Abstract
Puberty is an important phase of development when the neural circuit organization is transformed by sexual hormones, inducing sexual dimorphism in adult behavioural responses. The principal brain area responsible for the control of the receptive component of female sexual behaviour is the ventrolateral division of the ventromedial nucleus of the hypothalamus (VMHvl), which is known for its dependency on ovarian hormones. Inputs to the VMHvl originating from the medial preoptic nucleus (MPN) are responsible for conveying essential information that will trigger such behaviour. Here, we investigated the pattern of the projection of the MPN to the VMHvl in rats ovariectomized at the onset of puberty. Sprague Dawley rats were ovariectomized (OVX) at puberty and then subjected to iontophoretic injections of the neuronal anterograde tracer Phaseolus vulgaris leucoagglutinin into the MPN once they reached 90 days of age. This study analysed the connectivity pattern established between the MPN and the VMH that is involved in the neuronal circuit responsible for female sexual behaviour in control and OVX rats. The data show the changes in the organization of the connections observed in the OVX adult rats that displayed a reduced axonal length for the MPN fibres reaching the VMHvl, suggesting that peripubertal ovarian hormones are relevant to the organization of MPN connections with structures involved in the promotion of female sexual behaviour.
Keywords: female sexual behaviour, neural circuitry, Phaseolus vulgaris leucoagglutinin, puberty, steroids, three‐dimensional reconstruction
Schematic drawings comparing the MPN efferents between injected‐female (control) and injected‐OVX female groups. The interrupted lines show the decrease in the density of the projections in OVX group compared to the control group.
![]()
1. INTRODUCTION
The central nervous system (CNS) is modulated by internal and external signals that establish neuronal connections and physiological functioning in two critical periods of development, which will promote sexually dimorphic behavioural responses in the adult (Schwarz and McCarthy, 2008; Xu et al., 2012; McCarthy et al., 2015). One internal signalling molecule that is of great importance during the two key moments of CNS development is oestradiol (Schwarz and McCarthy, 2008). First, in the perinatal phase, low oestradiol levels in the female CNS prevent the organizational masculinization of neuronal systems, promoting the sexual differentiation of adult neuronal programming and behavioural responses (Romeo et al., 2002; Sisk and Zehr, 2005; Schulz and Sisk, 2006; Phoenix, 2009; Flanagan‐Cato, 2011). In the second moment, at puberty, increased oestradiol levels in the female CNS promote neural refinement, which is reflected in the volume and number of cells in some neural regions such as the hippocampal formation, the anteroventral periventricular nucleus (AVPV) and the medial nucleus of the amygdala (MeA; Woolley, 1998; Ahmed et al., 2008). This oestradiol increase activates the previously organized cortical and limbic circuits, modifying the structural and functional refinement that will ultimately influence adult decision‐making and cognitive strategies (Schulz et al., 2004, 2009a; Sisk and Zehr, 2005; Schulz and Sisk, 2006) as well as promoting the integration of endogenous and exogenous information to coordinate the appropriate expression of adulthood behaviours, such as aggression and reproduction (Romeo et al., 2002; Sisk and Zehr, 2005; Schulz and Sisk, 2006; Phoenix, 2009).
Several environmental factors and ovarian hormones (oestradiol and progesterone) affect the neuronal circuit that controls female sexual behaviour (Fahrbach et al., 1989; Canteras et al., 1994; Auger and Blaustein, 1995; Canteras, 2012). Within this neuronal circuit, the ventromedial nucleus of the hypothalamus (VMH), which is an area highly modulated by these hormones in the perinatal and peripubertal stages as well as in adulthood, is the area responsible for triggering the lordosis reflex, which is a receptive component of female sexual behaviour (Powers, 1970; Flanagan‐Cato, 2000; Sá et al., 2009; Griffin and Flanagan‐Cato, 2011). The VMH is a nucleus comprised of the dorsomedial (dm), central (c) and ventrolateral (vl) divisions located in the medial region of the tuberal hypothalamus. In coronal sections, the VMH is visible as a moderately dense group of polymorphic cells that form an oval region surrounded by a mesh of dendrites and axons, which form the lateral fibre complex. This fibre complex is relevant for the intra‐ and inter‐connectivity of the communicating fibres reaching the VMH and is enriched in neurotransmitters such as oxytocin and serotonin that are important modulators involved in behavioural responses (Flanagan‐Cato, 2000; Sá et al., 2009; Griffin and Flanagan‐Cato, 2011; McEwen et al., 2012). The ventrolateral division of the VMH (VMHvl) expresses abundant oestrogen (ER) and progesterone (PR) receptors, which enable these neurons to play a central role in the promotion of female sexual behaviour (Shughrue et al., 1992; McEwen et al., 2012). It was previously shown that in the VMHvl, priming by oestradiol followed by progesterone induces broad morphological and neurochemical changes (Madeira et al., 2001; Griffin and Flanagan‐Cato, 2011) that are necessary and sufficient for triggering the lordosis reflex (Powers, 1970; Rubinow et al., 2009; Sá et al., 2013). The VMHvl is connected to a network of nuclei that also express an abundance of ERs and PRs, which are composed of the medial preoptic nucleus (MPN), the MeA and the bed nucleus of the stria terminalis (BNST; Flanagan‐Cato, 2000; Sá et al., 2010; Shimogawa et al., 2015).
The MPN is a sexually dimorphic nucleus located in the medial preoptic area that abundantly expresses both types of ERs, ERα and ERβ (Shughrue et al., 1992, 1998; Hoshina et al., 1994; Maggi et al., 2004). Through its connections with the septal nuclei, the parahippocampal gyrus, the prefrontal cortex and limbic and prelimbic areas, the MPN is responsible for integrating sensitive visceral and olfactory information along with hormonal signals to coordinate the endocrine, autonomous and locomotor systems through its projections and to regulate adaptive, feeding, sexual and maternal behaviours (Simerly and Swanson, 1988; Xiao et al., 2005; Canteras, 2012).
Therefore, the morphological plasticity induced by oestradiol in the MPN and VMHvl is crucial for the modulation of female sexual behaviour (Flanagan‐Cato et al., 2006; Rubinow et al., 2009).
Additionally, the hormonal modulation of the CNS in the peripubertal stage of development is a topic that is very relevant to behavioural modulation in adulthood; however, its repercussions are not completely understood. Little is known about the peripubertal effects of oestradiol on the morphological changes of the organized neural circuits involved in the promotion of behaviours dependent on ovarian hormones in adulthood. Many of the studies on the action of gonadal hormones during puberty on the morphological and functional development of the CNS and the associated behaviours were conducted in males, resulting in a large gap in our knowledge of how these systems interact with one another in females (Romeo et al., 2002; Schulz et al., 2004, 2009a, 2009b).
In the present study, we aimed to determine the effects of ovarian hormones during the peripubertal period on the pattern of projections from the MPN to the VMHvl.
2. MATERIALS AND METHODS
2.1. Animals
Female Sprague Dawley rats were obtained from the Central Animal Facility of the ICB/USP (São Paulo, Brazil) and were kept in a facility with a 12‐h light/dark cycle (lights on at 07:00 h) with an ambient temperature of 22°C. Once they were approximately 35 days old, a set of animals (n = 15) was randomly selected and bilaterally ovariectomized (OVX group) under deep anaesthesia by intraperitoneal injection (0.2 ml/100 g body weight) of a solution containing ketamine (25 mg/ml), xylazine (5 mg/ml) and acepromazine (1 mg/ml). Another group of rats (n = 15) were kept intact to serve as controls. The oestrous cycle was monitored daily by vaginal smear cytology. The inclusion criteria for the control rats required them to exhibit at least two consecutive oestrous cycles, with the injection being performed on a random day of the cycle; for the OVX rats, it was required that they be in persistent dioestrus (Cora et al., 2015). Once they were 90 days old, the OVX and control animals were submitted to stereotaxic surgery under anaesthesia to perform unilateral iontophoretic injections of the neuronal anterograde tracer Phaseolus vulgaris leucoagglutinin (PHA‐L, 10% solution in 0.01 M PBS; 10.000 MW, Molecular Probes, Life Technologies Corporation) in the MPN according to the following stereotaxic coordinates: AP: −0.7; ML: ±0.4; DV: −7.0 (Swanson, 2018). The iontophoretic injection was applied by passing +10 µA current pulses (7 s on and 7 s off) using a digital current source (Midgard Precision Current Source, Stoelting Co.) for 10 min through a glass pipette with a 15 µm internal diameter. The pipette was left in place for an additional 10 min before being slowly withdrawn to avoid the overflow of the tracer. The experimental procedures were performed according to the guidelines of the Ethical Committee on the Use of Animals CEUA—ICB/USP (protocol 030/2015 and 032/2015/CEUA). All efforts were made to minimize the number of animals used and animal suffering.
