Abstract
Myelodysplastic syndromes (MDS) are a spectrum of clonal stem-cell disorders characterized clinically by bone-marrow failure. Resultant cytopenias are responsible for significant mortality and decreased quality of life in patients with MDS. In patients with low-risk MDS (LR-MDS), anemia is the most common cytopenia and erythropoiesis-stimulating agents (ESA) are usually used as first-line therapy. Those patients who become refractory to ESA have a poor survival. Available treatment options such as lenalidomide, hypomethylating agents, and immunosuppressive therapy can provide some hematologic response among selected subsets of patients, however durable responses are limited, and these agents can carry significant adverse effects. Chronic transfusions help to alleviate symptoms of anemia but still carry risks associated with transfusion and iron overload. Luspatercept, recently approved for those LR-MDS with ring sideroblasts refractory to ESA, was found to have an improvement in transfusion independence with a well-tolerated safety profile. While anemia is the most common cytopenia, thrombocytopenia and neutropenia management is challenging and the co-occurrence of these cytopenias with anemia may dictate the choice of therapy. In this article, we review LR-MDS and discuss the optimal use of current treatment options and explore new therapeutic options on the horizon.
Keywords: erythropoiesis-stimulating agents, hypomethylating agents, lenalidomide, low-risk myelodysplastic syndromes, luspatercept
Introduction
Myelodysplastic syndromes (MDS) are heterogeneous bone-marrow failure disorders which cause clonal ineffective hematopoiesis with dysplastic morphology. They are typically seen in patients in the seventh decade of life and usually without a predisposing factor.1 Owing to their heterogeneity, prognosis and progression to acute myelogenous leukemia (AML) can vary among patients depending upon the risk of their disease. Upon initial evaluation of de novo MDS, the International Prognostic Scoring System (IPSS) may be used. In the IPSS, identified factors are marrow blast, karyotype, and cytopenias, however, the more recent revised system, IPSS-R, incorporates the depth of cytopenias into its scoring system. Those scored as low/intermediate-1 in IPSS and very low/low/intermediate in IPSS- R are characterized as low-risk MDS.2,3 However, missing from the scoring system is the impact of somatic mutations, some of which identify a significantly worse prognostic risk. These are TP53, EZH2, ETV6, RUNX1, and ASXL1, whereas SF3B1 alone portends a favorable risk.1,4–6 Despite the type of somatic mutations, each additional acquired mutation increases the risk of disease. Patients with intermediate-risk disease scored by IPSS-R are further stratified into higher or lower risk groups based on the presence of high-risk somatic mutations, an IPSS-R score of >3.5, the presence of circulating myeloblasts, or transfusion dependency.7–10
In addition to the prognostic scoring system, morphology of the disease based on World Health Organization 2016 criteria can herald behavior. Patients with MDS-ring sideroblasts (RS)-single-lineage dysplasia/MDS-RS are considered to have better prognosis, especially those harboring the SF3B1 somatic mutation. Also associated with good prognosis is MDS with del(5q) alone or with one additional abnormality (not involving chromosome 7). This is seen in contrast to patients with MDS with excess blasts who carry a significantly poor prognosis with median overall survival ranging from 5 months to 12 months and high time-to-disease evolution to AML.11
The degree and symptoms of cytopenias and increasing blast percentage may call for the initiation of treatment. While low-risk disease may be indolent, the impact of even mild anemia on these elderly patients can disrupt quality of life and exacerbate underlying comorbid conditions.12,13
How to treat?
Our approach to evaluating patients is to first confirm the diagnosis. Studies suggest discrepancies in diagnosis due to the complexity of morphology.14 We use the IPSS-R complemented by data from somatic mutations identifying patients with low-risk MDS (LR-MDS). We consider LR-MDS for very low-risk IPSS-R +/– 1 high-risk somatic mutations, low-risk IPSS-R +/– 1 high-risk somatic mutations, very low/low/intermediate-risk IPSS-R with SF3B1 somatic mutations, intermediate-risk IPSS-R with no high-risk mutation or clinical adverse variables such as heavy transfusion dependency, circulating myeloblasts, or bone-marrow fibrosis.
If patients with LR-MDS are asymptomatic or have no profound cytopenia, we elect to observe those patients. In the majority of cases, anemia and red blood cell (RBC) transfusion dependency are the indications for treatment. Profound thrombocytopenia or neutropenia, if associated with recurrent infections, are rarely a sole indication for therapy in LR-MDS, however, if concomitant with anemia may dictate choice of therapy.
