEP, occlusion of the vertebral endplate marrow channels, or by reduced proteoglycan (PG) content of the disc (Kang et al., 2014). It is reasonable to suppose that micro‐damaged CEPs can lead to nutritional disturbance of the disc, which in turn accelerates disc degeneration.
Damaged CEPs may also influence discogenic pain. Because human intervertebral disc aggrecan (ACAN) has been shown to inhibit nerve growth in vitro (Johnson et al., 2002) and endothelial cell adhesion and cell migration in vitro (Johnson et al., 2005), aggrecan‐rich CEPs/NP could hinder new nerves and vessels from growing through the endplate into the disc (Lama et al., 2018). Also, NP homeostasis and intradiscal pressure depend on an intact AF and CEPs (Adams and Dolan, 2012; Vergroesen et al., 2015; Wang et al., 2011). If either structure is damaged, the NP becomes decompressed (Adams et al., 2000) and exposed to immune cells outside the disc (Cole et al., 2014). Delicate CEPs adjacent the NP are more easily damaged than vertebral endplates, especially in patients with Modic changes (Chen et al., 2014; Tang et al., 2016), so damage to a CEP may be a common initiating factor in disc degeneration, neoinnervation, and pain.
In the present study, we examine and classify micro‐damage in the human lumbar CEPs, and test the hypothesis that such damage is associated with disc degeneration, neoinnervation, and pain.
2. MATERIALS AND METHODS
2.1. Patient groups and tissue collection
Our study was approved by the Ethical Review Board of Sir Run Run Shaw Hospital and informed consent from each patient was obtained. CEP/NP samples were obtained from 35 patients (the “degenerative group”) with chronic LBP who underwent lumbar fusion surgery. Before surgery, these patients were scored for physical disability and LBP using the Oswestry Disability Index (ODI) (Fairbank et al., 1980), the Japanese Orthopedic Association scores (JOA) (Izumida and Inoue, 1986), and Japanese Orthopedic Association Back Pain Evaluation Questionnaire (JOABPEQ) (Yao et al., 2018). Additional specimens were obtained from 15 patients (the “control” group) with lumbar vertebral burst fractures who underwent anterior vertebral body excision and fusion. These patients did not show significant degenerative changes on MR images, or suffer from severe LBP. Clinical information was summarized in Table 1. Samples of CEP were taken from sites adjacent the nucleus pulposus in all discs, and in addition some samples were taken from sites adjacent the inner annulus fibrosus. Dissected samples were either frozen immediately in liquid nitrogen, or fixed in 4% paraformaldehyde for 24 hrs at room temperature and then decalcified in 10% EDTA for 1 month.
Table 1.
Patients’ clinical characteristics
| Characteristic | Control group | Study group |
|---|---|---|
| Number | 15 | 35 |
| Age (year) | 54.67 (26–67) | 60.91 (41–79) |
| Gender (male/female) | 9/6 | 20/15 |
| BMI (kg/m2±SD) | 23.09 ± 2.69 | 24.31 ± 3.41 |
2.2. Histology and immunohistochemistry
Decalcified CEP/NP tissues were dehydrated with a graded series of ethanol, embedded in paraffin, and then sliced into 4 μm sections as described previously (Tang et al., 2016). Sections were subsequently stained with hematoxylin & eosin (to evaluate morphology and micro‐damage), and either Safranin O/Fast Green or Alcian Blue (to evaluate matrix degeneration). Sections were immunostained to detect the matrix metalloproteinase‐13 (MMP13) (ab75606, Abcam), type II collagen (Col II) (ab34712, Abcam), Substance P (GB11412, Servicebio), TNF‐alpha (ab212899, Abcam), and protein gene product‐9.5 (PGP9.5) (EM1701‐86, Huabio), using an SP Rabbit & Mouse HRP Kit (DAB) (CW2069, CWBIO, China). Antibodies (IgG) against MMP13, Col II, Substance P, TNF‐alpha, and PGP9.5 were diluted at 1:100–1:200. Three pathologists who were blind to the clinical data assessed the CEP/NP tissues for cell density, and for the proportion of cells positive for each antibody. Three sections of each specimen (n = 3) were examined under high magnification (400×), and sections were recounted if the intraclass correlation coefficient was below 0.8.
2.3. RNA isolation and RT‐PCR, quantitative RT‐PCR
CEP/NP tissues were soaked in liquid nitrogen, finely powdered using a mortar and pestle, and then lysed in TRIzol (Invitrogen, Carlsbad, CA, USA), as described previously (Huang et al., 2019). Briefly, total RNA was extracted using Ultrapure RNA Kit (CW0581, CWBIO, China) and quantified using Nanodrop 2000. RNA was reverse transcribed using RT‐PCR, and complementary DNA was synthesized using PrimeScript RT MasterMix (Takara Bio, Otsu, Japan), according to the manufacturer's protocols. Then gene transcriptional levels (n = 3) were quantified by RT‐qPCR using the SYBR Green qPCR Master Mix (Takara Bio, Otsu, Japan), according to the manufacturer's protocols. Experimental reaction was conducted at 95°C for 10 minutes (pre‐incubation), 95°C for 15 seconds, 60°C for 60 seconds for 40 cycles (amplification), 95°C for 15 seconds, 60°C for 60 seconds (melting curves) and 40°C for 5 minutes (cooling). Whole qRT‐PCR reactions were performed in duplicate, and amplification signals were normalized relative to the succinate dehydrogenase complex subunit A (SDHA) signal in the same reaction. Primer sequences were summarized in Table 2. Relative mRNA levels were calculated as x = 2−∆∆Ct, in which ∆∆Ct = ∆Ct E‐∆Ct Ctrl, ∆Ct E = Ct exp‐Ct SDHA, and ∆Ct Ctrl = Ct Ctrl‐Ct SDHA.