2.2. Tissue preparation
Fifteen days after the stereotaxic surgery, the rats were anaesthetized by employing the same protocol used previously and perfused transcardially with 0.9% saline followed by a fixative solution containing 4% formaldehyde in 0.1 M sodium phosphate buffer, pH 7.4. The brains remained in the skull for 3 hr before they were removed, and they were trimmed in the coronal plane through the superior colliculus and immersed for approximately 12 hr in a 20% sucrose solution in 0.02 M potassium phosphate‐buffered saline (KPBS). After the brain tissue blocks were placed in a microtome, five sets of 40‐µm‐thick coronal sections were sequentially cut at regular intervals of 200 µm and stored at −20°C. One set of sections was used for immunohistochemical detection of the injection site, and the second and third sets were used for PHA‐L immunohistochemistry. The injection sites were verified by bright‐ and dark‐field microscopic examination. The inclusion criteria used to ensure adequate injection of the tracer were an injection site that was confined to the limits of the MPN that did not contaminate neighbouring neural structures beyond the established limits and confirmation of tracer transport to the VMHvl.
2.3. Immunohistochemistry
For PHA‐L immunostaining, one set of sections was incubated in rabbit anti‐PHA‐L primary antibody (1:5,000; L1110, Vector Laboratories) for 72 hr at 4°C. The sections were washed in KPBS, incubated for 2 hr in biotinylated goat anti‐rabbit secondary antibody (1:200; BA1000, Vector Laboratories), washed again and then incubated for 2 hr in the avidin‐biotin peroxidase complex (1:200; ABC Elite Kit, Vectastain PK‐6100 Elite, Vector Laboratories). Following a final wash, the sections were incubated for 5 min in 100 ml of a solution containing 3,3′‐diaminobenzidine tetrahydrochloride (50 mg; DAB; Sigma), glucose oxidase (0.6 mg; Sigma) and ammonium chloride (40 mg; Sigma) in sodium phosphate‐buffered saline (0.1 M). Then, β‐D‐glucose (MP Biomedicals, LLC) was added to the solution for 15 min, and the reaction was stopped by a KPBS wash. The stained sections were mounted on gelatine‐coated slides and dried at 37°C for 18 hr. Finally, the sections were treated with osmium tetroxide, counterstained with 0.25% thionin solution for 15–20 min and then dehydrated and coverslipped using dibutyl phthalate xylene (DPX; Aldrich Chemical Co.).
2.4. Histological assessments
2.4.1. Semi‐quantitative analysis
To confirm the accuracy and similarity of the tracer injection site in the MPN of all animals studied, for each animal, all sections showing the injection sites were ordered, sequenced and analysed throughout their entirety, and the boundaries of the injection site were delineated. Then, the volumes of the injection sites were measured using Neurolucida software and outlined using the Canvas™ program X 10.0 (Deneba Systems Inc.). The MPN has three divisions with dissimilar projection patterns (Simerly et al., 1984; Simerly and Swanson, 1986; Simerly and Swanson, 1988). Therefore, care was taken to confirm that the injection sites covered the entire nucleus, with the injection site beginning in the anterior portion, encompassing the ventral–dorsal extent and extending to the most posterior portions (Swanson, 2018). After these analyses were performed by an observer blinded to the experimental groups, three control and three OVX rats were selected that presented a similar injection location and volume. Therefore, these selected cases were used in all the quantitative determinations (n = 3 per group).
The semi‐quantitative analysis of the PHA‐L‐labelled fibres in the rostrocaudal extension of the VMHvl was performed by two different observers, who were blinded to the experimental groups, by employing bright‐ and dark‐field observation (Veenman et al., 1992; Reznitsky et al., 2016) with a Leica DMR microscope with a 20× objective (Leica Wetzlar, Germany). The fibre density was qualitatively classified as (Figure 1) sparse (+/−), low (+), moderate (++), high (+++) or very high (++++). Photomicrographs were acquired using a LEICA DMR optical microscope with NIS‐Elements BR 3.2 software (Nikon Corporation Japan) and Neurolucida image capture software using a 100× objective. The images were adjusted for brightness, contrast and focus. The Stack‐Focuser tool in ImageJ 1.50i software (Rasband, W.S., ImageJ, U. S. National Institutes of Health, https://imagej.nih.gov/ij/, 1997‐2018) was used to merge the photomicrographs of the stained fibres in the VMHvl. In all analyses, the stereotaxic atlas of the rat brain was used as a reference (Swanson, 2018).
FIGURE 1.
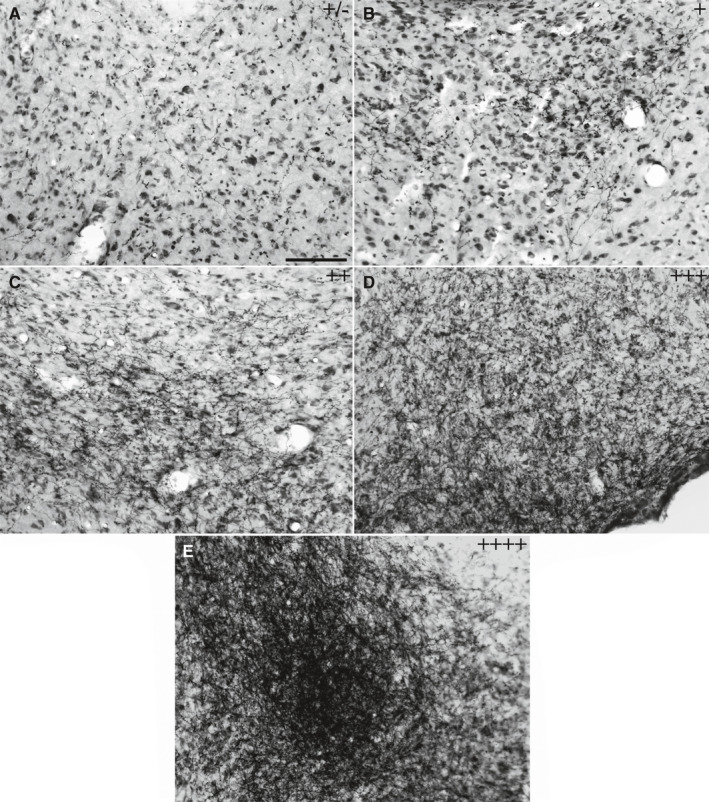
Bright‐field photomicrographs of representative coronal brain sections showing the density of anterograde‐labelled fibres immunoreactive for PHA‐L, which was used for the semi‐quantitative analysis of the projections. Images represent the sparse or inconsistent (±) (a), low (+) (b), moderate (++) (c), high (+++) (d) or very high (++++) (e) density of fibres. Scale bar = 100 µm
2.4.2. Stereological estimations
For the quantitative analyses, the density of the PHA‐L‐labelled fibres extending from the MPN to the VMHvl was estimated using a stereology method by employing spherical probes (Gundersen et al., 1999; Mouton et al., 2002; Bukhatwa et al., 2009; Chareyron et al., 2011; West, 2018). The sections were analysed using a bright‐field Nikon ECLIPSE 80 microscope connected to a computer equipped with StereoInvestigator™ software using a 40× oil immersion objective (West, 2013, 2018). To estimate the total axonal length, the Spaceball Workflow tool was used with the following parameters: the guard zones were 2 µm, the probe shape was a hemisphere with a radius of 10 µm, the volume associated with the sphere was 144,000 µm3 and the area of the sampling grid was 3,600 µm2. The entire extent of the VMHvl was analysed using a 100× oil immersion objective. The total axonal length was estimated using the following formula (Mouton et al., 2002):
where ∑Qi is the sum of the counted intersections; v is the volume (area of the grid × section thickness); a is the surface area of the sphere; and ssf is the section sampling fraction. In the stereological estimation, one set of sections previously stained for PHA‐L was used (n = 3 per group), with a sampling ratio of 1:5 and an average thickness of 10.4 µm.