Anemia
Erythropoiesis-stimulating agents
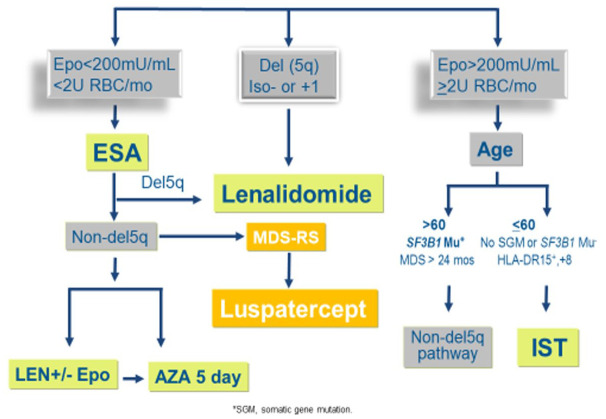
Anemia is usually the most common cytopenia present in LR-MDS. Low transfusion requirement and serum erythropoietin levels <500 can predict the probability of adequate response to erythropoiesis-stimulating agents (ESA), such as recombinant erythropoietin (EPO) or darbepoetin (DAR).12 Clinical studies in low-risk MDS have revealed response rates of 40–60% with improved quality of life.15–18 In a randomized controlled trial of EPO-alpha versus placebo in patients with LR-MDS, erythroid responses were evaluated by International working group (IWG) criteria. It was found that patients treated with EPO-alpha had a response rate of 31.8% versus 4.4% in placebo. However, an independent response review committee reviewed blinded data with modified IWG-2006 criteria which resulted in a 45.9% erythroid response in the EPO-alpha group and continued 4.4% in the placebo group.17 (Figure 1).
Figure 1.
Anemia management algorithm in LR-MDS 2020.
AZA, azacitidine; EPO, erythropoietin; ESA erythropoiesis-stimulating agents; IST, immunosuppressive therapy; LEN, lenalidomide; LR-MDS, low-risk myelodysplastic syndromes; MDS-RS, myelodysplastic syndromes-ring sideroblasts; RBC, red blood cell.
A phase III trial randomizing those with DAR-alpha versus placebo in LR-MDS found a significant decrease in transfusion and increased rates of erythroid response.18
There is no consensus about ESA, however, most responses have a median duration of 15–18 months. In a phase II study, DAR, with or without granulocyte colony-stimulating factor (G-CSF), was evaluated. DAR was given once every 2 weeks for 12 weeks with G-CSF given at 12 weeks to nonresponders. Erythroid response rate by IWG criteria was 48% and 56% at 12 months and 24 months, respectively. In nonresponders, the addition of G-CSF showed an erythroid response in 22% of these patients.19 Similarly, another phase II study evaluated the addition of G-CSF to DAR to nonresponders and showed a response rate of 47%, mostly seen in refractory anemia with ringed sideroblasts (RARS).20 Additional retrospective studies showed that ESA + G-CSF was safe and effective without increasing the rate of progression to AML.21,22 In those patients who have lost response or never had a response to ESA alone, the addition of G-CSF may be able to salvage a response.19,22
In a small cohort of patients with LR-MDS, somatic mutations were evaluated for an association with a response to ESA. In the results of a univariate analysis, the number of mutations correlated with lower hematologic improvement-erythroid (HI-E), however it was not significant.23 In addition, patients with LR-MDS who harbor specific somatic mutations such as ASXL1, RUNX1, ETV6, EZH2, or TP53 may ‘step up’ the risk of disease despite R-IPSS.5,24
How to treat
Our approach is to consider a trial of ESA as a first step for managing anemia in LR-MDS at 40,000–60,000 units epoetin dose equivalent for 8–12 weeks in patients with endogenous serum EPO level <500 or non-heavily RBC transfusion dependency. If a response is observed, we continue until loss of hematologic response. If there is no response, the addition of G-CSF is safe and may be appropriate to evoke a response.
Lenalidomide
In 2005, a phase I clinical trial evaluated the safety and efficacy of lenalidomide in patients with LS- MDS with refractory anemia, transfusion dependency, and ESA-resistance. Of those patients, 56% had a hematologic response, and 63% achieved transfusion independence. Cytogenetic responses were seen in 55% of patients. Complete cytogenetic response was seen in 75% of patients with long-arm deletion of 5q31.1, and a more rapid time to response was observed in this group as well.25 Further, a phase II trial, MDS-003, evaluated transfusion-dependent patients with LS-MDS with del(5q). Results showed 67% of patients achieved transfusion independence and 45% showed complete cytogenetic resolution with the use of lenalidmide.26 The phase III MDS-004 trial was a randomized, blinded, placebo-controlled study conducted to further assess findings in the MDS-003 trial. In the treatment group, the dose of lenalidomide was given at 5 mg or 10 mg. Transfusion independence in the lenalidomide group (5 mg and 10 mg) compared with placebo was 56.1% and 42.6% versus 5.9%, respectively. Cytogenetic response rates were seen in 50% and 25% of the lenalidomide group 10 mg versus 5 mg, respectively, and 0% in the placebo group. Risk reductions in AML progression and death were also seen in both lenalidomide groups.27 In patients with non-del(5q), use of lenalidomide alone resulted in transfusion independence rates of approximately 20–30%, especially in those who were refractory to ESA.28 Myelosuppression seen with neutropenia and thrombocytopenia is a common adverse effect and laboratory values need close monitoring during the initial weeks of treatment. While most patients with del(5q) respond to lenalidomide, approximately 50% lose the response or progress in 2–3 years.26
Lenalidomide was evaluated in combination with ESA in patients with LS-MDS who had a suboptimal response to lenalidomide monotherapy and ESA refractoriness. With combination therapy, erythroid response rate was 26%, and 22% achieved transfusion independence.29 A larger phase III confirmatory trial randomized patients to LR-MDS, non-del5(q), who were unresponsive to ESA or transfusion dependent with serum EPO (sEPO) >500 to receive lenalidomide or lenalidomide with EPO. Those with combination therapy had a significantly better major erythroid response than those with lenalidomide alone, 28.3% versus 11.5%, respectively. In addition, this response was durable with a median duration of 23.8 months versus 13 months. This demonstrated that lenalidomide restores sensitivity to those who are refractory to ESA.30
How to treat
Our approach is to consider lenalidomide at 10 mg daily for patients with del5(q) LR-MDS if there is a low chance of response/no response to ESA. A 3-month trial of lenalidomide is adequate to assess response in 90% of patients. More than two-thirds of patients will require dose interruption during the first month for 3–4 weeks and subsequent dose reduction to 5 mg daily after count recovery to 70% of baseline; cytopenias are predictive of response. Gastrointestinal toxicity, rash, and muscle cramps are common side effects. In non-del5(q) LR MDS, we restrict use of lenalidomide with or without ESA to those with pure anemia with platelets >100 and absolute neutrophil count (ANC) >1000 and no prior use of hypomethylating agents (HMA). Cytopenias in non-del5(q) LR-MDS are less severe compared with del5(q) and nonpredictive of response.
