Abstract
The efficacy of deep brain stimulation (DBS) for refractory Tourette syndrome (TS) is accepted, but whether the efficacy of DBS treatment in the Japanese population is equivalent to those reported internationally and whether adverse effects are comparable are not yet known. This study evaluated the clinical practice and outcome of DBS for TS in a Japanese institution. This study included 25 consecutive patients with refractory TS treated with thalamic centromedian-parafascicular nucleus DBS. The severity of tics was evaluated with the Yale Global Tic Severity Scale (YGTSS) before surgery, at 1 year after surgery, and at the last follow-up of 3 years or more after surgery. The occurrence of adverse events, active contact locations, and stimulation conditions were also evaluated. YGTSS tic severity score decreased by average 45.2% at 1 year, and by 56.6% at the last follow-up. The reduction was significant for all aspects of the scores including motor tics, phonic tics, and impairment. The mean coordinates of active contacts were 7.62 mm lateral to the midline, 3.28 mm posterior to the midcommissural point, and 3.41 mm above anterior commissure–posterior commissure plane. Efficacy and stimulation conditions were equivalent to international reports. The stimulation-induced side effects included dysarthria (32.0%) and paresthesia (12.0%). Device infection occurred in three patients (12.0%) as a surgical complication. The DBS device was removed because of infection in two patients. DBS is an effective treatment for refractory TS, although careful indication is necessary because of the surgical risks and unknown long-term outcome.
Keywords: Tourette syndrome, deep brain stimulation, centromedian-parafascicular nuclei, tic
Introduction
Tourette syndrome (TS) is a neurological condition characterized by severe motor and phonic tics, typically starting in childhood. Patients with TS usually experience relief of symptoms by late adolescence, but severe tics persist into adulthood in around 20% of patients. Such severe tics often hamper social activities at school and work, causing social isolation and reduced educational opportunities. Self-injury induced by severe motor tics may threaten life or result in physical disability. TS is commonly associated with other neuropsychiatric comorbidities such as attention deficit hyperactivity disorder and obsessive compulsive disorders.1)
Thalamic deep brain stimulation (DBS) was first reported in 1999,2) and multiple institutions around the world have established the effectiveness of DBS for refractory TS.3) DBS is now an important treatment option for TS. However, the operative indication of DBS is controversial because although refractory phonic and motor tics severely impair quality of life, these tics may resolve with age as a natural course of the disease. Therefore, whether DBS is a temporary or life-long therapy for TS remains unclear.4) Additionally, the optimal targets and stimulation conditions have not been standardized. Several stimulation targets, including the anteromedial globus pallidus internus, anterior limb of the internal capsule, and centromedian nucleus-substantia periventricularis-nucleus ventro-oralis (Vo) internus complex have shown comparative efficacy.3) The centromedian-parafascicular (CM-Pf) nucleus is most often selected as the stimulation target in Japan. To assess the efficacy and safety, a multinational registry of the DBS treatment for TS has been launched.5) However, whether the efficacy of DBS treatment in the Japanese population is equivalent to those reported internationally and whether adverse effects are comparable are not yet known.
This study reports the clinical practice and long-term outcome of DBS for TS in our institution, aiming to determine the reproducibility of the therapeutic effect in Japanese population.
Methods
This study included 25 consecutive patients (19 males and 6 females) with refractory TS who were treated with CM-Pf nucleus DBS from January 2008 to May 2019 in our hospital and were followed up for at least 1 year. Bilateral DBS therapy has been approved for severe tics or involuntary movements occurring in refractory TS by the ethics committee of the National Center of Neurology and Psychiatry since 2008. The severity of tics was evaluated by the Yale Global Tic Severity Scale (YGTSS).6) The YGTSS consists of motor and phonic tic scores (25 points maximum each) and impairment score (50 points maximum).
Indications for DBS therapy for TS was as follows: (1) Diagnosis of TS made by an experienced psychiatrist, (2) age 18 years or more, (3) severe tics with YGTSS tic score of 35 or higher, (4) 3 or more types of medications have been tried, (5) cognitive behavioral therapy has been offered if applicable, and (6) comorbid disorders should be stable with no active suicidal ideation for at least 6 months before surgery.