2.4.3. Three‐dimensional reconstruction method
To study the distribution pattern of MPN projections to the VMHvl, the three‐dimensional (3D) reconstruction method was used (Sauzeau et al., 2010). The slides were analysed using a Nikon ECLIPSE 80 optical light microscope equipped with an extraction tube and a digital camera connected to a computer with Neurolucida 360 software (MBF Bioscience, MicroBrightField Inc.). One representative animal (selected from among the animals that were analysed by the semi‐qualitative method) from both the control and OVX groups was used for the reconstruction of the fibres, collaterals and varicosities in the entirety of the VMHvl. The reconstruction was performed using a 100× oil immersion objective by covering the entire thickness of each slice and representing the fibres as segments of lines and the varicosities as points. A set of six sections of the VMHvl from each case, which were regularly spaced at intervals of 200 µm, was used to draw and reconstruct the tissue in three dimensions to compare the ipsilateral distribution of PHA‐L‐labelled fibres in OVX and control animals. The contours of the optic tract, the fornix, the third ventricle and the VMH were also reconstructed bilaterally to generate a spatial reference (Figures S1 and S2 of the supplementary material—3D interactive models). The three‐dimensional reconstructions were displayed and analysed using the Neuroexplorer program (MicroBrightField). The drawings of each section were exported as isolated figures to obtain data on axonal length and the quantity of varicosities. The varicosities were identified as dotted dilatations corresponding to the continuous fibres (Figure 3h,k).
FIGURE 3.
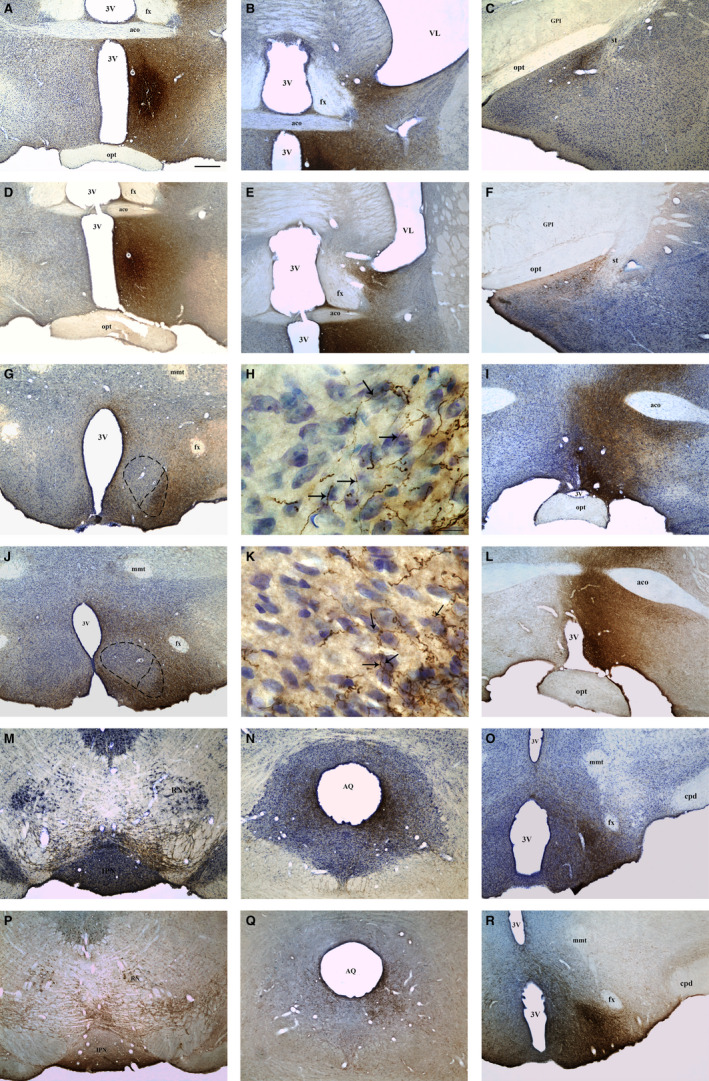
Bright‐field photomicrographs of coronal brain sections representative of projecting areas immunoreactive for PHA‐L upon tracer injection in the MPN. Photomicrographs showing the injection site in the MPN of control (a) and OVX rats (d). Photomicrographs showing anterograde PHA‐L‐labelled fibres in the BNST (b), MeA (c), VMH (g), AVPV (i), VTA (m), PAG (n) and PMv (o) of control rats and in the BNST (e), MeA (f), VMH (j), AVPV (l), VTA (p), PAG (q) and PMv (r) of OVX rats. h, k Higher magnification of the VMH (g, j), respectively, showing the overlap of anterograde PHA‐L‐labelled fibres (arrows) with Nissl‐stained neuronal cell bodies in the VMHvl of control (h) and OVX rats (k). Dashed lines represent the boundaries of the MPN (a, d) and VMHvl (g, j). 3V, third ventricle; aco, anterior commissure; AQ, cerebral aqueduct; AVPV, anteroventral periventricular nucleus; BNST, bed nucleus of the stria terminalis; cpd, cerebral peduncle; fx, fornix; GPm, medial part of globus pallidus; IPN, interpeduncular nucleus; MeA, medial nucleus of the amygdala; mmt, mammillothalamic tract; MPN, medial preoptic nucleus; opt, optic chiasm; PAG, periaqueductal grey matter; PMv, ventral premammillary nucleus; RN, red nucleus; st, stria terminalis; VL, lateral ventricle; VTA, ventral tegmental area. Scale bars = 400 µm (a–g/i–j/l–r), 20 µm (h, k)
2.5. Statistical analyses
The effects of peripubertal ovarian hormones on the total axonal length of efferents from the MPN to the VMHvl were assessed by descriptive analyses using JASP 0.8.6 software. The results are presented as the mean ± SD. The total axonal length that was quantified by the stereological analysis for each experimental group was plotted individually. Groups were considered different when the data distribution in the boxplot did not overlap with a confidence interval of 95% (Cumming et al., 2007). According to the individual values for each animal analysed in the stereological quantification, one‐way Bootstrap ANOVA was performed for the total axonal length to obtain better insight into what would result from an analysis of a larger sample.
One‐way bootstrap ANOVA with Welch correction was performed using SPSS® software and was used to compare the groups. The means of the analysed groups were considered significantly different when p < .05.
3. RESULTS
3.1. Determination of the neural fibre distribution pattern using semi‐quantitative analysis
Three control rats with injections centred in the MPN with no adjacent contamination were used in the semi‐quantitative analyses. The injection sites in the control rats encompassed all three divisions of MPN with similar volumes for all the animals (Table 1). The injection site in the control rat SD2380 (Figure 2a; Figure S3c in the supplementary material) was slightly rostral, with its centre in the lateral and central divisions of the MPN, while the injection sites in the control rats SD1923 and SD2386 were centred in the central and medial divisions of the MPN (Figure 2b,c; Figure S3a,b of the supplementary material). After the MPN injections (Figure 3a), the terminal fields of the nerve fibres were found in the main regions controlling female sexual behaviour, showing a high density in the BNST, VMHvl and PAGvl (Figure 3b,g,n; Table 2), a moderate density in the VTA (Figure 3m) and a low density in the MeApd (Figure 3c; Table 2). In addition, terminal fields were observed in other hypothalamic nuclei, with a very high density of ascending projections to the AVPV, a high density of descending projections to the ventral premammillary nuclei (PMv; Figure 3o; Table 2) and a moderate density of projections to the preoptic anteroventral (AVP) nuclei and the arcuate nucleus (ARH; Figure 3i; Table 2).
TABLE 1.
Volume of the PHA‐L antegrade tracer injection site in the MPN of intact control or OXV rats
| Animals | Control | Animals | OVX | |
|---|---|---|---|---|
| Volume (mm3) | SD 1923 | 26.5 | SD 2193 | 11.7 |
| SD 2380 | 14.7 | SD 2195 | 24.51 | |
| SD 2386 | 21.2 | SD 2201 | 24.7 | |
| Mean ± SD | 20.8 ± 5.9 | Mean ± SD | 20.3 ± 7.4 |
FIGURE 2.