HMA
In 2002, the CALGB reported a phase III clinical trial which showed HMA improved survival, decreased leukemic transformation, and improved quality of life.31 HMA use in patients with LR-MDS may be utilized after ESA and lenalidomide failure. The use of HMA resulted in hematologic responses in 30–40% of cases.32,33 Dosing strategies were also investigated for safety and efficacy. Lyons et al. developed a phase II trial which randomized patients to one of the three dosing schedules. 32 These schedules were azacitidine 75 mg/m2 at 5 days, 5 days with a 2-day break followed by an additional 2 days of treatment, and 5 days with a 2-day break followed by an additional 5 days of treatment. These were administered over a 28-day dosing schedule. This trial was not designed to compare statistical significance between the groups, however it did demonstrate transfusion independence (50–64%) and hematologic improvement (44–55%) in all groups within the first two cycles. In addition, azacitidine 5-day dosing showed better tolerability and more convenience in dosing compared with the other two dosing schedules.32 A further study investigated azacitidine 75 mg/m2 or decitabine 20 mg/m2 given over 3 days of a 28-day dosing schedule. Hematologic improvement was seen in 18% of patients and transfusion independence was seen in 25%. Decitabine however did show a higher overall response rate, transfusion independency, and cytogenetic response compared with azacitidine.34 In patients with LR-MDS, outcome after HMA failure is poor. One study evaluated 290 patients at the time of HMA failure and found that median transformation-free survival was 15 months and overall survival was 17 months.35 Some have evaluated the addition of ESA to HMA but have failed to show a significant improvement and thus combination is not recommended due to limited efficacy with potential increased toxicity.8,36,37
After failure of ESA, a retrospective study evaluated two groups of patients with LR-MDS: those who received lenalidomide before azacitidine and those who received it after azacitidine. It was found that HI-E was achieved in 38% of those who had received lenalidomide before azacitidine compared with 12% of those who had received lenalidomide after azacitidine.38 Further, a single institution, large cohort, retrospective study evaluated those who ultimately lose response to lenalidomide and the subsequent use of HMA. It was identified that HMA can result in a 50% response rate and those who had a response were found to have a median overall survival of 32 months.39
How to treat
Our approach is to consider 3–5 days of HMA regimens in patients with LR-MDS after ESA/lenalidomide failure or in those with concomitant profound thrombocytopenia or neutropenia. Neutrophil response is observed in 20% of treated patients only. It is reasonable to consider a clinical trial prior to HMA in patients with LR-MDS.
Immunosuppressive therapy
Disease-specific mechanisms of MDS have been reported as ineffective marrow hematopoiesis, bone-marrow apoptosis, and potential autoimmune T cell-mediated cell death. Immuno-suppressive therapy (IST) has been investigated for targeting the autoimmune T cell-mediated pathogenesis of MDS. Initial prospective studies evaluated antithymocyte globulin (ATG) in unselected patients with MDS. Response rate was seen in 34–50% of patients.40–42 An additional phase III study investigated the addition of cyclosporine (CSA) to ATG compared with the best supportive care in all subtypes of MDS. It was found that hematologic response was higher with IST than the supportive care, however transformation-free survival and overall survival were not impacted.43 In addition, treatment with horse ATG and cyclosporine induced improved response.44
Studies looked to identify factors predictive of response. Factors such as young age, limited transfusion requirements, normal karyotype, and HLA-DR15, were shown to predict better response.45 These studies however had small numbers of patients for evaluation. Further, a recent multicenter study involving other tertiary care cancer centers identified a large cohort of patients with MDS treated with IST. This study evaluated these predictive factors but found them to be less impactful, rather, patients with hypocellular bone marrow (<20%) and treatment naïvety were more likely to have increased rates of transfusion independence.44 Additionally, a recent meta-analysis reviewed the use of IST in LR-MDS and was unable to associate specific biomarkers predictive of response given the overall lack of prospective, randomized, controlled studies.46
How to treat
Our approach is to consider ATG/CSA in patients with LR-MDS early in the disease and if the patient is young, has no response or low chance of response to ESA, no high-risk somatic mutations, and no SF3B1 mutation. In our experience bone-marrow cellularity is not predictive of response and IST can offer an option for patients with trilineage cytopenias.