Table 2.
Primer sequences
| Gene | Stream | Sequence |
|---|---|---|
| Sox9 | Forward | 5′‐GGAATGTTTCAGCAGCCAAT−3′ |
| Reverse | 5′‐TGGTGTTCTGAGAGGCACAG−3′ | |
| Col2a1 | Forward | 5′‐CTGGAAAAGCTGGTGAAAGG−3′ |
| Reverse | 5′‐GGCCTGGATAACCTCTGTGA−3′ | |
| Aggrecan | Forward | 5′‐CTTCCGCTGGTCAGATGGAC−3′ |
| Reverse | 5′‐CGTTTGTAGGTGGTGGCTGT−3′ | |
| MMP13 | Forward | 5′‐ACTGAGAGGCTCCGAGAAATG−3′ |
| Reverse | 5′‐GAACCCCGCATCTTGGCTT−3′ | |
| MMP3 | Forward | 5′‐CTGGAATGGTCTTGGCTCAT−3′ |
| Reverse | 5′‐CTGACTGCATCGAAGGACAA−3′ | |
| ADAMTS5 | Forward | 5′‐TACTTGGCCTCTCCCATGAC−3′ |
| Reverse | 5′‐TTTGGACCAGGGCTTAGATG−3′ | |
| SDHA | Forward | 5′‐AGACCTAAAGCACCTGAAGACG−3′ |
| Reverse | 5′‐ATCAATCCGCACCTTGTAGTCT−3′ |
2.4. Statistical analysis
Data analyses were performed using SPSS 16.0 (SPSS, Chicago, IL, USA). Differences in gene expression levels, and in the percentage of immunopositive cells, were assessed using Student's t‐test (comparing two groups) or one‐way ANOVA followed by Tukey's post‐hoc test (comparing >two groups). All values were expressed as the mean and standard deviation. ‘N’ stands for the number of individual donors, while ‘n’ stands for the number of replicates. p‐values less than 0.05 were considered statistically significant.
3. RESULTS
3.1. Micro‐damage in human CEPs
Histology showed the normal structure of human lumbar CEPs (Figure 1a). In the degenerated group, however, H&E staining showed fibrotic and sclerotic extracellular matrix and fewer round chondrocytes (Figure 1b1–g2). Safranin O/Fast Green staining, and Alcian Blue staining, showed a reduction in the proteoglycan (PG) content of the cartilage matrix in the degenerated specimens. In addition, 14/35 (40%) of the ‘degenerated’ CEPs showed physical micro‐damage, which could be classified into six types: fissure (Figure 1b); vascular mimicry (blood vessel ingrowth after damage) (Figure 1c); NP herniation into the CEPs (Figure 1d); NP herniation and incorporation of bone tissue into the CEPs (Figure 1e); incorporation of bone tissue into the CEPs (Figure 1f); and the traumatic node (damaged/repaired CEPs) (Figure 1g). These are the six primary types of CEP micro‐damage found in patients with degenerated human lumbar IVDs.
Figure 1.
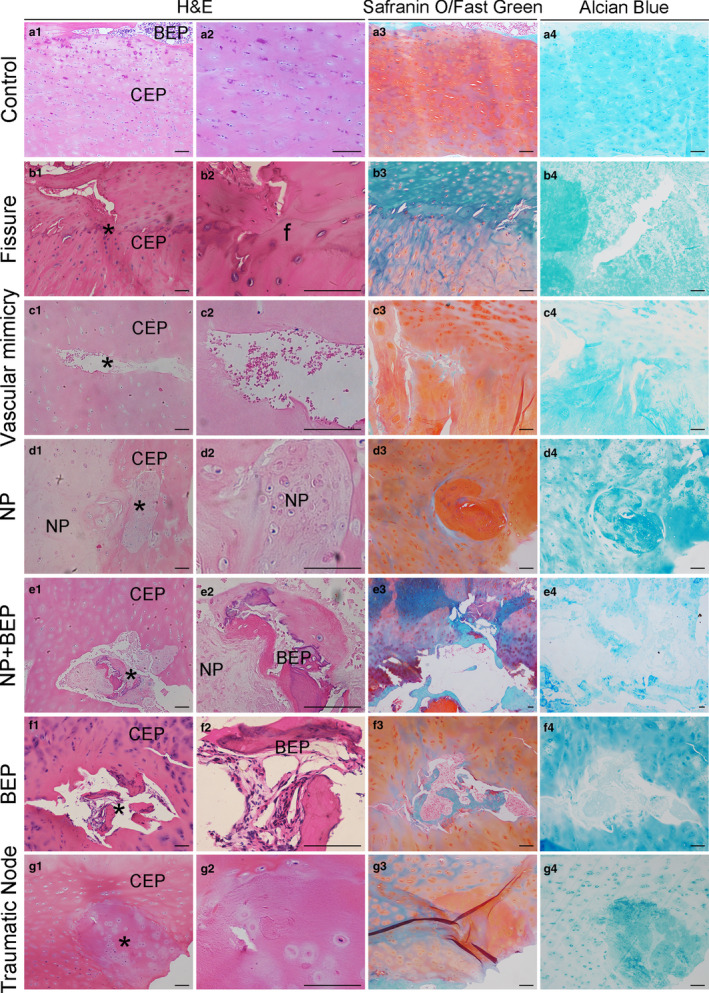
Histology of cartilage endplates (CEPs). (a1–a4) Control (normal) CEPs stained with H&E, Safranin O/Fast green, and Alcian Blue staining. Some bony endplate (BEP) is seen at the top of some images. (b1–b4) CEPs with fissures. The cartilage calcification ‘tidemark’ lies centrally in some images. (c1–c4) CEPs with ‘vascular mimicry’ suggesting blood vessel ingrowth after damage. (d1–d4) Nucleus pulposus (NP) herniation into the CEPs. (e1–e4) NP herniation and incorporation of bone tissue into the CEPs. (f1–f4) Incorporation of bone tissue into the CEPs. (g1–g4) The traumatic nodes (damaged/repaired CEPs) within CEPs. BEP, bony endplate; CEP, cartilaginous endplate; NP, nucleus pulposus; f, fissure; *The position of typical structural features. Scale bar: 100 μm
3.2. Expression of anabolic and catabolic molecules in CEP and NP tissues
To investigate degenerative processes within micro‐damaged CEPs and discs, we quantified the expression level of important anabolic and catabolic molecules in CEPs and NP tissue, using RT‐qPCR and immunohistochemistry (IHC). Each symbol represented the mean of replicates from a single donor. The percentage of MMP13‐positive cells was significantly higher in micro‐damaged CEP and NP tissue, than in control tissue (Figure 2b), whereas the concentration of cells positive for Collagen Type II was greatly decreased (Figure 2c). Furthermore, gene expression for the ‘anabolic’ molecules Sox9, aggrecan, and collagen type II was significantly decreased (to different degrees) in micro‐damaged CEPs and NP compared to the control group (Figure 3a–c). In contrast, gene expression for the ‘catabolic’ molecules MMP3, MMP13, and ADAMTS5 was significantly increased in micro‐damaged CEPs and NP compared to the normal controls (Figure 3d–f).
