Abstract
Children undergoing solid organ and hematopoietic stem cell transplantation are at high risk of morbidity and mortality from tuberculosis (TB) disease in the post-transplant period. Treatment of TB infection and disease in the post-transplant setting is complicated by immunosuppression and drug interactions. There are limited data that address the unique challenges for the management of TB in the pediatric transplant population. This review presents the current understanding of the epidemiology, clinical presentation, diagnosis, management, and prevention for pediatric transplant recipients with TB infection and disease. Further studies are needed to improve diagnosis of TB and optimize treatment outcomes for these patients.
Keywords: pediatric tuberculosis, solid organ transplant, hematopoietic stem cell transplant
Introduction
Tuberculosis (TB) is the leading cause of death worldwide from a single infectious agent. There were 10 million new TB cases with 1.5 million deaths in 2018.1 Though adults account for the majority of cases, TB remains a major health problem for children throughout the world. In 2018, 1.1 million children (<15 years old) developed TB disease, leading to more than 200 000 deaths.1 This number is likely an underestimation due to reporting and diagnostic challenges.2 Mathematical modeling studies estimate that up to 53 million children had TB infection in 2010.3
TB is caused by infection with the bacterium Mycobacterium tuberculosis (MTB). Children exposed to MTB can be classified into 3 main diagnostic categories: TB exposure, TB infection, and TB disease.4 TB exposure refers to recent close contact with an adult or adolescent with infectious pulmonary TB, without evidence of TB infection or disease.4 Children with TB infection have either a positive tuberculin skin test (TST) or interferon-gamma release assay (IGRA), without findings consistent with TB disease on physical exam or chest radiograph.5 Both the TST and IGRA are tests that measure the cell-mediated immune response to MTB antigens.6 Children with TB disease have symptoms, physical exam findings, or radiographic features suggestive of TB.7 In addition to pulmonary disease, children can have hematogenous and lymphatic spread resulting in TB disease at extrapulmonary sites, including the lymph nodes, central nervous system, intra-abdominal organs, bones, joints, and disseminated TB, also known as miliary TB.6-8
Children with TB are considered less infectious than adults, largely due to the paucibacillary nature of TB in young children.6 Hence, pediatric TB has historically been overlooked from a public health standpoint. Paucibacillary disease makes it difficult to detect mycobacteria in clinical specimens, often leading to delay in diagnosis or failure to diagnose TB.6 Despite these challenges, it is important to identify and treat children with TB, as young children are more likely to progress from TB infection to TB disease and are more prone to extra-pulmonary and life-threatening manifestations of TB than adults.5 Children who are immunocompromised, such as those living with human immunodeficiency virus (HIV), those with hematologic malignancies, or those who have received a solid organ transplant (SOT) or hematopoietic stem cell transplant (HSCT) are at increased risk for development of TB disease and are more prone to life-threatening manifestations of TB.7,9,10
Pediatric transplant recipients are a particularly vulnerable subpopulation with a high risk of morbidity and mortality from TB. There are an increased number of transplants performed worldwide, both in countries with high and low TB incidence. There were 126 670 solid organs transplanted in 2015 worldwide, with kidney and liver as the most common organs.11 In 2012, a total of 68 146 HSCT (36 220 autologous, 53%; 31 926 allogeneic, 47%) were reported worldwide; this was a relative increase of 46% total compared to 2006.12 Pediatric-specific data is lacking for both SOT and HSCT transplants on a global level. Additionally, increased travel and migration globally contribute to an expanded donor pool and increase the risk of exposure to MTB in the transplant population.13 Limited data indicate severe morbidity associated with post-transplant TB, particularly in the setting of delayed diagnosis, atypical presentations, medication interactions, and allograft rejection.14 A mortality rate of 20% to 30% in both adults and children with post-transplant TB disease has been observed in the SOT population.9 The mortality rate for post-transplant TB disease in HSCT patients is high, up to 50%, particularly in allogeneic recipients.15
There are limited data on the clinical presentation, diagnosis, management, and outcomes of TB infection and disease in pediatric SOT and HSCT recipients. This review serves to provide a summary of the literature on TB infection and disease in pediatric SOT and HSCT recipients. As data are limited, adult data have been extrapolated where no pediatric data exist.
Methods
Our review of the literature was conducted through a PubMed search from 1 November 2019 through 30 June 2020 using Medical Subject Headings for “tuberculosis,” “pediatric,” “solid organ transplant,” and “hematopoietic stem cell transplant.” We additionally sought out consensus statements, clinical practice guidelines, systematic reviews, case series, and case reports that are widely referenced by clinicians and pediatric infectious disease experts. The authors read individual articles and extracted information relevant to the review. Ethics approval was not required for this literature review.
Overview of TB Immunology
The immune response to MTB is complex and incompletely understood. This section serves as a brief overview of the immune mechanisms involved in the response to MTB infection and why immunocompromised hosts may be at greater risk for progression from TB infection to disease and to develop life-threatening manifestations of TB.
Both the innate and adaptive immune system play a role in defense against MTB. The innate response to MTB is primarily composed of macrophages and dendritic cells (antigen presenting cells, APCs) in the airway that play a role in phagocytosis of MTB. Within the phagosome, MTB is presented to T cells on major histocompatibility complex (MHC) class II molecules, triggering the adaptive immune response.16 T-cell immunity, particularly CD4 T cells, is essential for protection against MTB. These MTB-specific CD4 T cells produce Th1 cytokines, including interferon-gamma (IFN-ɣ), interleukin-2 (IL-2), and tumor necrosis factor alpha (TNF-ɑ).17 IFN-ɣ activates macrophages and TNF-ɑ facilitates mononuclear cell recruitment and activation.6 Additionally, interleukin-12 (IL-12) is secreted by APCs and leads to CD4 cell proliferation and IFN-ɣ production.17 This cell-mediated immune response is critical for granuloma formation and containment of MTB infection. Defects in the IFN-ɣ/IL-12 pathway have been identified in children with impaired granuloma formation and increased susceptibility to disseminated mycobacterial infections, known as “Mendelian Susceptibility to Mycobacterial Disease” (MSMD).6,18
Immunocompetent children under 5 and adolescents are at greater risk for progression from TB infection to disease.19 Young children are at risk for primary pulmonary infection, which can progress to meningitis and disseminated TB around 1 to 3 months after primary infection.6 One hypothesis for this phenomenon in young children is deficient macrophage phagocytosis in early childhood.17 Infants and young children are more likely to develop Th2-type CD4 T cells in response to pathogens compared to adults, which may result in more severe and disseminated disease.20 In addition to young children, adolescents are at higher risk to develop TB disease, often adult type cavitary lung disease.6 While age plays an important role in the immune response, it remains unclear whether certain ages are at higher risk within the immunocompromised population.
MTB has developed a unique host-pathogen relationship that has allowed its survival throughout history.21 MTB has a distinct pathogenic lifecycle within the host that involves granuloma formation, as well as certain virulence factors and differential mycobacterial load that can influence disease presentation.6,21 Recent studies recognize a spectrum of TB infection and disease states in the host, related to the host immune response and bacterial load.4,6,22 There are 3 main outcomes from infection with TB: spontaneous healing, containment, and disease, with the last outcome often occurring in the immunocompromised host.16 Most patients undergoing transplant have impaired T-cell function due to their conditioning regimens and immunosuppressive therapies, placing them at risk for development of TB disease.23
Epidemiology and Clinical Presentation
Epidemiology of TB in Transplant Recipients
The World Health Organization (WHO) has defined 30 high TB burden countries, 30 high TB/HIV burden countries, and 30 high multidrug-resistant TB (MDR-TB) burden countries worldwide.1 Given local epidemiology, pediatric transplant recipients in these countries are at increased risk for post-transplant TB. The incidence of TB disease in adult SOT recipients is estimated to be 20 to 74 times that of the general population.24 This differs according to the organ transplanted, with greatest risk in lung transplant recipients.9 Among HSCT recipients, the incidence of TB after HSCT is estimated to be 10 to 40 times higher than the general population.25 In comparison to SOT recipients, TB occurs less commonly in HSCT recipients.26 One hypothesis is that HSCT patients do not receive lifelong immunosuppression, allowing restoration of T-cell function over time.26
Risk factors for post-transplant TB in SOT recipients include social factors;27-29 infectious disease history and co-infections;24,27-31 underlying clinical conditions;24,27,28,30-32 type of organ transplanted33,34; immunosuppressive therapies, particularly T-cell depleting antibodies24,27,28,30,31,34-36; and transplant complications30 (Table 1).
