Abstract
Background
Steroid-induced osteonecrosis of the femoral head (SONFH) is the pathological process caused by the death of the active components of the head of the femur due to the high dose of hormones, which has become a common public health problem. BuShenHuoXue capsule (BSHXC) has been clinically proven to be effective against the SONFH, the main pharmacological action of BSHXC is tonifying kidney and promoting blood circulation, but the mechanism remains to be explored.
Methods
We established a rat SONFH model by injecting Methylprednisolone (MPS) into the right gluteus muscle 30 mg/kg/d, 3 days of continuous injection every week, 4 weeks in total. According to the clinical dosage of BSHXC (Herba epimedium 3 g, Eucommia ulmoides 15 g, Salvia miltiorrhizae 30 g, Chuanxiong 15 g, Paeonia lactiflora Pall 15 g, Poria cocos 12 g, Achyranthes bidentata 12 g, antler gum 10 g, Cyperus rotundus L. Nine g and Radix Glycyrrhizae 9 g), it was converted into the equivalent dose of rats, and gavage was performed at the weight of 10 mL/kg, once per day. The BSHXC was subjected to experiments in vivo, SONFH pharmacodynamics, bioinformatics, and network of pharmacology to determine the active ingredients, and its protective role against SONFH, Enrichment analysis was performed to explore the possible mechanism of BSHXC, and cell experiments were undertaken to analyze the impact of BSHXC on the hormones associated with bone marrow mesenchymal stem cells (BMSCs) between osteogenesis and apoptosis.
Results
Experiments confirmed that BSHXC could effectively reduce bone loss in SONFH rat models. From bioinformatics and a network constructed from 10 drugs-208 pharmacology-126 targets, the enrichment analysis showed that the core targets were inflammatory reaction, steroid hormones, estrogen receptors, osteoporosis, and adjustment of osteogenesis and osteoclast differentiation, among others. The cell proliferation and staining supported that the mechanism of BSHXC promoted osteogenesis and intervening in apoptosis.
Conclusions
The BSHXC reduced the inflammatory response, changed steroid response, regulated estrogen receptors, delayed osteoporosis, regulated osteoblast and osteoclast differentiation by regulating related targets, and improved the local microenvironment by a multi-component, multi-target, and multi-link process to delay or reverse the progression of SONFH.
Keywords: BuShenHuoXue capsule (BSHXC), steroid-induced osteonecrosis of the femoral head (SONFH), bioinformatics, molecular mechanisms of pharmacological action
Introduction
Steroid-induced osteonecrosis of the femoral head (SONFH) is the pathological process caused by the death of the active components of the head of the femur (bone tissue, bone marrow hematopoietic cells, and fat cells) due to the high dose of hormones (1). According to a recent multicenter survey, 55.75% of women and 26.35% of men with ONFH had developed the pathology due to glucocorticoids (GC) (2). Because SONFH tends to occur at a younger age and is difficult to treat, it has become one of the most common causes of hip disability in young adults (3). Risk factors for SONFH include hormones, alcohol, smoking and various chronic diseases (such as kidney disease, SLE and organ transplantation). The results of the interaction between genetic predisposition and specific risk factors may determine whether a specific population will eventually develop avascular necrosis of the femoral head. To date, Western medicine has conducted much research on pharmaceuticals treatments for early SONFH, which has mainly included anticoagulant drugs (4), lipid-lowering drugs (5), and anti-osteoporosis drugs (6), among others. There are many methods to treat ONFH, including hip replacement, hip preserving surgery and non-surgical treatment. Hip arthroplasty is also considered the last resort for ONFH patients who have failed in all other treatments or are secondary to the progression of advanced femoral head collapse and loss of functional activity and hip arthritis, but considering about 75% of ONFH The age of the patients is between 30 and 60 years old. This kind of young and middle-aged patients not only need to consider the functional activity of the hip joint, but also consider that the artificial hip joint prosthesis has a certain service life. The durability and revision of prosthesis after long-term use after joint replacement have limited its wide application to a certain extent. With the development of surgical techniques and concepts, hip salvage surgery includes core decompression of femoral head, bone grafting without blood supply, autologous bone transplantation with blood supply, osteotomy without reconstruction of bone marrow cavity and bone tissue engineering. If the above methods are applied properly, hip replacement can be postponed or avoided to a certain extent. In fact, most patients diagnosed with ONFH need more than one operation in their lifetime, so any need to postpone surgery is welcome. However, the majority of these experiments have been basic, and the clinical efficacy is still uncertain. In order to address the above problems, this paper used a Bioinformatics Analysis Tool for Molecular mechanism of Traditional Chinese Medicine (BATMAN-TCM), Gene Expression Omnibus (GEO), DrugBank, and other databases to collect drug and SONFH targets. The information was systematically analyzed to find the pharmacodynamic substance basis and mechanism of action, with the aim of assisting the development of a specific clinical medication. In addition, through pharmacodynamics and histological analysis of BuShenHuoXue Capsule (BSHXC), this paper organically combined traditional Chinese medicine (TCM) theory with modern network pharmacological research. This approach enabled the explanation of the intervention effect of BuShenHuoXue Capsule on SONFH from various aspects of “Prescription-Component-Target-Pathway-Activity”, which allowed the exploration of new research models of TCM and set a foundation for future research.
We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-7040).
Methods
Ethics statement
The experiment was implemented through the approval of ethics committee in Chinese People’s Liberation Army General Hospital and followed the tenets of the Declaration of Helsinki (as revised in 2013). All animal experiments were carried out with the approval of the animal ethics committee in Chinese People’s Liberation Army General Hospital, and the animals were treated in strict line with the International Code of Ethics and the National Health Guidelines for the maintenance and use of laboratory animals
Preparation of BSHXC drug powder
The powder of BSHXC was processed into concentrated powder at the Pharmaceutical Center of the Affiliated Hospital of Shandong University of Traditional Chinese Medicine, and stored at 4 °C to maintain freshness. The powder consisted of Herba epimedium (Yinyanghuo) 6 kg, Eucommia ulmoides (Duzhong) 3 kg, Salvia miltiorrhizae (Danshen) 6 kg, Chuanxiong (Chuanxiong) 3 kg, Paeonia lactiflora Pall (Baishao) 3 kg, Poria cocos (Fuling) 2.4 kg, Achyranthes bidentata (Niuxi) 2.4 kg, antler gum (Lujiao) 2 kg, Cyperus rotundus L. (Xiangfu) 1.8 kg and Radix Glycyrrhizae (Gancao) 1.8 kg. It is processed into concentrated powder at one time by the Pharmacy Center of the Affiliated Hospital of Shandong University of Traditional Chinese Medicine and stored at 4 °C for later use. Among them, the glue type Chinese medicine needs to be added to the liquid at no lower than 60 °C, and the mixture is stirred. Therefore, in this topic, the deerhorn gum will be powdered separately, and the remaining medicine will be concentrated 1:5 into powder for use. According to the 2010 edition of “The Pharmacopoeia of the People’s Republic of China” and the quality standards of traditional Chinese medicine in Shandong Province, the methods of Epimedium Thin Layer Chromatography (TLC) and Icariin High Performance Liquid Chromatography (HPLC) were used to invigorate the kidney. The quality of Huoxue Capsules concentrated powder is controlled. According to the equivalent dose coefficient conversion between human and rat, the equivalent dose of rats is 6.3 times that of humans. The clinical dose of Bushen Huoxue Capsule is 157 g (the herbal dose is 147 g, and the dose of deerhorn gum is 10 g). The concentration ratio of the drug is 1:5, so the dose of the herbal part of the rat is 2.826 g/kg; the body weight is 10 mL/kg gavage, 1 time/day, so the concentration of the herbal preparation should be 0.2646 g/mL. The amount of gavage for rats in 1 week (the concentration of herbal medicine is about 35 g). Therefore, take 39.69 g of the concentrated powder and dissolve it in 150 mL of water, heat it on a slow fire, add 13.5 g of antler gum to the liquid at no less than 60 °C, fully incubate for 5 min, stir well, and distribute to 50 mL clean centrifuge tubes In, indicate the concentration and time, and store in a refrigerator at 4 °C.