Figure 2.
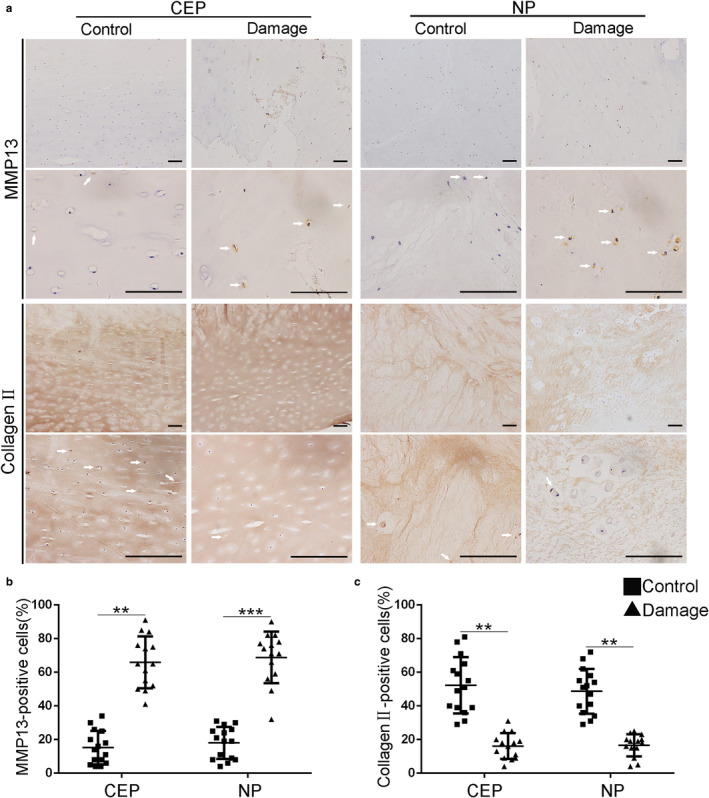
Micro‐damaged CEPs are associated with altered protein levels. (a) Immunopositive cells for MMP13 and Col II are compared visually in micro‐damaged (N = 14) and control (N = 15) tissues. Quantitative results show that damaged tissues contain a significantly higher % of cells positive for MMP13 (b) and a significantly lower % of cells positive for Col II (c). The white arrows represent the MMP13 or Collagen II‐positive cells. CEP, cartilaginous endplate; MMP, matrix metalloproteinase; Col II, type II collagen; NP, nucleus pulposus. Scale bar: 100 μm. *p < 0.05, **p < 0.01, ***p < 0.001
Figure 3.
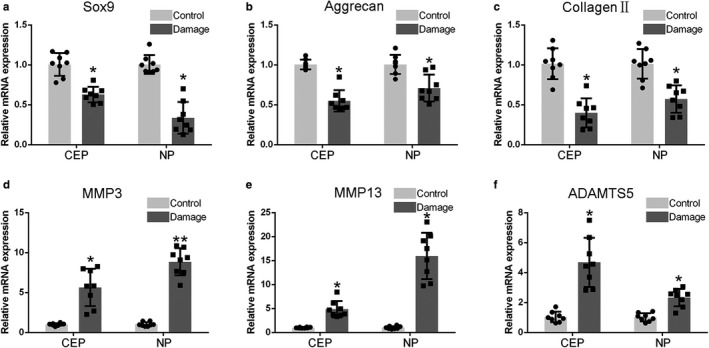
Micro‐damaged CEPs influence mRNA expression of anabolic and catabolic molecules. Transcriptional levels are shown for (a) Sox9, (b) Aggrecan, (c) Collagen type II, (d) MMP3, (e) MMP13, and (f) ADAMTS5. Compared to the control group (N = 8), the micro‐damaged CEP group (N = 8) showed decreased expression of molecules associated with anabolism (Sox9, aggrecan, and collagen type II), but increased expression of molecules associated with catabolism (MMP 3, MMP13, and ADAMTS5). RT‐qPCR data were normalized to SDHA. Sox9, SRY (sex‐determining region Y)‐box9; MMP, matrix metalloproteinase; ADAMTS, a disintegrin and metalloproteinase with thrombospondin motifs. *p < 0.05, **p < 0.01, ***p < 0.001
3.3. Inflammation and Substance P in micro‐damaged CEPs
In order to investigate whether inflammation and algogenesis are associated with micro‐damaged CEPs, we compared expression of the inflammatory factor TNF‐α, and the neuropeptide Substance P, in damaged and control tissues. As shown visually in Figure 4a, expression levels of both molecules were elevated in micro‐damaged CEPs compared to control specimens. These differences were highly significant in both CEP and NP tissues (Figure 4b,c).