Table 1.
| Solid organ transplant | Hematopoietic stem-cell transplant |
|---|---|
| Social factors | Social factors |
| Birth or residence in high-endemic area27-29 | Birth or residence in high-endemic area37 |
| Infectious Diseases History and Co-infections | Underlying indication for transplantation |
| Patient or donor history of TB infection or disease27-29 | Hematologic malignancy10 |
| HIV infection28 | Conditioning Regimen |
| Other coinfections: Mycoses, CMV, PJP or Nocardia24,27,30-31 | Busulfan10 |
| Non-infectious co-morbidities | T-cell depleting agents10,41 |
| Diabetes mellitus24,27,28,30-32 | Total body irradiation10,38,40,42,43 |
| Chronic liver disease24,27,28,30-32 | Type of stem-cell transplant |
| Chronic renal insufficiency or hemodialysis24,27 | Allogeneic transplant10,38-40 |
| Organ Transplanted | Mismatched allograft10 |
| Lung transplant33 | Transplant complications |
| Non-renal transplant34 | BOOP10 |
| Immunosuppressive therapies | Chronic GVHD10,25,38-41,43-48 |
| Cyclosporine27,34 | Graft failure48 |
| Intensification of immunosuppression for graft rejection27,28 | Other risk factors |
| Mycophenolate mofetil27 | Corticosteroid use10,46,48 |
| OKT33 or anti-T lymphocyte antibodies24,27,28,30,31,34-36 | |
| Tacrolimus27 | |
| Transplant Complications | |
| Allograft rejection30 |
Abbreviations: TB, Tuberculosis; HIV, Human Immunodeficiency virus; CMV, Cytomegalovirus; PJP, Pneumocystic jiroveci pneumonia; BOOP, bronchiolitis obliterans organizing pneumonia; GVHD, graft vs host disease.
Risk factors for post-transplant TB in HSCT recipients include social factors37; allogeneic transplantation10,38-40; underlying indication for transplantation10; pre-transplant conditioning therapies, particularly T-cell depleting agents10,41 and total body irradiation10,38,40,42,43; and transplant complications, particularly chronic graft versus host disease10,25,38-41,43-48 (Table 1).
Transmission
Transmission of MTB is airborne and occurs via entry of the pathogen into the host by inhalation of bacilli into the lungs.21 There are 3 mechanisms for developing TB in the post-transplant setting: progression from TB infection (also known as endogenous reactivation), donor-derived TB, and de novo infection.49,50 A major risk factor for endogenous reactivation is residence in or previous travel to an endemic region.28,29,51 Age impacts the risk of having TB infection at the time of transplant, which may progress to disease in the setting of immunosuppression; young children are less likely to have been exposed to MTB than older children.42,52 Donor-derived TB is another important mode of transmission, which can present early in the post-transplant period and should be considered in donors with residence in a TB-endemic area.29 Among HSCT recipients, development of TB disease primarily occurs through progression of TB infection or de novo infection; approximately one-fourth of MTB infections in HSCT recipients are from endogenous reactivation.10 The implementation of infection control measures in health care facilities is critical for prevention of de novo TB infection in transplant populations and health care workers.53 Such measures include developing an infection control plan, ensuring safe sputum collection, improving room ventilation, and using personal protective equipment.54
Clinical Presentation of TB Disease
The clinical presentation of TB in the post-transplant setting is variable and can be different from immunocompetent hosts. The majority of SOT and HSCT recipients present with pulmonary TB, but remain at greater risk for extrapulmonary and disseminated disease than the immunocompetent host.9,10 TB disease is generally defined as the following: pulmonary (involvement of pulmonary parenchyma), extrapulmonary (involvement of other organs), or disseminated (involvement of at least 2 noncontiguous organs).33
TB in SOT recipients can result in typical and atypical manifestations. In a literature review of TB in 476 SOT recipients (age 10-months-old to 71 years) between 1967 and 1997, 51% (244) had pulmonary TB, 33% (155) had disseminated TB, and 16% (77) had extrapulmonary TB (gastrointestinal (GI); skin, muscle, and osteoarticular TB; central nervous system (CNS); renal and genitourinary disease).34 In a case series of mycobacterial infections after pediatric liver transplantation, 2 children (19-months-old and 9-years-old) developed disseminated TB over a 12 month period.55 Atypical presentations have been reported in adults, including thyroid TB and TB mastitis post renal transplant,56,57 and primary liver TB post liver transplant.58 A case of hepatic graft TB was reported in a 10-month-old female with history of biliary atresia, likely transmitted from the living related donor; the transplant recipient died of pneumonia on day 273 after transplantation.59 Increased mortality in SOT recipients is associated with delayed diagnosis, disseminated disease, prior organ rejection, and receipt of anti-T-cell antibodies.14
Clinical presentation of TB disease in HSCT recipients can be variable. In a case series of 10 adult HSCT recipients, all were found to have pulmonary TB.38 Cases of pulmonary TB have been described in children with acute lymphoblastic leukemia (ALL) who received HSCT.60,61 Additionally, extrapulmonary and disseminated TB can occur in this population. In a literature review of TB disease in HSCT recipients from 10 countries between 1966 and 2000, 80% (38 patients) had pulmonary TB, 18% (8 patients) extrapulmonary TB (CNS, musculoskeletal, kidney, and oral cavity), and 2% (1 patient) disseminated TB.40 Atypical manifestations may also occur, including fever of unknown origin and non-specific clinical manifestations given immunological deficits.10 Imaging and tissue biopsies may not show typical findings due to impaired T cell function.10 Mortality in HSCT recipients is associated with multidrug-resistant strains, disseminated disease, and delayed initiation of therapy.10
Time to Diagnosis
Diagnosis of TB disease after transplantation requires a high index of suspicion, given atypical symptoms and other potential infectious and non-infectious causes of symptoms in the post-transplant setting. Diagnosis is often delayed, leading to increased morbidity and mortality.9
The majority of cases of TB disease in SOT recipients occur in the first year after transplant, with a median time for presentation of disease of 6 to 11 months.9 In a retrospective review on clinical characteristics and management of 51 adult SOT recipients in Spain who developed post-transplant TB disease, the majority (61%) were diagnosed in the first year after transplantation.62 In a review of 6 pediatric liver transplant recipients in the UK with TB disease, the median time to diagnosis was 8 months.63 In a review of post-transplant associated pediatric TB disease in Spain, 7 children (6 SOT, 1 HSCT) developed TB disease over 26 years; the median time from transplantation to diagnosis was 2.5 years.63 TB cases beyond 1 year post transplant have been described, which may be due to exposure to MTB in the community in countries with a high incidence of TB.64
Similar to SOT recipients, TB disease in HSCT recipients generally develops within the first year post-HSCT.10 Post-HSCT infectious complications are divided into the pre-engraftment phase, post-engraftment phase (prior to day 100) and late phase (after day 100), with most cases of post-transplant TB presenting in the late phase.26 In a retrospective review of mycobacterial infections following HSCT between 1974 and 1994 at University of Minnesota Hospital, 2 of 11 patients developed TB disease: 1 diagnosed before transplant, and one 70 days post-transplant.65 In a literature review of TB disease in 47 adult HSCT recipients between 2010 and 2018, the median time to clinical presentation was 4.6 months based on cohort data and 2.4 months based on individual case reports.44 Given variable clinical presentation and diagnostic delays, it is important to maintain a high index of suspicion for TB among HSCT recipients from endemic areas.10
Pre-transplant Evaluation
History and Exam
Children often undergo a rigorous pre-transplant evaluation to determine risk factors for development of infectious diseases prior to SOT and HSCT. Based on literature review and clinical experience, we recommend the following key steps in the pre-transplant evaluation to determine risk factors and prevent the development of TB disease. We additionally recommend that all providers consult their national TB guidelines for information regarding country-specific practices.