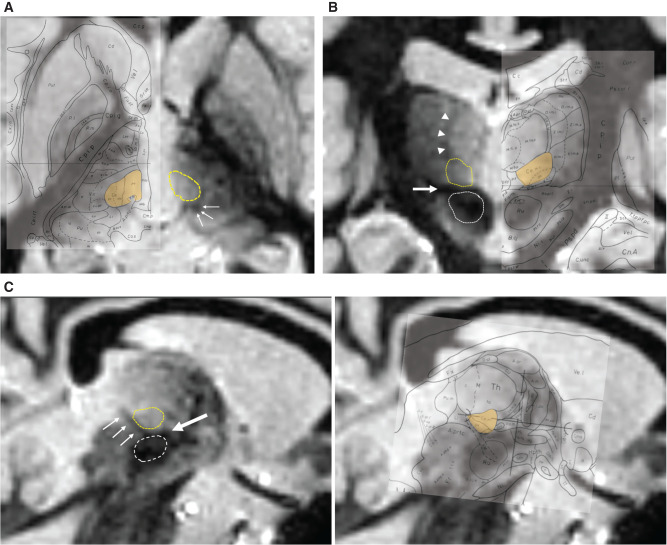
Video recordings of the tic symptoms were obtained before surgery. Neuropsychological tests including the Wechsler Adult Intelligence Scale III, Wechsler Memory Scale-Revised, Beck Depression Inventory-II, and Yale-Brown Obsessive-Compulsive Scale were performed, but were not the subject of this study. Brain magnetic resonance (MR) imaging was essential to plan the surgical targets as well as to evaluate co-morbidities and complications. All MR images were acquired with a 3 Tesla system (Achieva, Philips, Best, The Netherlands). Essential MR imaging included three-dimensional T1-weighted, fast gray matter acquisition T1 inversion recovery (FGATIR), T2-weighted, and diffusion-weighted sequences. The tentative target of the CM-Pf nucleus was determined as 5 mm lateral to the midline, 4 mm posterior to the midcommissural point, and on the anterior commissure–posterior commissure (AC-PC) plane according to Vandewalle’s original study.2) The final target was adjusted based on the patient’s MR images. The CM-Pf nucleus was identifiable as a round shaped structure of relatively high intensity located above the prelemniscal radiation and the red nucleus, and anterior to the habenulo-interpeduncular tract on the sagittal plane of FGATIR images (Fig. 1). The final coordinate was also adjusted laterally according to the size of the third ventricle. The entry point was made anteriorly to the coronal suture and laterally enough to avoid penetrating the lateral ventricle. The final trajectories were at an average angle of 55.8° from the AC-PC plane and 26.9° from the sagittal plane.
Fig. 1.
The direct identification of the CM-Pf nucleus. The FGATIR images show the axial section 1.5 mm above the AC-PC plane (A), the coronal section 6 mm posterior from the mid-commissural point (B), and the sagittal section 6.5 mm lateral from the midline (C). The CM-Pf complex (yellow dashed circle) is identified as a round-shaped structure with relatively high intensity located above the prelemniscal radiation (thick arrow) and red nucleus (white dashed circle), anterior to the habenulo-interpeduncular tract (thin arrow) and below the internal medullary lamina (arrowhead). The CM-Pf complex is also shown in yellow on the Schaltenbrand and Wahren atlas overlaid on the corresponding images in the right side. The figure was quoted from the plates 28, 40, and 53 in Atlas for Stereotaxy of the Human Brain, Second Revised and Enlarged Edition, 1977, Thieme Publishers, by Georges Schaltenbrand and Waldemar Wahren. AC-PC: anterior commissure–posterior commissure, CM-Pf: centromedian-parafascicular, FGATIR: fast gray matter acquisition T1 inversion recovery.
The DBS system was implanted under general anesthesia under microelectrode recording. The track was expected to pass the Vo and the ventral intermediate (Vim) nuclei, which could be confirmed by microelectrode recording. Responses to light touch were also recorded on the necessity of confirming that the ventral caudal (Vc) nucleus was entered.
The DBS electrodes (Model 3387, Medtronic, Minneapolis, MN, USA) were inserted into the bilateral thalamic CM-Pf nuclei as the target using the Leksell Stereotactic System (Elekta Instrument AB, Stockholm, Sweden). Implantable pulse generators (IPGs) (Activa, Medtronic) were implanted subcutaneously or into the subfascia of the pectoralis major muscle in the subclavicular region. Rechargeable IPG is often selected because high output is expected.