Figure 4.
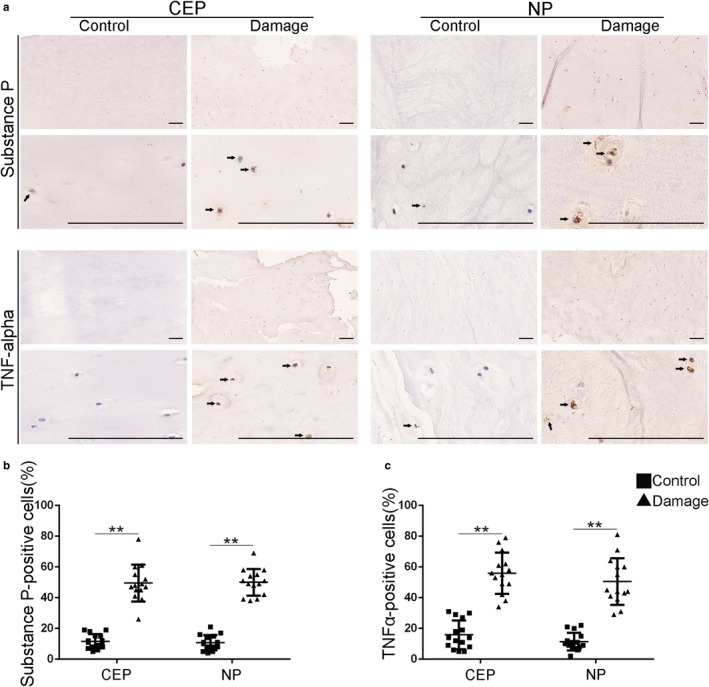
Inflammation and Substance P in damaged CEPs. (a) Cells immunopositive for Substance P and TNF‐α are compared visually for controls (N = 15) and patients with micro‐damaged endplates (N = 14). (b and c) Quantified results show that CEP micro‐damage is associated with an increased % of cells that are positive for Substance P and TNF‐α, both in the CEP and NP. The Black arrows represent the substance P or TNFα‐positive cells. Scale bar: 100 μm. *p < 0.05, **p < 0.01, ***p < 0.001
3.4. Patients with damaged CEPs have more severe disability and LBP
Clinical significance of the above results was investigated by comparing disability and back pain in patients with normal and damaged CEPs within the ‘degenerated’ group. Pain and disability were assessed using the ODI, JOA, and JOABPEQ scores. In addition, the potential for pain from the CEP was assessed immunohistochemically using the general nerve marker PGP9.5. LBP patients with micro‐damaged CEPs (N = 14) showed significantly higher ODI scores (Figure 5a); lower JOA scores (Figure 5b); and lower JOABPEQ scores (Figure 5c) than LBP patients with non‐damaged CEPs (N = 21). Taken together, these results imply that micro‐damaged CEPs can increase LBP and physical disability. In addition, preliminary evidence (Figure 5d) suggests that fine nerves, positive for PGP9.5, can be found in damaged CEPs showing vascular mimicry, or the ingrowth of bone (or NP and bone).
Figure 5.
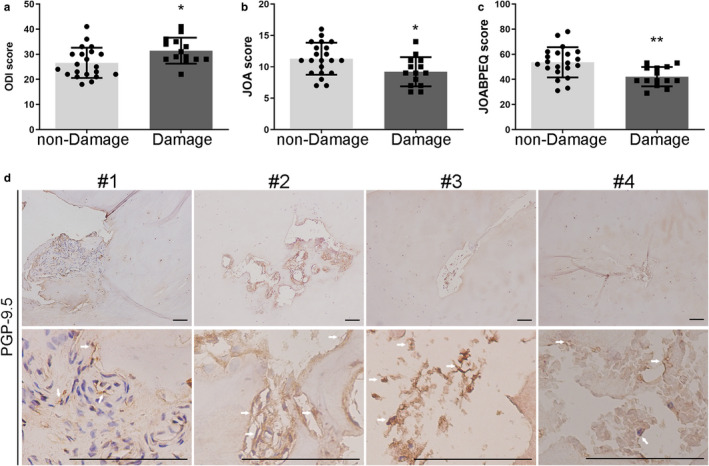
Damaged CEPs are associated with physical disability and LBP. Pain and disability scores were compared for patients in the “degeneration” group who had non‐damaged (N = 21) or micro‐damaged (N = 14) CEPs. (a) Oswestry Disability Index (ODI), (b) the Japanese Orthopedic Association scores (JOA), and (c) the Japanese Orthopedic Association Back Pain Evaluation Questionnaire (JOABPEQ). (d) Fine nerves immunopositive for protein gene product‐9.5 (PGP‐9.5) in four patients with micro‐damaged CEPs. The white arrows represent the PGP‐9.5‐positive cells. Scale bar: 100 μm. *p < 0.05, **p < 0.01, ***p < 0.001
4. DISCUSSION
This study identified micro‐damage in the cartilage endplate (CEP) in approximately 40% of patients with suspected discogenic LBP. Patterns of micro‐damage were used to propose a novel histological classification which included: cartilage fissures, “vascular mimicry,” “traumatic nodes,” and ingrowth within the CEP of nucleus pulposus (NP) and/or bone tissue. Additional experiments involving IHC and RT‐PCR showed that damaged CEPs were associated with increased matrix breakdown in the CEP and adjacent NP. Finally, LBP patients with damaged CEPs reported more back pain and disability, which may be linked to preliminary findings of increased innervation by PGP9.5‐positive nerves. It should be noted that the present study was unable to examine all regions of excised endplates, so the true proportion of CEPs with micro‐damage in the “degenerated” patient group may exceed the 40% reported here.