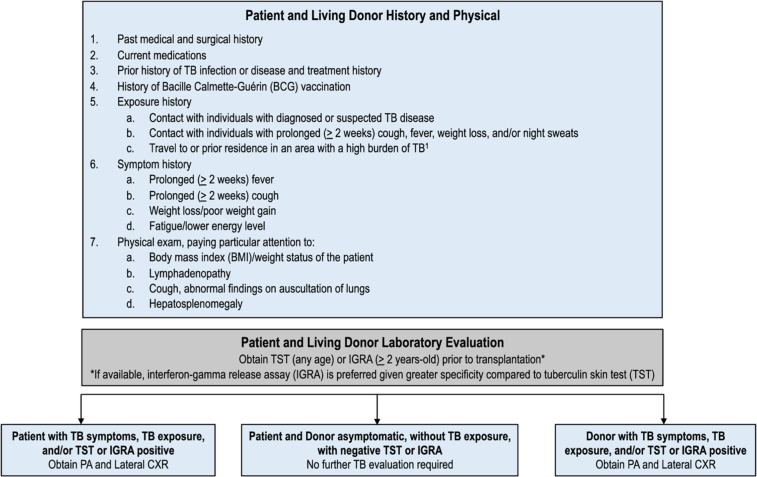
We recommend starting the evaluation by obtaining a detailed history about the patient: past medical history, current medications, prior history of TB infection or disease (details about prior TST/IGRA results if available), history of BCG vaccination, and exposure history (Figure 1). (World Health Organization 2019)1,7,9,26 In children, it is particularly important to inquire about contact with infectious TB source case, as children with TB infection and disease often have recent exposure to an adult with infectious pulmonary TB.66 Next, we recommend obtaining a symptom history for TB from the patient and/or caregiver, including a fever and/or cough for more than 2 weeks, weight loss, night sweats, or fatigue.53 Vital signs should be measured, with attention to temperature and weight. Lastly, a thorough physical exam should be performed, with particular attention to the respiratory exam and evaluation for lymphadenopathy and hepatosplenomegaly (Figure 1).
Figure 1.
Pre-transplant evaluation for TB for pediatric solid organ and hematopoietic stem-cell transplant candidates and living donors. (World Health Organization 2019)1(American Academy of Pediatrics)7(Subramanian and Theodoropoulos, 2019)9(Tomblyn, Chiller and Einsele, 2009)26
Diagnosis
All transplant candidates should then undergo evaluation for TB infection with TST or an IGRA test prior to transplantation.7 TST results should be interpreted based on diameter of induration in millimeters per local guidelines. The American Society of Transplantation uses the cutoff for a positive result as ≥5 mm of induration at 48 to 72 hours.9 The TST is the preferred method for evaluation of TB infection in children under 2-years-old primarily due to paucity of data for use of IGRAs in this age-group.7 However, studies have shown that there is a lower number of indeterminate IGRAs in children under 2-years-old than previously expected, which may allow its future use in this young population.67,68
There are 2 types of IGRAs: QuantiFERON-TB Gold In-Tube or the newer Gold Plus (QFT-GIT and QFT-Plus; Qiagen, Hilden, Germany) and T-SPOT.TB (Oxford Immunotec, Abingdon, UK). Both are approved by the Food and Drug Administration in the United States as blood tests that measure the cell-mediated immune response to MTB antigens.7 The T-SPOT.TB measures the percentage of lymphocytes releasing IFN-ɣ; the QFT measures the total quantity of IFN-ɣ released into the plasma.69 IGRAs are preferred over TST in patients with history of BCG vaccination because the antigens used in IGRAs are not found in the BCG vaccine, reducing the frequency of false-positive results.9 Both these tests, however, must be interpreted with caution, especially in immunocompromised patients. As patients undergoing HSCT or SOT are often on immunosuppressive drugs, there can be falsely negative or indeterminate results. T-SPOT.TB may be preferred in immunocompromised patients as it appears to have slightly higher sensitivity for detecting MTB infection compared to QFT.6,10,70 The test used varies by institution. It is important to note that a negative result from either the TST or IGRA test does not exclude TB infection or disease.7
For patients with evidence of TB exposure or TB infection, it is critical to further evaluate for TB disease prior to starting therapy. Initiation of treatment for TB infection when the child has TB disease can lead to under-treatment and development of resistance. If the patient has symptoms concerning for TB, TB exposure, and/or the TST or IGRA is positive, the child should be evaluated with a chest radiograph (Figure 1). Radiographic findings of TB in children include hilar or mediastinal lymphadenopathy, large airway compression, parenchymal infiltrates, Ghon complexes/foci, miliary picture, and cavitations.71 Apical cavitation as is seen in reactivation TB in adults is uncommon, though can be seen in adolescents who often develop adult type TB disease.7 For detailed evaluation of children with TST/IGRA results that are negative or indeterminate with epidemiologic risk for TB infection or disease, chest computed tomography (CT) scan can be considered to further evaluate lung pathology, which can show consolidations, nodules, cavitary lesions, and lymphadenopathy.9,72 Further evaluation is recommended for children under 12-months-old with suspected TB given their risk for TB meningitis and disseminated disease. For children this age, most experts recommend obtaining CSF for indices, acid fast bacilli (AFB) stain and culture, and MTB PCR given the risk of meningitis.7
When there is concern for TB disease, it is important to obtain a microbiological diagnosis to provide susceptibility data prior to initiation of treatment. Samples for laboratory isolation of MTB can be obtained by various methods, via sputum sample in older children (10 years of age or older), gastric aspirate for younger children (particularly those under age 5), and induced sputum samples for all ages as long as proper training and equipment are available.53 Laboratory testing for MTB is performed via smear microscopy for AFB, nucleic acid amplification test via Xpert® MTB/Rif assay (GeneXpert) if available, and mycobacterial culture, which remains the gold standard. At times, histological confirmation by biopsy is helpful, which can demonstrate the presence of AFB and histologic findings ranging from typical caseous necrosis with granulomas to poorly organized granulomas, depending on the degree of immune suppression.58 Though microbiological confirmation from a patient source is optimal, this is not often achievable in children. In these circumstances, the susceptibility pattern of the index case, if known, should be used to guide treatment of the child.7
Additional Considerations
Recommendations for pre-transplant evaluation for TB for transplant candidates and donors are based on limited data, largely consensus statements and expert opinion. If screening is negative for TB infection or disease, transplant candidates may proceed to transplant if there are no other contraindications. In endemic regions, empiric treatment for TB infection may be given with negative screening results due to high suspicion.73 If screening is positive for TB infection with negative evaluation for TB disease, the ideal approach is to treat for TB infection prior to transplantation, but this is not possible in every case.27 In liver transplant patients, it may be reasonable to delay initiation of therapy until liver function stabilization after transplantation in order to prevent further liver dysfunction from anti-tuberculosis therapy (ATT) or alternatively treat with an agent that is less hepatotoxic, such as a fluoroquinolone.27,28
If screening is positive for TB disease, transplant candidates should start treatment prior to transplant when possible, given challenges of treatment in the post-transplant setting. Some experts regard TB disease in the recipient as a relative contraindication to transplant, except when a transplant is deemed urgent and life-saving.73,74 Patients with pulmonary TB can be considered as candidates for non-pulmonary SOT if the patient is receiving TB treatment and if sputum is AFB smear negative prior to transplant.27 HSCT should be delayed until TB disease is deemed controlled based on clinical judgment.26
In addition to transplant candidates, living donors should undergo a similar pre-transplant evaluation for TB infection and disease by obtaining exposure and symptom history as well as TST or IGRA testing (Figure 1). For deceased donors, we recommend obtaining contact and exposure history as feasible. There are limited data on the performance of IGRAs in the deceased donor population.69 If a living or deceased donor is found to have TB disease, it is recommended that the organ not be used.9
In most countries, children receive the live Bacille Calmette-Guérin (BCG) vaccine at birth, which offers protection to infants and young children from meningitis and disseminated TB disease.7,75 We recommend consultation with national guidelines regarding recommendations for BCG vaccination at birth. In high endemic TB settings, the WHO recommends BCG vaccination for all infants. (World Health Organization) BCG vaccination can have a rare complication of disseminated BCG disease and should not be used in children with impaired immunity.6,53 Children with history of BCG vaccination and underlying immunodeficiency are at risk for disseminated BCG disease after transplant, as seen in the case report of a 23-month-old girl with Bare Lymphocyte Syndrome type II who underwent allogeneic HSCT with development of disseminated BCG infection.76
Management
Treatment of TB infection and disease in children is similar to adults, but there are several differences to highlight for children. Children in general are able to tolerate TB medications for infection and disease better than adults, and are less likely to have hepatotoxicity, although such side effects do occur in certain high risk groups, including children undergoing transplant. Children also have different psychosocial needs; it is important to provide education appropriate to the child’s age and encourage adherence to medications.6 Treatment decisions are guided by the drug susceptibility pattern. MDR-TB, defined as MTB resistant to isoniazid and rifampin, presents treatment challenges for children, but new data is emerging regarding safety and pharmacokinetic studies in children along with child-friendly formulations.77,78 (World Health Organization) Additionally, TB treatment varies by region based on national TB guidelines.