Animal models and groups
A total of 18 healthy male Sprague-Dawley (SD) rats given standard feed and free access to drinking water, purchased from SPF (Beijing) Biotechnology Co., Ltd., and raised in the SPF Laboratory Animal Center of the Chinese People’s Liberation Army General Hospital, weighing (285±15) g, the room temperature was controlled at 22–25 °C, and humidity was maintained at 40–60%. After 7 days of adaptive feeding, they were divided into control (CG), model (MG), and drug (DG) groups according to a random number table method. A rat model of SONFH was established using the methods mentioned in the references (7,8). All procedures involving animals were approved by the Institutional Animal Care Committee, license: SCXK (jing) 2016-0002. The specific grouping and intervention scheme are shown in Table 1.
Table 1. The number of rats in different groups and the intervention scheme.
| Group | Quantity | Experimental method |
|---|---|---|
| Control group (CG) | 6 | Inject saline into the right gluteus muscle 30 mg/kg/d, 3 days of continuous injection every week, 4 weeks in total, drinking water gavage, 10 mL/kg, once a day, for 6 weeks |
| Model group (MG) | 6 | Inject MPS into the right gluteus muscle 30 mg/kg/d, 3 days of continuous injection every week, 4 weeks in total, drinking water gavage, 10 mL/kg, once a day, for 6 weeks |
| Drug group (DG) | 6 | Inject MPS into the right gluteus muscle 30 mg/kg/d, 3 days of continuous injection every week, 4 weeks in total, BSHXC liquid gavage, 10 mL/kg, once a day, for 6 weeks |
BSHXC, BuShenHuoXue capsule; MPS, methylprednisolone.
Imageological examination
The rats were sacrificed after intraperitoneal injection of 3% pentobarbital sodium 45 mg/kg. The left femur was photographed by a small animal X-ray machine (Faxitron Bioptics, US). Subsequently, the proximal left femur specimens were immobilized in a 4% tissue-cell fixation solution, and the right sides were stored in liquid nitrogen, with the group and sampling time recorded. All animal carcasses were disposed of according to regulations.
A Micro-CT scanner (Locus SP, General Electric Inc.) was used to detect the tissue after 72 h of fixation. Each time, 6 specimens were scanned, with a layer thickness of 0.027 mm, layer spacing of 0.027 mm, pixel size of 0.027 mm, scanning current of 450 mA, scanning voltage of 80 kV, and single scanning time of 88 min. After scanning, the morphological changes of the rats were observed to evaluate the necrosis and repair of the femoral heads in the different groups.
Histological analysis
After decalcification and paraffin embedding, femoral heads were sectioned at a thickness of 5 µm in the coronal plane. Then, the sections in each group were stained with hematoxylin and eosin (H&E) to observe the histological changes in the femoral head. Under the microscope, the femoral head was selected as the center in the subchondral area and near the center point, and the number of bone lacunae and vacant bone lacunae in each field was calculated to obtain the rate of empty bone lacunae.
Cell proliferation assay
To investigate cytotoxicity, the effects of BSHXC on the proliferation of bone marrow mesenchymal stem cells (BMSCs) in rats were observed. Cell proliferation was determined using a cell counting kit 8 (CCK-8) assay. Take SPF grade 4-week-old SD rats. After being sacrificed, both lower limbs and abdomen are skinned and immersed in 75% ethanol for 15 minutes. The rats are supine on a clean petri dish on a clean bench. Scissors expose the muscles of the distal femur, clean and remove the soft tissue attached to the distal surface of the femur, cut off the epiphysis of the distal femur with diagonal cutting forceps, expose the bone marrow cavity, and use a 5 mL syringe to suck 2.0–3.0 mL of low-sugar containing 10% fetal bovine serum DMEM culture solution, directly inserted into the bone marrow cavity to flush out the bone marrow in the proximal femur and femoral shaft, transfer the bone marrow suspension to a 15 mL centrifuge tube, centrifuge at 800 r/min at room temperature for 5 min, discard the supernatant, and use 6 mL containing Resuspend in low-sugar DMEM culture medium with a volume fraction of 10% fetal bovine serum, gently pipette several times to make a single cell suspension, inoculate it in a cell culture flask, and place the cell culture flask at 37 °C with a volume fraction of 5% Cultivate in a CO2 saturated humidity incubator; after standing for 36–48 hours, observe the cell morphology and growth under an inverted microscope, and perform a half-volume exchange to remove floating blood cells; then, complete the medium exchange every two or three days until the cells proliferate to confluence.
When the cells are fused to 70–90%, start subculture, aspirate the culture medium, wash the cells gently with 3 mL of pre-warmed PBS 2 or 3 times, aspirate the PBS, add 1 mL of trypsin to digest for 2 or 3 minutes, observe under the microscope when the cells retracted and rounded, the digestion was terminated, the bottom wall of the culture flask was gently pipetted repeatedly, the cell suspension was transferred to a 15-mL centrifuge tube, centrifuged at 800 r/min for 5 min, the supernatant was discarded, and 3 mL containing the volume fraction was added. Resuspend the cells in low-sugar DMEM medium with 10% fetal bovine serum, gently pipette and shake to make a single cell suspension, culture in a 37 °C, 5% CO2 saturated humidity incubator, observe the cells with an inverted phase contrast microscope Morphology and growth.
When the cells are passaged to the third generation, wash 2 times with PBS, digest with 1mL trypsin, stop the digestion when the cells retract and turn round, centrifuge at 800 r/min for 5 min, wash 2 times with PBS, add PBS again to make a concentration of 1×106/mL cell suspension. Take out 2 copies of each sample, 100 µL each, add anti-CD29-FITC, anti-CD34-FITC, anti-CD45-FITC, anti-CD90-FITC and anti-CD105-FITC antibodies respectively, and incubate at room temperature for 15 min, using flow cytometry The expression of rat BMSCs surface marker antigens CD29, CD34, CD45, CD90 and CD105 were detected to identify their phenotype.