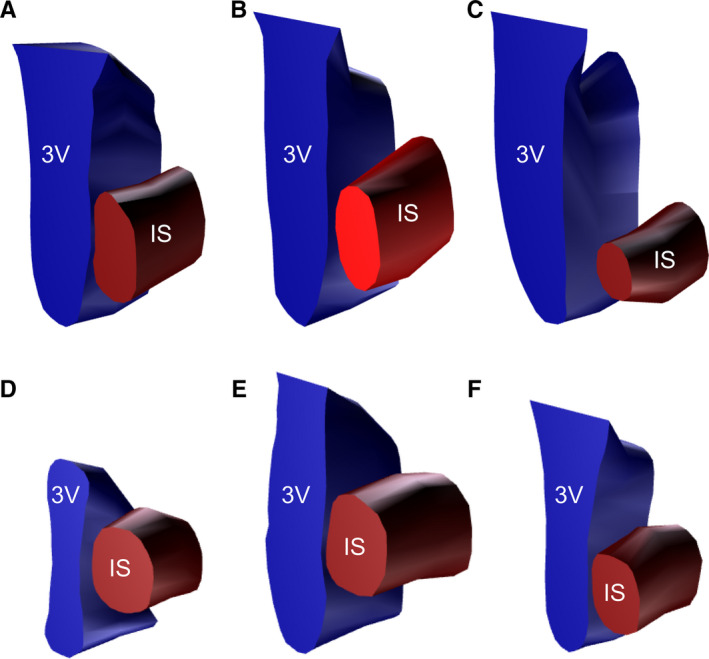
Schematic 3D representation of injection sites of PHA‐L in the MPN of three control (a–c) and three OVX rats (d–f). Red edge, outer border of the PHA‐L injection; blue edge, outer border of the third ventricle. IS, injection site; 3V, third ventricle
TABLE 2.
Semi‐quantitative analysis of the PHA‐L fibres density distributed in the main MPN efferents, in both Control and Ovx groups
| Brain nuclei | Control | OVX | |
|---|---|---|---|
| Control areas of female sexual behaviour | BNST | +++ | ++ |
| VMHvl | +++ | ++ | |
| PAGvl | +++ | ++ | |
| VTA | ++ | + | |
| MeApd | + | + | |
| Hypothalamic neuroendocrine control | PMv | +++ | +++ |
| AVPV | ++++ | ++++ | |
| AVP | ++ | ++ | |
| ARH | ++ | ++ |
Density of PHA‐L labelled fibres in the main MPN efferents regions of female brain rats. The density is described as low (+), moderate (++), high (+++) or very high (++++).
Abbreviations: ARH, arcuate nucleus of the hypothalamus; AVP, preoptic anteroventral nuclei; AVPV, anteroventral periventricular nucleus; BNST, bed nucleus of the stria terminalis; MeApd, medial nucleus of the amygdala, poterodorsal division; PAGvl, periaqueductal grey matter, ventrolateral division; PMv, ventral premammillary nuclei; VMHvl, ventromedial nucleus of the hypothalamus, ventrolateral division; VTA, ventral tegmental area.
In the OVX group, three rats with injection sites that encompassed all three divisions of the MPN with similar volumes among the control and OVX animals (Figure 3d; Table 1) were used in the analyses. The injection site in the OVX rat SD2193 (Figure 2f; Figure S3f of the supplementary material) was slightly rostral and centred in the central and lateral divisions of the MPN, while the injection sites in the OVX rats SD2195 and SD2201 were centred in the central and medial divisions of the MPN (Figure 2d,e; Figure S3d,e of the supplementary material). The terminal fields of nerve fibres extending to the main regions responsible for female reproductive behaviour were found, with a moderate density in the BNST, VMHvl and PAGvl (Figure 3e,j,q; Table 2) and a low density in the MeApd and VTA (Figure 3f,p; Table 2). In addition, terminal fields were observed in other hypothalamic nuclei, with a very high density of ascending projections to the AVPV, a high density of descending projections to the PMv (Figure 3r) and a moderate density of projections to the ARH and the AVP (Figure 3l; Table 2).
Table 2 summarizes the results of the semi‐quantitative analyses. By comparing the control and OVX animals, we observed an effect of peripubertal ovariectomy on the projections from the MPN only in the main regions controlling female sexual behaviour.
Varicosities corresponding to the terminal fields of nerve fibres were observed at densities similar to those of the fibres in the same regions as well as in all the extensions of these nuclei in both the control (Figure 3h) and the OVX animals (Figure 3k).
3.2. Quantitative and statistical analyses of neural fibre distribution patterns using the stereological method
The stereological estimations showed a decrease in the average total axonal length in the OVX group, which was 30,421.30 µm ± 7,026.60 in the control and 11,410.70 µm ± 1,420.05 in OVX rats with a mean coefficient of error (m = 1) of 0.03 for the control and 0.04 for the OVX rats (Figure 4). To complement our stereological analysis, one‐way ANOVA of the results for the total axonal length was performed by using the bootstrap method. The analysis showed a significant difference in the total axonal length of fibres in the OVX group compared with that in the control group (F(1, 4) = 21.098; p = .038; η2 = 0.841; Table S1).
FIGURE 4.
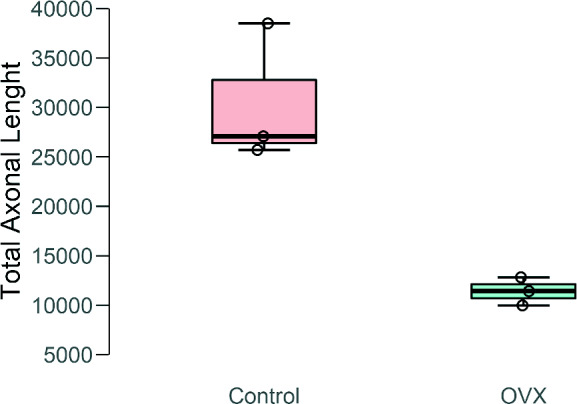
Box and whisker plot of data obtained from the stereological estimation of the total axonal length of fibres reaching the VMHvl after PHA‐L injection in the MPN using the space ball method
3.3. Determination of the neural fibre distribution pattern using the 3D reconstruction method
Data obtained from analyses performed with Neurolucida 360 of the distribution pattern of PHA‐L fibres in the VMHvl from the MPN using the 3D reconstruction method (Figure 5a,b) showed a difference between the OVX and control groups, especially in the VMHvl, where we observed an important reduction in the fibre density and the extension of labelling in the OVX group (Figure 5; Figures S1 and S2). The total axonal length of nerve fibres reaching the VMHvl in the control rats was 107,044.00 µm, and the number of varicosities was 10,264. The total axonal length of nerve fibres reaching the VMHvl in the OVX animals was 42,665.90 µm, and the number of varicosities was 8,150.
FIGURE 5.