Luspatercept
Treatment failure after ESA, HMA, and lenalidomide is an unmet need in LR-MDS. In preclinical data, transcription growth factor (TGF)-beta has proved to have an important role in MDS pathogenesis. The TGF-beta receptor is grouped into three types. Activin II receptors, part of the type II TGF-beta superfamily, allows for recruitment and phosphorylation of downstream targets including activation of inhibitory SMAD proteins. In MDS, TGF-beta is constitutively activated which contributes to ineffective erythropoiesis. Activin receptor II ligand traps have been found to neutralize the TGF-beta superfamily and inhibition of SMAD proteins to allow for effective erythropoiesis. These novel agents, luspatercept and sotatercept, have been studied and found to be of benefit in LR-MDS.47,48
The phase II PACE-MDS trial was a single-arm study which evaluated the safety and efficacy of luspatercept in low or intermediate 1 risk MDS and nonproliferative chronic myelomonocytic leukemia with anemia regardless of RBC transfusion need. Response rate of HI-E was 63% and transfusion independence was 38%. Although HI-E and RBC transfusion independence were higher in those with RS (69% and 42%), SF3B1 somatic mutation (77% and 44%), and sEPO <200 IU/L (76% and 53%), patients with contrasting characteristics still achieved HI-E and RBC transfusion independence: no RS (43% and 29%), no SF3B1 mutation (40% and 39%), and with sEPO level 200–500 IU/L (58% and 44%) and >500 IU/L (43% and 14%), at 58% and 43%, respectively. The safety profile was found to be favorable with a significant difference in adverse effects at increased doses.49
In further investigation, the MEDALIST trial randomized patients to receive placebo or luspatercept subcutaneously every 3 weeks. Those included in the trial were patients with very low, low, and intermediate-risk MDS-RS, had been receiving frequent transfusions, and were refractory or unlikely to respond to ESA. Result showed that 38% of the patients in the luspatercept group were transfusion independent for 8 weeks or longer, compared with 13% of those in the placebo group. Further, higher responses were observed in patients in the luspatercept group with a low transfusion burden,.50 Luspatercept was also associated with a very tolerable side-effect profile with infrequent discontinuation of treatment.49,50
In addition, a phase II study evaluated sotatercept in patients with LR-MDS who were transfusion dependent and ESA refractory. Further, previously half had received HMA or lenalidomide. Patients enrolled were stratified to 0.1, 0.3, 0.5, 1.0, or 2.0 mg/kg once every 3 weeks with a primary endpoint of rate of erythroid hematologic improvement. Results demonstrated 49% HI-E according to IWG-2006. Patients were further classified as high transfusion burden (HTB) (⩾4 RBC units in 56 days) or low transfusion burden (LTB) (<4 RBC units in 56 days). Results demonstrated HI-E in 49% of patients, and 47% and 58% in those with HTB and LTB with a median duration of response of 169 days and 652 days, respectively. It is important to note that a morphological subset of patients may have greater benefit with sotatercept as 59% of patients with at least 15% RS achieved HI-E compared with 22% with less than 15% RS.51
How to treat
Our approach is to consider luspatercept as first line after ESA failure or with a low chance of response in those patients with MDS-RS or MDS/myeloproliferative neoplasm (MPN)-RS-thrombocytosis. Responses seem to be higher if introduced before heavy RBC transfusion dependency. Responses can be observed early and dose titration may be required every third dose. We monitor blood pressure prior to therapy and fatigue is commonly observed in first two cycles. No data exist on use following HMA failure, however responses were observed in the sotatercept study.
Thrombocytopenia
Thrombocytopenia in MDS can cause significant bleeding and substantially impacts morbidity. Thrombopoietin agonists have been evaluated for use in this setting. In a phase I/II study, eltrombopag was compared with placebo in patients with LR-MDS and severe thrombocytopenia. Platelet responses were observed in 47% of eltrombopag recipients. Of these responses, 11 were partial responses and 17 complete responses. In the placebo group, 3% of patients had a platelet response. Of those who were platelet transfusion dependent in the eltrombopag group, 54% had transfusion independence whereas no patients in the placebo group had transfusion independence. Evolution to AML was seen more frequently in patients with refractory anemia with excess blasts 1. However, there was no significant evidence of an increased risk in eltrombopag recipients.52
How to treat
Our approach is to consider eltrombopag as a single agent for patients with isolated thrombocytopenia LR-MDS.