The cells were divided into three groups, namely CG, MG, and DG. They were then digested by trypsin and resuspended into a single cell suspension. Cells in each group were counted 3 times with the help of the blood count board, and the mean value was taken. After cell count, the concentration of the cells was diluted to 2,000/mL. They were placed in a 96-well plate with 6 wells in each group and 100 µL in each well. The cells were set at a time point of 1, 3, 5, and 7 d, and each time point was repeated 3 times. A CCK-8 solution of 10 L was added into the corresponding hole at the corresponding time, and placed in the incubator for 3 h. The absorbance value was measured at 450 nm with the enzyme marker, and the difference of the optical density (OD) value of the cells in the medium minus the absorbance of the blank medium was used to indicate the proliferation of the cells.
Alizarin red stain
Osteogenic differentiation was induced by SD rat BMSCs complete osteogenic differentiation medium. The third-generation rat BMSCs trypsin was used to digest and resuspend into a single cell suspension, and 2 mL of SD rat BMSCs osteogenic induction and differentiation medium containing CG and drug serum were added. After 3 weeks, the morphological changes and growth of the cells were observed, cell fixation and alizarin red staining were performed, and the osteogenic staining was observed under a microscope (Olympus, Japan).
Apoptosis detection
In order to detect the influence of the BSHXC on rat BMSCs cell apoptosis, BMSCs cells were digested with trypsin, collected, centrifuged followed by disposal of the supernatant, precooled, rinsed twice in phosphate buffered saline (PBS), a suitable amount of the combination of the buffer was added to gently blow heavy suspension cells, and cell concentration was adjusted to 1×109/L. This resulted in a 100 µL cell suspension with 5 µL Annexin V-FITC for blending which was then incubated for 15 min at room temperature away from light, 5 µL propidium iodide (PI) stain was added, and the apoptosis of each BMSCs cell was measured using a flow cytometry instrument (Becton Dickinson, US).
Screening of active ingredients of BSHXC into blood
The Bioinformatics Analysis Tool for Molecular mechanism of Traditional Chinese Medicine [BATMAN-TCM (http://bionet.ncpsb.org/batman-tcm/)] is the first online analysis tool to systematically explain the action and mechanism of TCM compounds by combining the prediction of the action target of important compound chemical components with the network pharmacological analysis. All components of BSHXC [Herba epimedium (Yinyanghuo), Eucommia ulmoides (Duzhing), Salvia miltiorrhiza (Danshen), Chuanxiong (Chuanxiong), Paeonia suffruticosa (Mudanpi), Poria cocos (Fuling), Achyranthes bidentata (Huainiuxi), antler gum (Lurong), and Radix glycyrrhiza (Gancao)] were input into the BATMAN-TCM online analysis tool, with scores ≥20 and P<0.05 as critical values, and the composition and potential target of the compound were predicted.
SONFH candidate target screening
The SONFH-related mRNA chips were retrieved from the Gene Expression Omnibus (GEO) database (9); the original chip data numbered GSE123568 and the chip Gene annotation file of GPL15207 were obtained. The original chip data file contained 40 serum samples, including 10 control and 30 SONFH samples uploaded by Zhang Y. (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE123568). R language version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.) was used for secondary analysis of the original chip files, and the screening criteria for significantly different genes were P<0.05 and fold change (FC) >2. Database retrieval of SONFH related targets was conducted through the DrugBank (https://www.drugbank.ca/), GeneCards (https://www.genecards.org/) and Kyoto Encyclopedia of Genes and Genomes (KEGG, https://www.kegg.jp/), and the Universal Protein Resource (UniProt) database (https://uniprot.org/) was used to retrieve the uniform number of disease targets, and the duplicates were deleted to obtain the known SONFH-related targets.
Network construction and core target screening of BSHXC for SONFH
The targets of active components of BSHXC were mapped to SONFH disease-related targets to obtain key targets. The search tool for the retrieval of interacting genes/proteins (STRING) database (https://string-db.org) was used to mine related target proteins of key targets. Cytoscape software (https://cytoscape.org) was used to construct the protein-protein interaction (PPI) network to eliminate repeated interactions; only the connected nodes were retained for further analysis.
KEGG signaling pathway enrichment and GO biological function analysis
The analysis of KEGG (10) signaling pathways and gene ontology (GO) enrichment was performed using the R language clusterProfiler package (v3.14.3, https://www.rdocumentation.org/packages/clusterProfiler/versions/) (11), and KEGG and GO bubble diagrams were plotted.
Results
Pharmacodynamic analysis of BSHXC on SONFH
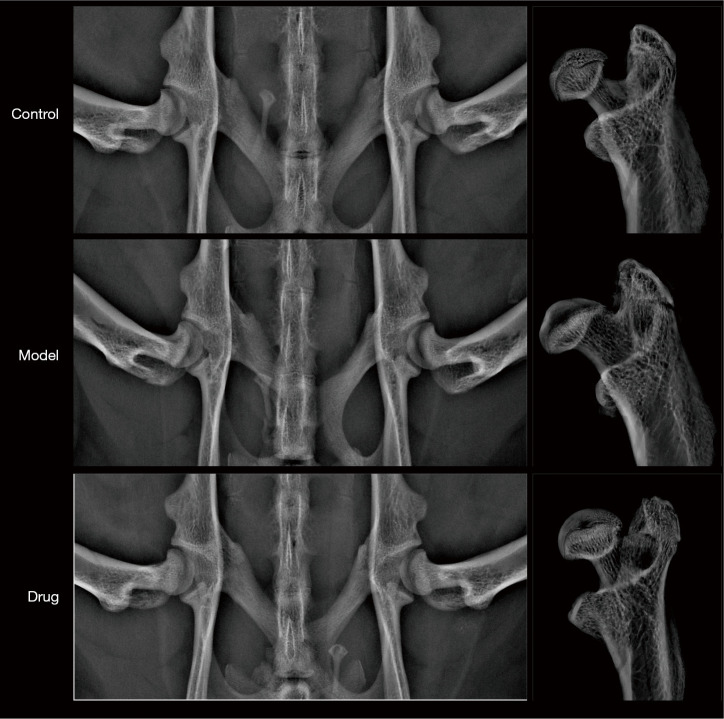
All experimental animals underwent orthotopic X-ray examination of the hip joint in vivo and in vitro. The results showed that the femoral head of rats in each group was spherical, and there was no significant difference in the diameter, height, and area of the femoral heads, as shown in Figure 1.
Figure 1.
In vivo and in vitro femoral head X-ray images of different groups of rats.