Previous work has focused mainly on the bony vertebral endplates, in cadaveric or animal tissues, and on MRI (Adams and Dolan, 2012; Adams et al., 2000; Berg‐Johansen et al., 2018b; Berg‐Johansen et al., 2018a; Feng et al., 2018; Fields et al., 2014; Rade et al., 2018; Wang et al., 2012). Some defects in cadaveric tissues may be artefactual, and defects in young animal tissues may not accurately represent those in ex vivo tissues obtained from living humans, as identified in the present study. Also, detection of CEP defects in MRI studies is limited by image resolution (typically in the region of 1 mm). Therefore, the present study is the first to consider human CEP defects at the histological (µm) scale, and their relationship with disc degeneration and pain. Previous histological studies have reported (as incidental findings) that defects in CEPs can be filled with transformed or necrotic tissue (Lama et al., 2014), or bone tissue (Zehra et al., 2015), but no systematic classification of micro‐damaged CEPs was attempted. Small‐scale damage may well arise before the larger lesions visible on MRI or radiographs.
The main strength is that human tissues were examined at high resolution, and that clinical data were obtained from the same human donors. The exact anatomic location of the CEP specimens could not be confirmed, but they were all adjacent degenerated discs that were believed to be painful. It is not possible to avoid any tissue damage during excision and sectioning, but the risk of artifacts was minimized by requiring micro‐damage to be identified on at least three sequential sections, in order to exclude the result error caused by excision and sectioning.
CEP fissures, with or without tidemark damage, are probably generated by shearing forces at the cartilage‐bone junction, possibly in response to axial rotation of the vertebral column. “Vascular mimicry” involves blood cells within a void in the CEP and may represent neovascularization of damaged and proteoglycan‐depleted tissue, as seen in intervertebral discs (Lama et al., 2018). Such tissue may also be susceptible to the incorporation of fragments of bone and/or NP from an adjacent degenerated IVD. Tissue fragments could be generated when the lumbar spine is loaded severely in bending, which can cause the disc annulus to pull fragments of CEP and bone from the adjacent vertebral body (Lama et al., 2014). “The nodes” may be formed similarly following more severe trauma. The present cross‐sectional study cannot identify the mechanisms leading to micro‐damaged CEPs. However, high compressive loading of the spine causes gross damage to an endplate (cartilage and bone) (Adams et al., 2000), and this is known to decompress the adjacent disc nucleus and lead to disc degeneration (Adams and Dolan, 2012).
Alternatively, or in addition, focal damage to a CEP could lead to disc degeneration by direct biological means. An intact CEP represents an effective biological barrier which would obstruct the transport of inflammatory mediators and bacteria between the disc nucleus and the vertebral body. By allowing increased transport of these substances, micro‐damaged CEPs could therefore promote disc degeneration or Modic changes (Adams et al., 2000; Berg‐Johansen et al., 2018b; Fields et al., 2010; Rutges et al., 2013; Shan et al., 2017). Previously, we have shown that disc degeneration is associated with anabolic and catabolic molecules, including inflammatory mediators, entering a disc in the CEP/NP regions (Chen et al., 2019; Dudli et al., 2015; Henry et al., 2018; Monchaux et al., 2017; Nakazawa et al., 2018; Tang et al., 2016). Hence, progressive disc degeneration could be explained by a ‘vicious circle’ involving endplate damage, catabolic responses of disc cells, reduced water binding by the disc matrix, and further overloading and damage to the endplate (Adams and Dolan, 2012; Vergroesen et al., 2015). We suggest that micro‐damage to a CEP could be a common initiating factor of this process.
CEP damage could also lead to ‘discogenic’ pain. Vertebral endplates are more innervated than the intervertebral disc (Fields et al., 2014), although nerves in the latter are not detectable on MRI. Histological studies have shown that nociceptive nerves can grow into damaged and proteoglycan‐depleted regions of annulus fibrosus (Lama et al., 2018) and evidence from the present study (Figures 4 and 5) suggests that this occurs in the CEP also. Defects in the CEP probably provide adequate space, and allow sufficient nutrient transport from the vertebral bodies, for neoinnervation and/or neovascularization to be possible. Furthermore, cytokines such as IL‐1β which are generated during IVD degeneration can stimulate the expression of angiogenic and neurotrophic factors (Lee et al., 2011), so that endplate abnormalities are associated with inflammation and axon growth induced by TNF‐α (Ohtori et al., (2006)). LBP can be generated from the local dorsal root ganglion (You et al., 2013) and thalamus, temporal lobes, insula, or dorsolateral prefrontal cortex (Schmidt‐Wilcke et al., 2006; Vlaeyen et al., 2018). Clinical results from the present study are consistent with LBP and disability arising from a damaged CEP following nerve ingrowth and sensitization.
Tissue engineering can influence disc cell metabolism, and may eventually provide an effective treatment for disc degeneration in humans (Yuan et al., (2017); Li et al., 2016; Silva‐Correia et al., 2013). However, this approach is unlikely to succeed unless allowance is made for the effects of CEP micro‐damage when gels or tissues are introduced into the degenerating disc space. This is a new objective for tissue engineering of IVDs.