Iron overload
Patients with MDS are at risk for iron overload not only from transfusions but also from ineffective iron metabolism due to ineffective erythropoiesis and inflammation. Iron overload in these patients has been associated with reduced survival. Iron deposition may be seen in vital organs including the liver, pancreas, and heart causing endorgan damage. Though many trials evaluating the use of iron chelation therapy (ICT) in LR-MDS have been retrospective or nonrandomized, improved outcomes were often seen.53–56 The TELESTO trial was the first prospective randomized control trial to evaluate the event-free survival and safety of ICT with deferasirox in patients with LR-MDS. Patients with LR-MDS and ferritin levels >1000 were enrolled and randomized to ICT or placebo. The primary endpoint of event-free survival was defined as time to randomization to nonfatal or death. Results showed that patients who were treated with ICT had a median event-free survival of 3.9 years compared with 3 years in the placebo group. Most of the benefit was observed in reducing hospitalization for cardiac events (0.7% versus 3.9%). Unfortunately, this trial did require a redesign from phase III to phase II due to slow accrual causing it to be underpowered in determining overall survival. The side-effect profile was similar in both groups except for an increase in serum creatinine in the ICT group.57
How to treat
Our approach is to consider oral ICT in patients with ferritin levels >1000 ng/ml and >15–20 units RBC transfusion history with a goal of a ferritin level <500 ng/ml. It is important to monitor kidney function during treatment. We individualize the decision to start treatment based on risks and potential benefit.
Novel therapies
Roxadustat
The expression of EPO is regulated through oxygen tension by oxygen-sensing enzymes, prolyl hydroxylase enzymes. When oxygen is decreased, prolyl hydroxylase activity decreases and allows for accumulation and transcription of hypoxia-inducible factor (HIF) leading to upregulation of EPO. Roxadustat is a reversible HIF prolyl hydroxylase inhibitor which promotes the physiologic response to hypoxia to increase EPO. It has been used for the treatment of anemia in patients with chronic kidney disease.58 In an open-label, dose-finding study, roxadustat was administered to 24 transfusion-dependent but LTB patients with LR-MDS. Patients who had an sEPO level >400 were not included in this trial. Results showed 9 out of 24 patients (38%) had achieved transfusion independence and 58% had achieved ⩾50% reduction in RBC units in any 8-week period compared with baseline.59 The study is continuing in a phase III trial (NCT03263091).
Imetelstat
Telomerases help to protect regions of chromosomes from genomic instability causing potentially catastrophic events. Telomerases are not constitutively active in normal tissue, however they have been found to be upregulated in human cancers.60 Imetelstat is a novel telomerase inhibitor which, in preclinical data, showed activity in hematologic and solid tumors.61–64 With regard to MPNs, two pilot studies found that with imetelstat treatment patients were able to achieve durable hematologic responses with some achieving complete or partial remission.6,7 In one study, it was suggested that those who harbored SF3B1 or U2AF1 mutations had higher response rates than those who did not.7 The most common adverse effect was myelosuppression.65,66 The use of imetelstat was evaluated in a phase II/III trial in patients with LR-MDS who were heavily transfusion dependent and refractory to ESA. Although data are preliminary, hematologic improvement was seen in 68%, RBC transfusion independence was seen in 42%, and 24% achieved either a complete remission (CR) or marrow CR. Myelosuppression again was seen as the most common adverse effect. Those with the SF3B1 mutation were associated with prolonged responses with reduction of variant allele frequency suggesting the potential for disease-modifying activity with use of imetelstat.65
Conclusion
As we have seen over the last decade, the treatment landscape of LR-MDS is evolving from its backbone of ESA and HMA to becoming further personalized based on cytogenetics, somatic mutations, and MDS morphology. Those with del5(q) have had impressive responses to lenalidomide allowing for its frontline use.6,25,26,29,38 Current investigations with luspatercept in those with MDS-RS and SF3B1 mutation have also shown improved hematologic response47,49,50 leading to its US Food and Drug Administration approval and now clinical trials evaluating its frontline use in this population (NCT03682536). As we continue on this trajectory to identifying novel therapies and molecular diagnostics, the future for improved LR-MDS treatment is promising.
Footnotes
Conflict of interest statement: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Geron: Honoraria; Novartis: Honoraria; Incyte: Honoraria; JAZZ: Honoraria, Speakers Bureau; AbbVie: Honoraria; Agios: Honoraria, Speakers Bureau; Acceleron: Honoraria; BMS: Honoraria, Speakers Bureau.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Virginia O. Volpe  https://orcid.org/0000-0002-9872-1919
https://orcid.org/0000-0002-9872-1919
Contributor Information
Virginia O. Volpe, H. Lee Moffitt Cancer Center and Research Institute, 12902 Magnolia Drive, Tampa, FL 33612, USA.
Rami S. Komrokji, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA
References
- 1. Adès L, Itzykson R, Fenaux P. Myelodysplastic syndromes. Lancet 2014; 383: 2239–2252. [DOI] [PubMed] [Google Scholar]
- 2. Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood 2012; 120: 2454–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 1997; 89: 2079–2088. [PubMed] [Google Scholar]
- 4. Della Porta MG, Tuechler H, Malcovati L, et al. Validation of WHO classification-based Prognostic Scoring System (WPSS) for myelodysplastic syndromes and comparison with the revised International Prognostic Scoring System (IPSS-R). A study of the International Working Group for Prognosis in Myelodysplasia (IWG-PM). Leukemia 2015; 29: 1502–1513. [DOI] [PubMed] [Google Scholar]
- 5. Bejar R, Stevenson K, Abdel-Wahab O, et al. Clinical effect of point mutations in myelodysplastic syndromes. N Engl J Med 2011; 364: 2496–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fenaux P, Adès L. How we treat lower-risk myelodysplastic syndromes. Blood 2013; 121: 4280–4286. [DOI] [PubMed] [Google Scholar]
- 7. Benton CB, Khan M, Sallman D, et al. Prognosis of patients with intermediate risk IPSS-R myelodysplastic syndrome indicates variable outcomes and need for models beyond IPSS-R. Am J Hematol 2018; 93: 1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Montoro J, Pomares H, Villacampa G, et al. Dichotomization of the new revised international prognostic scoring system for a better clinical stratification of patients with myelodysplastic syndromes. Leuk Lymphoma 2019; 60: 1522–1527. [DOI] [PubMed] [Google Scholar]
- 9. Nazha A, Sekeres MA. Improving prognostic modeling in myelodysplastic syndromes. Curr Hematol Malig Rep 2016; 11: 395–401. [DOI] [PubMed] [Google Scholar]
- 10. Nazha A, Sekeres MA. Precision medicine in myelodysplastic syndromes and leukemias: lessons from sequential mutations. Annu Rev Med 2017; 68: 127–137. [DOI] [PubMed] [Google Scholar]
- 11. National Comprehensive Cancer Network. Myelodysplastic syndrome (version 2.2020), https://www.nccn.org/professionals/physician_gls/pdf/mds.pdf (accessed 11 May 2020).