Three-dimensional computed tomography (CT) was performed after DBS implantation to evaluate the electrode position. The postoperative CT images were then fused with the preoperative MR images to identify the actual stereotactic coordinates of the lead contacts using iPlan (Brainlab AG, Munich, Germany). The locations of the active contacts were evaluated for 24 leads in 12 patients in whom postoperative thin slice CT data were available. Initial programming began at a few days after DBS implantation. Tolerability was evaluated for each electrode using constant voltage stimulation up to 5 V, with pulse width of 60 μs, and frequency of 130 Hz.
Tic symptoms were evaluated with YGTSS and video recording 1 year after surgery and were followed up on a yearly basis. In this study, YGTSS scores, active contact locations, and stimulus conditions at 1 year and at the last follow-up of 3 years or more after surgery were compared.
Repeated measures analysis of variance (ANOVA) was used to evaluate the statistical difference of YGTSS over time. Post-hoc pairwise comparison was performed by a paired t-test with Bonferroni correction using R version 3.5.2 (The R Foundation for Statistical Computing).
Results
The ages at the onset of tics and at surgery were 7.67 ± 4.45 and 26.07 ± 6.08 years (range, 19–39 years), respectively. The preoperative YGTSS scores were 19.89 ± 3.72 for motor tics, 19.39 ± 3.30 for phonic tics, and 40.64 ± 13.91 for impairment. Motor and phonic tic scores were reduced to 11.64 ± 3.92 and 9.92 ± 3.95 at 1 year after surgery (n = 25), respectively, and to 8.78 ± 5.49 and 8.28 ± 5.41 at the last follow-up (n = 14), respectively (Fig. 2). Impairment score of YGTSS was decreased to 19.61 ± 11.59 at 1 year after surgery and to 15.71 ± 12.47 at the last follow-up. Total YGTSS decreased by average 45.2% at 1 year, and by 56.6% at the last follow-up. The postoperative change in the total YGTSS score was statistically significant (p <0.01). The post-hoc analysis revealed that the difference was significant between preoperative and postoperative scores (p <0.01) but was not between scores at 1 year after surgery and at the last follow-up.
Fig. 2.
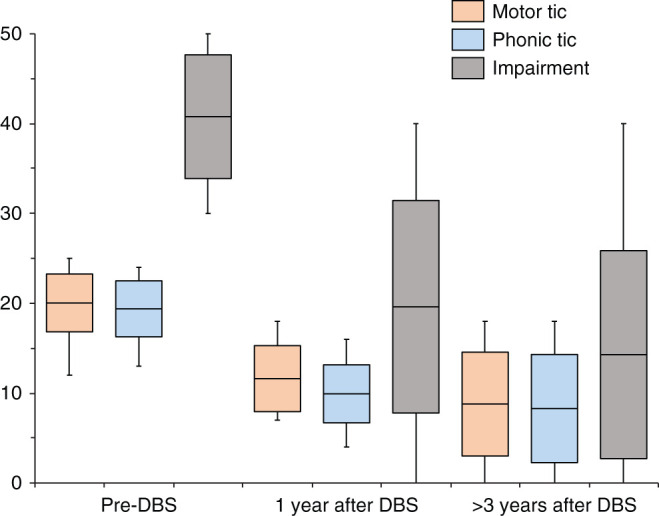
Postoperative changes in YGTSS motor tic, phonic tic, and impairment scores. The maximum scores for the motor tic, photic tic, and impairment are 25, 25, and 50, respectively. Box shows the average and standard deviation. Whisker shows the range. All scores were reduced after surgery and stable over 3 years. YGTSS: Yale Global Tic Severity Scale.