In summary, we have characterized the histological features of damaged CEPs, and present a novel classification system. We have also identified nerves within micro‐damaged CEPs, and shown that CEP micro‐damage is significantly associated with features of disc degeneration, with LBP, and with disability. We conclude that CEP micro‐damage could initiate disc degeneration, and so should be considered in the development of tissue‐engineering approaches to treat it.
CONFLICTS OF INTEREST
Each of us does not have any financial support or other benefits from commercial sources for this work reported on the manuscript and there is no conflict or potential conflict of interest.
AUTHOR CONTRIBUTIONS
Concept/design: BH, JHL, JC, and FDZ. Acquisition of data: BH, JHL, and XAW. Data analysis/interpretation: BH, SWL, YFX, and XAW. Data interpretation: HW, JC, and FDZ. Drafting of the manuscript: BH, HW, and JC. Revision of the manuscript: JC and FDZ. All authors take responsibility for the integrity of the data analysis.
ACKNOWLEDGMENTS
This study is sponsored by the Natural Science Foundation of China (no. 81871796) and Zhejiang Provincial Natural Science Foundation of China (LQ19H060005). We thank Mike and Trish for revising the manuscript.
Huang B, Liu J, Wei X, et al. Damage to the human lumbar cartilage endplate and its clinical implications. J. Anat. 2021;238:338–348. 10.1111/joa.13321.
Bao Huang and Junhui Liu contributed to this work equally and should be regarded as the co‐first authors.
The material contained in the manuscript has not been previously published and is not being concurrently submitted elsewhere.
Contributor Information
Jian Chen, Email: chenjian-bio@zju.edu.cn.
Fengdong Zhao, Email: zhaofengdong@zju.edu.cn.
REFERENCES
- Adams, M.A. & Dolan, P. (2012) Intervertebral disc degeneration: Evidence for two distinct phenotypes. Journal of Anatomy, 221, 497–506. 10.1111/j.1469-7580.2012.01551.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams, M.A. , Freeman, B.J. , Morrison, H.P. , Nelson, I.W. & Dolan, P. (2000) Mechanical initiation of intervertebral disc degeneration. Spine, 25, 1625–1636. [DOI] [PubMed] [Google Scholar]
- Berg‐Johansen, B. , Fields, A.J. , Liebenberg, E.C. , Li, A. & Lotz, J.C. (2018) Structure‐function relationships at the human spinal disc‐vertebra interface. Journal of Orthopaedic Research, 36, 192–201. 10.1002/jor.23657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg‐Johansen, B. , Jain, D. , Liebenberg, E.C. , Fields, A.J. , Link, T.M. , O'Neill, C.W. et al. (2018) Tidemark avulsions are a predominant form of endplate irregularity. Spine, 43, 1095–1101. 10.1097/BRS.0000000000002545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boos, N. , Weissbach, S. , Rohrbach, H. , Weiler, C. , Spratt, K.F. & Nerlich, A.G. (2002) Classification of age‐related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine, 27, 2631–2644. [DOI] [PubMed] [Google Scholar]
- Chen, J. , Mei, Z. , Huang, B. , Zhang, X. , Liu, J. , Shan, Z. et al. (2019) IL‐6/YAP1/beta‐catenin signaling is involved in intervertebral disc degeneration. Journal of Cellular Physiology, 234, 5964–5971. 10.1002/jcp.27065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, S. , Huang, Y. , Zhou, Z.J. , Hu, Z.J. , Wang, J.Y. , Xu, W.B. et al. (2014) Upregulation of tumor necrosis factor alpha and ADAMTS‐5, but not ADAMTS‐4, in human intervertebral cartilage endplate with modic changes. Spine, 39, E817–E825. 10.1097/BRS.0000000000000362 [DOI] [PubMed] [Google Scholar]
- Chou, R. & Shekelle, P. (2010) Will this patient develop persistent disabling low back pain? JAMA, 303, 1295–1302. [DOI] [PubMed] [Google Scholar]
- Cole, A.A. , Breakwell, L.M. , Michael, A.L. , Chiverton, N. , Cross, A.K. & Le Maitre, C.L. (2014) Expression and regulation of neurotrophic and angiogenic factors during human intervertebral disc degeneration. Arthritis Research & Therapy, 16, 416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyo, R.A. & Weinstein, J.N. (2001) Low back pain. The New England Journal of Medicine, 344, 363–370. [DOI] [PubMed] [Google Scholar]
- Dudli, S. , Boffa, D.B. , Ferguson, S.J. & Haschtmann, D. (2015) Leukocytes enhance inflammatory and catabolic degenerative changes in the intervertebral disc after endplate fracture in vitro without infiltrating the disc. Spine, 40, 1799–1806. 10.1097/BRS.0000000000001186 [DOI] [PubMed] [Google Scholar]
- Fairbank, J.C. , Couper, J. , Davies, J.B. & O'Brien, J.P. (1980) The Oswestry low back pain disability questionnaire. Physiotherapy, 66, 271–273. [PubMed] [Google Scholar]
- Feng, Z. , Liu, Y. , Yang, G. , Battie, M.C. & Wang, Y. (2018) Lumbar vertebral endplate defects on magnetic resonance images: Classification, distribution patterns, and associations with modic changes and disc degeneration. Spine, 43, 919–927. 10.1097/BRS.0000000000002450 [DOI] [PubMed] [Google Scholar]