- 12. Hellström-Lindberg E, Gulbrandsen N, Lindberg G, et al. A validated decision model for treating the anaemia of myelodysplastic syndromes with erythropoietinc+ granulocyte colony-stimulating factor: significant effects on quality of life. Br J Haematol 2003; 120: 1037–1046. [DOI] [PubMed] [Google Scholar]
- 13. Steensma DP. Myelodysplastic syndromes current treatment algorithm 2018. Blood Cancer J 2018; 8: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Naqvi K, Garcia-Manero G, Bueso-Ramos CE, et al. Discrepancy in diagnosis of myelodysplastic syndrome (MDS) between referral and tertiary care centers: experience at MD Anderson Cancer Center (MDACC). Blood 2010; 116: 1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stauder R, Valent P, Theurl I. Anemia at older age: etiologies, clinical implications, and management. Blood 2018; 131: 505–514. [DOI] [PubMed] [Google Scholar]
- 16. Park S, Greenberg P, Yucel A, et al. Clinical effectiveness and safety of erythropoietin-stimulating agents for the treatment of low- and intermediate-1-risk myelodysplastic syndrome: a systematic literature review. Br J Haematol 2019; 184: 134–160. [DOI] [PubMed] [Google Scholar]
- 17. Fenaux P, Santini V, Spiriti MAA, et al. A phase 3 randomized, placebo-controlled study assessing the efficacy and safety of epoetin-α in anemic patients with low-risk MDS. Leukemia 2018; 32: 2648–2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Platzbecker U, Symeonidis A, Oliva EN, et al. A phase 3 randomized placebo-controlled trial of darbepoetin alfa in patients with anemia and lower-risk myelodysplastic syndromes. Leukemia 2017; 31: 1944–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kelaidi C, Park S, Sapena R, et al. Long-term outcome of anemic lower-risk myelodysplastic syndromes without 5q deletion refractory to or relapsing after erythropoiesis-stimulating agents. Leukemia 2013; 27: 1283–1290. [DOI] [PubMed] [Google Scholar]
- 20. Gotlib J, Lavori P, Quesada S, et al. A phase II intra-patient dose-escalation trial of weight-based darbepoetin alfa with or without granulocyte-colony stimulating factor in myelodysplastic syndromes. Am J Hematol 2009; 84: 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Park S, Grabar S, Kelaidi C, et al. Predictive factors of response and survival in myelodysplastic syndrome treated with erythropoietin and G-CSF: the GFM experience. Blood 2008; 111: 574–582. [DOI] [PubMed] [Google Scholar]
- 22. Jädersten M, Malcovati L, Dybedal I, et al. Erythropoietin and granulocyte-colony stimulating factor treatment associated with improved survival in myelodysplastic syndrome. J Clin Oncol 2008; 26: 3607–3613. [DOI] [PubMed] [Google Scholar]
- 23. Kosmider O, Passet M, Santini V, et al. Are somatic mutations predictive of response to erythropoiesis stimulating agents in lower risk myelodysplastic syndromes? Haematologica 2016; 101: e280–e283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bejar R, Papaemmanuil E, Haferlach T, et al. Somatic mutations in MDS patients are associated with clinical features and predict prognosis independent of the IPSS-R: analysis of combined datasets from the international working group for prognosis in MDS-molecular committee. Blood 2015; 126: 907. [Google Scholar]
- 25. List A, Kurtin S, Roe DJ, et al. Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med 2005; 352: 549–557. [DOI] [PubMed] [Google Scholar]
- 26. List A, Dewald G, Bennett J, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med 2006; 355: 1456–1465. [DOI] [PubMed] [Google Scholar]
- 27. Fenaux P, Giagounidis A, Selleslag D, et al. A randomized phase 3 study of lenalidomide versus placebo in RBC transfusion-dependent patients with low-/intermediate-1-risk myelodysplastic syndromes with del5q. Blood 2011; 118: 3765–3776. [DOI] [PubMed] [Google Scholar]
- 28. Santini V, Almeida A, Giagounidis A, et al. Randomized phase III study of lenalidomide versus placebo in RBC transfusion-dependent patients with lower-risk non-del(5q) myelodysplastic syndromes and ineligible for or refractory to erythropoiesis-stimulating agents. J Clin Oncol 2016; 34: 2988–2996. [DOI] [PubMed] [Google Scholar]
- 29. Komrokji RS, Lancet JE, Swern AS, et al. Combined treatment with lenalidomide and epoetin alfa in lower-risk patients with myelodysplastic syndrome. Blood 2012; 120: 3419–3424. [DOI] [PubMed] [Google Scholar]