The effect of DBS was observed immediately after the initiation of stimulation in many patients. Patients frequently reported sensations such as “no compulsion for tics” and “feeling physically easier without tics.” Their symptoms continued to improve over the long term. As a stimulation-induced side effect, dysarthria was transiently observed in eight patients. Dizziness and paresthesia were observed in three cases, usually transient and improved by adjusting the stimulus voltage. As a surgical complication, device infection occurred in three patients including two cases that required removal of the DBS. Two of them occurred after the first implantation, but another occurred after IPG replacement. DBS was withdrawn in two patients on their request. Both patients had obtained significant reduction of tics in the long term and felt the burden of receiving multiple surgeries for changing batteries.4)
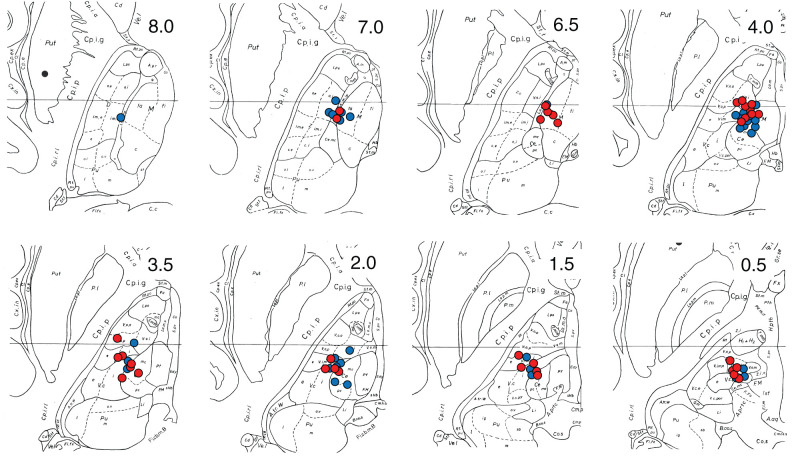
The number of active contacts per a side was 3 in 14 hemispheres and 4 in 10 hemispheres. The active contact coordinates were 7.62 ±1.74 mm lateral to the midline (7.80 ± 1.70 on the left side and 7.54 ± 1.75 on the right side), 3.28 ± 2.08 mm posterior to the midcommissural point (3.30 ± 1.88 on the left side and 3.27 ± 2.27 on the right side), and 3.41 ± 2.50 mm above AC-PC plane (3.00 ± 2.55 on the left side and 3.85 ± 2.37 on the right side). Figure 3 shows the distribution of all active contacts. These positions did not change over time. The above coordinates were comparable to the previous findings in 51 cases of CM-Pf nucleus DBS for TS.7) These average coordinates were reported as 6.6 (left) and 6.1 mm (right) lateral to the midline, 3.3 (left) and 3.1 mm (right) posterior to the midcommissural point, and 3.0 (left) and 3.2 mm (right) above the AC-PC plane.
Fig. 3.
The active contact coordinates are plotted on the axial plane of the Schaltenbrand–Wahren atlas. The number on the upper right corner shows the distance (mm) from the AC-PC plane. The contacts in the left and right sides are shown in blue and red, respectively. The active contacts were located not only in the CM-Pf but also in the Vo, Vim, and Medial dorsal nucleus. Quoted and revised from plates 52 and 53, Atlas for Stereotaxy of the Human Brain, Second Revised and Enlarged Edition, 1977, Thieme Publishers, by Georges Schaltenbrand and Waldemar Wahren. AC-PC: anterior commissure–posterior commissure.
DBS setting per electrode at 1 year after surgery was as follows: 1.5–4.3 V (left, median 3.3), 1.4–4.3 V (right, median 2.9), 80–210 Hz (both sides, median 145), and 90–320 μs pulse width (both sides, median 210). Interleaving stimulation was performed in four cases. The stimulation parameters were within a similar range to the above setting during the following years in all patients.
Representative Case 1
The patient was a 37-year-old man. He had developed a tic with repeated blinks at the age of 7 years. Symptoms fluctuated, but he poked pencils into his eyes from around the age of 15 years, and injured himself by hitting the back of his neck. He was diagnosed with TS at the age of 19 years and received multiple medical treatments, but the symptoms did not improve. He suffered cervical spinal cord injury caused by blows to the occipital region and neck induced by motor tics. Preoperative evaluation was performed at the age of 37 years. His YGTSS motor and phonic tic scores were 23/25 and 20/25, respectively.
Bilateral CM-Pf nucleus DBS devices were implanted under general anesthesia. Within a few days after DBS was turned on, he felt “safe without tics” and said “I can control the phonic tics by myself.” One year after the surgery, the YGTSS motor and phonic tic scores were reduced to 8/25 and 9/25, respectively. Symptoms were further alleviated over the long term, and the tics causing self-injury had completely disappeared by 26 months after the operation. Although tics appear at home, he can work as a construction worker, and tics are almost never recognized during outpatient visits. He has slight numbness in his face, which may be a sensory symptom due to the spread of stimulus to the nucleus ventralis caudalis.
