Abstract
Nonhuman primates have a highly diverse locomotor repertoire defined by an equally diverse hand use. Based on how primates use their hands during locomotion, we can distinguish between terrestrial and arboreal taxa. The ‘arboreal’ hand is likely adapted towards high wrist mobility and grasping, whereas the ‘terrestrial’ hand will show adaptations to loading. While the morphology of the forearm and hand bones have been studied extensively, functional adaptations in the forearm and hand musculature to locomotor behaviour have been documented only scarcely. In this paper, we investigate the forelimb musculature of the highly arboreal gibbons (including Hylobates lar,Hylobates pileatus,Nomascus leucogenys,Nomascus concolor and Symphalangus syndactylus) and compare this with the musculature of the semi‐terrestrial rhesus macaques (Macaca mulatta). Anatomical data from previous dissections on knuckle‐walking bonobos (Pan paniscus) and bipedal humans (Homo sapiens) are also included to further integrate the analyses in the scope of catarrhine hand adaptation. This study indicates that the overall configuration of the arm and hand musculature of these primates is very similar but there are some apparent differences in relative size which can be linked to differences in forelimb function and which might be related to their specific locomotor behaviour. In macaques, there is a large development of wrist deviators, wrist and digital flexors, and m. triceps brachii, as these muscles are important during the different phases of palmi‐ and digitigrade quadrupedal walking to stabilize the wrist and elbow. In addition, their m. flexor carpi ulnaris is the most important contributor to the total force‐generating capacity of the wrist flexors and deviators, and is needed to counteract the adducting torque at the elbow joint during quadrupedal walking. Gibbons show a relatively high force‐generating capacity in their forearm rotators, wrist and digital flexors, which are important muscles in brachiation to actively regulate forward movement of the body. The results also stress the importance of the digital flexors in bonobos, during climbing and clambering, and in humans, which is likely linked to our advanced manipulation skills.
Keywords: adaptation, anatomy, hylobatids, locomotion, macaques, primates
This study indicates that the overall configuration of the arm and hand musculature of these primates is very similar but there are some apparent differences in relative size which can be linked to differences in forelimb function and which might be related to their specific locomotor behavior.

1. INTRODUCTION
Primates live in diverse environments and, as a consequence, have an equally diverse locomotor repertoire (Fleagle, Janson & Reed, 1999). As the primate hand interacts with the superstrate and/or substrate during locomotion, its morphology will most likely reflect differences in behaviour (Kikuchi, Takemoto & Kuraoka, 2012). Therefore, it is expected that the primate hand is functionally adapted to its specific use during locomotion. Several studies have indeed shown a relation between locomotion and forelimb muscle properties in different primate taxa. Japanese macaques (Macaca fuscata) exhibit large wrist and digital flexor muscles, possibly as an adaptation for weight bearing during quadrupedal locomotion and forceful grasping of arboreal supports (Ogihara & Oishi, 2012), while the highly arboreal orangutans exhibit elbow flexors with a high potential for force production and forearm muscles that allow a large range of wrist mobility (Oishi, Ogihara, Endo, et al., 2008). Capuchin monkeys show climbing and suspensory behaviour similar to that of chimpanzees, and their deep wrist and digital flexor and extensor muscles show high similarities, suggesting a possible link between locomotor behaviour and forearm musculature (Aversi‐Ferreira, Maior, Carneiro‐e‐Silva, et al., 2011; Ogihara, Kunai & Nakatsukasa, 2005). According to Leischner et al. (2018), arboreal primates have forearm muscles with significantly longer fascicle lengths compared to terrestrial primates, suggesting that arboreal primates are adapted for greater speed and/or flexibility in the trees (Leischner, Crouch, Allen, et al., 2018). Similar results were found by Anapol & Gray (2003), as fibre architecture of the intrinsic shoulder and arm muscles of the semi‐terrestrial vervets is largely suited for higher velocity when running on the ground, while the fibre architecture in red‐tailed guenons implies passive storage of elastic strain energy for exploitation of the forest canopy (Anapol & Gray, 2003).
In contrast, in an earlier study on great ape forelimb musculature (Myatt, Crompton, Payne‐Davis, et al., 2012), no large differences were found in muscle architecture between orangutans, chimpanzees, bonobos and gorillas despite marked differences in locomotor repertoire. This made the authors conclude that a shared evolutionary origin might lead to an overall consistency in muscle architecture. When studying functional adaption in the primate forelimb it is therefore important to take phylogeny into account.
For this study, we selected two primate taxa with a different phylogenetic position (belonging to a different family) and a contrasting locomotor behaviour, namely the semi‐terrestrial rhesus macaques (Fam. Cercopithecidea,Macaca mulatta) and the highly arboreal hylobatids (Fam. Hylobatidae, six different species, further referred to as ‘gibbons’). The aim of the study is to evaluate if anatomical adaptations to locomotor behaviour can be found in their forelimb musculature. The paper provides a full quantification of the gibbon and macaque forelimb muscle architecture and is a sequel of a descriptive paper studying the configuration of the forelimb musculature of the same primate species (Vanhoof, van Leeuwen & Vereecke, 2020).
Macaques are a primarily terrestrial genus, yet different macaque species display a different degree of terrestriality (Kikuchi, 2004; Rodman, 1979). The locomotor repertoire of macaques includes quadrupedal walking, running, climbing and leaping, with quadrupedalism being the dominant locomotor behaviour during travel (Wells & Turnquist, 2001). Hand postures are distinct between macaque species, with rhesus macaques mostly adopting a digitigrade posture (i.e. walking on the palmar side of the digits with the metacarpals elevated off the ground) when walking quadrupedally at slow speeds (Hayama, Chatani & Nakatsukasa, 1994; Patel & Carlson, 2007; Patel, 2009; Patel & Polk, 2010; Richmond, 2006; Schmitt, 2003; Tuttle, 1969; Zeininger, Shapiro & Raichlen, 2017), while Japanese macaques typically adopt a palmigrade posture (i.e. palm of the hand also makes contact with the ground) (Higurashi, Goto & Kumakura, 2018). Nevertheless, rhesus macaques also retain enough mobility at the wrist to use palmigrade postures on arboreal supports, uneven substrates and when walking at high speeds (Patel, 2009). Although macaques are mostly terrestrial, they also engage in arboreal locomotion, using climbing and quadrupedalism (Prime & Ford, 2016). On branches with a large diameter, quadrupedalism is similar to that on the ground. If the diameter of the support decreases, the forelimb joints become more flexed and the hands grasp the support (Dunbar & Badam, 1998; Hayama, Chatani & Nakatsukasa, 1994; Roy, Paulignan, Farnè, et al., 2000; Wells & Turnquist, 2001).
In contrast to macaques, gibbons navigate through the forest canopy primarily by arm‐swinging or brachiation (Michilsens, Vereecke, D’Août, et al., 2010; Orr, 2017; Rein, Harvati and Harrison, 2015; Reichard, Barelli, Hirai, et al., 2016; Tocheri, Orr, Jacofsky, et al., 2008). During brachiation, they can use a highly specialized form of brachiation that includes a true flight phase between each contact with a handhold, called ricochetal brachiation (Chang, Bertram & Lee, 2000; Fleagle, 1976; Prime & Ford, 2016; Reichard, Barelli, Hirai, et al., 2016; Tuttle, 1969; Turnquist, Schmitt, Rose, et al., 1999; Usherwood, Larson & Bertram, 2003). As an adaptation for brachiation, gibbons possess specialized morphological traits, including long arms, slender hook‐like hands with extremely elongated fingers, a unique ball‐and‐socket wrist joint, and specific muscle characteristics (e.g. powerful elbow flexors) (Almécija, Smaers & Jungers, 2015; Bartlett, Light & Brockelman, 2016; Marzke, 2009; Michilsens, Vereecke, D’août, et al., 2009; Michilsens, Vereecke, D’Août, et al., 2010; Reichard, Barelli, Hirai, et al., 2016; Susman, Jungers & Stern, 1982). However, gibbons are not only skilled brachiators, they are also able to use a wide variety of other locomotor modes during arboreal travel, such as bipedalism, quadrupedalism, leaping and vertical climbing (Channon, Günther, Crompton, et al., 2009; Fleagle, 1976; Preuschoft, Schönwasser & Witzel, 2016; Vereecke, D’Août & Aerts, 2006). Within the hylobatid family, there are also some differences in locomotor behaviour, with white‐handed gibbons (Hylobates lar) using more leaping and rapid ricochetal brachiation during travel compared to siamangs (genus Symphalangus) who use more climbing and for whom brachiation is slower and ricochetal brachiation is rare (Fleagle, 1976).
Macaques and gibbons use their hands not only in locomotion but also in manipulation, for example during grooming and foraging. Gibbon hands have a deep cleft separating the thumb from the index finger, allowing their relatively short thumb to be widely opposable and enabling grasping large objects (Prime & Ford, 2016). Compared to gibbons, the macaque hand is more ‘human‐like’, with short fingers and a relatively long opposable thumb which allows high dexterity and even pad‐to‐pad gripping (Kivell, 2015; Moyà‐Solà, Köhler and Rook, 1999; Marzke, 2013), although pad‐to‐side gripping is more commonly used (Feix, Kivell, Pouydebat, et al., 2015; Pouydebat, Gorce, Coppens, et al., 2009). Despite having an opposable thumb, and although captive macaques and gibbons have been observed using tools (Cunningham, Anderson & Mootnick, 2006; Parks & Novak, 1993; Prime & Ford, 2016; Tuttle, 1975), both wild gibbons and rhesus macaques have not been observed to use complex manipulative tasks in daily life (Prime & Ford, 2016; Santos, Miller & Hauser, 2003; Tomasello & Call, 1997), but note that tool use has been observed for long‐tailed macaques (Macaca fascicularis) (Gumert, Kluck & Malaivijitnond, 2009). Even though the gibbon and macaque hand might represent a compromise between locomotor and manipulation functions (Higurashi, Goto & Kumakura, 2018), the high compressive and tensile loads involved in locomotion are expected to have the largest effect on hand morphology (Carlson, Doran‐Sheehy, Hunt, et al., 2006; Dunmore, 2019; Lemelin & Schmitt, 1998; Marzke, 1997; Kikuchi & Hamada, 2009; Orr, 2017; Richmond, 2006). We therefore expect that the differences in locomotor behaviour between gibbons and macaques will lead to differences in upper arm, forearm and hand musculature. There are very few studies about the musculature of the macaque and gibbon forelimb, and most studies use small datasets or report results based on only one primate taxon (Chan & Moran, 2006; Michilsens, Vereecke, D’août, et al., 2009; Ogihara, Makishima, Aoi, et al., 2009). In this paper, newly collected gibbon data including the intrinsic hand muscles are added to the dataset of Michilsens et al. (2009) and compared to newly collected macaque data, as well as to previously published data of bonobos and humans (Michilsens, Vereecke, D’août, et al., 2009; van Leeuwen, Vanhoof, Kerkhof, et al., 2018).