- 30. List AF, Sun Z, Verma A, et al. Combined treatment with lenalidomide (LEN) and epoetin alfa (EA) is superior to lenalidomide alone in patients with erythropoietin (Epo)-refractory, lower risk (LR) non-deletion 5q [Del(5q)] myelodysplastic syndrome (MDS): results of the E2905 intergroup study-an ECOG-ACRIN cancer research group study, grant CA180820, and the national cancer institute of the national institutes of health. Blood 2016; 128: 223. [Google Scholar]
- 31. Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol 2002; 20: 2429–2440. [DOI] [PubMed] [Google Scholar]
- 32. Lyons RM, Cosgriff TM, Modi SS, et al. Hematologic response to three alternative dosing schedules of azacitidine in patients with myelodysplastic syndromes. J Clin Oncol 2009; 27: 1850–1856. [DOI] [PubMed] [Google Scholar]
- 33. Gurion R, Vidal L, Gafter-Gvili A, et al. 5-azacitidine prolongs overall survival in patients with myelodysplastic syndrome–a systematic review and meta-analysis. Haematologica 2010; 95: 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jabbour E, Short NJ, Montalban-Bravo G, et al. Randomized phase 2 study of low-dose decitabine vs low-dose azacitidine in lower-risk MDS and MDS/MPN. Blood 2017; 130: 1514–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jabbour EJ, Garcia-Manero G, Strati P, et al. Outcome of patients with low-risk and intermediate-1-risk myelodysplastic syndrome after hypomethylating agent failure: a report on behalf of the MDS clinical research consortium. Cancer 2015; 121: 876–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thepot S, Ben Abdelali R, Chevret S, et al. A randomized phase II trial of azacitidine +/–epoetin-beta in lower-risk myelodysplastic syndromes resistant to erythropoietic stimulating agents. Haematologica 2016; 101: 918–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tobiasson M, Dybedahl I, Holm MS, et al. Limited clinical efficacy of azacitidine in transfusion-dependent, growth factor-resistant, low- and Int-1-risk MDS: results from the Nordic NMDSG08A phase II trial. Blood Cancer J 2014; 4: e189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zeidan AM, Al Ali NH, Padron E, et al. Lenalidomide treatment for lower risk nondeletion 5q myelodysplastic syndromes patients yields higher response rates when used before azacitidine. Clin Lymphoma Myeloma Leuk 2015; 15: 705–710. [DOI] [PubMed] [Google Scholar]
- 39. Komrokji RS, Bally C, Itzykson R, et al. Azacitidine treatment for lenalidomide (LEN)-resistant myelodysplastic syndrome (MDS) with del 5q. Blood 2012; 120: 3833. [Google Scholar]
- 40. Killick SB, Mufti G, Cavenagh JD, et al. A pilot study of antithymocyte globulin (ATG) in the treatment of patients with ‘low-risk’ myelodysplasia. Br J Haematol 2003; 120: 679–684. [DOI] [PubMed] [Google Scholar]
- 41. Stadler M, Germing U, Kliche KO, et al. A prospective, randomised, phase II study of horse antithymocyte globulin vs rabbit antithymocyte globulin as immune-modulating therapy in patients with low-risk myelodysplastic syndromes. Leukemia 2004; 18: 460–465. [DOI] [PubMed] [Google Scholar]
- 42. Steensma DP, Dispenzieri A, Moore SB, et al. Antithymocyte globulin has limited efficacy and substantial toxicity in unselected anemic patients with myelodysplastic syndrome. Blood 2003; 101: 2156–2158. [DOI] [PubMed] [Google Scholar]
- 43. Passweg JR, Giagounidis AA, Simcock M, et al. Immunosuppressive therapy for patients with myelodysplastic syndrome: a prospective randomized multicenter phase III trial comparing antithymocyte globulin plus cyclosporine with best supportive care–SAKK 33/99. J Clin Oncol 2011; 29: 303–309. [DOI] [PubMed] [Google Scholar]
- 44. Stahl M, DeVeaux M, de Witte T, et al. The use of immunosuppressive therapy in MDS: clinical outcomes and their predictors in a large international patient cohort. Blood Adv 2018; 2: 1765–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Saunthararajah Y, Nakamura R, Wesley R, et al. A simple method to predict response to immunosuppressive therapy in patients with myelodysplastic syndrome. Blood 2003; 102: 3025–3027. [DOI] [PubMed] [Google Scholar]
- 46. Maximilian S, Jan Philipp B, Smith G, et al. Use of immunosuppressive therapy for management of myelodysplastic syndromes: a systematic review and meta-analysis. Haematologica 2020; 105: 102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Komrokji RS. Activin receptor II ligand traps: new treatment paradigm for low-risk MDS. Curr Hematol Malig Rep 2019; 14: 346–351. [DOI] [PubMed] [Google Scholar]