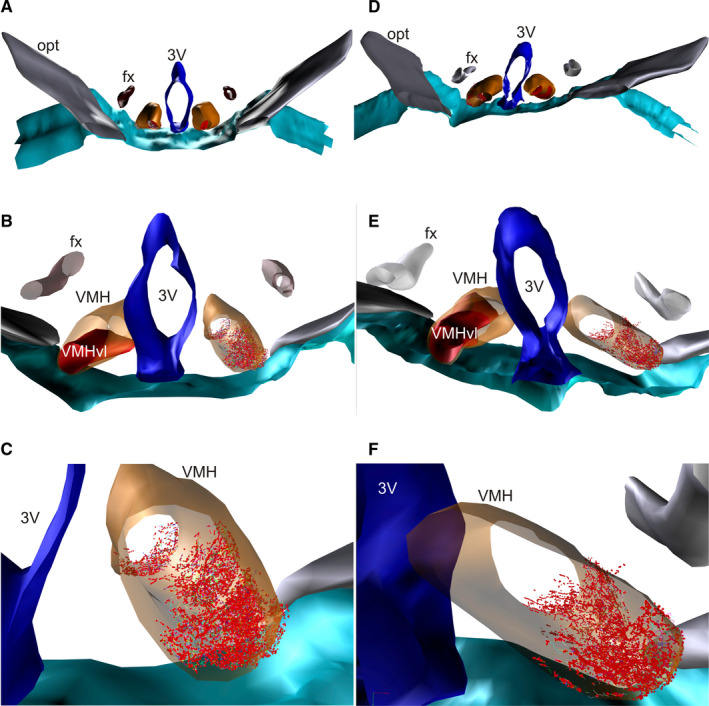
Schematic 3D reconstruction of PHA‐L‐labelled terminals in the VMHvl used for the quantification of fibre and varicosity numbers. Labelled terminals in the VMHvl (in red) after PHA‐L injection in the MPN of control (a–c) and OVX rats (d–f). 3V, third ventricle; fx, fornix; opt, optic tract; VMH in orange
4. DISCUSSION
By examining the specific periods of oestradiol activity and the effects on the organization and activation of neural circuits (Romeo et al., 2002) in this work, we showed, for the first time, that peripubertal ovarian hormones modulate some connections within the female sexual behaviour network. The present data highlight the changes in the projection patterns of efferents from the MPN to the VMHvl, showing a decrease in the total axonal length and varicosity number in adult female rats when gonadal hormones were absent in the peripubertal phase (Figure 6).
FIGURE 6.
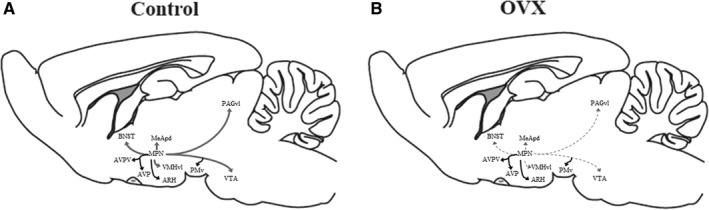
Schematic graphic comparing the MPN efferences between the control (a) and OVX (b) groups. A decrease in the density of the projections to regions related to female sexual behaviour in the OVX group compared to the control group was observed (arrows in grey in a and dotted arrows in b). ARH, arcuate nucleus of the hypothalamus; AVP, preoptic anteroventral nuclei; AVPV, anteroventral periventricular nucleus; BNST, bed nucleus of the stria terminalis; MeApd, medial nucleus of the amygdala, posterodorsal division; MPN, medial preoptic nucleus; PAGvl, periaqueductal grey matter, ventrolateral division; PMv, ventral premammillary nuclei; VMHvl, ventromedial nucleus of the hypothalamus, ventrolateral division; VTA, ventral tegmental area
A high degree of caution was taken to ensure that the PHA‐L injection sites in the control and OVX rats were similar to each other to prevent interference in the comparative analyses. As a result, the high number of animals used is due to the need to obtain similar injection results in the PHA‐L in the MPN of both groups in addition to the complex and indispensable methodology used to perform these injections and the postoperative losses of OVX animals, as they were subjected to two surgeries.
Therefore, because the MPN can be divided into several areas with dissimilar projection patterns (Simerly et al., 1984; Simerly and Swanson, 1986; Simerly and Swanson, 1988), care was taken to analyse experimental cases with injection sites in the MPN that began in the anterior portion and covered the full ventral–dorsal and rostrocaudal extent.
4.1. MPN efference
The results of the analyses performed on the injections of PHA‐L centred on the MPN are in accordance with others reported in the literature, especially for the areas that are involved in the control of female sexual behaviour, such as the VMHvl, VTA and PAGvl (Simerly and Swanson, 1988; Fahrbach et al., 1989; Delville and Blaustein, 1993). A striking number of efferents was also observed in the PMv, which is a nucleus that coordinates ovulation with lordosis behaviour through its influence on luteinizing hormone secretion (Beltramino and Taleisnik, 1985; Donato et al., 2009). Efferents were also observed in the ARH, AVPV and AVP, which, along with the MPN, are nuclei that establish a parallel neuroendocrine microcircuit involved in sexual control and the activation of the hypothalamic–pituitary–gonadal (HPG) axis at the onset of puberty (Sinchak et al., 2013).
In the sexual behaviour control circuit, the MPN is responsible for the integration of behavioural planning by promoting sensory, motor and hormonal integration, as it receives information from the septohippocampal complex, prefrontal cortex, and prelimbic and infralimbic areas in addition to the sensory integration information derived from the MeA and BNST (Xiao et al., 2005; Canteras, 2012). Thus, under the action of ovarian hormones, the NPM plays a fundamental role in female sexual behaviour because when it is activated, it sends projections to the VTA and nucleus accumbens to promote the proceptive component of female sexual behaviour while also sending projections to VMHvl to inhibit the receptive component (Takeo et al., 1993; Kato and Sakuma, 2000).
In sensory integration, the MPN efferences for the VMHvl receive all the pheromone information and all the proprioceptive information that is derived from the stimulation of the cervical portion of the vagina, which is carried directly by the subparafascicular nucleus [SPFp] (Coolen et al., 2003). In motor integration, the MPN projects itself to the VMHvl so that it adequately coordinates lordosis behaviour through the efferences for the PAGvl (Canteras et al., 1994; Flanagan‐Cato et al., 2006) when hormone levels allow the female to become receptive to the male.
All of these integrating processes are required to take place for reproduction to be successful (Takeo et al., 1993; Kato and Sakuma, 2000).
4.2. MPN efference without ovarian hormones
Ovarian hormonal action upon the morphological remodelling of the neural circuit that is accountable for the triggering of female sexual behaviour, including the reduction of the neuronal density, number, and size and the induction of protein expression, is well known (Madeira and Lieberman, 1995; Romeo et al., 2002; Xue et al., 2014). Therefore, the morphological changes occurring at puberty in the MPN and the VMH seem to induce significant physiological changes in the transition from puberty to adulthood.
The present data show that efferents to the micro‐circuitry involved in the modulation of female sexual behaviour (namely, the VMHvl, VTA, PAGvl, BNST and MeApd) were conserved after peripubertal ovariectomy; however, there was an important reduction in fibre density and the extension of labelling in the referred areas, especially in the VMHvl. In contrast, no changes were detected in the pattern of efferents to nuclei related to modulation of hormone secretion (namely, the PMv, ARH and AVPV/AVP; Beltramino and Taleisnik, 1985; Donato et al., 2009; Sinchak et al., 2013). This process occurs because at the onset of puberty, the initial activation is influenced by mechanisms that are not dependent on steroids, such as genomic mechanisms, and the absence of hormonal action has little impact on this efferent pattern (Xu et al., 2012; Armoskus et al., 2014; Lowe et al., 2015; McCarthy et al., 2015).
Thus, this study showed that there was a change in the pattern of projections when the rats were subjected to ovariectomy at the onset of puberty; therefore, the action of oestradiol before puberty may be needed to establish a directional pattern of VMHvl afferents that, upon puberty, will be reorganized according to the fluctuating hormonal levels during the oestrous cycle, which will become most relevant to the action of neurons in the modulation of physiological and behavioural responses. This is because puberty is a period when the neural circuits are refined and finalized to allow for their full maturation and for the expression of typical sexual behaviour in adulthood (Schulz et al., 2004, 2009b; Schulz and Sisk, 2006).
Throughout neural development, there are two windows of greater activity by ovarian hormones. First, during prenatal and postnatal development, steroids have an organizational effect because they have the ability to permanently sculpt neural structures (Phoenix et al., 1959; Romeo et al., 2002; Sisk and Zehr, 2005). In females, these organizational effects occur in the absence of or in the presence of low levels of oestradiol (Schwarz and McCarthy, 2008). Second, the action of steroids on neural structures will be more focused on the refinement of neural circuits in terms of the cell number, the volume of cell groups and even the triggering of cell death (Schulz et al., 2009), resulting in long‐lasting structural changes due to the effects of steroids that will determine sex behavioural responses in adults to hormonal and sensory stimuli (Sisk and Zehr, 2005).