We hypothesize that gibbons will have relatively slender extrinsic hand muscles compared to macaques (i.e. long fascicle lengths), allowing fast contraction and a wide range of motion. In contrast, we expect rhesus macaques to have more bulky extrinsic arm muscles (high physiological cross‐sectional area (PCSA) and short fascicle lengths) to generate large propulsive forces with a more restricted range of motion, as needed in quadrupedalism. Secondly, as the wrist and digital flexors of both gibbons and macaques are continuously active during brachiation and terrestrial digitigrady, respectively (Bertram, 2004; Courtine, Roy, Hodgson, et al., 2005; Fleagle, 1974; Michilsens, D’Août & Aerts, 2011; Patel, Larson & Stern, 2012; Swartz, Bertram & Biewener, 1989), we expect that both primates will have a larger proportion of wrist and digital flexors compared to wrist and digital extensors. Third, due to the importance of rotation during brachiation in gibbons, we hypothesize that the forearm rotators will have a larger PCSA in gibbons than in macaques. Fourth, we expect that the m. triceps brachii will be better developed in macaques than in gibbons as it is important for torque production at the elbow joint during quadrupedal walking (Manter, 1938), while in gibbons, we expect that the m. biceps brachii will be stronger developed than in macaques given its important function as elbow flexor during brachiation (Jungers & Stern, 1980; Michilsens, Vereecke, D’août, et al., 2009). Finally, we hypothesize that the flexor muscles of gibbons will have relatively longer tendons compared to those of macaques. One of the crucial correlates with brachiation appears to lie in flexor tendonization (Corruccini, 1978) (i.e. tendon length relative to muscle‐tendon‐unit length) as these relatively longer tendons can act as elastic springs, facilitating storage and release of elastic strain energy during brachiation (Alexander, 2002; Michilsens, Vereecke, D’août, et al., 2009; Usherwood, Larson & Bertram, 2003). Given the higher amount of brachiation in white‐handed gibbons compared to siamangs, we also predict relatively longer tendons in the flexor muscles in the genus Hylobates compared to the genus Symphalangus.
2. METHODS
2.1. Specimen collection
The data presented in this study are based on a detailed dissection of upper arm, forearm and hand of eight hylobatid specimens, belonging to six species within the family Hylobatidae (Hylobates lar,Hylobates pileatus,Hylobates moloch,Nomascus leucogenys,Nomascus concolor and Symphalangus syndactylus), further referred to as ‘gibbons’, and seven rhesus macaque specimens (Macaca mulatta, Fam. Cercopithecidae), further referred to as ‘macaques’. Both gibbons and macaques have a different phylogenetic position relative to modern humans and were selected because of their distinct locomotor behaviour. The gibbon specimens were obtained via collaborations with different European Zoos and institutes: the National Museum of Scotland (Edinburgh, UK), Ghent University (campus Merelbeke, Belgium), the Zoological and Botanical Park of Mulhouse (France), Pakawi Park (Belgium). The rhesus macaque specimens were obtained via collaboration with Ghent University (campus Merelbeke, Belgium). Both the macaque and gibbon specimens were housed in large enclosures and were still able to adopt their preferred locomotor behaviour. All specimens were collected opportunistically, no animals were sacrificed for this study. The raw data of the forearm musculature of 10 gibbon specimens collected in the scope of an earlier publication (Michilsens, Vereecke, D’août, et al., 2009) are also included in the analyses as these were collected using the same methodology. The entire gibbon dataset (n = 18) and macaque dataset (n = 7) is compared with the anatomical data of five bonobos (Pan paniscus) and one human cadaver (Homo sapiens) obtained in a previous study (van Leeuwen, Vanhoof, Kerkhof, et al., 2018). The specimen details are provided in Table 1.
TABLE 1.
Specimen details
| Code | Species | Sex | Age | Injury | Sample | Collection |
|---|---|---|---|---|---|---|
| Hl1 | Hylobates lar | F | young* | X, + | L | NMS, Edinburgh, UK |
| Hl2 | Hylobates lar | U | young* | X, + | R | NMS, Edinburgh, UK |
| Hl3 | Hylobates lar | U | young* | R | Ghent University, campus Merelbeke, Belgium | |
| Hl4 | Hylobates lar | M | young* | U | RZSA, Antwerp, Belgium | |
| Hl5 | Hylobates lar | M | adult | U | NMS, Edinburgh, UK | |
| Hp1 | Hylobates pileated | M | adult | X, + | R | NMS, Edinburgh, UK |
| Hp2 | Hylobates pileated | M | adult | U | NMS, Edinburgh, UK | |
| Hp3 | Hylobates pileated | F | adult | U | NMS, Edinburgh, UK | |
| Hm1 | Hylobates moloch | M | adult | U | NMS, Edinburgh, UK | |
| Hm2 | Hylobates moloch | M | adult | U | NMS, Edinburgh, UK | |
| Nc1 | Nomascus concolor | M | adult | L | Pakawi Park, Belgium | |
| Nl1 | Nomascus leucogenys | M | adult | R | Zoological and Botanical Park of Mulhouse, France | |
| Ss1 | Symphalangus syndactylus | M | adult | X | R | RZSA, Antwerp, Belgium |
| Ss2 | Symphalangus syndactylus | M | adult | X | L | NMS, Edinburgh, UK |
| Ss3 | Symphalangus syndactylus | F | adult | U | RZSA, Antwerp, Belgium | |
| Ss4 | Symphalangus syndactylus | F | young* | U | NMS, Edinburgh, UK | |
| Ss5 | Symphalangus syndactylus | F | adult | U | NMS, Edinburgh, UK | |
| Ss6 | Symphalangus syndactylus | M | adult | U | NMS, Edinburgh, UK | |
| Mm1 | Macaca mulatta | U | adult | R | Ghent University, campus Merelbeke, Belgium | |
| Mm2 | Macaca mulatta | F | adult | DP 4 | L | Ghent University, campus Merelbeke, Belgium |
| Mm3 | Macaca mulatta | U | adult | L | Ghent University, campus Merelbeke, Belgium | |
| Mm4 | Macaca mulatta | U | adult | L | Ghent University, campus Merelbeke, Belgium | |
| Mm5 | Macaca mulatta | U | adult | PIP 3 joint | L | Ghent University, campus Merelbeke, Belgium |
| Mm6 | Macaca mulatta | M | adult | L | Ghent University, campus Merelbeke, Belgium | |
| Mm7 | Macaca mulatta | U | adult |
IP 2 and 3 joints |
R | KU Leuven, campus Gasthuisberg, Belgium |
*, (young) subadult based on presence of unfused growth plates; +, tendons extrinsic muscles damaged due to skinning post‐mortem; DP, distal phalanx; F, female; IP, interphalangeal joint; M, male; Nl1, wild born, new data (other data from Michilsens et al. (2009); NMS, National Museum of Scotland; PIP, proximal interphalangeal joint; RZSA, Royal Zoological Society of Antwerp; U, unknown; X, thenar muscles damaged due to skinning post‐mortem.
2.2. Dissection procedure
We performed a detailed dissection on the forelimb and hand of the primate specimens, using the procedure described in Vanhoof et al. (Vanhoof, van Leeuwen & Vereecke, 2020). All specimens were stored at −18°C and were thawed at room temperature 24 hr before starting the dissection. To quantify muscle architecture, the following parameters were measured for each muscle (Lieber & Fridén, 2000): (a) muscle mass (m); (b) muscle volume (V); (c) muscle‐tendon‐unit length (MTU), measured from the most proximal muscle fibres or tendon to the most distal muscle fibres or tendon; (d) muscle fascicle length (FL), which is the approximate length of the muscle fibres; (e) external tendon length (ETL), the distance from the most distal muscle fibres to the end of the tendon, and (f) internal tendon length (ITL), the part of the tendon enveloped by muscle fibres. Length measurements are taken to the nearest 0.1 mm with a digital calliper (Mitutoyo, UK, accurate to 0.01 mm) and muscle volume is determined to the nearest 0.1 ml by submersion in physiological saline solution (0.9% NaCl). Muscles are cut lengthwise along the tendon to determine muscle fascicle length and tendon length. The data provided for fascicle length are average values of at least three measurements taken on different places along the muscle belly. FL was measured as this value is needed to calculate physiological cross‐sectional area (PCSA; see below). Moreover, FL can give us information about muscle function (Lieber & Fridén, 2000): long fascicle lengths allow fast contraction and large excursions at low force, while shorter fascicle lengths in a pennate organization can generate large propulsive forces with small excursion.
2.3. Data analysis
Physiological cross‐sectional area (PCSA) of a muscle is calculated using Equation (1).
| (1) |
PCSA is related to the force‐generating capacity of a muscle and is therefore a more functionally relevant parameter to report than muscle mass (Lieber & Fridén, 2000). We chose to omit pennation angle (PA, the angle between a fascicle's orientation and the internal tendon axis (Lee, Li, Sohail, et al., 2015)) from the PCSA equation as (a) there were difficulties in obtaining accurate PA measurements during the dissections, (b) the in vitro measurements are not fully representative of the PA in vivo given that PA changes during muscle contraction and (c) the PA of most muscles ranges between 0 and 30 degrees, the cosine of which ranges between 1 and 0.87, having only a minor influence on PCSA calculation (Payne, Crompton, Isler, et al., 2006; Vereecke, D’Août, Payne, et al., 2005; van Leeuwen, Vanhoof, Kerkhof, et al., 2018). If muscles consisted of multiple muscle bellies that were easily separable (e.g. m. triceps brachii, m. flexor digitorum superficialis), the PCSA was calculated as the sum of the PCSA of the separate muscle bellies.
For the small intrinsic hand muscles we were not able to accurately determine the muscle volume using the submersion method. Therefore, we calculated the muscle density only for the extrinsic muscles of all specimens using Equation (2).
| (2) |
For both gibbons and macaques, the average muscle density is 0.0011 g/mm3 (SD <0.0001 g/mm3), which is almost equal to the density defined for human muscles (0.00106 g/mm3) (Ward and Lieber, 2005). Therefore, the density value of 0.0011 g/mm3 is used in the calculation of the PCSA for all muscles in this study.
To calculate the relative length of tendons, Equation (3) is used.
| (3) |
with total tendon length (TTL) being the sum of ETL and ITL. This measure allows us to investigate ‘tendonization’ of muscles, and was calculated for the inserting tendons as these are typically most pronounced.
To facilitate comparison between gibbons and macaques, we categorized the muscles into functional groups with respect to their main function at the elbow, wrist and fingers (Table 2). The m. biceps brachii and m. triceps brachii were only listed as elbow flexor and extensor, respectively, as we did not measure other shoulder muscles. Scaling of the anatomical data was necessary as the primate sample included specimens of different size (ranging from 4.5 kg for small white‐handed gibbons to adult male siamangs of 12 kg). Body mass at time of death was unknown for most specimens, therefore scaling was done using total arm, forearm or hand muscle mass. The PCSAs of the m.biceps and triceps brachii, and the rotators were scaled to the total arm PCSA (see Table 2). For the forearm muscles, the PCSA of the other functional muscle groups was scaled to the total PCSA of all extrinsic forearm muscles. For the intrinsic hand muscles, the PCSA was scaled to the total PCSA of all intrinsic hand muscles. The FL was scaled to the total forearm muscle mass to one third (FLMM1/3) (Channon, Günther, Crompton, et al., 2009). In addition, we calculated a set of dimensionless ratios (i.e. ratio of wrist flexors to wrist extensors, ratio of radial deviators to ulnar deviators, …) that allow comparison of relevant anatomical traits between different‐sized animals.