- Fields, A.J. , Lee, G.L. & Keaveny, T.M. (2010) Mechanisms of initial endplate failure in the human vertebral body. Journal of Biomechanics, 43, 3126–3131. 10.1016/j.jbiomech.2010.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields, A.J. , Liebenberg, E.C. & Lotz, J.C. (2014) Innervation of pathologies in the lumbar vertebral end plate and intervertebral disc. The Spine Journal, 14, 513–521. 10.1016/j.spinee.2013.06.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giers, M.B. , Munter, B.T. , Eyster, K.J. , Ide, G.D. , Newcomb, A.G.U.S. , Lehrman, J.N. et al. (2017) Biomechanical and endplate effects on nutrient transport in the intervertebral disc. World Neurosurgery, 99, 395–402. 10.1016/j.wneu.2016.12.041 [DOI] [PubMed] [Google Scholar]
- Henry, N. , Clouet, J. , Le Bideau, J. , Le Visage, C. & Guicheux, J. (2018) Innovative strategies for intervertebral disc regenerative medicine: From cell therapies to multiscale delivery systems. Biotechnology Advances, 36, 281–294. 10.1016/j.biotechadv.2017.11.009 [DOI] [PubMed] [Google Scholar]
- Huang, B. , Chen, J. , Zhang, X. , Wang, J. , Zheng, Z. , Shan, Z. et al. (2019) Alpha 2‐macroglobulin as dual regulator for both anabolism and catabolism in the cartilaginous endplate of intervertebral disc. Spine, 44, E338–E347. 10.1097/BRS.0000000000002852 [DOI] [PubMed] [Google Scholar]
- Izumida, S. & Inoue, S. (1986) Assessment of treatment for low back pain. Journal of Orthopaedic Science, 60, 391–394. 10.5483/BMBRep.2018.51.5.198 [DOI] [Google Scholar]
- Johnson, W.E. , Caterson, B. , Eisenstein, S.M. , Hynds, D.L. , Snow, D.M. & Roberts, S. (2002) Human intervertebral disc aggrecan inhibits nerve growth in vitro. Arthritis and Rheumatism, 46, 2658–2664. 10.1002/art.10585 [DOI] [PubMed] [Google Scholar]
- Johnson, W.E. , Caterson, B. , Eisenstein, S.M. & Roberts, S. (2005) Human intervertebral disc aggrecan inhibits endothelial cell adhesion and cell migration in vitro. Spine, 30, 1139–1147. [DOI] [PubMed] [Google Scholar]
- Kakitsubata, Y. , Theodorou, D.J. , Theodorou, S.J. , Tamura, S. , Nabeshima, K. , Trudell, D. et al. (2002) Cartilaginous endplates of the spine MRI with anatomic correlation in cadavers. Journal of Computer Assisted Tomography, 26, 933–940. [DOI] [PubMed] [Google Scholar]
- Kang, R. , Li, H. , Ringgaard, S. , Rickers, K. , Sun, H. , Chen, M. et al. (2014) Interference in the endplate nutritional pathway causes intervertebral disc degeneration in an immature porcine model. International Orthopaedics, 38, 1011–1017. 10.1007/s00264-014-2319-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lama, P. , Le Maitre, C.L. , Harding, I.J. , Dolan, P. & Adams, M.A. (2018) Nerves and blood vessels in degenerated intervertebral discs are confined to physically disrupted tissue. Journal of Anatomy, 233, 86–97. 10.1111/joa.12817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lama, P. , Zehra, U. , Balkovec, C. , Claireaux, H.A. , Flower, L. , Harding, I.J. et al. (2014) Significance of cartilage endplate within herniated disc tissue. European Spine Journal, 23, 1869–1877. 10.1007/s00586-014-3399-3 [DOI] [PubMed] [Google Scholar]
- Lee, J.M. , Song, J.Y. , Baek, M. , Jung, H.Y. , Kang, H. , Han, I.B. et al. (2011) Interleukin‐1beta induces angiogenesis and innervation in human intervertebral disc degeneration. Journal of Orthopaedic Research, 29, 265–269. 10.1002/jor.21210 [DOI] [PubMed] [Google Scholar]
- Li, Z. , Lang, G. , Chen, X. , Sacks, H. , Mantzur, C. , Tropp, U. et al. (2016) Polyurethane scaffold with in situ swelling capacity for nucleus pulposus replacement. Biomaterials, 84, 196–209. 10.1016/j.biomaterials.2016.01.040 [DOI] [PubMed] [Google Scholar]
- Monchaux, M. , Forterre, S. , Spreng, D. , Karol, A. , Forterre, F. & Wuertz‐Kozak, K. (2017) Inflammatory processes associated with canine intervertebral disc herniation. Frontiers in Immunology, 8, 1681 10.3389/fimmu.2017.01681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa, K.R. , Walter, B.A. , Laudier, D.M. , Krishnamoorthy, D. , Mosley, G.E. , Spiller, K.L. et al. (2018) Accumulation and localization of macrophage phenotypes with human intervertebral disc degeneration. The Spine Journal, 18, 343–356. 10.1016/j.spinee.2017.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtori, S. , Inoue, G. , Ito, T. , Koshi, T. , Ozawa, T. , Doya, H. et al. (2006) Tumor necrosis factor‐immunoreactive cells and PGP 9.5‐immunoreactive nerve fibers in vertebral endplates of patients with discogenic low back Pain and Modic Type 1 or Type 2 changes on MRI. Spine, 31, 1026–1031. [DOI] [PubMed] [Google Scholar]