TB Infection
There are several regimens, each with different toxicities, available for treatment of TB infection (Table 2).7,79-81 Three preferred regimens include 3 months of once-weekly isoniazid and rifapentine for children above 2 years of age; 4 months of daily rifampin monotherapy; and 3 months of daily isoniazid plus rifampin therapy.81-83 Given shorter treatment duration and higher completion rates, these 3 regimens are preferred for patients who are able to tolerate it.81 Alternative regimens include 6 or 9 months of isoniazid monotherapy. Other regimens can be used in select circumstances, including fluoroquinolones as a treatment regimen for TB infection in liver transplant patients and in patients with MDR-TB contacts.28,78 Preventative therapy for TB is recommended for exposed contacts with impaired immunity, particularly immunosuppressed children and children younger than 5-years-old.7
Table 2.
| Medication | Dose range (mg/kg) | Duration/frequency |
|---|---|---|
| A) Isoniazid-susceptiblea | ||
| Isoniazid (INH) and Rifapentine (RPT)80 | 1) Isoniazid: | 3 months/once weekly |
| Children age 2-11 year: 25 mg/kg | ||
| Children age 12 and older: 15 mg/kg | ||
| Max dose: 900 mg | ||
| 2) Rifapentine: | ||
| 10-14 kg: 300 mg | ||
| 14.1-25 kg: 450 mg | ||
| 25.1-32 kg: 600 mg | ||
| 32.1-49.9 kg: 750 mg | ||
| >50 kg: 900 mg | ||
| Max dose 900 mg | ||
| Rifampin (RIF)7 | 15-20 mg/kgb | 4 months/daily |
| Max dose: 600mg | ||
| Isoniazid (INH) and Rifampin (RIF)80 | 1) Isoniazid: | 3 months/daily |
| 10 mg/kg | ||
| Max 300 mg | ||
| 2) Rifampin | ||
| 15 mg/kg | ||
| Max dose: 600 mg | ||
| Isoniazid (INH)79 | 10 mg/kg | 9 months/dailyc |
| Max 300 mg | ||
| 20-30 mg/kg | 9 months/twice weeklyd | |
| Max 900 mg | ||
| B) Isoniazid-rifampin resistant | ||
| Recommend consultation with an ID specialist. Recommend regimen based on drug resistance profile of source case if available. Consider use of fluoroquinolone (levofloxacin or moxifloxacin).80 | ||
Isoniazid (INH) and rifapentine (RPT), rifampin (RIF), and isoniazid (INH) and rifampin (RIF) are preferred regimens. Isoniazid monotherapy is an alternative regimen.81
A daily rifampin dose of 20-30 mg/kg/day is recommended for infants and toddlers.7
WHO recommends 6 or 9 months daily of isoniazid.80
The INH twice weekly regimen must be provided via directly observed therapy (DOT).79
TB Disease
Treatment recommendations for TB disease depend on whether the organism is drug-susceptible or drug-resistant (Table 3).6,7,78,84-87 The treatment of drug-susceptible TB (DS-TB) disease is typically 6 to 9 months of therapy for pulmonary and extrapulmonary TB, with 2 months of a 4-drug regimen (rifampin, isoniazid, pyrazinamide, and ethambutol) followed by 4 to 7 months of a 2-drug regimen (rifampin and isoniazid). If there is low concern for isoniazid-resistant TB in the community, some experts recommend an initial regimen of rifampin, isoniazid, and pyrazinamide, without ethambutol.7 Given risk of peripheral neuropathy with isoniazid, pyridoxine (vitamin B6) supplementation is recommended for children in certain circumstances, particularly those with nutritional deficiencies.7 This duration of therapy depends on immunocompromised status and location of disease; for example, patients with TB meningitis or osteoarticular disease are typically treated with 9 to 12 months of anti-TB therapy.6
Table 3.
| Medication | Dose range (mg/kg) | Maximum daily dose (mg) | Adverse reactions |
|---|---|---|---|
| Drug susceptible TB | |||
| Rifampin7 | 15-20 mg/kg daily | 600 | Orange discoloration of secretions, hepatitis, drug-drug interactions |
| Isoniazid7 | 10-15 mg/kg daily | 300 | Hepatitis, peripheral neuropathy |
| Pyrazinamide7 | 30-40 mg/kg daily | 2000 | Hepatitis, GI upset, arthralgia, pruritus |
| Ethambutol7 | 15-25 mg/kg daily | 1000 | Optic neuritis, decreased red-green color discrimination |
| Drug resistant TBa | |||
| Group A Drugs | |||
| Levofloxacin/Moxifloxacin87 | 15-20 mg/kg daily | 1500 | Tendonitis, QTc prolongation, GI upset |
| 10-15 mg/kg daily | 800 | Tendonitis, QTc prolongation, GI upset | |
| Bedaquiline87 | >6 years: 200 mg/day for 2 weeks, then 100 mg 3 times/week for 22 weeksb | 400 | Hepatitis, possible QTC prolongation |
| >12 years: 400 mg/day for 2 weeks, then 200 mg 3 times/week for 22 weeks | |||
| Linezolid86 | <16 kg: 15 mg/kg daily | 1200 | Bone marrow suppression, peripheral neuropathy, optic neuritis |
| >16 kg: 10-12 mg/kg daily | |||
| Group B Drugs | |||
| Clofazimine87 | 2-5 mg/kg/day | 100 | Hepatitis, possible QTC prolongation |
| Cycloserine/terizidone87 | 15-20 mg/kg daily | 1000 | Neurologic/psychiatric effects |
| Group C Drugsc | |||
| Delamanid87 | >3-5 years and 10-20 kg: 25 mg twice daily | 200 | Hepatitis, Possible QTC prolongation |
| 6-11 years and 20-34 kg: 50 mg/dose twice daily | |||
| 12-17 years: 100 mg/dose twice daily | |||
| Imipenem-cilastatin87 | 15-25 mg/kg/dose IV 4 times daily (imipenem component) | — | GI upset, rash, nephrotoxicity, seizures |
| Meropenem87 | 20-40 mg/kg/dose IV 3 times daily | — | GI upset, rash, nephrotoxicity |
| Amoxicillin-clavulanate86,d | 40 mg/kg given twice daily based on amoxicillin component | — | GI upset, rash |
| Amikacin/Streptomycin87 | 15-20 mg/kg daily | 1000 | Auditory and vestibular toxic effects, nephrotoxic effects |
| Ethionamide/Prothionamide87 | 15-20 mg/kg daily (divided into 2 daily doses) | 1000 | GI upset, hepatitis, thyroid dysfunction |
| P-aminosalicylic acid (PAS)78 | 8-12 g/day (in 2-3 divided doses) | 12 000 | Electrolyte monitoring, hepatitis, thyroid dysfunction |
Abbreviations: mg/kg, milligram/kilogram; GI, gastrointestinal; QTc, Corrected QT interval.