TABLE 2.
List of functional muscle groups and their abbreviations
| Muscle group | Muscle | Abbreviation |
|---|---|---|
| Upper arm muscles | ||
| Shoulder flexor | m. coracobrachialis | CB |
| Elbow flexors | m. biceps brachii | Bb |
| m. brachialis | B | |
| m. brachioradialis | BR | |
| Elbow extensors | m. triceps brachii | Tb |
| m. dorso‐epitrochlearis | DET | |
| Forearm musculature | ||
| Wrist flexors | m. flexor digitorum superficialis | FDS |
| m. flexor digitorum profundus | FDP | |
| m. flexor carpi radialis | FCR | |
| m. flexor carpi ulnaris | FCU | |
| m. palmaris longus | PL | |
| Digital flexors | m. flexor digitorum superficialis | FDS |
| m. flexor digitorum profundus | FDP | |
| m. flexor pollicis longus | FPL | |
| Wrist extensors | m. extensor digitorum communis | EDC |
| m. extensor digitorum brevis | EDB | |
| m. extensor carpi radialis longus | ECRL | |
| m. extensor carpi radialis brevis | ECRB | |
| m. extensor digiti minimi | EDM | |
| m. extensor carpi ulnaris | ECU | |
| m. extensor indicis | EI | |
| m. extensor digiti secundi et tertii proprius | EDST | |
| m. extensor digiti quarti et quinti proprius | EDQQ | |
| Digital extensors | m. extensor digitorum communis | EDC |
| m. extensor digitorum brevis | EDB | |
| m. extensor digiti minimi | EDM | |
| m. extensor indicis | EI | |
| m. extensor pollicis longus | EPL | |
| m. extensor digiti secundi et tertii proprius | EDST | |
| m. extensor digiti quattro et quinti proprius | EDQQ | |
| Arm rotators | m. pronator teres | PT |
| m. pronator quadratus | PQ | |
| m. supinator | SUP | |
| m. brachioradialis | BR | |
| m. biceps brachii | Bb | |
| Radial deviators | m. flexor carpi radialis | FCR |
| m. extensor carpi radialis longus | ECRL | |
| m. extensor carpi radialis brevis | ECRB | |
| Ulnar deviators | m. flexor carpi ulnaris | FCU |
| m. extensor carpi ulnaris | ECU | |
| Extrinsic thumb | m. abductor pollicis longus | APL |
| m. extensor pollicis longus | EPL | |
| Intrinsic hand muscles | ||
| Thenar | m. flexor pollicis brevis | FPB |
| m. abductor pollicis brevis | APB | |
| m. adductor pollicis | ADP | |
| m. opponens pollicis | OPP | |
| Intermediate | m. intermetacarpalis I, II, III, IV | IM |
| m. flexor brevis profundi III, IV, V, VI, VII, IIX, IX | FBP | |
| m. interosseous dorsalis I, II, III | IOD | |
| m. lumbricalis II, III, IV, V | LUMB | |
| m. lumbricalis accessorius | LUMBa | |
| m. contrahens 2 | C2 | |
| m. contrahens 4 | C4 | |
| m. contrahens 5 | C5 | |
| m. contrahentes digitorum | CD | |
| Hypothenar | m. palmaris brevis | PB |
| m. abductor digiti minimi | ADM | |
| m. flexor digiti minimi | FDM | |
| m. opponens digiti minimi | ODM | |
2.4. Statistical analysis
For all relevant parameters, an analysis of variance (ANOVA) was used to test for significant differences between the primate groups and Tukey HSD tests were used for pairwise post‐hoc comparisons. All statistical analyses were run in R (version 4.0.2), and the significance value was set at 0.05. Within the hylobatids, no significant differences were found for all tested parameters. Therefore, all hylobatids were taken together as one group in the analyses and box plots.
3. RESULTS
The muscle parameters discussed below are based on the analysis of the newly collected data from the macaque (n = 7) and gibbon (n = 8) sample, and are supplemented by new analyses of previously published data on the forelimb anatomy of gibbons (n = 10) (Michilsens, Vereecke, D’août, et al., 2009), bonobos (n = 5) and humans (n = 1) (van Leeuwen, Vanhoof, Kerkhof, et al., 2018). The different functional muscles groups and their associated muscles and abbreviations can be found in Table 2. Detailed documentation of the raw muscle parameters discussed below is provided in the supplementary material (Tables S1 and S2). In the graphical presentation of the results, siamang data are presented using a different symbol than the other gibbons because of their markedly higher size and body weight, and the differences in locomotor behaviour compared to the other hylobatids.
3.1. Characteristics of the upper arm and forearm muscles
Below, we present the results on FL, PCSA and tendonization of the forelimb muscles of the studied specimens. Unless stated otherwise, values given are always group means and standard deviation (SD).
3.1.1. Fascicle length
There is no significant difference between macaques, gibbons, and bonobos for the scaled FL of the wrist flexors (0.32 vs. 0.51 vs. 0.33), wrist extensors (0.40 vs. 0.48 vs. 0.38) and radioulnar deviators (0.42 vs. 0.48 vs. 0.35) (Figure 1a‐c). For the rotators there is no significant difference between macaques and gibbons (0.60 vs. 0.74) (p > .05) (Figure 1d). In macaques, the scaled FL of the rotators is significantly longer than that of their flexors (p < .001), extensors (p < .001) and deviators (p < .01) (Figure 1e). In gibbons, the scaled FL of the rotators also appears longer than that of the other functional muscle groups, but this difference is not significant (p > .05) (Figure 1f). In bonobos, no significant difference is found between the functional muscle groups (p > .05) (Figure 1g).
FIGURE 1.
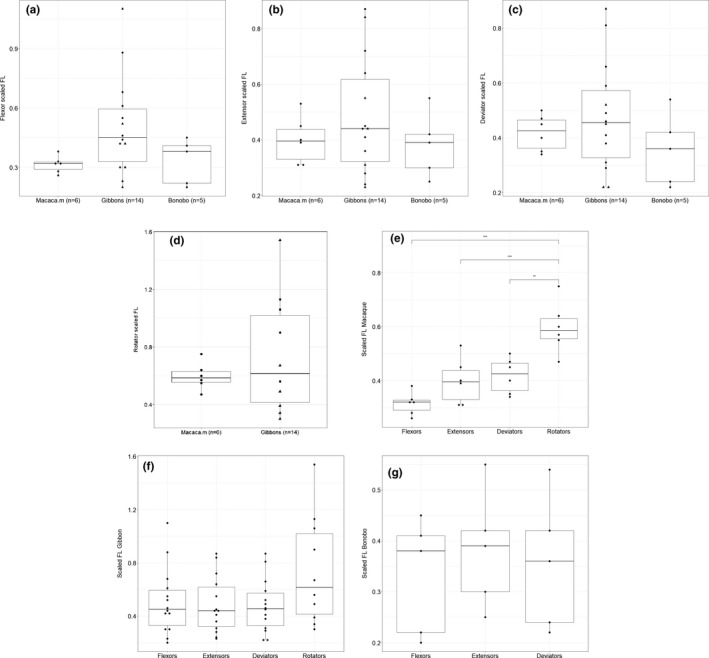
Boxplot of the scaled fascicle length (FL) of the (a) flexors, (b) extensors, (c) deviators and (d) rotators. Within the hylobatid family, the triangles represent the siamangs. For none of these groups, there is a significant difference between macaques, gibbons and bonobos. (e) In macaques, the scaled FL of the rotators is significantly different from that of their flexors (p < .001), extensors (p < .001) and deviators (p < .01). In gibbons (f) and bonobos (g), the scaled FL of the functional muscle groups are not significantly different from one another (p > .05)
3.1.2. Elbow flexors and extensors
The ratio of elbow flexors over elbow extensors is significantly lower in macaques (0.40, SD: 0.07) compared to gibbons (1.35, SD: 0.35) (p < .001) (Figure 2a). We can observe that the m.triceps brachii (Tb) has a significantly higher PCSA, as proportion of the total arm PCSA, in macaques (30.7%, SD: 2.8%) compared to gibbons (15.0%, SD: 4.9%) (p < .001) (Figure 2b), while the m.biceps brachii (Bb) is somewhat larger in gibbons (8.9%, SD: 2.1%) than in macaques (6.6%, SD: 1.0%) (p < .05) (Figure 2c).
FIGURE 2.
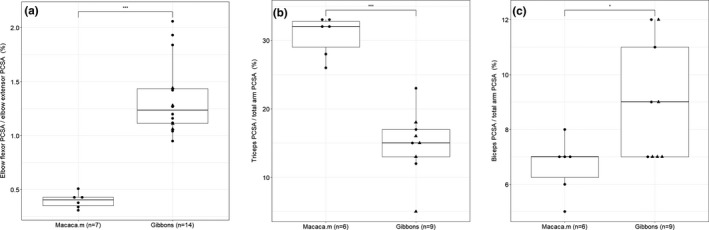
(a) Boxplot of the ratio of elbow flexors over elbow extensors is low in macaques compared to gibbons (p < .001). (b, c) Boxplots of the relative PCSA of the biceps and triceps brachii. Within the hylobatid group, the triangles represent the siamangs. (b) The m. triceps brachii has a significantly higher PCSA in macaques compared to gibbons (p < .001). (c) The m. biceps brachii has a slightly higher relative PCSA in gibbons compared to macaques (p < .05)
3.1.3. Wrist and digital flexors and extensors
Both macaques and gibbons have a high ratio of wrist flexors over wrist extensors (3.1 (SD: 0.4) and 3.7 (SD: 0.8), resp.) (p > .05), yet there is an apparent variability in gibbons (range: 2.0‐5.0). The flexor to extensor ratio is only significantly different between gibbons and humans (1.5), with gibbons having a significantly higher flexor to extensor ratio (p < .05) (Figure 3a). For the relative PCSA of wrist flexors as percentage of the total forearm muscle PCSA, we observe a similar proportion of wrist flexor PCSA in macaques and gibbons (p > .05) (Figure 3b), accounting for more than half of the forearm muscle PCSA (macaques: 55.0% (SD: 2.0%) and gibbons: 56.6% (SD: 4.5%)). In contrast, the extensor PCSA only makes up less than 20% of the forearm muscle PCSA (macaques; 18.0% (SD: 2.0%), gibbons: 16.5% (SD: 3.8%), resp.). Significant differences in relative proportion of wrist flexor PCSA can, however, be observed between the other primate taxa (gibbon‐human: p < .001, gibbon‐bonobo: p < .01, macaque‐human: p < .01, macaque‐bonobo: p < .05) (Figure 3b). When looking at the relative PCSA of different flexors, we also observe some interesting differences between the four taxa. In macaques, the PCSA of the digital flexors makes up 54.0% (SD: 3.0%) of the total wrist flexor PCSA, which is significantly lower compared to gibbons (73.4%, SD: 7.6% (p < .001)) and humans (86.1%, p < .01), but not to bonobos (65.0%, SD: 13.9% (p > .05)). In macaques, the PCSA of the m. flexor carpi ulnaris (FCU) makes up on average 27.3% (SD: 3.7%) of the total wrist flexor PCSA, which is significantly higher compared to gibbons (9.4%, SD: 2.7% (p < .001)), bonobos (21.0%, SD: 3.6% (p < .05)) and humans (11.4%, p < .01).