- Rade, M. , Maatta, J.H. , Freidin, M.B. , Airaksinen, O. , Karppinen, J. & Williams, F.M.K. (2018) Vertebral endplate defect as initiating factor in intervertebral disc degeneration: Strong association between endplate defect and disc degeneration in the general population. Spine, 43, 412–419. 10.1097/BRS.0000000000002352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutges, J.P. , Duit, R.A. , Kummer, J.A. , Bekkers, J.E. , Oner, F.C. , Castelein, R.M. et al. (2013) A validated new histological classification for intervertebral disc degeneration. Osteoarthritis and Cartilage, 21, 2039–2047. 10.1016/j.joca.2013.10.001 [DOI] [PubMed] [Google Scholar]
- Schmidt‐Wilcke, T. , Leinisch, E. , Ganssbauer, S. , Draganski, B. , Bogdahn, U. , Altmeppen, J. et al. (2006) Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. Pain, 125, 89–97. 10.1016/j.pain.2006.05.004 [DOI] [PubMed] [Google Scholar]
- Shan, Z. , Zhang, X. , Li, S. , Yu, T. , Mamuti, M. & Zhao, F. (2017) The influence of direct inoculation of propionibacterium acnes on modic changes in the spine: Evidence from a rabbit model. The Journal of Bone and Joint Surgery, 99, 472–481. 10.2106/JBJS.16.00146 [DOI] [PubMed] [Google Scholar]
- Silva‐Correia, J. , Correia, S.I. , Oliveira, J.M. & Reis, R.L. (2013) Tissue engineering strategies applied in the regeneration of the human intervertebral disk. Biotechnology Advances, 31, 1514–1531. 10.1016/j.biotechadv.2013.07.010 [DOI] [PubMed] [Google Scholar]
- Tanaka, M. , Nakahara, S. & Inoue, H. (1993) A pathologic study of discs in the elderly. Separation between the cartilaginous endplate and the vertebral body. Spine, 18, 1456–1462. [PubMed] [Google Scholar]
- Tang, P. , Zhu, R. , Ji, W.P. , Wang, J.Y. , Chen, S. , Fan, S.W. et al. (2016) The NLRP3/Caspase‐1/interleukin‐1beta axis is active in human lumbar cartilaginous endplate degeneration. Clinical Orthopaedics and Related Research, 474, 1818–1826. 10.1007/s11999-016-4866-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergroesen, P.P. , Kingma, I. , Emanuel, K.S. , Hoogendoorn, R.J. , Welting, T.J. , van Royen, B.J. et al. (2015) Mechanics and biology in intervertebral disc degeneration: A vicious circle. Osteoarthritis and Cartilage, 23, 1057–1070. 10.1016/j.joca.2015.03.028 [DOI] [PubMed] [Google Scholar]
- Vlaeyen, J.W.S. , Maher, C.G. , Wiech, K. , Van Zundert, J. , Meloto, C.B. , Diatchenko, L. et al. (2018) Low back pain. Nature Reviews Disease Primers, 4, 52 10.1038/s41572-018-0052-1 [DOI] [PubMed] [Google Scholar]
- Wade, K.R. , Robertson, P.A. , Thambyah, A. & Broom, N.D. (2014) How healthy discs herniate: A biomechanical and microstructural study investigating the combined effects of compression rate and flexion. Spine, 39, 1018–1028. [DOI] [PubMed] [Google Scholar]
- Wang, P. , Yang, L. & Hsieh, A.H. (2011) Nucleus pulposus cell response to confined and unconfined compression implicates mechanoregulation by fluid shear stress. Annals of Biomedical Engineering, 39, 1101–1111. 10.1007/s10439-010-0221-1 [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Videman, T. & Battie, M.C. (2012) Lumbar vertebral endplate lesions: Prevalence, classification, and association with age. Spine, 37, 1432–1439. 10.1097/BRS.0b013e31824dd20a [DOI] [PubMed] [Google Scholar]
- Yao, M. , Li, Z.J. , Zhu, S. , Wang, J.Y. , Pan, Y.F. , Tian, Z.R. et al. (2018) Simplified Chinese version of the Japanese orthopaedic association back pain evaluation questionnaire: Cross‐cultural adaptation, reliability, and validity for patients with low back pain. Spine, 43, E357–E364. 10.1097/BRS.0000000000002424 [DOI] [PubMed] [Google Scholar]
- You, C. , Zhu, K. , Liu, X. , Xi, C. , Zhang, Z. , Xu, G. et al. (2013) Tumor necrosis factor‐α‐dependent infiltration of macrophages into the dorsal root ganglion in a rat disc herniation model. Spine, 38, 2003–2007. 10.1097/BRS.ObOI [DOI] [PubMed] [Google Scholar]
- Yuan, D. , Chen, Z. , Zhou, Y. , Xiao, D. , Liu, K. , Xiang, X. et al. (2017) A regenerative intervertebral disc endplate based on biomimetic 3‐dimensional scaffolds. Spine, 42, E260–E266. 10.1097/BRS.0000000000001791 [DOI] [PubMed] [Google Scholar]
- Zehra, U. , Noel‐Barker, N. , Marshall, J. , Adams, M.A. & Dolan, P. (2019) Associations between intervertebral disc degeneration grading schemes and measures of disc function. Journal of Orthopaedic Research, 37(9), 1946–1955. 10.1002/jor.24326 [DOI] [PubMed] [Google Scholar]
- Zehra, U. , Robson‐Brown, K. , Adams, M.A. & Dolan, P. (2015) Porosity and thickness of the vertebral endplate depend on local mechanical loading. Spine, 40, 1173–1180. 10.1097/BRS.0000000000000925 [DOI] [PubMed] [Google Scholar]
- Zhao, F. , Pollintine, P. , Hole, B.D. , Dolan, P. & Adams, M.A. (2005) Discogenic origins of spinal instability. Spine, 30, 2621–2630. 10.1097/01.brs.0000188203.71182.c0 [DOI] [PubMed] [Google Scholar]