Previous studies addressing this subject have ovariectomized the rats upon adulthood, when the development of the CNS was already completed. In contrast, the results reflecting the performance of ovariectomy earlier in development in the present study suggest that the observed hormonal changes are established earlier in development during the organizational period, when the female brain has not yet experienced the activating effects of oestradiol. Earlier studies have shown that the activation of ERs and PRs by increased ovarian hormone levels induces a reduction in ERα expression in the MPNm (Shughrue et al., 1992; Zhou et al., 1995; Martins et al., 2015), which indicates that OVX rats present more ERα‐expressing neurons than control rats.
Taken together, the results of the previous studies suggest that the observed decrease in MPN afferents to the VMN is not related to morphological or neurochemical changes induced in the MPN by the lack of ovarian hormones and further corroborate the present hypothesis of a hormone‐dependent reorganization of the connectivity pattern between the two nuclei.
4.3. Analysis techniques
The present data obtained from both methodologies, 3D reconstruction and stereology analysis, report a reduction in the total length of axonal fibres in OVX rats, corroborating, along with the statistical and semi‐quantitative analyses, the results that suggested a reduction in efferences established between the MPN and VMH. Although, presently, there is a vast selection of image software that can be used to quantify neuronal cell bodies, one of the main limitations of these software programs in studies with anterograde tracers is the quantification of axon fibres (Dickstein et al., 2016; Glaser and Glaser, 1990). To quantify the variation in the density of fibres and varicosities in the present study, a new methodology of 3D reconstruction was used (Sauzeau et al., 2010). Despite the indisputable relevance of this technique for fibre analysis, in the present study, the quantification of the reconstructed fibres and varicosities was very time‐consuming due to the high number of fibres observed, making it very challenging to use in the other animals of each group. In this way, in the present study, the 3D reconstruction method was used as a prospective approach, attempting to compare the distribution pattern of nerve fibres. To compare the estimation of the total length of nerve fibres, we used the stereology method, a more time‐efficient, highly precise and unbiased method, by intersecting each axonal fibre with a space ball probe. Notably, although the injection site analyses found a small number of animals to be acceptable for quantitative assessments, previous studies have found the number to be suitable for this type of study (Chareyron et al., 2011). In addition, the bootstrap analysis, which estimated the statistical outcome of an extrapolated data set predicted by the distribution of the observed data (Efron and Tibshirani, 1993; Efron, 2003; Schomaker and Heumann, 2018), corroborated the present analysis by reporting a significant difference between the total axonal length of both groups studied. Nevertheless, new studies with a higher sample size should be performed to further corroborate the present data.
The present results, using both qualitative and quantitative approaches, showed that the withdrawal of ovarian hormones during the onset of the peripubertal period induced a 60% reduction in the length of axonal fibres from the MPN to the VMHvl. However, despite the low number of animals used, both methods noticed the same changes, which were also observed in the semi‐quantitative analysis. The data also report a decrease of approximately 20% in the number of varicosities seen in the fibres reaching the VMHvl. A more detailed analysis of the distribution of these markers along the sections revealed that the observed changes were proportional to the morphological increase in the rostrocaudal volume and neuronal number of the VMHvl. These results should be interpreted with caution because only a small sample of animals was used; however, the observed fibre distribution is in accordance with the observed rostrocaudal increase in the neuronal density and ER expression, further corroborating the notion that afferents reach the VMHvl mostly at the caudal level and that the processed information leaves the nucleus through the rostral end (Madeira et al., 2001; Sá et al., 2009, 2013).
Therefore, this study aimed to provide new insights into the role of ovarian hormones in the connectivity pattern of the MPN, mainly in terms of its projections to the VMHvl and other nuclei that participate in the circuitry of female sexual behaviour control.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Supporting information
Fig S1
Fig S2
Fig S3
Table S1
ACKNOWLEDGEMENTS
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo [São Paulo Research Foundation—FAPESP] grants #2010/52068‐0 and #2016/02224‐1 [JCB], grant #2015/05990‐4 [JGPF] and FAPESP #2016/13136‐6 [JACHJ]. We would also like to thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior [Agency for the Advancement of Higher Education—CAPES grant #848/15]. JCB is an Investigator with the Conselho Nacional de Desenvolvimento Científico e Tecnológico [National Council for Scientific and Technological Development—CNPq] under grant #426378/2016‐4. We are also thankful to the Programa Operacional Competitividade e Internacionalização—COMPETE2020; National Funds through FCT—Fundação para a Ciência e a Tecnologia within CINTESIS (UID/IC/4255/2013).
Pereira LDS, Gobbo DR, Ferreira JGP, Horta‐Junior JDADC, Sá SI, Bittencourt JC. Effects of ovariectomy on inputs from the medial preoptic area to the ventromedial nucleus of the hypothalamus of young adult rats. J. Anat. 2021;238:467–479. 10.1111/joa.13304
Contributor Information
Susana Isabel Sá, Email: sasusana@med.up.pt.
Jackson Cioni Bittencourt, Email: sasusana@med.up.pt, Email: jcbitten@icb.usp.br.
REFERENCES
- Ahmed, E.I. , Zehr, J.L. , Schulz, K.M. , Lorenz, B.H. , DonCarlos, L.L. & Sisk, C.L. (2008) Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nature Neuroscience, 11(9), 995–997. 10.1038/nn.2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armoskus, C. , Moreira, D. , Bollinger, K. , Jimenez, O. , Taniguchi, S. & Tsai, H.W. (2014) Identification of sexually dimorphic genes in the neonatal mouse cortex and hippocampus. Brain Research, 1562, 23–38. 10.1016/j.brainres.2014.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger, A.P. & Blaustein, J.D. (1995) Progesterone enhances an estradiol‐induced increase in Fos immunoreactivity in localized regions of female rat forebrain. Journal of Neuroscience, 15(3), 2272–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltramino, C. & Taleisnik, S. (1985) Ventral premammillary nuclei mediate pheromonal‐induced LH release stimuli in the rat. Neuroendocrinology, 41(2), 119–124. 10.1159/000124164 [DOI] [PubMed] [Google Scholar]
- Bukhatwa, S. , Iravani, M.M. , Zeng, B.Y. , Cooper, J.D. , Rose, S. & Jenner, P. (2009) An immunohistochemical and stereological analysis of PSI‐induced nigral neuronal degeneration in the rat. Journal of Neurochemistry, 109(1), 52–59. 10.1111/j.1471-4159.2009.05956.x [DOI] [PubMed] [Google Scholar]
- Canteras, N.S. (2012) Hypothalamic goal‐directed behavior ‐Ingestive, reproductive and defensive In: The Mouse Nervous System (pp. 539–562). San Diego: Academic Press; 10.1016/B978-0-12-369497-3.10020-2 [DOI] [Google Scholar]
- Canteras, N.S. , Simerly, R.B. & Swanson, L.W. (1994) Organization of projections from the ventromedial nucleus of the hypothalamus: A Phaseolus vulgaris‐Leucoagglutinin study in the rat. The Journal of Comparative Neurology, 348(1), 41–79. 10.1002/cne.903480103 [DOI] [PubMed] [Google Scholar]