FIGURE 3.
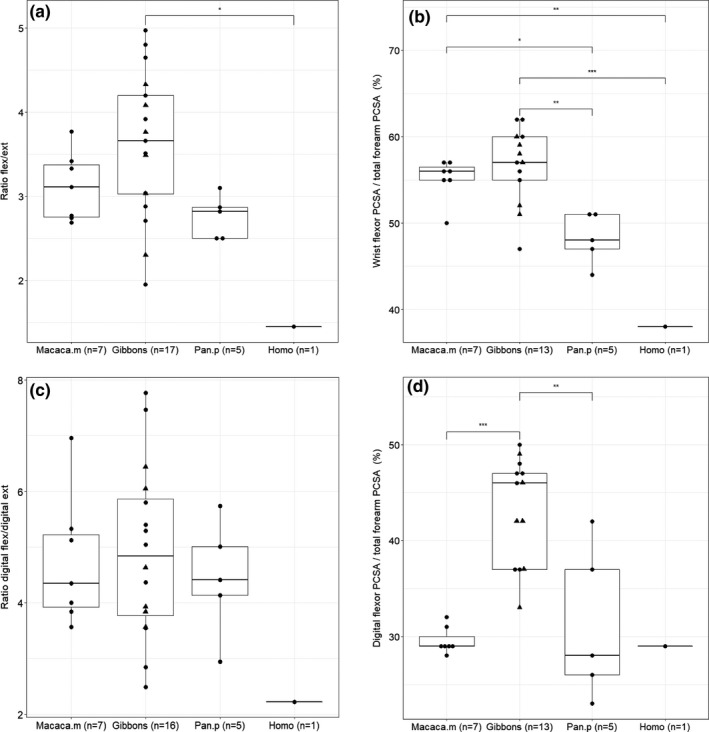
Boxplot of the relative size of the flexors in the forearm. Within the hylobatid group, the triangles represent the siamangs. (a) The ratio of flexors over extensors is significantly higher in gibbons compared to humans (p < .05); (b) the relative PCSA of the wrist flexors is similar in macaques and gibbons (p > .05), while significant differences can be observed between the other primate taxa; (c) the ratio of digital flexors over digital extensors is similar in gibbons, macaques and bonobos; (d) the relative PCSA of the digital flexors is significantly higher in gibbons compared to macaques (p < .001) and bonobos (p < .01)
The ratio of digital flexors over digital extensors is not significantly different between the different primate taxa, with gibbons, macaques and bonobos having a high ratio (4.9 (SD: 1.5), 4.7 (SD: 1.2) and 4.5 (SD: 1.1), resp.), while the ratio of humans is much smaller (2.2) (Figure 3c). For the PCSA of the digital flexors as proportion of the forearm PCSA, we can observe that the digital flexors have a significantly higher relative PCSA in gibbons (43.7%, SD: 5.5%) compared to macaques (29.6%, s.d: 1.4%; p < .001) and bonobos (31.2%, SD: 8.0%; p < .01), with macaques, bonobos and humans showing a similar proportion of digital flexors (Figure 3d). The PCSA of the digital extensors accounts for on average 54.3% (SD: 9.9%) of the total extensor PCSA in gibbons, which is comparable to humans (56.3%), and is significantly different from macaques (36.3%, SD: 4.1%; p < .001) and bonobos (41.4%, SD: 8.8%; p < .05). In macaques and bonobos the PCSA of the m.extensor carpi radialis longus (ECRL) and m.extensor carpi radialis brevis (ECRB) accounts for more than 40% of the total extensor PCSA (43.4% and 40.6%, resp.), while in gibbons and humans the digital extensors have the largest PCSA of the extensor group (48.8% and 46.3%, resp.).
3.1.4. Wrist deviators
The wrist deviators have a significantly larger PCSA in macaques (38.3%, SD: 3.3%) compared to gibbons (25.4%, SD: 5.2%; p < .001). The wrist deviator PCSA of bonobos (30.7%, SD: 4.8%) and humans (32.9%) falls in between the macaque and gibbon values, but only the bonobos are significantly different from macaques (p < .05) (Figure 4a). In macaques, the FCU is the most important contributor to the total wrist deviator PCSA (38.9%) and has a much higher PCSA compared to the m.flexor carpi radialis (FCR) (17.3%) (p < .001). In gibbons and bonobos, however, the PCSA of FCU (21.4% and 31.7%, resp.) and FCR (26.1% and 26.3, resp.) are very similar, together accounting for more than half of the total wrist deviator PCSA. Humans are notably different in this aspect, as the extensors (ECU (23.9%), ECRL (22.0%), ECRB (22.0%)) make up the largest proportion of the wrist deviator PCSA.
FIGURE 4.
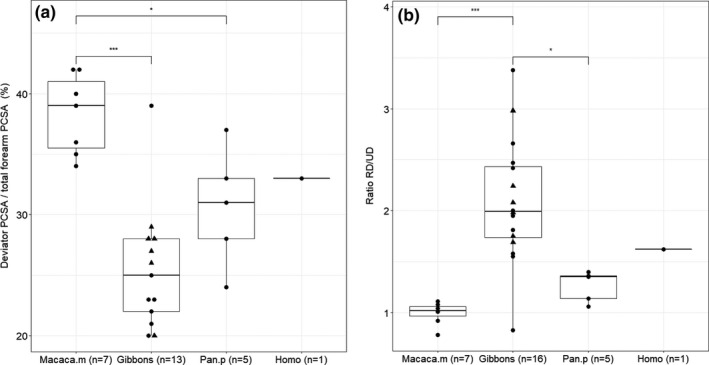
Boxplot of the relative size of the radioulnar deviators. Within the hylobatid group, the triangles represent the siamangs. (a) The wrist deviators have a significantly higher PCSA in macaques compared to gibbons (p < .001) and bonobos (p < .05); (b) the ratio of radial deviators over ulnar deviators of gibbons is significantly higher than the ratio observed in macaques (p < .001) and bonobos (p < .05)
The ratio of radial deviators (RD) over ulnar deviators (UD) is 1.0 in macaques, with RD and UD having a similar PCSA relative to the total forearm muscle PCSA (19.0% (sd.: 1.6%) and 19.3% (SD: 2.3%), resp.). Gibbons, on the other hand, have a high RD/UD ratio of on average 2.1 (SD: 0.61), which is significantly higher than the ratio observed in macaques (p < .001) and bonobos (1.3) (p < .05) (Figure 4b). This is mainly due to the small PCSA of the UD relative to total forearm PCSA in gibbons (10.4%, SD: 5.7%), whereas the RD (16.6%, SD: 2.4%) have a similar PCSA as seen in macaques. In humans, the ratio of RD over UD is 1.6 but this is not significantly different from that of macaques, gibbons or bonobos.
3.1.5. Forearm rotators
The proportion of rotator PCSA is higher in gibbons (23.2%, SD: 2.8%) compared to macaques (17.7%, SD: 1.8%), and this difference is highly significant (p < .01) (Figure 5). In both macaques and gibbons, the Bb is the most important contributor to total rotator PCSA (37% and 41%, respectively), while the m.bracioradialis (BR) only accounts for 10% of the total rotator PCSA. In both primates, supination is the dominant movement as the supinator muscles account for more than 65% of the total rotator PCSA.
FIGURE 5.
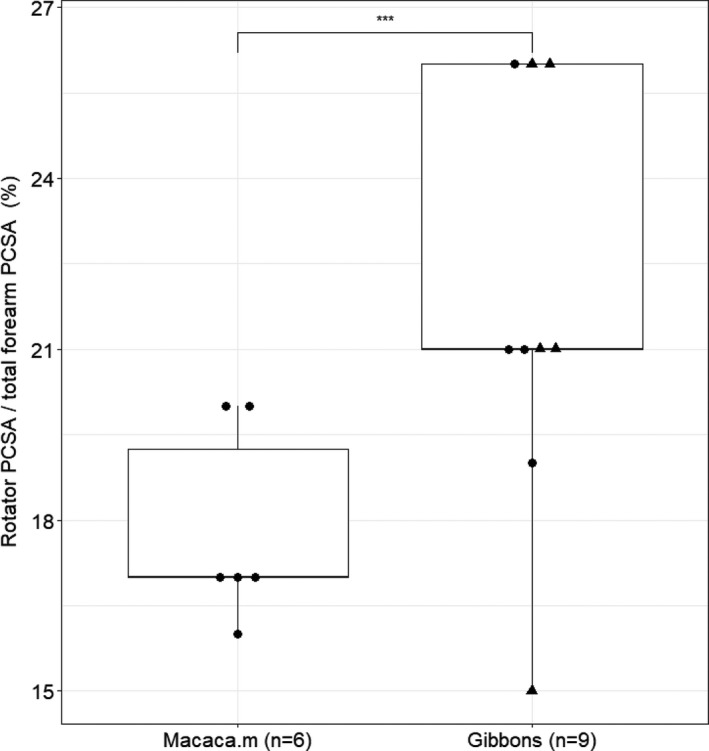
Boxplot of the relative PCSA of the forearm rotators. Within the hylobatid group, the triangles represent the siamangs. The proportion of the rotator PCSA is significantly higher in gibbons compared to macaques (p < .01)
3.1.6. Tendonization
The relative length of the flexor tendons (‘tendonization’) is significantly longer in gibbons (80.1%, SD: 7.7%) compared to that of bonobos (68.8%, SD: 7.3%; p < .05), while there is no significant difference between gibbons and macaques (70.8%, SD: 4.4%; p > .05) or humans (65.0%; p > .05) (Figure 6a). There is also no significant difference between the relative length of the tendons of white‐handed gibbons (Hylobates lar) compared to siamangs (Symphalangus syndactylus) (p > .05) (Figure 6b).
FIGURE 6.
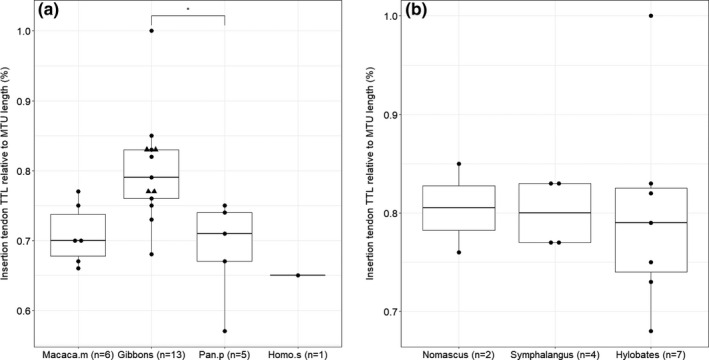
Results on tendonization of the flexor muscles. Within the hylobatid family, the triangles represent the siamangs. (a) The relative length of the tendons is significantly longer in gibbons compared to bonobos (p < .05) but not to macaques (p > .05); (b) within the hylobatid family, there is no significant difference between the relative length of the tendons between the different genera (Nomascus, Hylobates, Symphalangus) (p > .05)
3.2. Characteristics of the intrinsic hand muscles
The proportion of intrinsic hand muscle PCSA relative to total forearm muscle PCSA is remarkably similar between macaques (14.7%, SD: 3.2%), gibbons (14.5%, SD: 4.2%), humans (14.5%) and bonobos (18.4%, SD: 4.6%) (p > .05). The composition of the intrinsic hand muscles is similar in gibbons and macaques, with a dominant development of the intermediate hand muscles (~59% and ~51% of hand muscle PCSA, respectively), while the thenar PCSA takes up approximately 30% of the total intrinsic PCSA, and the hypothenar muscle amounting to only 10.0% and 17.5% of the hand muscle PCSA. In bonobos, the intermediate hand muscles take up a slightly larger proportion of the total intrinsic PCSA (66.1%, SD: 4.6%), although this is not significantly different compared to the other primate groups, while in humans the thenar PCSA is significantly more prominent (46.7%) compared to bonobos (p < .05) (Figure 7).