Classification adapted from WHO Consolidated Guidelines on Drug-Resistant Tuberculosis Treatment78 and ATS/CDC/ERS/IDSA Clinical Practice Guideline on Treatment of Drug-Resistant Tuberculosis.87
Dose is based on expert opinion. Studies are ongoing in lower age groups and weights.87
Pyrazinamide and Ethambutol are included in group C drugs.78
Amoxicillin-clavulanate should be co-administered with carbapenems. It should be given 30 minutes prior to IV infusion of imipenem or meropenem.86
MDR-TB is typically treated with at least 4 to 5 drugs based on the susceptibility pattern of the patient’s isolate. The WHO has classified the second-line TB treatment drugs into 3 groups: Group A (include all 3 drugs, unless they cannot be used), Group B (add both drugs, unless they cannot be used), and Group C (add to complete regimen and when drugs from Groups A and B cannot be used).78,86 In children, it is recommended that all-oral regimens be used.78 More recently, newer medications have been recommended in children and adults, including bedaquiline (children aged 6 years and above) and delamanid (children aged 3 years and above); however, dosing and safety data remain unknown in younger children.86,88 Historically, drug-resistant TB has been treated with long regimens lasting about 12 to 24 months.6 Shorter regimens are available in adults, for example the 9 to 12 month “Bangladesh regimen,” which consists of an initial 4 to 6 months of kanamycin, moxifloxacin, prothionamide, clofazimine, pyrazinamide, high-dose isoniazid, and ethambutol, followed by 5 months of moxifloxacin, clofazimine, pyrazinamide, and ethambutol.89,90 Another regimen, the BPaL regimen, is now being used for adults with extensively drug-resistant TB (XDR-TB), which consists of oral bedaquiline, pretomanid, and linezolid for 6 to 9 months.91 While these shorter regimens are promising, there are limited data in children and immunocompromised patients to date; hence their utility in the pediatric transplant population remains uncertain.
In immunocompromised patients, the treatment duration can be variable, often prolonged, and tailored to the individual’s condition and complications.63 The American Thoracic Society (ATS), Centers for Disease Control and Prevention (CDC), and Infectious Diseases Society of America (IDSA) recommend extending the total duration of DS-TB treatment to at least 9 months for SOT recipients.93 In a retrospective review of MTB in pediatric liver transplant recipients from the UK between 1991 and 1998, TB treatment duration ranged between 9 and 18 months.63 In a case series of 10 adult HSCT patients with pulmonary TB, five patients received treatment with the standard 4-drug regimen in Hong Kong for 6 months and 2 or 3 drugs for another 6 months, which was well-tolerated.38 There remains scarce data on treatment duration in cases of drug-resistant TB in pediatric transplant recipients; one case series in adult transplant recipients with MDR-TB showed a treatment duration ranging between 18 and 24 months.94
Treatment Challenges
There are numerous treatment challenges when treating TB in pediatric transplant recipients, including medication adverse effects that can be difficult to monitor in children and interactions with other treatment medications. Rifampin and isoniazid, along with other agents used for ATT, can cause hepatotoxicity, which can be particularly concerning in liver transplant patients.30
Two significant treatment challenges include drug-drug interactions, particularly with rifampin, and hepatotoxicity (Table 4). Rifampin is a strong inducer of microsomal enzymes that metabolize immunosuppressive agents, including calcineurin inhibitors, mTOR inhibitors, and corticosteroids.9 Therefore, doses of these medications often need to be increased at least 2 to 5 fold when used concurrently with rifampin.63 Rifampin also interacts with other drug classes, including antiretroviral agents, other anti-infectives, hormone therapy, cardiovascular agents, anticoagulants, and anti-convulsants.93 Rifabutin can be substituted for rifampin in some circumstances, particularly for those on antiretroviral therapy for HIV, as it is a less strong inducer of cytochrome p450 activity.7,95 If available, measurement of drug concentrations for medications that interact with rifampin may be a helpful monitoring strategy to ensure therapeutic levels and prevent toxicity, particularly for children.93 An additional treatment challenge is hepatotoxicity, which is associated with rifampin, isoniazid, pyrazinamide, and other TB medications (Table 4). Use of hepatotoxic drugs should be suspended if ALT or AST increase 3-fold in symptomatic patients and 5-fold in asymptomatic patients.27,93 Once liver enzymes normalize, it is then possible to reintroduce medications one-by-one, starting with the least hepatotoxic drugs, or consider using alternative agents that are not hepatotoxic.86 For patients with prolonged or severe hepatotoxicity, treatment with isoniazid and rifampin without pyrazinamide with treatment extension to 9 months may be considered.96 It is important to exclude other causes of abnormal liver function tests, such as viral hepatitis, biliary tract disease, and other hepatotoxic drugs, prior to attributing drug-induced hepatotoxicity to ATT.93
Table 4.
| Adverse effects | Medications | Management considerations |
|---|---|---|
| Drug-drug Interactions with Rifampin9,93,a | Antiretroviral agents: | Rifabutin is preferred with protease inhibitors, as it is a less strong inducer of cytochrome p450 activity. Dose adjustments may be necessary when using rifampin with NNRTIs and INSTIs.93 |
| HIV-1 protease inhibitors, NNRTIs, INSTIs | ||
| Anti-microbials: | ||
| 1) Macrolide antibiotics (azithromycin, clarithromycin, erythromycin) | 1) Azithromycin has no interaction with. Clarithromycin and erythromycin have interactions with rifampin; recommend use of alternative agents. | |
| 2) Azole antifungal agents (fluconazole, voriconazole, itraconazole) | 2) Dosing of azoles may be sub-therapeutic with rifampin. Recommend laboratory monitoring and considering increase in azole dose with co-administration of rifampin. | |
| 3) Doxycycline | 3) Rifampin may decrease serum concentration of doxycycline; consider alternative agent. | |
| 4) Atovaquone | 4) Use alternate drug for PJP prophylaxis. | |
| Immunosuppressive agents: | ||
| 1) Calcineurin inhibitors (cyclosporine, tacrolimus) | 1) Monitoring of calcineurin inhibitor serum concentrations may assist with dosing. Consider replacing rifampin with rifabutin to help maintain immunosuppressant levels. | |
| 2) mTOR inhibitors (sirolimus) | 2) Monitoring of mTOR inhibitor serum concentrations may assist with dosing. | |
| 3) Corticosteroids | 3) Recommend clinical monitoring, as may require 2-3 fold increase in corticosteroid dose. | |
| Hormone therapy: Ethinylestradiol, norethindrone | Women on oral contraceptives should use a barrier method of contraception while on rifampin. | |
| Cardiovascular agents:90 Propanolol, metroprolol | Clinical monitoring recommended; may require alternate cardiovascular drug. | |
| Anticoagulants: Warfarin | Monitor prothrombin time; may require 2-3 fold warfarin dose increase. | |
| Anticonvulsants: Phenytoin, lamotrigine | TDM recommended; may require dose increase in anticonvulsant dose. | |
| Hepatoxicity84,86 | Rifampin, Pyrazinamide, Ethionamide/Prothionamide, Bedaquiline, Clofazimine, Delamanid, PAS | Stop all drugs if ALT/AST >5 times upper limit of normal. Allow liver enzymes to normalize. Re-introduce drugs one-by-one, starting with least hepatotoxic drugs. If symptoms recur or ALT increases, the last drug added should be stopped. |
Table adapted from ATS/CDC/IDSA Clinical Practice Guidelines: Treatment of Drug-Susceptible Tuberculosis.93
Abbreviations: NNRTI, non-nucleoside reverse transcriptase inhibitor; INSTI, integrase strand transfer inhibitor; TDM, therapeutic drug monitoring; PJP, Pneumocystis jiroveci pneumonia.
Not all available drug-drug interactions with rifampin are listed here.