- Chareyron, L.J. , Banta Lavenex, P. , Amaral, D.G. & Lavenex, P. (2011) Stereological analysis of the rat and monkey amygdala. The Journal of Comparative Neurology, 519(16), 3218–3239. 10.1002/cne.22677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coolen, L.M. , Veening, J.G. , Petersen, D.W. & Shipley, M.T. (2003) Parvocellular subparafascicular thalamic nucleus in the rat: Anatomical and functional compartmentalization. The Journal of Comparative Neurology, 463(2), 117–131. 10.1002/cne.10740 [DOI] [PubMed] [Google Scholar]
- Cora, M.C. , Kooistra, L. & Travlos, G. (2015) Vaginal cytology of the laboratory rat and mouse: review and criteria for the staging of the estrous cycle using stained vaginal smears. Toxicologic Pathology, 43(6), 776–793. 10.1177/0192623315570339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumming, G. , Fidler, F. & Vaux, D.L. (2007) Error bars in experimental biology. Journal of Cell Biology, 177(1), 7–11. 10.1083/jcb.200611141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delville, Y. & Blaustein, J.D. (1993) Estrogen receptor‐immunoreactive forebrain neurons project to the ventrolateral hypothalamus in female guinea pigs. The Journal of Comparative Neurology, 334(4), 571–589. 10.1002/cne.903340406 [DOI] [PubMed] [Google Scholar]
- Dickstein, D.L. , Dickstein, D.R. , Janssen, W.G.M. , Hof, P.R. , Glaser, J.R. , Rodriguez, A. et al (2016) Automatic dendritic spine quantification from confocal data with neurolucida 360. Current Protocols in Neuroscience, 77, 1.27.1–1.27.21. 10.1002/cpns.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato, J. , Silva, R.J. , Sita, L.V. , Lee, S. , Lee, C. , Lacchini, S. et al (2009) The ventral premammillary nucleus links fasting‐induced changes in leptin levels and coordinated luteinizing hormone secretion. Journal of Neuroscience, 29(16), 5240–5250. 10.1523/JNEUROSCI.0405-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron, B. (2003) Second Thoughts on the Bootstrap. Statist. Sci, 18(2), 135–140. 10.1214/ss/1063994968 [DOI] [Google Scholar]
- Efron, B. & Tibshirani, R.J. (1993). An Introduction to the Bootstrap. New York: Chapman and Hall; 10.1007/978-1-4899-4541-9 [DOI] [Google Scholar]
- Fahrbach, S.E. , Morrell, J.I. & Pfaff, D.W. (1989) Studies of ventromedial hypothalamic afferents in the rat using three methods of HRP application. Experimental Brain Research, 77(2), 221–233. 10.1007/BF00274980 [DOI] [PubMed] [Google Scholar]
- Flanagan‐Cato, L.M. (2011) Sex differences in the neural circuit that mediates female sexual receptivity. Frontiers in Neuroendocrinology, 32(2), 124–136. 10.1016/j.yfrne.2011.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan‐Cato, L.M. (2000) Estrogen‐induced remodeling of hypothalamic neural circuitry. Frontiers in Neuroendocrinology, 21(4), 309–329. 10.1006/frne.2000.0204 [DOI] [PubMed] [Google Scholar]
- Flanagan‐Cato, L.M. , Lee, B.J. & Calizo, L.H. (2006) Co‐localization of midbrain projections, progestin receptors, and mating‐induced Fos in the hypothalamic ventromedial nucleus of the female rat. Hormones and Behavior, 50(1), 52–60. 10.1016/j.yhbeh.2006.01.012 [DOI] [PubMed] [Google Scholar]
- Glaser, J.R. & Glaser, E.M. (1990) Neuron imaging with neurolucida ‐ A PC‐based system for image combining microscopy. Computerized Medical Imaging and Graphics, 14(5), 307–317. 10.1016/0895-6111(90)90105-K [DOI] [PubMed] [Google Scholar]
- Griffin, G.D. & Flanagan‐Cato, L.M. (2011) Ovarian hormone action in the hypothalamic ventromedial nucleus: Remodelling to regulate reproduction. Journal of Neuroendocrinology, 23(6), 465–471. 10.1111/j.1365-2826.2011.02143.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen, H.J.G. , Jensen, E.B.V. , Kiêu, K. & Nielsen, J. (1999) The efficiency of systematic sampling in stereology ‐ Reconsidered. Journal of Microscopy, 193(3), 199–211. 10.1046/j.1365-2818.1999.00457.x [DOI] [PubMed] [Google Scholar]
- Hoshina, Y. , Takeo, T. , Nakano, K. , Sato, T. & Sakuma, Y. (1994) Axon‐sparing lesion of the preoptic area enhances receptivity and diminishes proceptivity among components of female rat sexual behavior. Behavioural Brain Research, 61(2), 197–204. 10.1016/0166-4328(94)90160-0 [DOI] [PubMed] [Google Scholar]
- Kato, A. & Sakuma, Y. (2000) Neuronal activity in female rat preoptic area associated with sexually motivated behavior. Brain Research, 862(1–2), 90–102. 10.1016/S0006-8993(00)02076-X [DOI] [PubMed] [Google Scholar]
- Lowe, R. , Gemma, C. , Rakyan, V.K. & Holland, M.L. (2015) Sexually dimorphic gene expression emerges with embryonic genome activation and is dynamic throughout development. BMC Genomics, 16(1), 295 10.1186/s12864-015-1506-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira, M.D. , Ferreira‐Silva, L. & Paula‐Barbosa, M.M. (2001) Influence of sex and estrus cycle on the sexual dimorphisms of the hypothalamic ventromedial nucleus: Stereological evaluation and Golgi study. The Journal of Comparative Neurology, 432(3), 329–345. 10.1002/cne.1106 [DOI] [PubMed] [Google Scholar]
- Madeira, M.D. & Lieberman, A.R. (1995) Sexual dimorphism in the mammalian limbic system. Progress in Neurobiology, 45(4), 275–333. 10.1016/0301-0082(94)00052-J [DOI] [PubMed] [Google Scholar]
- Maggi, A. , Ciana, P. , Belcredito, S. & Vegeto, E. (2004) Estrogens in the nervous system: Mechanisms and nonreproductive functions. Annual Review of Physiology, 66(1), 291–313. 10.1146/annurev.physiol.66.032802.154945 [DOI] [PubMed] [Google Scholar]
- Martins, S.I. , Madeira, M.D. & Sá, S.I. (2015) Effects of gonadal steroids and of estrogen receptor agonists on the expression of estrogen receptor alpha in the medial preoptic nucleus of female rats. Neuroscience, 310, 63–72. 10.1016/j.neuroscience.2015.09.030 [DOI] [PubMed] [Google Scholar]
- McCarthy, M.M. , Pickett, L.A. , VanRyzin, J.W. & Kight, K.E. (2015) Surprising origins of sex differences in the brain. Hormones and Behavior, 76, 3–10. 10.1016/j.yhbeh.2015.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen, B.S. , Akama, K.T. , Spencer‐Segal, J.L. , Milner, T.A. & Waters, E.M. (2012) Estrogen effects on the brain: Actions beyond the hypothalamus via novel mechanisms. Behavioral Neuroscience, 126(1), 4–16. 10.1037/a0026708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton, P.R. , Gokhale, A.M. , Ward, N.L. & West, M.J. (2002) Stereological length estimation using spherical probes. Journal of Microscopy, 206(1), 54–64. 10.1046/j.1365-2818.2002.01006.x [DOI] [PubMed] [Google Scholar]
- Phoenix, C.H. (2009) Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Hormones and Behavior, 55(5), 566 10.1016/j.yhbeh.2009.01.004 [DOI] [PubMed] [Google Scholar]
- Phoenix, C.H. , Goy, R.W. , Gerall, A.A. & Young, W.C. (1959) Organizing action of prenatally administered testosterone propionate on the Tissues mediating mating behavior in the female guinea pig. 10.1210/endo-65-3-369 [DOI] [PubMed]
- Powers, J.B. (1970) Hormonal control of sexual receptivity during the estrous cycle of the rat. Physiology and Behavior, 5(8), 831–835. 10.1016/0031-9384(70)90167-8 [DOI] [PubMed] [Google Scholar]
- Reznitsky, M. , Plenge, P. & Hay‐Schmidt, A. (2016) Serotonergic projections from the raphe nuclei to the subthalamic nucleus; a retrograde‐ and anterograde neuronal tracing study. Neuroscience Letters, 612, 172–177. 10.1016/j.neulet.2015.11.035 [DOI] [PubMed] [Google Scholar]
- Romeo, R.D. , Richardson, H.N. & Sisk, C.L. (2002) Puberty and the maturation of the male brain and sexual behavior: Recasting a behavioral potential. Neuroscience and Biobehavioral Reviews, 26(3), 381–391. 10.1016/S0149-7634(02)00009-X [DOI] [PubMed] [Google Scholar]