FIGURE 7.
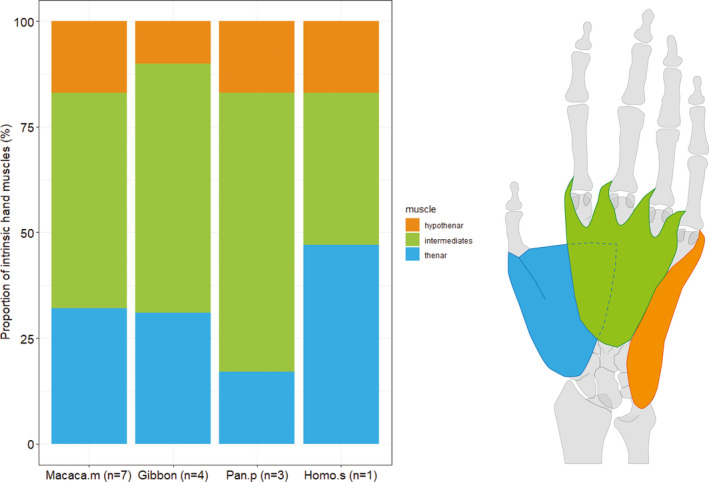
The composition of the intrinsic hand muscles is very similar in gibbons and macaques, with a dominant development (%PCSA) of the intermediate hand muscles (~59% and ~51% respectively), the thenar PCSA taking up approximately 30% of the total intrinsic PCSA and the hypothenar muscle PCSA amounting to only 10% and 18%. In bonobos, the intermediate hand muscles take up a larger proportion of the total intrinsic PCSA (~66%), while in humans, the thenar PCSA is relatively more prominent (~47%). The proportion of the intrinsic hand muscle PCSA relative to total forearm muscle PCSA is 14.7% in macaques, 14.5% in gibbons and humans and 18.4% in bonobos (p > .05)
4. DISCUSSION
In this study, the forelimb musculature of macaques and gibbons is compared based on a detailed quantification of their forelimb muscle architecture. Anatomical data from previous dissections of different gibbon species (Michilsens, Vereecke, D’août, et al., 2009) are included to increase the sample size, and these data are compared with anatomical data of bonobos and humans (van Leeuwen, Vanhoof, Kerkhof, et al., 2018) to allow a broader functional comparison of the forelimb musculature. The results are summarized in Figure 8.
FIGURE 8.
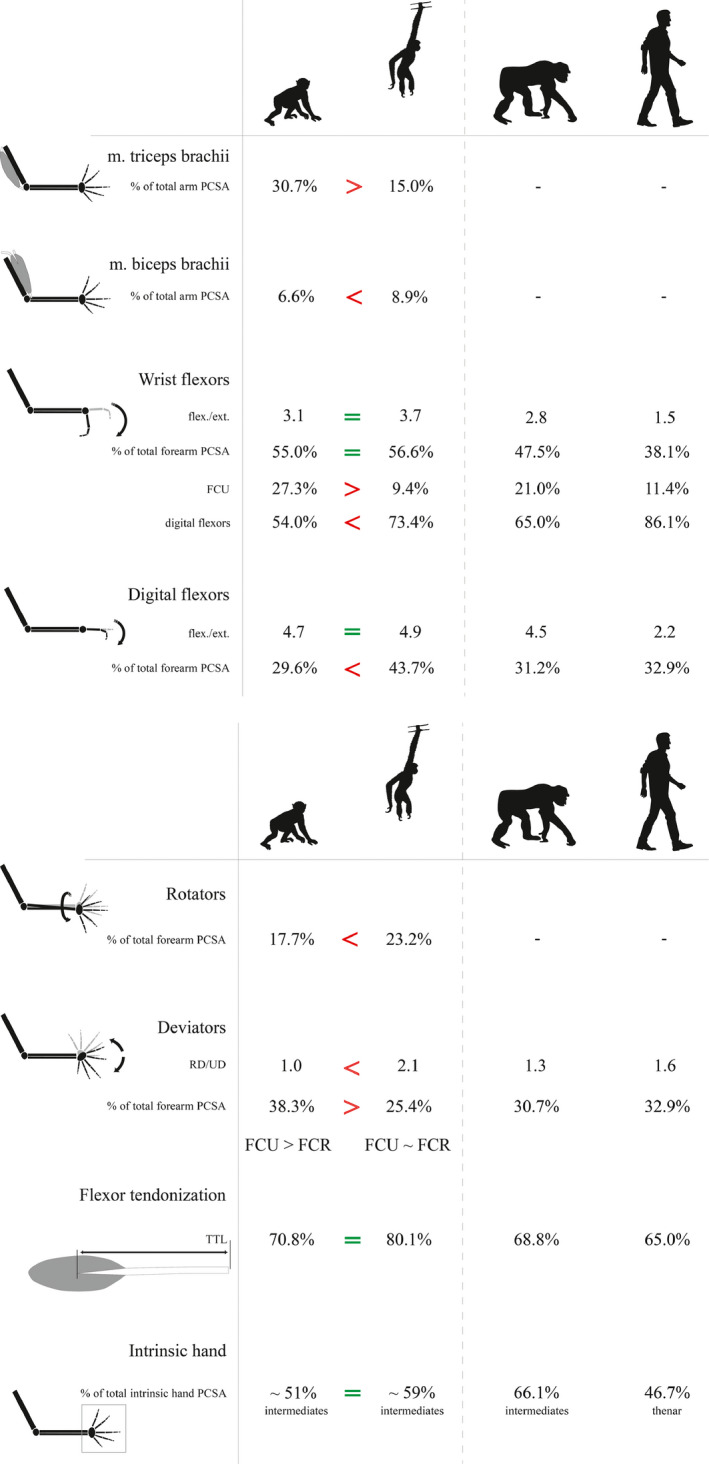
Overview of the different muscle parameters that were measured for this study and the corresponding values for macaques, gibbons, bonobos and humans. Significant differences are only shown between macaques and gibbons
4.1. Fascicle length
Due to the different locomotor behaviour of gibbons and macaques, we hypothesized that gibbons would have relatively slender forearm muscles, with a relatively long FL and high tendonization, compared to macaques, for which we expected more bulky forearm muscles, with shorter FL and a higher PCSA. However, we found no significant difference in FL when comparing the functional muscle groups (i.e. the wrist flexors, wrist extensors, radioulnar deviators and rotators) between both primates. In contrast, Leischner et al. (2018) found a difference in relative fascicle lengths between terrestrial and arboreal primates (Leischner, Crouch, Allen, et al., 2018). This might be explained in the context of inertia, as we only look at distal forelimb muscles. Forearms that are too muscular would be energetically inefficient for quadrupeds like macaques. Myatt, Crompton, Payne‐Davis, et al. (2012) also showed that FL were generally longer in the proximal muscles of the forelimb in great apes (Myatt, Crompton, Payne‐Davis, et al., 2012), so maybe larger differences can be found in the FL of the macaque and gibbon upper arm musculature. Another reason for not finding a difference between the FL of gibbons and macaques is that we looked at functional muscle groups, not at differences between individual muscles. When individual muscles are compared, we find that gibbons do have significantly longer FL in the m.biceps brachii (Bb), m.flexor digitorum superficialis (FDS), m.supinator (SUP) and m.palmaris longus (PL) compared to macaques, while macaques show longer FL only for the m.brachioradialis (BR) and m.extensor carpi radialis longus (ECRL). The lack of differences in the other muscles might have singled out a difference at the level of the muscle groups. When looking at the different primate taxa, we can see that in macaques the rotator FL is significantly different from the other functional muscles groups, while this is not observed for gibbons, bonobos and humans. The long FL of the rotators in macaques might be important for running at high speeds on the ground, while the long FL of the different individual muscles in gibbons might enable high speed and flexibility in the trees during brachiation (Anapol & Gray, 2003; Leischner, Crouch, Allen, et al., 2018). Nevertheless, the functional interpretation of these results remains difficult.
4.2. Elbow flexors and extensors
The ratio of elbow flexors over extensors is significantly lower in macaques compared to gibbons due to the significantly larger PCSA of the m.triceps brachii (TB) in macaques compared to gibbons. This can be understood from the quadrupedal gait mechanics, as the TB is recruited during the first three quarters of a step to produce the torque at the elbow joint (Manter, 1938; Demes, Stern, Hausman, et al., 1998). As predicted, the m.biceps brachii PCSA is higher in gibbons compared to macaques, which is likely related to its important function as elbow flexor during brachiation (Michilsens, Vereecke, D’août, et al., 2009; Reichard, Barelli, Hirai, et al., 2016). Moreover, in gibbons, the origin of the short head of the Bb attaches on the lesser tubercle of the humerus and, as it is mono‐articular, it can be fully recruited for elbow flexion which might be an adaptation to brachiation during which the arms are used to hoist the body by extending the arm at the shoulder and flexing it at the elbow (Michilsens, Vereecke, D’août, et al., 2009). Note that the PCSA value for the elbow extensors of three gibbon specimens is likely a slight underestimation as the contribution of the DET, which is also an elbow extensor and inserts onto the oleocranon in these three specimens, is not accounted for.
4.3. Wrist and digital flexors and extensors
Both gibbons and macaques show a proportion of wrist flexor PCSA that is approximately three times larger than the wrist extensor PCSA, and a proportion of digital flexor PCSA that is more than four times larger than the digital extensor PCSA. Such configuration is also seen in bonobos, while in modern humans the extensors are more prominent and these ratios are much smaller (wrist flexor/extensor: 1.45; digital flexor/extensor: 2.22). The wrist flexor PCSA makes up more than half of the forearm muscle PCSA in macaques and gibbons, and this proportion is significantly higher compared to bonobos and humans. The relatively large flexor PCSA in macaques, combined with a small FL gives a high force‐generating capacity which can be related to their locomotor behaviour as the wrist and digital flexors are continuously active during terrestrial digitigrady (Courtine, Roy, Hodgson, et al., 2005; Patel, Larson & Stern, 2012). In gibbons, the flexors have relatively longer FLs and together with the high flexor PCSA this results in a capacity to produce high power, whereby these muscles are capable of producing high levels of work over a wider range of motion. Being able to produce high power is probably necessary to counteract the gravitational forces during brachiation (Bertram, 2004; Michilsens, D’Août & Aerts, 2011; Swartz, Bertram & Biewener, 1989), while moving the limbs over a wide range of motion during the rapid locomotion of gibbons during brachiation likely has advantages for reaching a branch and avoiding a fall (Channon, Günther, Crompton, et al., 2009; Channon, Crompton, Günther, et al., 2010; Oishi, Ogihara, Endo, et al., 2008). The relatively lower PCSA of the wrist flexors in bonobos and humans compared to macaques and gibbons might indicate that bonobos and humans rely less on wrist flexion, although the higher flexor to extensor ratio in bonobos compared to humans indicates that wrist and digital flexion is more important than extension in bonobos compared to humans. We suggest that the wrist and digital flexors might be important in bonobos during climbing and clambering, but less so during knuckle‐walking as the wrist and digital flexors are not required to maintain a stable knuckle‐walking stance pose (pers. obs., unpublished data) (Simpson, Latimer & Lovejoy, 2018). In humans, there are high wrist and digital extensor requirements during complex activities such as knapping, dart‐throwing and hammering (Wolfe, Crisco, Orr, et al., 2006; Williams, Gordon & Richmond, 2010).