Representative Case 2
This patient was a 23-year-old woman. She had developed screaming phonic tics and was unable to sit still at school from the age of 7 years. The severe tics caused her to hit her head against a wall or desk. She always wore headgear to protect herself. She vocalized loud screams and was not able to write or illustrate because she could not help breaking pencils. She was admitted to a psychiatric hospital for a long time, and her life had almost collapsed. Preoperative YGTSS score was the most severe, with motor and phonic tic scores both 24/25 and social disability score 50.
She received bilateral CM-Pf nucleus DBS devices, then she stopped hitting her head and she could live without headgear by 1 month after the operation. She was hospitalized for IPG exchange 1 year after the DBS surgery, when the phonic tic score had decreased to 9/25 and the motor tic score to 11/25. Her tics did not completely disappear, but she was able to live without tics in public (Supplementary video). She works in day-care and enjoys drawing illustrations which she loves.
Discussion
Our study showed that CM-Pf nucleus DBS for refractory TS was effective in the Japanese population comparable to previous international studies. The total YGTSS scores were significantly decreased after DBS, and the improvement was sustained for the long term. The operative indications accorded to the international guidelines3) in this study. The clinical effect was mediated by CM-Pf nucleus stimulation targeted to similar coordinates as reported previously.
The most beneficial DBS target for TS is not yet determined. The CM-Pf nucleus is the most common target among various targets including the CM-Pf, anteromedial globus pallidus internus, and anterior limb of the internal capsule.3) About half of the patients received DBS of the CM-Pf nucleus in the previous report, followed by DBS of the anteromedial globus pallidus internus.7)
Our experience with the patient responses to CM-Pf nucleus DBS is quite similar to previous reports. Average 47% improvement in YGTSS score was reported at 3 months to 4 years after CM-Pf nucleus DBS.8) A 73% improvement was found at an average follow-up of 5 years.9) More than 200 DBS cases are currently registered by the International Tourette Deep Brain Stimulation Database and Registry.10) CM-Pf nucleus DBS achieved average 44.15% reduction in YGTSS score at 1 year after surgery according to the database. DBS is effective for the long term and even shows sustained improvement over years.
The mechanism of action of DBS for TS is unclear, although dysfunction of the cortico-striato-thalamo-cortical circuits is suspected to be critical. The thalamus shows a relatively hyperexcited state in this circuit in patients with TS. DBS of the CM-Pf thalamic nuclei may generate its effect by modulating and controlling this loop.11) The International Tourette Deep Brain Stimulation Database and Registry10) reports average programming settings at 1 year after DBS are 3.21 V, 132 Hz, and 114 μs pulse width. Although direct comparison is difficult, strong stimulation is generally required for treating TS compared with Parkinson’s disease.
The most common side effects induced by stimulation in our study were dysarthria (32.0%), which was usually transient and dependent on DBS output, and is probably similar to the dysarthria that occurs after bilateral Vim nucleus DBS performed for essential tremor. Dysarthria is the most common side effect in DBS for TS.12) Paresthesia was observed in three patients (12.0%) in this study, and occurred in 34.78% of patients in the International Registry at 6 months after CM-Pf nucleus DBS. Overspreading current to the nucleus ventralis caudalis, located outside and posterior to the CM-Pf thalamus, is the most likely cause of this side effect.
Concerning surgical complications, the risk of infection is higher in TS compared to other diseases treated with DBS.13) Abnormal immune system response is suspected in the pathogenesis of TS. It was reported that the changes in lymphocyte subpopulations and gene expression profiles of peripheral immune cells causes increased immune responses in TS.14) However, the relationship of the abnormal immune response to the risk of infection is unclear. Excessive physical contact with the wound due to obsessive compulsive behavior may increase the chance of infection.12) We also speculate that high daily activity and sweating are related with the risk of infection in TS patients who are apparently younger than other DBS patients. Frequent exchange of IPG, required when using non-rechargeable batteries, is another risk for infection.
A multicenter study is required to establish the operative indication, efficacy, and risks in the DBS therapy of TS in Japan. Limited case series are only available to date, although DBS therapy for TS has been performed in about 40 patients with TS so far in Japan (personal communication). We need to accumulate experience with the appropriate target selection, DBS settings, device maintenance, and adverse events.