Immunocompromised patients are at risk for complications of TB and increased mortality, secondary to their immunosuppression and underlying disease. SOT recipients can develop immune reconstitution syndrome, similar to patients living with HIV.27,36,95,97 After HSCT, children may be at risk for opportunistic infections that require concurrent treatment, such as fungal disease. A case series from Korea reports 2 children with ALL who developed co-infections with pulmonary TB and invasive pulmonary fungal infection after allogeneic HSCT.61 It was difficult to determine patient response to ATT in these patients given presence of co-infection.61 Drug interactions have also been reported with ATT and antifungals, particularly the azole class. Isoniazid (INH) is well tolerated post-HSCT with fluconazole, but itraconazole is not recommended and the safety of concurrent use of voriconazole or posaconazole is not known.26 Transplant recipients are additionally at risk for graft rejection and failure in the setting of drug-drug interactions between ATT and immunosuppressants (Table 4).30,48
Another treatment challenge is the delay in obtaining drug susceptibility testing, beyond the rapid rifampin resistance testing provided by the Xpert-MTB/RIF. It is important to diagnose drug-resistant TB presumptively if the known TB source case has drug-resistant TB. Drug-resistant TB should be considered if a patient is clinically worsening on appropriate therapy in a setting where MDR-TB is endemic.41 Patients on therapy for drug-resistant TB may require additional monitoring, as medications used to treat MDR-TB are often associated with toxicities (Table 3).
Discussion
Children undergoing SOT and HSCT have unique vulnerabilities and challenges in the post-transplant setting. Their immune response is impaired, due to decreased T-cell function from conditioning regimens and immunosuppressive regimens. Pediatric transplant recipients are at greatest risk for pulmonary TB, but can additionally have extrapulmonary and disseminated disease with atypical presentations. It is important to maintain a high degree of suspicion for TB in children with relevant medical and social risk factors, particularly those residing in or traveling to an endemic region. Children should undergo comprehensive pre-transplant evaluation to screen for TB infection and disease in order to reduce the risk of post-transplant TB. Patient characteristics and drug susceptibility patterns should be considered when choosing a treatment regimen. Treatment challenges, particularly due to medication interactions and toxicity, can lead to morbidity and mortality in this immunocompromised population. There remains limited data on TB in pediatric transplant recipients, with a need for further research related to diagnosis, treatment, and prevention.
There are limitations on the sensitivity of current diagnostic modalities to diagnose TB infection and disease. Young children remain vulnerable, as it is difficult to obtain a microbiological diagnosis in this age group. Since immunosuppression may alter TST and IGRA responses, it is important to develop diagnostic tests for TB infection that do not rely on an intact T-cell response.6 Further, a test that distinguishes TB infection and disease is needed.6 Given increased risk of extrapulmonary and disseminated disease in this population, further research to develop diagnostic testing for MTB using other clinical specimens (in addition to sputum) is vital.51
There is also a need for medications with less toxicities, child-friendly formulations, and shortened regimens. Given the hepatotoxicity from ATT, many liver transplant recipients are at risk for interruptions in therapy and graft rejection. Shorter regimens for TB infection and disease are key to preventing these complications and improving completion rates. MDR-TB treatment remains a particular challenge in many settings worldwide, with a need for clinical trials in children and treatment availability in low-resource settings worldwide.
Finally, there is a need for further implementation of therapy for TB infection in vulnerable populations worldwide to prevent progression to TB disease. Screening of TB in deceased donors with IGRAs is another area of research that will be helpful to prevent TB transmission to SOT recipients. Infection control to prevent transmission of TB remains critical in all settings, both in the clinic and hospital setting.
Conclusion
This review serves to provide a summary of the literature to date on TB infection and disease in pediatric SOT and HSCT recipients. There are many challenges related to diagnosis, treatment and prevention of TB in this population. Further research is needed to improve diagnosis and treatment in order to reduce morbidity and mortality of this disease.
Acknowledgments
We would like to thank Dr. Tanvi Sharma and Dr. Robert Husson for their support and guidance throughout the writing process.
Footnotes
Author Contributions: Melanie M. Dubois: Contributed to conception and design; contributed to analysis; drafted the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Avika Dixit: Contributed to conception and design; contributed to analysis; critically revised the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Gabriella S. Lamb: Contributed to conception and design; contributed to analysis; critically revised the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr. Dixit is supported through a Boston Children’s Hospital OFD/BTREC/CTREC Faculty Career Development Fellowship and the Bushrod H. Campbell and Adah F. Hall Charity Fund/Charles A. King Trust Postdoctoral Fellowship. Dr. Lamb and Dr. Dubois do not have funding sources to report.
ORCID iD: Melanie Dubois  https://orcid.org/0000-0001-9972-8533
https://orcid.org/0000-0001-9972-8533
References
- 1. World Health Organization. Global tuberculosis report 2019. 2019. Accessed November 23, 2019 https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-eng.pdf?ua=1
- 2. World Health Organization. Estimating TB incidence among children. Published online 2020. Accessed October 1, 2020 https://www.who.int/tb/areas-of-work/children/childhoodTBestimates/en/
- 3. Dodd PJ, Gardiner E, Coghlan R, Seddon J. Burden of childhood tuberculosis in 22 high-burden countries: a mathematical modeling study. Lancet Glob Health. 2014;2:e453- e459. [DOI] [PubMed] [Google Scholar]
- 4. Roya-Pabon CL, Perez-Velez CM. Tuberculosis exposure, infection, and disease in children: a systematic diagnostic approach. Pneumonia. 2016;8:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cruz AT, Starke JR. Pediatric tuberculosis. Pediatr Rev. 2010;31:13-26. [DOI] [PubMed] [Google Scholar]
- 6. Starke JR, Donald PR, eds. Handbook of Child and Adolescent TB. Oxford University Press; 2016. [Google Scholar]
- 7. American Academy of Pediatrics. Tuberculosis. In: Kimberlin D, Brady M, Jackson M, Long S, eds. Red Book: 2018 Report of the Committee on Infectious Diseases. 31st ed American Academy of Pediatrics; 2018:829-853. [Google Scholar]
- 8. Diagnostic standards and classification of tuberculosis in adults and children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med. 2000;161:1376-1395. [DOI] [PubMed] [Google Scholar]
- 9. Subramanian AK, Theodoropoulos NM, on behalf of the Infectious Diseases Community of Practice of the American Society of Transplantation. Mycobacterium tuberculosis infections in solid organ transplantation: guidelines from the Infectious Disease Community of Practice of the American Society of Transplantation. Clin Transpl. 2019;33:e13513. [DOI] [PubMed] [Google Scholar]
- 10. Al-Anazi KA, Al-Jasser AM, Alsaleh K. Infections caused by Mycobacterium tuberculosis in recipients of hematopoietic stem cell transplantation. Front Oncol. 2014;4: 1-11. doi: 10.3389/fonc.2014.00231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Global Observatory on Donation and Transplantation. Organ donation and transplantation activities: 2015 report. Published online September 2017. Accessed June 15, 2020 http://www.transplant-observatory.org/reports/