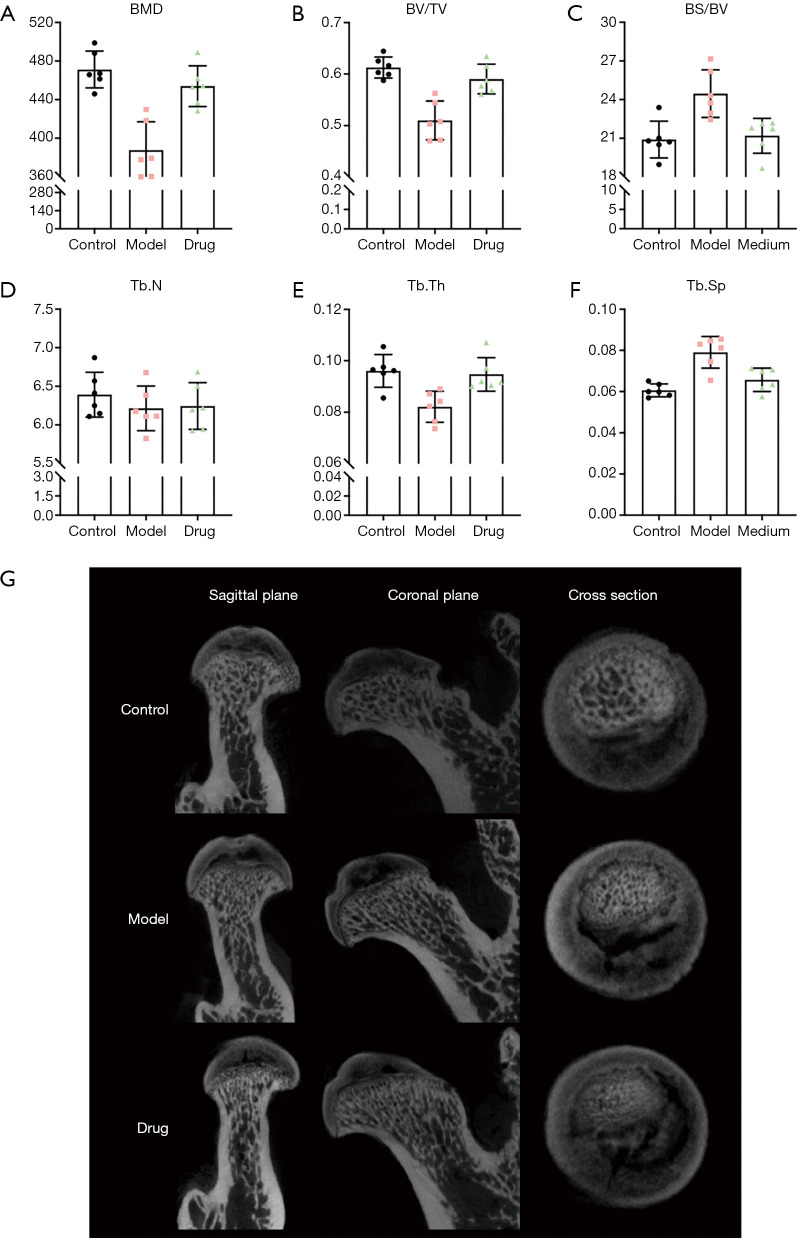
In order to further determine whether Bushen Huoxue Capsule treatment improves bone loss in GC-related ONFH rats, Micro-CT was used to analyze the changes in the trabecular bone in the subchondral area of the femoral head and quantitative analysis of its bone morphometric parameters, as shown in the Figure 2. After 6 weeks, rats in the model group and the Bushen Huoxue Capsule group showed different degrees of damage to the trabecular bone morphology of the subchondral area in the control group, but the rats after the intervention of Bushen Huoxue Capsule were better than the rats with MPS injection. The degree of morphological damage is light.
Figure 2.
Comparison of BMD, BV/TV, BS/BV, Tb.N, Tb.Th and Tb.Sp results under MicroCT scan (A,B,C,D,E,F). Micro CT images of the femoral head in coronal plane, cross section, and sagittal position of different groups of rats (G).
Quantitative evaluation of the trabecular bone parameters of the femoral head in each group showed that the BMD (387.62±29.55 mg/cm3) of the model group was significantly lower than that of the control group (471.22±19.12 mg/cm3), and the difference was statistically significant, while Bushen Huoxue Capsule The BMD of rats in the group (453.93±21.11 mg/cm3) was significantly higher than that in the model group, and the difference was statistically significant, and there was no significant difference in BMD from the control group.
In addition, the differences in other bone parameters, such as BV/TV, BS/TV, Tb.Th and Tb.Sp, between the groups are similar to the difference in BMD, and the difference is statistically significant (P<0.05), while Tb.N is There was no difference between different groups (P>0.05).
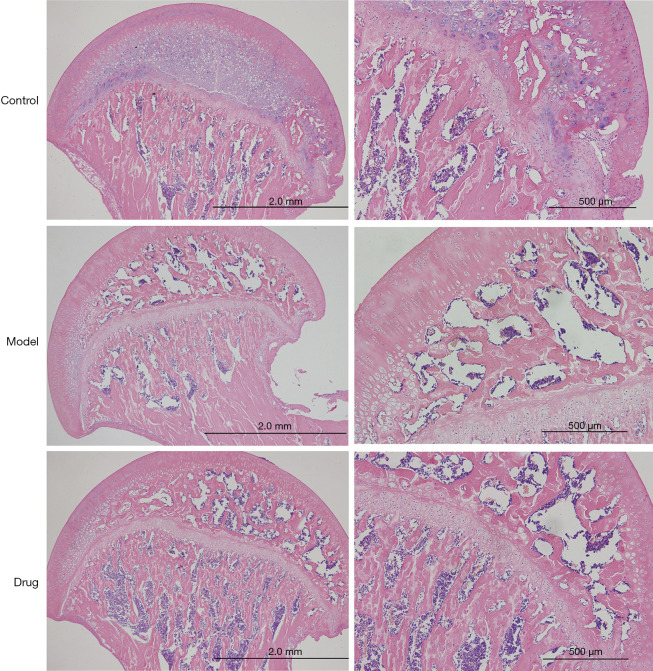
The histological evidence of H&E staining showed that in the normal group, bone trabecular structure and morphology were intact, bone cells in trabecular bone were clearly visible, surrounding osteoblasts were abundant, bone lacunae were rare, hematopoietic cells were abundant and normal in the bone marrow cavity, and the proportion of fat cells and hematopoietic tissue was uniform. In the model group, the trabeculae of the femoral head became thinner, the spacing increased, the structure was disorganized, the bone marrow fat cells increased, and the number of vacant bone lacunae increased significantly. In the BSHXC group, pathological changes of bone trabecular structure were significantly reduced, few bone trabecular structures or bone marrow were replaced by necrotic tissue, no proliferation of fat cells was observed, most of the bone cells were normal, and few empty bone lacunae were found, as shown in Figure 3.
Figure 3.
H&E staining of the coronal plane of the femoral head in different groups. H&E, hematoxylin and eosin.
Proliferation detection of BMSCs in rats
The results of CCK-8 showed that the serum containing BSHXC had no cytotoxic effect on BMSCs in normal rats, and significantly promoted the proliferative activity of BMSCs in rats to a certain extent, as shown in Figure 4. A certain dose of GC continuous intervention could significantly reduce the proliferation ability of BMSCs in rats, while intervention with the BSHXC containing drug serum could significantly improve the proliferation ability of BMSCs in rats, as shown in Figure 4. The above results indicated that the serum containing BSHXC not only promoted the proliferation of BMSCs in normal rats to a certain extent, but also significantly increased the proliferation activity of BMSCs in rats induced by GC.