When looking at each wrist flexor, in macaques the m. flexor carpi ulnaris (FCU) is the most important contributor to total wrist flexor PCSA accounting for on average 27%, while in gibbons the PCSA of the digital flexors makes up on average 73% of the total wrist flexor PCSA, a configuration also seen in modern humans (86%). In bonobos, the digital flexors are also the most important flexors, although the relative proportion (59%) is smaller compared to gibbons and humans. These results stress the importance of digital flexors in the locomotor behaviour of gibbons (during brachiation) and bonobos (climbing and clambering), whereas their importance in humans is likely linked to our advanced manipulation skills, for example, during tool making and tool use (Kivell, 2015; Marzke, 1997; Skinner, Stephens, Tsegai, et al., 2015; Wolfe, Crisco, Orr, et al., 2006). In macaques, the FCU is the not only the most important flexor, but also the most important contributor to the total deviator PCSA. Demes et al. (1998) observed that rhesus macaques closely align their forearms with the substrate reaction force vector in the sagittal plane, especially around the midstance when the reaction forces are high. Their elbows are positioned lateral to the point of substrate contact, and the substrate reaction force vector is inclined medially. As the force vector passes medial to the forearm, it produces medial bending (i.e. in the frontal plane) of the ulna. This bending direction is somewhat counterintuitive as other in vivo studies report anteroposterior bending (i.e. in the sagittal plane) (Demes, Stern, Hausman, et al., 1998). The medial bending of the ulna causes an adducting torque at the elbow joint, which causes stress on the lateral wrist. This stress, and therefore the risk of collapsing, is likely counteracted by the FCU. The action of the FCU is enhanced by the orientation and size of the pisiform, giving the FCU an optimal lever arm (Sarmiento, 1988).
4.4. Wrist deviators
The proportion of wrist deviators is significantly higher in macaques compared to gibbons. The combination of high PCSA and small FL enables the deviators of macaques to produce high levels of force to counteract the stress on the wrist during quadrupedal walking (see above). In gibbons and bonobos, when looking at the wrist deviators, the proportions of the FCU and m. flexor carpi radialis (FCR) are very similar, while in modern humans the m. extensor carpi ulnaris (ECU), m. extensor carpi radialis longus (ECRL) and m. extensor carpi radialis brevis (ECRB) make up the largest proportion of the deviator PCSA. In both gibbons and humans, the radial deviators (RD) have a relatively higher force‐generating capacity compared to the ulnar deviators (UD) (contrary to the situation in macaques). In gibbons, during brachiation considerable radial and ulnar deviation of the wrist—hence the similar development of the FCR and FCU—takes place at the beginning and end of the support phase, respectively (Sarmiento, 1988), but the relatively larger size of the RD suggests that these are more actively recruited during brachiation. In humans, radial and ulnar deviation of the wrist is important during tool making and tool use (Rainbow, Wolff, Crisco, et al., 2016; Wolfe, Crisco, Orr, et al., 2006; Williams, Gordon & Richmond, 2010), again with a dominance of RD (Vanswearingen, 1983). The fact that the extensors make up the largest proportion of the deviator PCSA might be an adaptation for the so‐called dart‐throw‐motion (i.e. oblique motion of the wrist, from radial extension to ulnar flexion), which is used during most activities of daily living (Edirisinghe, Troupis, Patel, et al., 2014; Wolfe, Crisco, Orr, et al., 2006). However, note that the PCSA value for the wrist deviators of gibbons, macaques and bonobos is likely a slight underestimation as the contribution of m.abductor pollicis longus (APL II) is not accounted for. Because of the insertion of the APL II on the prepollex in macaques and the trapezium in gibbons, the APL II functions as radial deviator of the wrist and has no function on the thumb (Vanhoof, van Leeuwen & Vereecke, 2020).
4.5. Forearm rotators
The proportion of forearm rotator PCSA is significantly higher in gibbons compared to macaques, and in combination with the relatively long FL of the rotators this can be linked to the importance of powerful forearm rotation during brachiation in gibbons. During a complete swing cycle of brachiation, the body rotates through approximately 180° about a vertical axis (Fleagle, 1974). In brachiation, gibbons try to maximize their forward momentum, and the centre of mass should travel in the same vertical plane as the centre of rotation. Lateral motion of the centre of mass between handholds is limited by extensive rotation at the wrist, elbow and shoulder, necessitating strong forearm rotators (Fleagle, 1974; Michilsens, D’Août & Aerts, 2011). In macaques, the rotators show a combination of long FL and low PCSA, probably to allow a wide range of motion for shifting the weight of the body to help change the direction of travel and maintain balance on a narrow branch during arboreal locomotion (Larson & Stern, 2006).
4.6. Flexor tendonization
The relative tendon length of the flexors appears on average somewhat higher in gibbons compared to macaques (80.1% and 70.8%, respectively), but this difference is not significant. In bonobos, the relative tendon length is 68.8% which is similar to macaques and significantly lower than that observed in gibbons. The relatively high ‘tendonization’ in the wrist and digital flexors of gibbons might indicate that elastic storage is indeed important during brachiation (Corruccini, 1978), and probably more so than in macaque and bonobo locomotion. Humans have the lowest flexor tendonization (65.0%), which could be related to absence of a locomotor function of arms and hands. In addition, there is no significant difference between siamangs and other gibbons, despite the lower percentage of brachiation in the locomotor repertoire of siamangs. Also in the other functional parameters of forearm and hand musculature, we found no differences between siamangs and the other gibbon genera.
4.7. Intrinsic hand muscles
Another example that may reflect differences in locomotion is found in the intrinsic hand muscles. The intermediate hand muscles are relatively more developed in macaques, gibbons, and bonobos compared to humans, while in humans the thenar muscles account for almost 50% of the total intrinsic hand PCSA, which is significantly more than in bonobos. The prominence of the thenar muscles in the human hand is not very surprising given its high dexterity and the importance of the thumb in tool making and tool use (i.e. power squeeze grips) (Kivell, 2015). The relatively strong development of the intermediate hand muscles in the studied nonhuman primates could be explained in the context of locomotion. It might be linked to the importance of grasping in an arboreal milieu, either for brachiation as seen in gibbons or for vertical climbing as seen in bonobos. The intermediate hand muscles might be equally important for palmi‐ or digitigrade macaques as they could aid in efficient positioning of the hand and fingers on uneven substrates.
4.7.1. Critical considerations
Our findings are based on a detailed dissection of 18 gibbon and seven macaque specimens. Although this is a limited sample size compared to human studies, it forms a unique sample and a valuable addition to the scarce information on forelimb muscle architecture in non‐human primates. Inherent to working with primate cadavers is the lack of an equal distribution across species, sexes or ages, and most importantly, sampling from captivity. Muscle is a dynamic tissue, so captivity will influence muscle dimensions and the values reported in this study might deviate from that of wild populations. However, both the macaque and gibbon specimens were housed in large enclosures and were still able to adopt their preferred locomotor behaviour. While this certainly deviates from their locomotor behaviour in the wild, the differences in locomotion between gibbons and macaques persist in captivity. It should also be noted that by comparing two primate groups, we are not able to discern between differences due to functional adaptation or differences due to genetic distance. Differences on species‐level might be more difficult to capture, which—together with low sample size—could be the reason for the lack of differences in muscle dimensions between siamangs and other gibbon species. Also, sampling from a broader range of primate taxa is needed to further substantiate the functional adaptations in the forelimb. Note that in addition to the quantification of the muscle architecture, information on fibre type, sarcomere length and muscle moment arms are important for a full interpretation of muscle function. Finally, scaling of fascicle length was done using total forelimb muscle mass to the one‐third, as body mass was not available for every specimen. However, this does not appear to have an effect on the results as we also did the same analysis with unscaled data, given that the body mass of gibbons (ranging from 4 to 12 kg) and macaques (ranging from 5 to 8 kg) is very similar, and we obtained comparable significance levels.
5. CONCLUSIONS
This study identifies important features of the forelimb and hand musculature in macaques and gibbons based on the detailed dissections of six gibbon species (Hylobates lar,Hylobates pileatus,Hylobates moloch,Nomascus leucogenys,Nomascus concolor and Symphalangus syndactylus) and one macaque species (Macaca mulatta), in combination with complete anatomical data from previous dissections of ten gibbons (Michilsens, Vereecke, D’août, et al., 2009), five bonobos (Pan paniscus) and one human cadaver (Homo sapiens) (van Leeuwen, Vanhoof, Kerkhof, et al., 2018).
Overall, most of the identified differences in forelimb muscle architecture between the primate groups can be linked to their specific locomotor behaviour. In macaques, the wrist deviators, and wrist and digital flexors have a relatively large PCSA and small FL, and thus a high force‐generating capacity, as is seen for the m.triceps brachii. These muscles are important during the different phases of quadrupedal walking to stabilize wrist and elbow. Gibbons have powerful forearm rotators and wrist and digital flexors, and an elbow flexor with a high force‐generating capacity. These muscles are important in brachiation to actively regulate the forward movement of the body. However, given the genetic distance between macaques and gibbons, we cannot be certain that these differences are due to differences in locomotor behaviour and not phylogenetic position. This is challenging to test, but should not go unremarked as only two taxa are being compared, and there is no relative context of the anatomical variation across other arboreal and terrestrial primate taxa.
In a preceding paper (Part I), we provided an extensive description of the extrinsic and intrinsic hand muscles to fully document their configuration and to evaluate if there are specific adaptations in forelimb musculature to locomotor behaviour. This sequel (Part II) provides a full quantification of the forelimb and hand muscle architecture of macaques and gibbons and a comparative analysis between both primate groups. Not only is this research important to obtain a detailed insight in the macaque and gibbon anatomy, but in combination with in vivo research and behavioural studies, it can be translated to complete form‐function relationships of the hand and advance current concepts of the evolutionary history of the forearm and hand of modern humans.
AUTHOR CONTRIBUTIONS
EEV conceived the study; EEV, MJMV and TvL further designed the study; MJMV and TvL performed the dissections; MJVM and LG set up the statistical protocol and designed the data plots; MJMV, EEV and LG analysed the data; MJMV and EEV wrote the manuscript; all authors reviewed and approved the manuscript.