- 12. Niederwieser D, Baldomero H, Szer J, et al. Hematopoietic stem cell transplantation activity worldwide in 2012 and a SWOT analysis of the Worldwide Network for Blood and Marrow Transplantation Group (WBMT) including the global survey. Bone Marrow Transpl. 2016;51:778-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Munoz L, Santin M. Prevention and management of tuberculosis in transplant recipients: from guidelines to clinical practice. Transplantation. 2016;100:1840-1852. [DOI] [PubMed] [Google Scholar]
- 14. Horne DJ, Narita M, Spitters CL, Parimi S, Dodson S, Limaye AP. Challenging issues in tuberculosis in solid organ transplantation. Clin Infect Dis. 2013;57:1473-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ramos J, Batista M, Costa S. Tuberculosis in hematopoietic stem cell transplant recipients. Mediterr J Hematol Infect Dis. 2013;5:e2013061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaufmann SHE. How can immunology contribute to the control of tuberculosis? Nat Rev Immunol. 2001;1:20-30. [DOI] [PubMed] [Google Scholar]
- 17. Jones C, Whittaker E, Bamford A, Kampmann B. Immunology and pathogenesis of childhood TB. Paediatr Respir Rev. 2011;12:3-8. [DOI] [PubMed] [Google Scholar]
- 18. Bustamante J, Boisson-Dupuis S, Abel L, Casanova J-L. Mendelian susceptibility to mycobacterial disease: genetic, immunological, and clinical features of inborn errors of IFN-γ immunity. Semin Immunol. 2014;26:454-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marais BJ, Gie RP, Schaaf HS, et al. The clinical epidemiology of childhood pulmonary tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis. 2004;8:278-285. [PubMed] [Google Scholar]
- 20. Lewinsohn D, Gennaro M, Scholvinck L, Lewinsohn D. Tuberculosis immunology in children: diagnostic and therapeutic challenges and opportunities. Int J Tuberc Lung Dis. 2004;8:658-674. [PubMed] [Google Scholar]
- 21. Cambier C, Falkow S, Ramakrishnan L. Host evasion and exploitation schemes of Mycobacterium tuberculosis. Cell. 2014;159:1497-1509. [DOI] [PubMed] [Google Scholar]
- 22. Casadevall A, Pirofski L. The damage-response framework of microbial pathogenesis. Nat Rev Microbiol. 2003;1:17-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tavil B, Gulhan B, Ozcelik U, et al. Tuberculin skin test positivity in pediatric allogeneic BMT recipients and donors in Turkey. Pediatr Transplant. 2007;11:414-418 [DOI] [PubMed] [Google Scholar]
- 24. Aguado JM, Silva JT, Samanta P, Singh N. Tuberculosis and transplantation. Microbiol Spectr. 2016;4:1-14. [DOI] [PubMed] [Google Scholar]
- 25. de la Camara R, Martino R, Granados E, et al. Tuberculosis after hematopoietic stem cell transplantation: incidence, clinical characteristics and outcome. Bone Marrow Transplant. 2000;26:291-298. [DOI] [PubMed] [Google Scholar]
- 26. Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15:1143-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aguado JM, Torre-Cisneros J, Fortún J, et al. Tuberculosis in solid-organ transplant recipients: consensus statement of the Group for the Study of Infection in Transplant Recipients (GESITRA) of the Spanish Society of Infectious Diseases and Clinical Microbiology. Clin Infect Dis. 2009;48:1276-1284. [DOI] [PubMed] [Google Scholar]
- 28. Silva JT, San-Juan R, Fernández-Ruiz M, Aguado JM. Fluoroquinolones for the treatment of latent Mycobacterium tuberculosis infection in liver transplantation. World J Gastroenterol. 2019;25:3291-3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Abad CLR, Razonable RR. Donor derived Mycobacterium tuberculosis infection after solid-organ transplantation: a comprehensive review. Transpl Infect Dis. 2018;20:e12971. [DOI] [PubMed] [Google Scholar]
- 30. Yehia BR, Blumberg EA. Mycobacterium tuberculosis infection in liver transplantation. Liver Transpl. 2010;16:1129-1135. [DOI] [PubMed] [Google Scholar]
- 31. Munoz P, Rodriguez C, Bouza E. Mycobacterium tuberculosis infection in recipients of solid organ transplants. Clin Infect Dis. 2005;40:581-587. [DOI] [PubMed] [Google Scholar]
- 32. John GT, Shankar V, Abraham AM, Mukundan U, Thomas PP, Jacob CK. Risk factors for post-transplant tuberculosis. Kidney Int. 2001;60:1148-1153. [DOI] [PubMed] [Google Scholar]
- 33. Torre-Cisneros J, Doblas A, Aguado JM, et al. Tuberculosis after solid-organ transplant: incidence, risk factors, and clinical characteristics in the RESITRA (Spanish Network of Infection in Transplantation) cohort. Clin Infect Dis Off Publ Infect Dis Soc Am. 2009;48:1657-1665. [DOI] [PubMed] [Google Scholar]
- 34. Singh N, Paterson DL. Mycobacterium tuberculosis infection in solid-organ transplant recipients: impact and implications for management. Clin Infect Dis. 1998;27:1266-1277. [DOI] [PubMed] [Google Scholar]
- 35. Iglesias J, Ledesma KJ, Couto PJ, Liu J. Immune reconstitution inflammatory syndrome occurring in a kidney transplant patient with extrapulmonary tuberculosis. Case Rep Transplant. 2017;2017:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Baghban A, Azar MM, Bernardo RM, Malinis M. Disseminated Mycobacterium tuberculosis following renal transplant with alemtuzumab induction. BMJ Case Rep. 2016;2016:bcr2016217998. doi: 10.1136/bcr-2016-217998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cruz AT, Airewele G, Starke JR. Tuberculosis in pediatric oncology and bone marrow transplantation patients. Pediatr Blood Cancer. 2014;61:1484-1485. [DOI] [PubMed] [Google Scholar]
- 38. Ip MS, Yuen KY, Woo PC, et al. Risk factors for pulmonary TB in bone marrow transplant recipients. Am J Respir Crit Care Med. 1998;158:1173-1177. [DOI] [PubMed] [Google Scholar]
- 39. Fan W-C, Liu C-J, Hong Y-C, et al. Long-term risk of tuberculosis in haematopoietic stem cell transplant recipients: a 10-year nationwide study. Int J Tuberc Lung Dis. 2015;19:58-64. [DOI] [PubMed] [Google Scholar]
- 40. Yuen KY, Woo PCY. Tuberculosis in blood and marrow transplant recipients. Hematol Oncol. 2002;20:51-62. [DOI] [PubMed] [Google Scholar]
- 41. Martino R, Martinez C, Brunet S, Sureda A, Lopez R, Domingo-Albos A. Tuberculosis in bone marrow transplant recipients: report of two cases and review of the literature. Bone Marrow Transpl. 1996;18:809-812. [PubMed] [Google Scholar]
- 42. McCulloch M, Lin PL. Globalization of pediatric transplantation: the risk of tuberculosis or not tuberculosis. Pediatr Transplant. 2017;21:e12891. [DOI] [PubMed] [Google Scholar]
- 43. George B, Mathews V, Srivastava V, Srivastava A, Chandy M. Tuberculosis among allogeneic bone marrow transplant recipients in India. Bone Marrow Transplant. 2001;27:973-975. [DOI] [PubMed] [Google Scholar]
- 44. Abad CLR, Razonable RR. An update on Mycobacterium tuberculosis infection after hematopoietic stem cell transplantation in adults. Clin Transplant. 2018;32:e13430. [DOI] [PubMed] [Google Scholar]
- 45. Liu M, Yang C, Liu L, et al. Hematopoietic stem cell transplantation for treatment of patients with leukemia concomitant with active tuberculosis infection. Med Sci Monit. 2014;20:2484-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Maeda T, Wake A, Kawabata M, et al. Disseminated tuberculosis following reduced-intensity cord blood transplantation for adult patients with hematological diseases. Bone Marrow Transplant. 2005;35:91-97. [DOI] [PubMed] [Google Scholar]
- 47. Agrawal N, Aggarwal M, Kapoor J, et al. Incidence and clinical profile of tuberculosis after allogeneic stem cell transplantation. Transpl Infect Dis. 2018;20:e12794. [DOI] [PubMed] [Google Scholar]
- 48. Aljurf M, Gyger M, Alrajhi A, et al. Mycobacterium tuberculosis infection in allogeneic bone marrow transplantation patients. Bone Marrow Transplant. 1999;24:551-554. [DOI] [PubMed] [Google Scholar]
- 49. Krishnamoorthy S, Kumaresan N, Zumla A. Latent tuberculosis infection and renal transplantation—Diagnosis and management. Int J Infect Dis. 2019;80:S73-S76. [DOI] [PubMed] [Google Scholar]
- 50. Epstein DJ, Subramanian AK. Prevention and management of tuberculosis in solid organ transplant recipients. Infect Dis Clin North Am. 2018;32:703-718. [DOI] [PubMed] [Google Scholar]
- 51. Anand M, Nayyar E, Concepcion B, Salani M, Schaefer H. Tuberculosis in kidney transplant recipients: a case series. World J Transplant. 2017;7:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Green M, Michaels MG. Infections in pediatric solid organ transplant recipients. J Pediatr Infect Dis Soc. 2012;1:144-151. [DOI] [PubMed] [Google Scholar]