- 48. Bewersdorf JP, Zeidan AM. Transforming growth factor (TGF)-β pathway as a therapeutic target in lower risk myelodysplastic syndromes. Leukemia 2019; 33: 1303–1312. [DOI] [PubMed] [Google Scholar]
- 49. Platzbecker U, Germing U, Götze KS, et al. Luspatercept for the treatment of anaemia in patients with lower-risk myelodysplastic syndromes (PACE-MDS): a multicentre, open-label phase 2 dose-finding study with long-term extension study. Lancet Oncol 2017; 18: 1338–1347. [DOI] [PubMed] [Google Scholar]
- 50. Fenaux P, Platzbecker U, Mufti GJ, et al. Luspatercept in patients with lower-risk myelodysplastic syndromes. N Engl J Med 2020; 382: 140–151. [DOI] [PubMed] [Google Scholar]
- 51. Komrokji R, Garcia-Manero G, Ades L, et al. Sotatercept with long-term extension for the treatment of anaemia in patients with lower-risk myelodysplastic syndromes: a phase 2, dose-ranging trial. Lancet Haematol 2018; 5: e63–e72. [DOI] [PubMed] [Google Scholar]
- 52. Oliva EN, Alati C, Santini V, et al. Eltrombopag versus placebo for low-risk myelodysplastic syndromes with thrombocytopenia (EQoL-MDS): phase 1 results of a single-blind, randomised, controlled, phase 2 superiority trial. Lancet Haematol 2017; 4: e127–e136. [DOI] [PubMed] [Google Scholar]
- 53. Angelucci E, Santini V, Di Tucci AA, et al. Deferasirox for transfusion-dependent patients with myelodysplastic syndromes: safety, efficacy, and beyond (GIMEMA MDS0306 Trial). Eur J Haematol 2014; 92: 527–536. [DOI] [PubMed] [Google Scholar]
- 54. Delforge M, Selleslag D, Beguin Y, et al. Adequate iron chelation therapy for at least six months improves survival in transfusion-dependent patients with lower risk myelodysplastic syndromes. Leuk Res 2014; 38: 557–563. [DOI] [PubMed] [Google Scholar]
- 55. Lyons RM, Marek BJ, Paley C, et al. Relation between chelation and clinical outcomes in lower-risk patients with myelodysplastic syndromes: registry analysis at 5 years. Leuk Res 2017; 56: 88–95. [DOI] [PubMed] [Google Scholar]
- 56. Rose C, Brechignac S, Vassilief D, et al. Does iron chelation therapy improve survival in regularly transfused lower risk MDS patients? A multicenter study by the GFM (Groupe Francophone des Myélodysplasies). Leuk Res 2010; 34: 864–870. [DOI] [PubMed] [Google Scholar]
- 57. Angelucci E, Li J, Greenberg P, et al. Iron chelation in transfusion-dependent patients with low- to intermediate-1-risk myelodysplastic syndromes: a randomized trial. Ann Intern Med 2020; 172: 513–522. [DOI] [PubMed] [Google Scholar]
- 58. Chen N, Hao C, Liu B-C, et al. Roxadustat treatment for anemia in patients undergoing long-term dialysis. N Engl J Med 2019; 381: 1011–1022. [DOI] [PubMed] [Google Scholar]
- 59. Henry DH, Glaspy J, Harrup RA, et al. Roxadustat (FG4592; ASP1517; AZD9941) in the treatment of anemia in patients with lower risk myelodysplastic syndrome (LR-MDS) and low red blood cell (RBC) transfusion burden (LTB). Blood 2019; 134(Suppl. 1): 843.31488460 [Google Scholar]
- 60. Blackburn EH. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett 2005; 579: 859–862. [DOI] [PubMed] [Google Scholar]
- 61. Gürkan E, Tanriverdi K, Basşlamisşli F. Telomerase activity in myelodysplastic syndromes. Leuk Res 2005; 29: 1131–1139. [DOI] [PubMed] [Google Scholar]
- 62. Bruedigam C, Bagger FO, Heidel FH, et al. Telomerase inhibition effectively targets mouse and human AML stem cells and delays relapse following chemotherapy. Cell Stem Cell 2014; 15: 775–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Burchett KM, Yan Y, Ouellette MM. Telomerase inhibitor imetelstat (GRN163L) limits the lifespan of human pancreatic cancer cells. PLoS One 2014; 9: e85155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hochreiter AE, Xiao H, Goldblatt EM, et al. Telomerase template antagonist GRN163L disrupts telomere maintenance, tumor growth, and metastasis of breast cancer. Clin Cancer Res 2006; 12: 3184–3192. [DOI] [PubMed] [Google Scholar]
- 65. Baerlocher GM, Oppliger Leibundgut E, Ottmann OG, et al. Telomerase inhibitor imetelstat in patients with essential thrombocythemia. N Engl J Med 2015; 373: 920–928. [DOI] [PubMed] [Google Scholar]
- 66. Tefferi A, Lasho TL, Begna KH, et al. A pilot study of the telomerase inhibitor imetelstat for myelofibrosis. N Engl J Med 2015; 373: 908–919. [DOI] [PubMed] [Google Scholar]