Supporting information
TableS1
TableS2
ACKNOWLEDGEMENTS
The authors thank the different zoos and institutes which provided additional primate specimens: Georg Hantke (National Museum of Scotland, Edinburgh), Pieter Cornillie (Ghent University, campus Merelbeke), Koen Nelissen (KU Leuven, campus Gasthuisberg), François Druelle (Zoological and Botanical Park of Mulhouse, France) and Robby Van der Velden (Pakawi Park, Belgium). Furthermore, we thank Dr. Olivier Vanovermeire and Henk Lacaeyse from the Medical Imaging Department, AZ Groeninge (Kortrijk, Belgium) for CT‐scanning of the specimens. Finally, we would like to thank one of the students who assisted during the dissections. Funding for this project was obtained from KU Leuven (project C14/16/082).
Vanhoof MJM, van Leeuwen T, Galletta L, Vereecke EE, et al. The forearm and hand musculature of semi‐terrestrial rhesus macaques (Macaca mulatta) and arboreal gibbons (fam.Hylobatidae). Part II. Quantitative analysi. J. Anat.2021;238:321–337. 10.1111/joa.13314
REFERENCES
- Alexander, R.M.N. (2002) Tendon elasticity and muscle function. Comparative Biochemistry and Physiology ‐ A Molecular and Integrative Physiology, 133(4), 1001–1011. [DOI] [PubMed] [Google Scholar]
- Almécija, S. , Smaers, J.B. & Jungers, W.L. (2015) The evolution of human and ape hand proportions. Nature Communications, 6(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anapol, F. & Gray, J.P. (2003) Fiber architecture of the intrinsic muscles of the shoulder and arm in semiterrestrial and arboreal guenons. American Journal of Physical Anthropology, 122(1), 51–65. [DOI] [PubMed] [Google Scholar]
- Aversi‐Ferreira, T.A. , Maior, R.S. , Carneiro‐e‐Silva, F.O. , Aversi‐Ferreira, R.A. , Tavares, M.C. , Nishijo, H. et al. (2011) Comparative anatomical analyses of the forearm muscles of Cebus libidinosus (Rylands et al. 2000): Manipulatory behavior and tool use, PLoS One, 6(7), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett, T.Q. , Light, L.E.O. & Brockelman, W.Y. (2016) Long‐term home range use in white‐handed gibbons (Hylobates lar) in Khao Yai National Park, Thailand. American Journal of Primatology, 78(2), 192–203. [DOI] [PubMed] [Google Scholar]
- Bertram, J.E.A. (2004) New perspectives on brachiation mechanics. Yearbook of Physical Anthropology, 47, 100–117. [DOI] [PubMed] [Google Scholar]
- Carlson, K.J. , Doran‐Sheehy, D.M. , Hunt, K.D. , Nishida, T. , Yamanaka, A. & Boesch, C. (2006) Locomotor behavior and long bone morphology in individual free‐ranging chimpanzees. Journal of Human Evolution, 50(4), 394–404. [DOI] [PubMed] [Google Scholar]
- Chan, S.S. & Moran, D.W. (2006) Computational model of a primate arm: from hand position to joint angles, joint torques and muscle forces. Journal of Neural Engineering, 3(4), 327–337. [DOI] [PubMed] [Google Scholar]
- Chang, Y. , Bertram, J.E.A. & Lee, D.V. (2000) External forces and torques generated by the brachiating white‐handed gibbon (Hylobates lar). American Journal of Physical Anthropology, 216, 201–216. [DOI] [PubMed] [Google Scholar]
- Channon, A.J. , Crompton, R.H. , Günther, M.M. , D’Août, K. & Vereecke, E.E. (2010) The biomechanics of leaping in gibbons. American Journal of Physical Anthropology, 143(3), 403–416. [DOI] [PubMed] [Google Scholar]
- Channon, A.J. , Günther, M.M. , Crompton, R.H. & Vereecke, E.E. (2009) Mechanical constraints on the functional morphology of the gibbon hind limb. Journal of Anatomy, 215(4), 383–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corruccini, R.S. (1978) Comparative osteometrics of the hominoid wrist joint, with special reference to knuckle‐walking. Journal of Human Evolution, 7(4), 307–321. [Google Scholar]
- Courtine, G. , Roy, R.R. , Hodgson, J. , McKay, H. , Raven, J. , Zhong, H. et al. (2005) Kinematic and EMG determinants in quadrupedal locomotion of a non‐human primate (Rhesus). Journal of Neurophysiology, 93(6), 3127–3145. [DOI] [PubMed] [Google Scholar]
- Cunningham, C.L. , Anderson, J.R. & Mootnick, A.R. (2006) Object manipulation to obtain a food reward in hoolock gibbons, Bunopithecus hoolock. Animal Behaviour, 71(3), 621–629. [Google Scholar]
- Demes, B. , Stern, J.T. , Hausman, M.R. , Larson, S.G. , McLeod, K.J. & Rubin, C.T. (1998) Patterns of strain in the macaque ulna during functional activity. American Journal of Physical Anthropology, 106(1), 87–100. [DOI] [PubMed] [Google Scholar]
- Dunbar, D.C. & Badam, G.L. (1998) Development of posture and locomotion in free‐ranging primates. Neuroscience and Biobehavioral Reviews, 22(4), 541–546. [DOI] [PubMed] [Google Scholar]
- Dunmore, C.J. (2019) Evolution in the palm of the human hand : Functional inferences from internal bone architecture in great apes and fossil. Canterbury, UK: University of Kent. [Google Scholar]
- Edirisinghe, Y. , Troupis, J.M. , Patel, M. , Smith, J. & Crossett, M. (2014) Dynamic motion analysis of dart throwers motion visualized through computerized tomography and calculation of the axis of rotation. Journal of Hand Surgery: European, 39(4), 364–372. [DOI] [PubMed] [Google Scholar]
- Feix, T. , Kivell, T.L. , Pouydebat, E. & Dollar, A.M. (2015) Estimating thumb–index finger precision grip and manipulation potential in extant and fossil primates. Journal of The Royal Society Interface, 12(106), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleagle, J. (1974) Dynamics of a brachiating siamang [hylobates (symphalangus) syndactylus]. Nature, 248(5445), 259–260. [DOI] [PubMed] [Google Scholar]
- Fleagle, J.G. (1976) ‘Locomotion and posture of the Malayan siamang and implications for hominoid evolution’, Folia Primatol (Basel) . Karger Publishers, 26(4), 245–269. [DOI] [PubMed] [Google Scholar]
- Fleagle, J.G. , Janson, C. & Reed, K.E. (1999) Primate communities. Edited by Fleagle J.G., Janson C., and Reed K.E. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Gumert, M.D. , Kluck, M. & Malaivijitnond, S. (2009) The physical characteristics and usage patterns of stone axe and pounding hammers used by long‐tailed macaques in the Andaman sea region of Thailand. American Journal of Primatology, 71(7), 594–608. [DOI] [PubMed] [Google Scholar]
- Hayama, S. , Chatani, K. & Nakatsukasa, M. (1994) The digitigrade hand and terrestrial adaptation in Japanese Macaques. Anthropological Science, 102, 115–125. [Google Scholar]
- Higurashi, Y. , Goto, R. & Kumakura, H. (2018) Intra‐individual variation in hand postures during terrestrial locomotion in Japanese macaques (Macaca fuscata). Primates. Springer Japan, 59(1), 61–68. [DOI] [PubMed] [Google Scholar]
- Jungers, W.L. & Stern, J.T. (1980) Telemetered electromyography of forelimb muscle chains in gibbons (Hylobates lar). Science, 208(4444), 617–619. [DOI] [PubMed] [Google Scholar]
- Kikuchi, Y. (2004) Quantitative analyses of cross‐sectional shape of the distal radius in three species of macaques. Primates, 45(2), 129–134. [DOI] [PubMed] [Google Scholar]
- Kikuchi, Y. & Hamada, Y. (2009) Geometric characters of the radius and tibia in Macaca mulatta and Macaca fascicularis. Primates, 50(2), 169–183. [DOI] [PubMed] [Google Scholar]
- Kikuchi, Y. , Takemoto, H. & Kuraoka, A. (2012) Relationship between humeral geometry and shoulder muscle power among suspensory, knuckle‐walking, and digitigrade/palmigrade quadrupedal primates. Journal of Anatomy, 220(1), 29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivell, T.L. (2015) Evidence in hand: recent discoveries and the early evolution of human manual manipulation. Philosophical Transactions of the Royal Society B: Biological Sciences, 370(1682), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson, S.G. & Stern, J.T. (2006) Maintenance of above‐branch balance during primate arboreal quadrupedalism: Coordinated use of forearm rotators and tail motion. American Journal of Physical Anthropology, 129(1), 71–81. [DOI] [PubMed] [Google Scholar]
- Lee, D. , Li, Z. , Sohail, Q.Z. , Jackson, K. , Fiume, E. & Agur, A. (2015) A three‐dimensional approach to pennation angle estimation for human skeletal muscle. Computer Methods in Biomechanics and Biomedical Engineering, 18(13), 1474–1484. [DOI] [PubMed] [Google Scholar]
- van Leeuwen, T. , Vanhoof, M.J.M. , Kerkhof, F.D. , Stevens, J.M.G. & Vereecke, E.E. (2018) Insights into the musculature of the bonobo hand. Journal of Anatomy, 233(3), 328–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leischner, C.L. , Crouch, M. , Allen, K.L. , Marchi, D. , Pastor, F. & Hartstone‐Rose, A. (2018) Scaling of primate forearm muscle architecture as it relates to locomotion and posture. Anatomical Record, 301(3), 484–495. [DOI] [PubMed] [Google Scholar]
- Lemelin, P. & Schmitt, D. (1998) The relation between hand morphology and quadrupedalism in primates. American Journal of Physical Anthropology, 105(2), 185–197. [DOI] [PubMed] [Google Scholar]
- Lieber, R.L. & Fridén, J. (2000) Functional and clinical significance of skeletal muscle architecture. Muscle and Nerve, 23(November), 1647–1666. [DOI] [PubMed] [Google Scholar]
- Manter, J.T. (1938) The dynamics of quadrupedal locomotion. Journal of Experimental Biology, 15, 522–540. [Google Scholar]
- Marzke, M.W. (1997) Precision grips, hand morphology, and tools. American Journal of Physical Anthropology, 102(1), 91–110. [DOI] [PubMed] [Google Scholar]
- Marzke, M.W. (2009) Upper‐limb evolution and development. Journal of Bone and Joint Surgery ‐ Series A, 91‐A, 26–30. [DOI] [PubMed] [Google Scholar]
- Marzke, M.W. (2013) Tool making, hand morphology and fossil hominins. Philosophical Transactions of the Royal Society B: Biological Sciences, 368(1630), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michilsens, F. , D’Août, K. & Aerts, P. (2011) How pendulum‐like are siamangs? Energy exchange during brachiation. American Journal of Physical Anthropology, 145(4), 581–591. [DOI] [PubMed] [Google Scholar]