Figure 4.
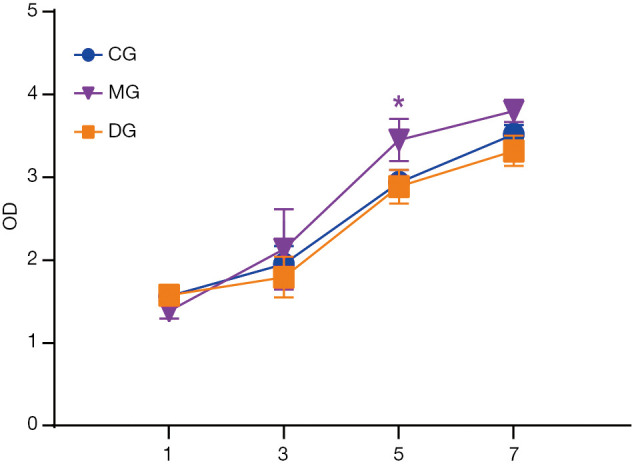
Detection of BMSCs cell proliferation in different groups of rats. *, P<0.05. BMSCs, bone marrow mesenchymal stem cells.
Screening of active compounds of BSHXC into blood
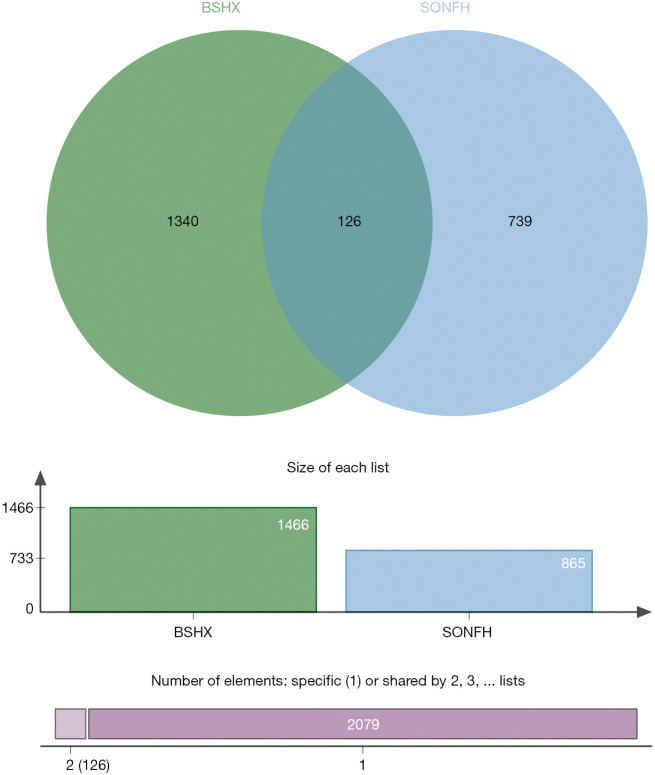
All the TCM ingredients in BSHXC were retrieved from the BATMAN-TCM database, and 293 components with scores ≥20 and P<0.05 were found. According to the prediction of BATMAN-TCM targets, 1,466 potential targets were obtained.
Screening of SONFH related targets
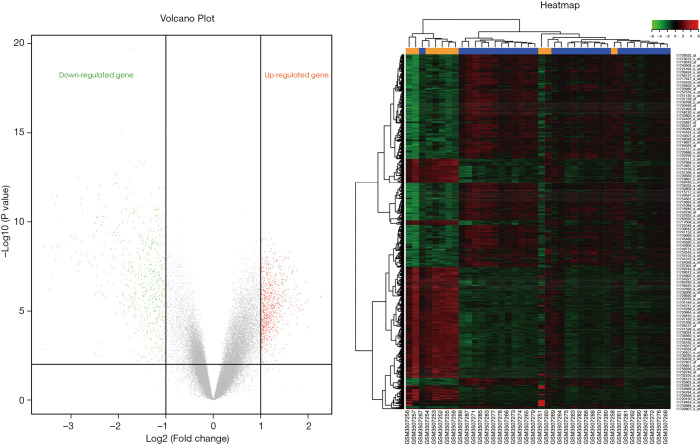
According to the screening conditions, the Illumina package of R language was used for the secondary analysis of the genes in the GEO chip database to obtain 813 differentially expressed genes, as shown in Figure 5, which may be closely related to the occurrence and development of SONFH. A total of 61 significant SONFH targets were collected through DrugBank, GeneCards, and KEGG databases, and 865 candidate gene targets were included after deletion of duplicates.
Figure 5.
Volcanogram and cluster diagram of SONFH differentially expressed mRNA. Orange and blue represent SONFH and the control group, respectively. Red represents up-regulated genes, and green represents down-regulated genes. SONFH, steroid-induced osteonecrosis of the femoral head.
Selection of key targets for the prevention and treatment of SONFH with BSHXC
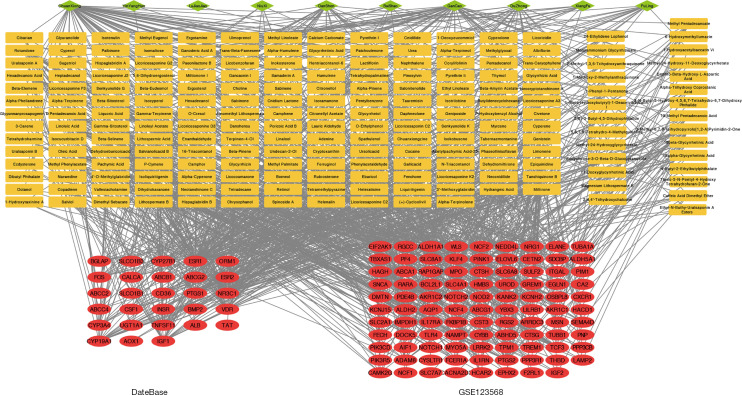
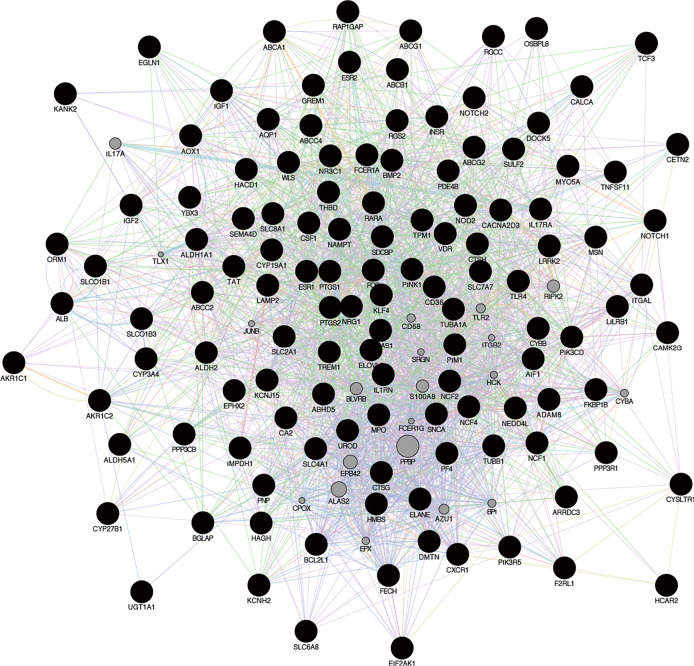
A total of 126 genes were obtained by mapping the SONFH-related targets of into the BSHXC active components of blood, as shown in Figure 6; these were the key targets of BSHXC for SONFH prevention and treatment. Cytoscape was used to construct a network of 10 drugs-208 components-126 targets, as shown in Figure 7. With the help of the STRING database, 2 proteins were mined to interact with the above 126 key targets, and Cytoscape was used to construct a PPI network consisting of 116 proteins and 454 mutual relationships, as shown in Figure 8. The maximal clique centrality (MCC) ranking method on cytoHubba (a Cytoscape App) showed that ALB, IGF1, FOS, PTGS2, NOTCH1, TNFSF11, ESR1, BMP2, BGLAP, and TLR4 were the core targets of BSHXC for SONFH prevention and treatment.