DBS has clear therapeutic effects, but should be carefully indicated for TS considering the potential for spontaneous remission and the risk of surgical complications. Multidisciplinary teams including psychiatrists and neurosurgeons should be involved to consider the differential diagnosis of other involuntary movements and psychiatric comorbidities, and to judge whether sufficient medication has been performed and whether the severity is enough to consider DBS. However, DBS can be a life-changing treatment option for patients with TS living severely impaired live.
Informed Consent
Written informed consent was obtained from the patient for publication of the supplementary video.
Acknowledgments
We would like to thank Mr. Naotake Shoji for data analysis. This study was supported by Japan Agency for Medical Research and Development (AMED), Tokyo, Japan under grant number JP19ek0109262.
Footnotes
Conflicts of Interest Disclosure
The authors have no conflict of interest to disclose.
References
- 1).Müller-Vahl KR: Surgical treatment of Tourette syndrome. Neurosci Biobehav Rev 37: 1178–1185, 2013 [DOI] [PubMed] [Google Scholar]
- 2).Vandewalle V, van der Linden C, Groenewegen HJ, Caemaert J: Stereotactic treatment of Gilles de la Tourette syndrome by high frequency stimulation of thalamus. Lancet 353: 724, 1999 [DOI] [PubMed] [Google Scholar]
- 3).Schrock LE, Mink JW, Woods DW, et al. : Tourette Syndrome Association International Deep Brain Stimulation (DBS) Database and Registry Study Group: Tourette syndrome deep brain stimulation: a review and updated recommendations. Mov Disord 30: 448–471, 2015 [DOI] [PubMed] [Google Scholar]
- 4).Kimura Y, Ikegaya N, Iijima K, et al. : Withdrawal of deep brain stimulation in patients with Gilles de la Tourette syndrome. Mov Disord 34: 1925–1926, 2019 [DOI] [PubMed] [Google Scholar]
- 5).Martinez-Ramirez D, Jimenez-Shahed J, Leckman JF, et al. : Efficacy and safety of deep brain stimulation in Tourette syndrome: The International Tourette Syndrome Deep Brain Stimulation Public Database and Registry. JAMA Neurol 75: 353–359, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Leckman JF, Riddle MA, Hardin MT, et al. : The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry 28: 566–573, 1989 [DOI] [PubMed] [Google Scholar]
- 7).Johnson KA, Fletcher PT, Servello D, et al. : Image-based analysis and long-term clinical outcomes of deep brain stimulation for Tourette syndrome: a multisite study. J Neurol Neurosurg Psychiatry 90: 1078–1090, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Servello D, Sassi M, Brambilla A, Defendi S, Porta M: Long-term, post-deep brain stimulation management of a series of 36 patients affected with refractory Gilles de la Tourette syndrome. Neuromodulation 13: 187–194, 2010 [DOI] [PubMed] [Google Scholar]
- 9).Porta M, Servello D, Zanaboni C, et al. : Deep brain stimulation for treatment of refractory Tourette syndrome: long-term follow-up. Acta Neurochir (Wien) 154: 2029–2041, 2012 [DOI] [PubMed] [Google Scholar]
- 10).The University of Florida Health Center for Movement Disorders and Neurorestoration: International Tourette Deep Brain Stimulation Database and Registry , (Accessed on 2020 June 2) Available at https://tourettedeepbrainstimulationregistry.ese.uf-health.org/.
- 11).Baldermann JC, Schüller T, Huys D, et al. : Deep brain stimulation for Tourette-syndrome: a systematic review and meta-analysis. Brain Stimul 9: 296–304, 2016 [DOI] [PubMed] [Google Scholar]
- 12).Martinez-Ramirez D, Jimenez-Shahed J, Leckman JF, et al. : Efficacy and safety of deep brain stimulation in Tourette syndrome: The International Tourette Syndrome Deep Brain Stimulation Public Database and Registry. JAMA Neurol 75: 353–359, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Servello D, Sassi M, Gaeta M, Ricci C, Porta M: Tourette syndrome (TS) bears a higher rate of inflammatory complications at the implanted hardware in deep brain stimulation (DBS). Acta Neurochir (Wien) 153: 629–632, 2011 [DOI] [PubMed] [Google Scholar]
- 14).Martino D, Dale RC, Gilbert DL, Giovannoni G, Leckman JF: Immunopathogenic mechanisms in Tourette syndrome: a critical review. Mov Disord 24: 1267–1279, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]