- Michilsens, F. , Vereecke, E.E. D'Août, K. & Aerts, P. (2009) Functional anatomy of the gibbon forelimb: Adaptations to a brachiating lifestyle. Journal of Anatomy, 215(3), 335–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michilsens, F. , Vereecke, E.E. , D’Août, K. & Aerts, P. (2010) Muscle moment arms and function of the siamang forelimb during brachiation. Journal of Anatomy, 217(5), 521–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyà‐Solà, S. , Köhler, M. & Rook, L. (1999) Evidence of hominid‐like precision grip capability in the hand of the Miocene ape Oreopithecus. Proceedings of the National Academy of Sciences of the United States of America, 96(1), 313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myatt, J.P. , Crompton, R.H. , Payne‐Davis, R.C. , Vereecke, E.E. , Isler, K. , Savage, R. et al. (2012) Functional adaptations in the forelimb muscles of non‐human great apes. Journal of Anatomy, 220(1), 13–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogihara, N. , Kunai, T. & Nakatsukasa, M. (2005) Muscle dimensions in the chimpanzee hand. Primates, 46(4), 275–280. [DOI] [PubMed] [Google Scholar]
- Ogihara, N. , Makishima, H. , Aoi, S. , Sugimoto, Y. , Tsuchiya, K. & Nakatsukasa, M. (2009) Development of an anatomically based whole‐body musculoskeletal model of the Japanese macaque (Macaca fuscata). American Journal of Physical Anthropology, 139(3), 323–338. [DOI] [PubMed] [Google Scholar]
- Ogihara, N. & Oishi, M. (2012) Muscle dimensions in the Japanese macaque hand. Primates, 53(4), 391–396. [DOI] [PubMed] [Google Scholar]
- Oishi, M. , Ogihara, N. , Endo, H. & Asari, M. (2008) Muscle architecture of the upper limb in the orangutan. Primates, 49(3), 204–209. [DOI] [PubMed] [Google Scholar]
- Orr, C.M. (2017) Locomotor hand postures, carpal kinematics during wrist extension, and associated morphology in anthropoid primates. Anatomical Record, 300(2), 382–401. [DOI] [PubMed] [Google Scholar]
- Parks, K.A. & Novak, M.A. (1993) Observations of increased activity and tool use in captive rhesus monkeys exposed to troughs of water. American Journal of Primatology, 29(1), 13–25. [DOI] [PubMed] [Google Scholar]
- Patel, B.A. (2009) The interplay between speed, kinetics, and hand postures during primate terrestrial locomotion. American Journal of Physical Anthropology, 234, 222–234. [DOI] [PubMed] [Google Scholar]
- Patel, B.A. & Carlson, K.J. (2007) Bone density spatial patterns in the distal radius reflect habitual hand postures adopted by quadrupedal primates. Journal of Human Evolution, 52(2), 130–141. [DOI] [PubMed] [Google Scholar]
- Patel, B.A. , Larson, S.G. & Stern, J.T. (2012) Electromyography of wrist and finger flexor muscles in olive baboons (Papio anubis). Journal of Experimental Biology, 215(1), 115–123. [DOI] [PubMed] [Google Scholar]
- Patel, B.A. & Polk, J.D. (2010) Distal forelimb kinematics in erythrocebus patas and papio anubis during walking and galloping. International Journal of Primatology, 31(2), 191–207. [Google Scholar]
- Payne, R.C. , Crompton, R.H. , Isler, K. , Savage, R. , Vereecke, E.E. , Günther, M.M. et al. (2006) Morphological analysis of the hindlimb in apes and humans. I. Muscle architecture. Journal of Anatomy, 208(6), 709–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouydebat, E. , Gorce, P. , Coppens, Y. & Bels, V. (2009) Biomechanical study of grasping according to the volume of the object: Human versus non‐human primates. Journal of Biomechanics, 42(3), 266–272. [DOI] [PubMed] [Google Scholar]
- Preuschoft, H. , Schönwasser, K.‐H. & Witzel, U. (2016) Selective value of characteristic size parameters in Hylobatids. A biomechanical approach to small ape size and morphology In: Preuschoft H., Schönwasser K.‐H. and Witzel U. (Eds.) Evolution of Gibbons and Siamang. Springer Science & Business Media, pp. 229–265. [Google Scholar]
- Prime, J.M. & Ford, S.M. (2016) Hand manipulation skills in hylobatids In: Reichard U.H., Barelli C., Hirohisa H. & Nowak M.G. (Eds.), Evolution of Gibbons and Siamang. Berlin, Germany: Springer Science & Business Media, pp. 269–289. [Google Scholar]
- Rainbow, M.J. , Wolff, A.L. , Crisco, J.J. & Wolfe, S.W. (2016) Functional kinematics of the wrist. Journal of Hand Surgery: European, 41(1), 7–21. [DOI] [PubMed] [Google Scholar]
- Reichard, U.H. , Barelli, C. , Hirai, H. & Nowak, M.G. (2016) The evolution of Gibbons and Siamang In: Reichard U.H., Barelli C., Hirai H. and Nowak M.G. (Eds.) Evolution of Gibbons and Siamang. Berlin, Germany: Springer Science & Business Media, pp. 3–41. [Google Scholar]
- Rein, T.R. , Harvati, K. & Harrison, T. (2015) Inferring the use of forelimb suspensory locomotion by extinct primate species via shape exploration of the ulna. Journal of Human Evolution. 78, 70–79. [DOI] [PubMed] [Google Scholar]
- Richmond, B.G. (2006) Functional morphology of the midcarpal joint in Knuckle‐Walkers and terrestrial quadrupeds In: Ishida H., Tuttle R., Pickford M., Ogihara N. & Nakatsukasa M. (Eds.), Human Origins and Environmental Backgrounds. New York, NY: Springer, pp. 105–122. [Google Scholar]
- Rodman, P.S. (1979) Skeletal differentiation of Macaca fascicularis and Macaca nemestrina in relation to arboreal and terrestrial quadrupedalism. American Journal of Physical Anthropology, 51(1), 51–62. [Google Scholar]
- Roy, A.C. , Paulignan, Y. , Farnè, A. , Jouffrais, C. & Boussaoud, D. (2000) Hand kinematics during reaching and grasping in the macaque monkey. Behavioural Brain Research, 117(1–2), 75–82. [DOI] [PubMed] [Google Scholar]
- Santos, L.R. , Miller, C.T. & Hauser, M.D. (2003) Representing tools: how two non‐human primate species distinguish between the functionally relevant and irrelevant features of a tool. Animal Cognition, 6(4), 269–281. [DOI] [PubMed] [Google Scholar]
- Sarmiento, E.E. (1988) Anatomy of the hominoid wrist joint: Its evolutionary and functional implications. International Journal of Primatology, 14(A), 1–345. [Google Scholar]
- Schmitt, D. (2003) Mediolateral reaction forces and forelimb anatomy in quadrupedal primates: Implications for interpreting locomotor behavior in fossil primates. Journal of Human Evolution, 44(1), 47–58. [DOI] [PubMed] [Google Scholar]
- Simpson, S.W. , Latimer, B. & Lovejoy, C.O. (2018) Why do knuckle‐walking african apes knuckle‐walk? Anatomical Record, 301(3), 496–514. [DOI] [PubMed] [Google Scholar]
- Skinner, M.M. , Stephens, N.B. , Tsegai, Z.J. , Foote, A.C. , Nguyen, N.H. , Gross, T. et al. (2015) Human‐like hand use in Australopithecus africanus . Science, 347(6220), 395–399. [DOI] [PubMed] [Google Scholar]
- Susman, R.L. , Jungers, W.L. & Stern, J.T. (1982) The functional morphology of the accessory interosseous muscle in the gibbon Hand: determination of locomotor and manipulatory compromises. Journal of Anatomy, 134(1), 111–120. [PMC free article] [PubMed] [Google Scholar]
- Swartz, S.M. , Bertram, J.E.A. & Biewener, A.A. (1989) Telemetered in vivo strain analysis of locomotor mechanics of brachiating gibbons. Nature, 342(6247), 270–272. [DOI] [PubMed] [Google Scholar]
- Tocheri, M.W. , Orr, C.M. , Jacofsky, M.C. & Marzke, M.W. (2008) The evolutionary history of the hominin hand since the last common ancestor of Pan and Homo. Journal of Anatomy, 212(4), 544–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasello, M. & Call, J. (1997) Primate cognition. New York, NY: Oxford University Press. [Google Scholar]
- Turnquist, J.E. , Schmitt, D. , Rose, M.D. & Cant, J.G.H. (1999) Pendular motion in the brachiation of captive Lagothrix and Ateles. American Journal of Primatology, 48(4), 263–281. [DOI] [PubMed] [Google Scholar]
- Tuttle, R.H. (1969) Quantitative and functional studies on the hands of the anthropoidea. I. the hominoidea. Journal of Morphology, 128(3), 309–363. [DOI] [PubMed] [Google Scholar]
- Tuttle, R.H. (1975) Socioecology and psychology of primates. The Hague, the Netherlands; Paris, France: Mouton Publishers. [Google Scholar]
- Usherwood, J.R. , Larson, S.G. & Bertram, J.E.A. (2003) Mechanisms of force and power production in unsteady ricochetal brachiation. American Journal of Physical Anthropology, 120(4), 364–372. [DOI] [PubMed] [Google Scholar]
- Vanhoof, M.J.M. , Leeuwen, T. & Vereecke, E.E. (2020) The forearm and hand musculature of semi‐terrestrial rhesus macaques (Macaca mulatta) and arboreal gibbons (Fam. Hylobatidae). Part I. Description and comparison of the muscle configuration. Journal of Anatomy, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanswearingen, J.M. (1983) Measuring wrist muscle strength. Journal of Orthopaedic & Sports Physical Therapy, 4(4), 217–228. [DOI] [PubMed] [Google Scholar]
- Vereecke, E.E. , D’Août, K. & Aerts, P. (2006) Locomotor versatility in the white‐handed gibbon (Hylobates lar): a spatiotemporal analysis of the bipedal, tripedal, and quadrupedal gaits. Journal of Human Evolution, 50(5), 552–567. [DOI] [PubMed] [Google Scholar]
- Vereecke, E.E. , D’Août, K. , Payne, R. & Aerts, P. (2005) Functional analysis of the foot and ankle myology of gibbons and bonobos. Journal of anatomy, 206(5), 453–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, S.R. & Lieber, R.L. (2005) Density and hydration of fresh and fixed human skeletal muscle. Journal of Biomechanics, 38(11), 2317–2320. [DOI] [PubMed] [Google Scholar]
- Wells, J.P. & Turnquist, J.E. (2001) Ontogeny of locomotion in rhesus macaques (macaca mulatta): II. Postural and locomotor behavior and habitat use in a free‐ranging colony. American Journal of Physical Anthropology, 115(1), 80–94. [DOI] [PubMed] [Google Scholar]
- Williams, E.M. , Gordon, A.D. & Richmond, B.G. (2010) Upper limb kinematics and the role of the wrist during stone tool production. American Journal of Physical Anthropology, 143(1), 134–145. [DOI] [PubMed] [Google Scholar]
- Wolfe, S.W. , Crisco, J.J. , Orr, C.M. & Marzke, M.W. (2006) The dart‐throwing motion of the wrist. Is it unique to humans? The Journal of hand surgery, 31A(5), 1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeininger, A. , Shapiro, L.J. & Raichlen, D.A. (2017) Ontogenetic changes in limb postures and their impact on effective limb length in baboons (Papio cynocephalus). American Journal of Physical Anthropology, 163(2), 231–241. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TableS1
TableS2