Figure 6.
Venn diagram of SONFH-related targets of BSHXC into the active components of blood. SONFH, steroid-induced osteonecrosis of the femoral head; BSHXC, BuShenHuoXue capsule.
Figure 7.
Network diagram of active components and targets in BSHXC. Green represents TCM information; yellow represents the information of active ingredients. Red represents target information. BSHXC, BuShenHuoXue capsule; TCM, traditional Chinese medicine.
Figure 8.
PPI network diagram of SONFH joint targets treated with BSHXC. PPI, protein-protein interaction; SONFH, steroid-induced osteonecrosis of the femoral head; BSHXC, BuShenHuoXue capsule.
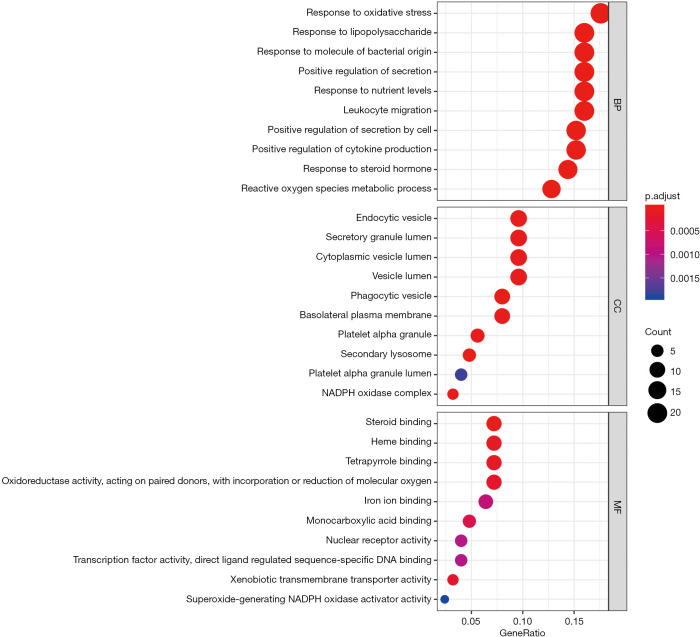
KEGG pathway and GO enrichment analysis
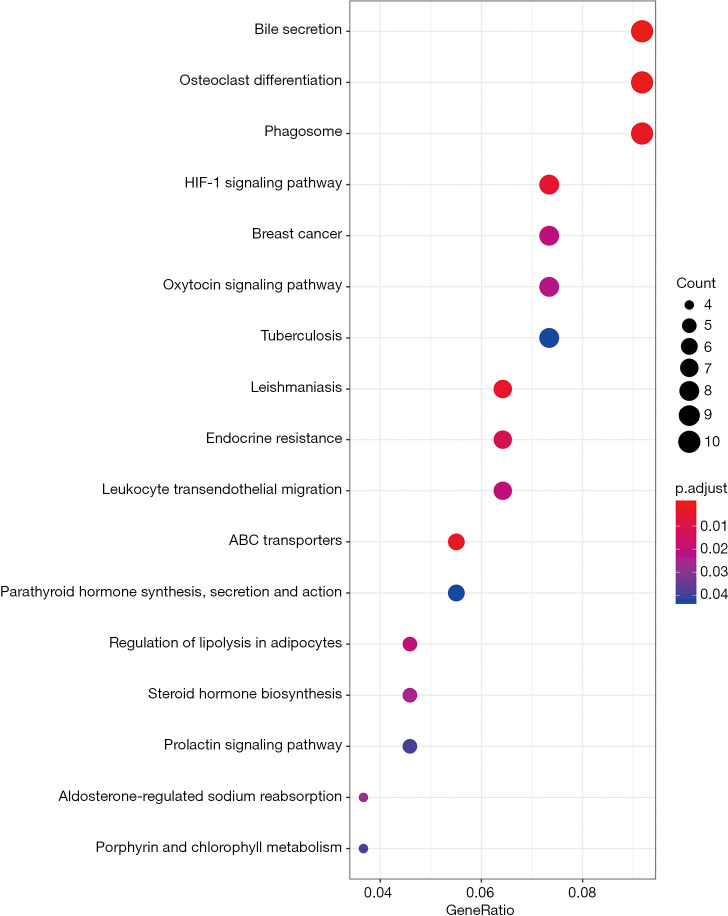
The possible mechanism of BSHXC to prevent SONFH was further elucidated with the help of the R language clusterProfiler package (Figure 9). The 126 key targets above were analyzed to explore the major signaling pathways, biological processes (BP), molecular function (MF), and cellular component (CC) that they may be involved in, as shown in the Figure 10. Analysis results showed that the key target signaling pathways and main functions involved were bile secretion, osteoclast differentiation, phagosome, breast cancer, oxytocin signaling pathway, tuberculosis, leishmaniasis, endocrine resistance, leukocyte transendothelial migration, steroid hormone biosynthesis, and prolactin signaling pathways, which were similar to those previously discovered (11). The BP, molecular composition, and functions involved in the key targets were mainly related to reducing the inflammatory response, changing steroid response, regulating estrogen receptors, delaying osteoporosis, and regulating osteoblast and osteoclast differentiation.
Figure 9.
Bubble diagram of BSHXC for SONFH signaling pathway predicted by the clusterProfiler package. BSHXC, BuShenHuoXue capsule; SONFH, steroid-induced osteonecrosis of the femoral head.
Figure 10.
Prediction of SONFH-related BP, MF, and CC bubble plots with BSHXC according to the clusterProfiler package. SONFH, steroid-induced osteonecrosis of the femoral head; BP, biological processes; MF, molecular function; CC, cellular component; BSHXC, BuShenHuoXue capsule.