- Rubinow, D.R. , Schmidt, P.J. , Meltzer‐Brody, S. & Harsh, V.L. (2009). Gonadal hormones and behavior in women: Concentrations versus context In: Pfaff D.W., Arnold A.P., Fahrbach S.E., Etgen A.M., Rubin R.T. (Eds.) Hormones, Brain and Behavior (pp. 37–73). 10.1016/B978-012532104-4/50086-X [DOI] [Google Scholar]
- Sá, S.I. , Lukoyanova, E. & Madeira, M.D. (2009) Effects of estrogens and progesterone on the synaptic organization of the hypothalamic ventromedial nucleus. Neuroscience, 162(2), 307–316. 10.1016/j.neuroscience.2009.04.066 [DOI] [PubMed] [Google Scholar]
- Sá, S.I. , Pereira, P.A. , Malikov, V. & Madeira, M.D. (2013) Role of estrogen receptor α and β in the induction of progesterone receptors in hypothalamic ventromedial neurons. Neuroscience, 238, 159–167. 10.1016/j.neuroscience.2013.02.023 [DOI] [PubMed] [Google Scholar]
- Sá, S.I. , Pereira, P.A. , Paula‐Barbosa, M.M. & Madeira, M.D. (2010) Role of neural afferents as mediators of estrogen effects on the hypothalamic ventromedial nucleus. Brain Research, 1366, 60–70. 10.1016/j.brainres.2010.10.043 [DOI] [PubMed] [Google Scholar]
- Sauzeau, V. , Horta‐Junior, J.A.C. , Riolobos, A.S. , Fernández, G. , Sevilla, M.A. , López, D.E. et al (2010) Vav3 is involved in GABAergic axon guidance events important for the proper function of brainstem neurons controlling cardiovascular, respiratory, and renal parameters. Molecular Biology of the Cell, 21(23), 4251–4263. 10.1091/mbc.e10-07-0639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schomaker, M. & Heumann, C. (2018) Bootstrap inference when using multiple imputation. Stat. Med. 10.1002/sim.7654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz, K.M. , Molenda‐Figueira, H.A. & Sisk, C.L. (2009a) Back to the future: The organizational‐activational hypothesis adapted to puberty and adolescence. Hormones and Behavior, 55(5), 597–604. 10.1016/j.yhbeh.2009.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz, K.M. , Richardson, H.N. , Zehr, J.L. , Osetek, A.J. , Menard, T.A. & Sisk, C.L. (2004) Gonadal hormones masculinize and defeminize reproductive behaviors during puberty in the male Syrian hamster. Hormones and Behavior, 45(4), 242–249. 10.1016/j.yhbeh.2003.12.007 [DOI] [PubMed] [Google Scholar]
- Schulz, K.M. & Sisk, C.L. (2006) Pubertal hormones, the adolescent brain, and the maturation of social behaviors: Lessons from the Syrian hamster. Molecular and Cellular Endocrinology, 254–255, 120–126. 10.1016/j.mce.2006.04.025 [DOI] [PubMed] [Google Scholar]
- Schulz, K.M. , Zehr, J.L. , Salas‐Ramirez, K.Y. & Sisk, C.L. (2009b) Testosterone programs adult social behavior before and during, but not after, adolescence. Endocrinology, 150(8), 3690–3698. 10.1210/en.2008-1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz, J.M. & McCarthy, M.M. (2008) Steroid‐induced sexual differentiation of the developing brain: Multiple pathways, one goal. Journal of Neurochemistry, 105(5), 1561–1572. 10.1111/j.1471-4159.2008.05384.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimogawa, Y. , Sakuma, Y. & Yamanouchi, K. (2015) Efferent and afferent connections of the ventromedial hypothalamic nucleus determined by neural tracer analysis: Implications for lordosis regulation in female rats. Neuroscience Research, 91, 19–33. 10.1016/j.neures.2014.10.016 [DOI] [PubMed] [Google Scholar]
- Shughrue, P.J. , Bushnell, C.D. & Dorsa, D.M. (1992) Estrogen receptor messenger ribonucleic acid in female rat brain during the estrous cycle: A comparison with ovariectomized females and intact males. Endocrinology, 131(1), 381–388. 10.1210/endo.131.1.1612018 [DOI] [PubMed] [Google Scholar]
- Shughrue, P.J. , Scrimo, P.J. & Merchenthaler, I. (1998) Evidence for the colocalization of estrogen receptor‐β mRNA and estrogen receptor‐α immunoreactivity in neurons of the rat forebrain. Endocrinology, 139(12), 5267–5270. 10.1210/endo.139.12.6525 [DOI] [PubMed] [Google Scholar]
- Simerly, R.B. & Swanson, L.W. (1988) Projections of the medial preoptic nucleus: A Phaseolus vulgaris leucoagglutinin anterograde tract‐tracing study in the rat. The Journal of Comparative Neurology, 270(2), 209–242. 10.1002/cne.902700205 [DOI] [PubMed] [Google Scholar]
- Simerly, R.B. & Swanson, L.W. (1986) The organization of neural inputs to the medial preoptic nucleus of the rat. The Journal of Comparative Neurology, 246(3), 312–342. 10.1002/cne.902460304 [DOI] [PubMed] [Google Scholar]
- Simerly, R.B. , Swanson, L.W. & Gorski, R.A. (1984) The cells of origin of a sexually dimorphic serotonergic input to the medial preoptic nucleus of the rat. Brain Research, 324(1), 185–189. 10.1016/0006-8993(84)90641-3 [DOI] [PubMed] [Google Scholar]
- Sinchak, K. , Dewing, P. , Ponce, L. , Gomez, L. , Christensen, A. , Berger, M. et al (2013) Modulation of the arcuate nucleus‐medial preoptic nucleus lordosis regulating circuit: A role for GABAB receptors. Hormones and Behavior, 64(1), 136–143. 10.1016/j.yhbeh.2013.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk, C.L. & Zehr, J.L. (2005) Pubertal hormones organize the adolescent brain and behavior. Frontiers in Neuroendocrinology, 26(3–4), 163–174. 10.1016/j.yfrne.2005.10.003 [DOI] [PubMed] [Google Scholar]
- Swanson, L.W. (2018) Brain maps 4.0—Structure of the rat brain: An open access atlas with global nervous system nomenclature ontology and flatmaps. The Journal of Comparative Neurology, 526(6), 935–943. 10.1002/cne.24381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeo, T. , Chiba, Y. & Sakuma, Y. (1993) Suppression of the lordosis reflex of female rats by efferents of the medial preoptic area. Physiology and Behavior, 53(5), 831–838. 10.1016/0031-9384(93)90258-H [DOI] [PubMed] [Google Scholar]
- Veenman, C.L. , Reiner, A. & Honig, M.G. (1992) Biotinylated dextran amine as an anterograde tracer for single‐ and double‐labeling studies. Journal of Neuroscience Methods, 41(3), 239–254. 10.1016/0165-0270(92)90089-V [DOI] [PubMed] [Google Scholar]
- West, M.J. (2018) Space balls revisited: Stereological estimates of length with virtual isotropic surface probes. Frontiers in Neuroanatomy, 12, 49 10.3389/fnana.2018.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West, M.J. (2013) Estimating length in biological structures. Cold Spring Harbor Protocols, 2013, 412–420. 10.1101/pdb.top071811 [DOI] [PubMed] [Google Scholar]
- Woolley, C.S. (1998) Estrogen‐mediated structural and functional synaptic plasticity in the female rat hippocampus. Hormones and Behavior, 34(2), 140–148. 10.1006/hbeh.1998.1466 [DOI] [PubMed] [Google Scholar]
- Xiao, K. , Kondo, Y. & Sakuma, Y. (2005) Differential regulation of female rat olfactory preference and copulatory pacing by the lateral septum and medial preoptic area. Neuroendocrinology, 81(1), 56–62. 10.1159/000084893 [DOI] [PubMed] [Google Scholar]
- Xu, X. , Coats, J.K. , Yang, C.F. , Wang, A. , Ahmed, O.M. , Alvarado, M. et al (2012) Modular genetic control of sexually dimorphic behaviors. Cell, 148(3), 596–607. 10.1016/j.cell.2011.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue, H.G. , Gai, X.D. , Sun, W.Q. , Li, C. & Liu, Q. (2014) Morphological changes of gonadotropin‐releasing hormone neurons in the rat preoptic area across puberty. Neural Regeneration Research, 9(13), 1303–1312. 10.4103/1673-5374.137578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y. , Shughrue, P.J. & Dorsa, D.M. (1995) Estrogen receptor protein is differentially regulated in the preoptic area of the brain and in the uterus during the rat estrous cycle. Neuroendocrinology, 61(3), 276–283. 10.1159/000126849 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Table S1