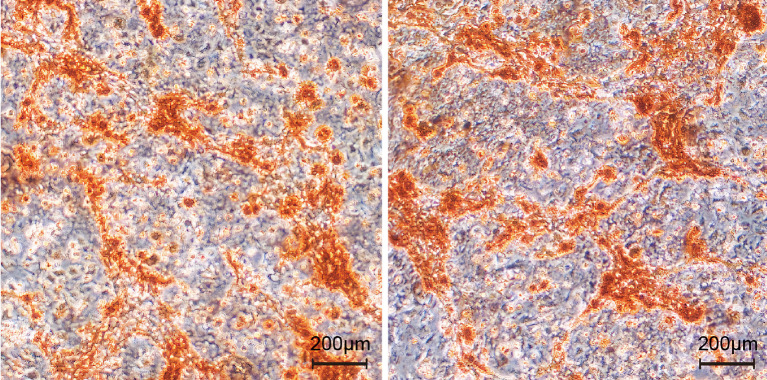
Alizarin red staining of BMSCs in rats
Alizarin red staining showed that, compared with the control group, the serum containing BSHXC could promote the formation and deposition of extracellular mineralized nodules and increase the positive staining area, among which the serum containing BSHXC showed higher activity, as shown in Figure 11. The results were consistent with those in vivo, and showed that the serum containing BSHXC could promote the osteogenic differentiation of BMSCs in rats.
Figure 11.
Alizarin red staining of BMSCs in different groups of rats. BMSCs, bone marrow mesenchymal stem cells.
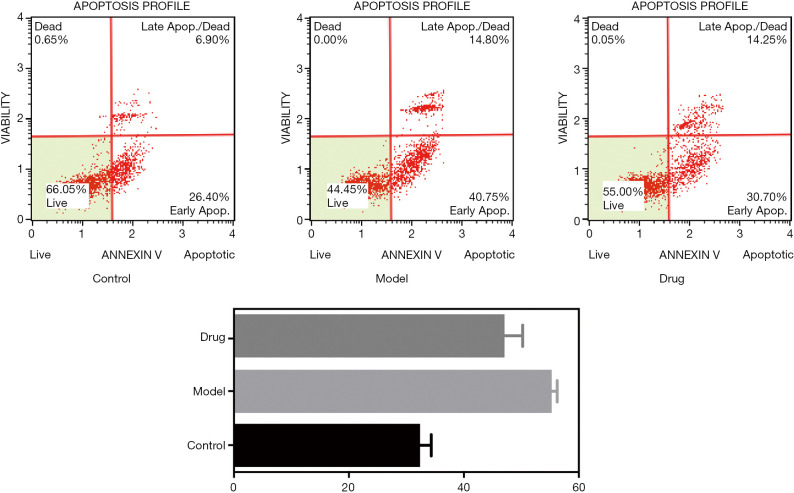
Apoptosis of BMSCs in rats was detected
Compared with the control group, GCs significantly induced the apoptosis of BMSCs, and BSHXC significantly reduced the apoptosis caused by GCs, as shown in Figure 12.
Figure 12.
Apoptosis detection of BMSCs in different groups of rats. BMSCs, bone marrow mesenchymal stem cells.
Discussion
Since Pietrogrande first reported that GC was associated with femoral head necrosis in 1953 (12), SONFH has attracted extensive attention in the field of orthopedics worldwide, with its research continuously deepening. At present, long-term or heavy use of hormones has become the main cause of femoral head ischemic necrosis (13). A large number of studies have shown that SONFH is caused by multiple factors, such as lipid metabolism disorders (14); lipogenic differentiation of BMSCs (15); intravascular coagulation (16); and apoptosis (17). Although the theory of apoptosis is scientific to some extent, it does not fully explain the mechanism of SONFH.
This study confirmed that BSHXC could effectively improve the bone loss of SONFH rats in vivo, but it was challenging to employ conventional methods to describe the scientific basis and mechanism of BSHXC in the prevention and treatment of SONFH. Therefore, this study used bioinformatics to explore disease targets and network pharmacological means to analyze the active ingredients and therapeutic targets of BSHXC in blood. After obtaining the key targets, the function of BSHXC was enriched and analyzed using the R language clusterProfiler package.
The core target TLR4 obtained through screening is mainly expressed in monocytes/macrophages and dendritic cells, and it recognizes the molecular pattern of pathogens and plays a role in immune recognition and response. Studies have suggested that TLR4 is also expressed in osteocytes, and its activation is involved in TLR-mediated osteoclast formation, affecting the differentiation of osteoclasts. It is the bridge between the immune response and bone metabolism disorders. Activated by the TLR4 pathway, monocytes/macrophages can also cause a series of inflammatory reactions, leading to the proliferation of fibrous vascular tissue in the femoral head and the destruction of bone structure, which is also one of the possible mechanisms of femoral head necrosis (18). The abnormal activation of the TLR4 signaling pathway and interference with the immune response can be caused by GCs, resulting in the disruption of balance of normal bone metabolism, proliferation of a large number of monocytes/macrophages, proliferation and activation of osteoclasts, reduction of bone mass in the femoral head, osteoporosis, and finally femoral head necrosis (19). Notch1-mediated signaling is an important factor in determining the differentiation potential of BMSCs (20). The Notch1-mediated signaling pathway plays a role in promoting osteogenic differentiation and inhibiting lipogenic differentiation. Impaired activity of the Notch signaling pathway in human BMSCs may be one of the factors influencing the occurrence of osteoporosis, while the possible mechanism of SONFH includes osteoporosis. Studies have shown that the core target IGF-1 can stimulate the proliferation and differentiation of osteoblasts and the production of extracellular matrix, and promote the recruitment of osteoclasts to enhance the activity of bone resorption (21). Estrogen receptor 1 (ESR1/ERα) is a ligand-dependent transcription factor and a major mediator of the biological effects of estrogen and selective estrogen receptor modulators. As far as the skeletal system is concerned, ESR1 can be expressed in osteoblasts, osteoclasts, chondrocytes, bone marrow stromal cells, and osteocytes (22), and estrogen can directly bind to the nuclear estrogen receptor ESR1 to activate the transcription of target genes, which is the classic estrogen receptor signal transduction pathway. Bone morphogenetic protein 2 (BMP2), is currently the most widely used bone morphogenetic protein in clinical applications. It can promote the differentiation of mesenchymal stem cells and BMSCs into osteoblasts by activating its downstream classical BMP/Smad signaling pathway and non-classical BMP-MAPK (mitogen-activated protein kinases) signaling pathway (23).
Despite these important findings, this study still had some limitations. First, the small number of SONFH samples collected in the database may have led to some disease target bias. Second, it is difficult to directly identify specific targets and key active ingredients from TCM compounds with complex chemistry and little cognition. Third, although network pharmacology can reflect the interrelationship between drugs and diseases at multiple levels, it cannot fully reflect the characteristics of all real cellular networks in the organism, such as some counterintuitive or abnormal system responses. In addition, this study focuses on the overall efficacy of BSHXC in an in vivo experiment and the pathological process involved in the prevention and treatment of key targets in ONFH. For future research, the role of important monomer components and specific targets in BSHXC in the pathological process requires attention.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This work was supported by a grant from the National Natural Science Foundation of China (81774333). Support from this grant contributed to the development of the study design, the collection, analysis and interpretation of data, writing of the report, and the decision to submit the article for publication.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The experiment was implemented through the approval of ethics committee in Chinese People’s Liberation Army General Hospital and followed the tenets of the Declaration of Helsinki (as revised in 2013). All animal experiments were carried out with the approval of the animal ethics committee in Chinese People’s Liberation Army General Hospital, and the animals were treated in strict line with the International Code of Ethics and the National Health Guidelines for the maintenance and use of laboratory animals
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-7040
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-7040
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-7040). The authors have no conflicts of interest to declare.
(English Language Editor: J. Jones)
References
- 1.Chang C, Greenspan A, Gershwin ME. The pathogenesis, diagnosis and clinical manifestations of steroid-induced osteonecrosis. J Autoimmun 2020;110:102460. 10.1016/j.jaut.2020.102460 [DOI] [PubMed] [Google Scholar]
- 2.Kang P, Xie X, Tan Z, et al. Repairing defect and preventing collapse of femoral head in a steroid-induced osteonecrotic of femoral head animal model using strontium-doped calcium polyphosphate combined BM-MNCs. J Mater Sci Mater Med 2015;26:80. 10.1007/s10856-015-5402-x [DOI] [PubMed] [Google Scholar]
- 3.Jin T, Zhang Y, Sun Y, et al. IL-4 gene polymorphisms and their relation to steroid-induced osteonecrosis of the femoral head in Chinese population. Mol Genet Genomic Med 2019;7:e563. 10.1002/mgg3.563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao F, Liu G, Wang W, et al. Combined Treatment with an Anticoagulant and a Vasodilator Prevents Steroid-Associated Osteonecrosis of Rabbit Femoral Heads by Improving Hypercoagulability. Biomed Res Int 2017;2017:1624074. 10.1155/2017/1624074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nozaki Y, Kumagai K, Miyata N, et al. Pravastatin reduces steroid-induced osteonecrosis of the femoral head in SHRSP rats. Acta Orthopaedica 2012;83:87-92. 10.3109/17453674.2011.641103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arai R, Takahashi D, Inoue M, et al. Efficacy of teriparatide in the treatment of nontraumatic osteonecrosis of the femoral head: a retrospective comparative study with alendronate. Bmc Musculoskeletal Disorders 2017;18:24. 10.1186/s12891-016-1379-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang YL, Yin JH, Ding H, et al. Vitamin K2 Prevents Glucocorticoid-induced Osteonecrosis of the Femoral Head in Rats. Int J Biol Sci 2016;12:347-58. 10.7150/ijbs.13269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tao SC, Yuan T, Rui BY, et al. Exosomes derived from human platelet-rich plasma prevent apoptosis induced by glucocorticoid-associated endoplasmic reticulum stress in rat osteonecrosis of the femoral head via the Akt/Bad/Bcl-2 signal pathway. Theranostics 2017;7:733-50. 10.7150/thno.17450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu H, Ma WL, Zheng WL. GEO Gene Expression Omnibus: High-throughput Gene Expression Database. Chinese Journal of Biochemistry & Molecular Biology 2007;23:236-44. [Google Scholar]
- 10.Altermann E, Klaenhammer TR. PathwayVoyager: pathway mapping using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database. BMC Genomics 2005;6:60. 10.1186/1471-2164-6-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu G, Wang LG, Han Y, et al. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 2012;16:284-7. 10.1089/omi.2011.0118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pietrogrande V, Marino V. Study of the circulation of the femoral head in some patients with arthritis deformans of the hip. Reumatismo 1953;5:219-22. [PubMed] [Google Scholar]
- 13.Guo S, Mao L, Ji F, et al. Activating AMP-activated protein kinase by an α1 selective activator Compound 13 attenuates dexamethasone-induced osteoblast cell death. Biochem Biophys Res Commun 2016;471:545-52. 10.1016/j.bbrc.2016.02.036 [DOI] [PubMed] [Google Scholar]
- 14.Jiang Y, Liu D, Kong X, et al. Huogu I formula prevents steroid-induced osteonecrosis in rats by down-regulating PPARγ expression and activating Wnt/LRP5/β-catenin signaling. Journal of Traditional Chinese Medicine 2014;(3):342-50. 10.1016/S0254-6272(14)60100-X [DOI] [PubMed] [Google Scholar]
- 15.Li J, Li Y, Wang Y, et al. Preventive effects of siRNA targeting PPARγ gene on steroid-induced osteonecrosis in rabbits. Connect Tissue Res 2014;55:322-30. 10.3109/03008207.2014.941106 [DOI] [PubMed] [Google Scholar]
- 16.Li J, Fan L, Yu Z, et al. The effect of deferoxamine on angiogenesis and bone repair in steroid-induced osteonecrosis of rabbit femoral heads. Exp Biol Med (Maywood) 2015;240:273-80. 10.1177/1535370214553906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu X, Wen H, Hu Y, et al. STAT1-caspase 3 pathway in the apoptotic process associated with steroid-induced necrosis of the femoral head. J Mol Histol 2014;45:473-85. 10.1007/s10735-014-9571-6 [DOI] [PubMed] [Google Scholar]
- 18.Adapala NS, Yamaguchi R, Phipps M, et al. Necrotic Bone Stimulates Proinflammatory Responses in Macrophages through the Activation of Toll-Like Receptor 4. Am J Pathol 2016;186:2987-99. 10.1016/j.ajpath.2016.06.024 [DOI] [PubMed] [Google Scholar]
- 19.Tian L, Zhou DS, Wang KZ, et al. Association of Toll-like receptor 4 Signaling Pathway with Steroid-induced Femoral Head Osteonecrosis in Rats. J Huazhong Univ Sci Technolog Med Sci 2014;34:679-86. 10.1007/s11596-014-1336-7 [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Pan J, Zhang Y, et al. Wnt and Notch signaling pathways in calcium phosphate-enhanced osteogenic differentiation: A pilot study. J Biomed Mater Res B Appl Biomater 2019;107:149-60. 10.1002/jbm.b.34105 [DOI] [PubMed] [Google Scholar]
- 21.Ulu MA, Batmaz B, Dilek B, et al. Prevalence of osteoporosis and vertebral fractures and related factors in patients with ankylosing spondylitis. Chinese Medical Journal 2014;127:2740-7. [PubMed] [Google Scholar]
- 22.Yang X, Guo Y, He J, et al. Estrogen and estrogen receptors in the modulation of gastrointestinal epithelial secretion. Oncotarget 2017;8:97683-92. 10.18632/oncotarget.18313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mont MA, Ragland PS, Biggins B, et al. Use of Bone Morphogenetic Proteins for Musculoskeletal Applications. J Bone Joint Surg Am 2004;86-A Suppl 2:41-55. 10.2106/00004623-200412002-00008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as