- 53. World Health Organization. Guidance for national tuberculosis programmes on the management of tuberculosis in children. Second edition Published online 2014. Accessed June 7, 2020 https://www.ncbi.nlm.nih.gov/books/NBK214448/pdf/Bookshelf_NBK214448.pdf [PubMed]
- 54. World Health Organization. WHO policy on TB infection control in health-care facilities, congregate settings and households. Published online 2009. Accessed November 1, 2020 https://apps.who.int/iris/bitstream/handle/10665/44148/9789241598323_eng.pdf?sequence=1 [PubMed]
- 55. McDiarmid SV, Blumberg DA, Remotti H, et al. Mycobacterial infections after pediatric liver transplantation: a report of three cases and review of the literature. J Pediatr Gastroenterol Nutr. 1995;20:425-431. [DOI] [PubMed] [Google Scholar]
- 56. Levitt DL, Mesmar B, Munir KM. Renal transplant-associated thyroid tuberculosis. J Endocr Soc. 2017;1:553-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Saribeyliler G, Sacli Alimoglu S, Mirioglu S, Demir E, Cagatay A, Yazici H. Tuberculosis mastitis: fever of unknown origin in a kidney transplant recipient. Eur J Breast Health. 2019;15:272-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Geramizadeh B, Nikeghbalian S, Janghorban P, Malekhosseini SA. Isolated tuberculosis of transplanted liver, a case report and review of the literature. Hepat Mon. 2013;13: e6691. doi: 10.5812/hepatmon.6691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kiuchi T, Inomata Y, Uemoto S, et al. A hepatic graft tuberculosis transmitted from a living-related donor. Transplantation. 1997;63:905-907. [DOI] [PubMed] [Google Scholar]
- 60. Chen CC, Huang LM, Chang YL, King CC, Lin KH. Acute respiratory distress syndrome due to tuberculosis in a child after allogeneic bone marrow transplantation for acute lymphoblastic leukemia. J Formos Med Assoc Taiwan Yi Zhi. 1999;98:701-704. [PubMed] [Google Scholar]
- 61. Lee JW, Kwon H-J, Jang P-S, et al. Two children with differing outcomes after treatment for pulmonary tuberculosis diagnosed after allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis. 2011;13:520-523. [DOI] [PubMed] [Google Scholar]
- 62. Aguado JM, Herrero JA, Gavalda J, et al. Clinical presentation and outcome of tuberculosis in kidney, liver, and heart transplant recipients in Spain. Spanish Transplantation Infection Study Group, GESITRA. Transplantation. 1997;63:1278-1286. [DOI] [PubMed] [Google Scholar]
- 63. Verma A, Dhawan A, Wade JJ, et al. Mycobacterium tuberculosis infection in pediatric liver transplant recipients. Pediatr Infect J. 2000;19:625-630. [DOI] [PubMed] [Google Scholar]
- 64. Vecino R, Santiago B, Baquero-Artigao F, et al. Tuberculosis in pediatric solid organ and hematopoietic stem cell transplant recipients. Pediatr Infect J. 2012;31:774-777. [DOI] [PubMed] [Google Scholar]
- 65. Viana LA, Cristelli MP, Santos DW, et al. Influence of epidemiology, immunosuppressive regimens, clinical presentation, and treatment on kidney transplant outcomes of patients diagnosed with tuberculosis: a retrospective cohort analysis. Am J Transpl. 2019;19:1421-1431. [DOI] [PubMed] [Google Scholar]
- 66. Roy V, Weisdorf D. Mycobacterial infections following bone marrow transplantation: a 20 year retrospective review. Bone Marrow Transplant. 1997;19:467-470. [DOI] [PubMed] [Google Scholar]
- 67. Starke JR. Pediatric tuberculosis: time for a new approach. Tuberculosis. 2003;83:208-212. [DOI] [PubMed] [Google Scholar]
- 68. Starke JR. Tuberculin skin test versus the interferon-gamma release assays: out with the old, in with the new. Pediatrics. 2020;145:e20193021. [DOI] [PubMed] [Google Scholar]
- 69. Ahmed A, Feng P-JI, Gaensbauer JT, et al. Interferon-gamma release assays in children < 15 years of age. Pediatrics. 2020;145:e20191930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Morris MI, Daly JS, Blumberg E, et al. Diagnosis and management of tuberculosis in transplant donors: a donor-derived infections consensus conference report. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2012;12:2288-2300. [DOI] [PubMed] [Google Scholar]
- 71. Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008;149:177-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Seddon JA, Padayachee T, Plessis A-MD. Teaching chest X-ray reading for child tuberculosis suspects. Int J Tuberc Lung Dis. 2014;18:763-769. [DOI] [PubMed] [Google Scholar]
- 73. Jung JI, Lee D-G, Kim Y-J, Yoon HK, Kim CC, Park SH. Pulmonary tuberculosis after hematopoietic stem cell transplantation: radiologic findings. J Thorac Imaging. 2009;24:10-16. [DOI] [PubMed] [Google Scholar]
- 74. Bumbacea D, Arend SM, Eyuboglu F, et al. The risk of tuberculosis in transplant candidates and recipients: a TBNET consensus statement. Eur Respir J. 2012;40:990-1013. [DOI] [PubMed] [Google Scholar]
- 75. Salvador NGA, Wee S-Y, Lin C-C, et al. Clinical outcomes of tuberculosis in recipients after living donor liver transplantation. Ann Transplant. 2018;23:733-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. The BCG World Atlas, 2nd edition. Accessed June 7, 2020 http://www.bcgatlas.org/index.php
- 77. Abu-Arja RF, Gonzalez BE, Jacobs MR, et al. Disseminated Bacillus Calmette-Guerin (BCG) infection following allogeneic hematopoietic stem cell transplant in a patient with Bare Lymphocyte Syndrome type II. Transpl Infect Dis. 2014;16:830-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Holland DP, Sanders GD, Hamilton CD, Stout JE. Strategies for treating latent multiple-drug resistant tuberculosis: a decision analysis. PloS One. 2012;7:e30194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. World Health Organization. WHO consolidated guidelines on drug-resistant tuberculosis treatment. Published online 2019. Accessed May 28, 2020 https://www.ncbi.nlm.nih.gov/books/NBK539517/pdf/Bookshelf_NBK539517.pdf [PubMed]
- 80. Rutgers Global Tuberculosis Institute. Management of latent tuberculosis infection in children and adolescents: a guide for the primary care provider. Published online 2020:1-44. Accessed May 1, 2020. http://globaltb.njms.rutgers.edu/educationalmaterials/Products/2020%20Peds%20LTBI%20Guide/Pediatric%20LTBI%20Handbook%202020.pdf
- 81. World Health Organization. Latent tuberculosis infection: updated and consolidated guidelines for programmatic management. Published online 2018. Accessed June 15, 2020 https://www.ncbi.nlm.nih.gov/books/NBK531235/pdf/Bookshelf_NBK531235.pdf [PubMed]
- 82. Sterling TR, Njie G, Zenner D, et al. Guidelines for treatment of latent TB infection: recommendations from the National Tuberculosis Controllers Association and CDC, 2020. Morb Mortal Wkly Rep. 2020;69:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Villarino ME, Scott NA, Weis SE, et al. Treatment for preventing tuberculosis in children and adolescents: a randomized clinical trial of a 3-month, 12-dose regimen of a combination of rifapentine and isoniazid. JAMA Pediatr. 2015;169:247-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sterling TR, Villarino ME, Borisov AS, et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011;365:2155-2166. [DOI] [PubMed] [Google Scholar]
- 85. Mase S, Chorba T, Lobue P, Castro K. Provisional CDC guidelines for the use and safety monitoring of bedaquiline fumarate (Sirturo) for the treatment of multidrug-resistant tuberculosis. Morb Mortal Wkly Rep. 2013;62:1-13. [PubMed] [Google Scholar]
- 86. World Health Organization. The use of delamanid in the treatment of multidrug-resistant tuberculosis in children and adolescents: interim policy guidance. Published online 2016. Accessed June 15, 2020 https://pubmed.ncbi.nlm.nih.gov/27854402/ [PubMed]
- 87. Management of Multidrug-Resistant Tuberculosis in Children: A Field Guide. 4th ed The Sentinel Project for Pediatric Drug-Resistant Tuberculosis, Boston. 2018. Accessed October 28, 2020 http://sentinel-project.org/wp-content/uploads/2019/02/Updated_DRTB-Field-Guide-2019-V3.pdf
- 88. Nahid P, Mase SR, Migliori GB, et al. Treatment of drug-resistant tuberculosis: an official ATS/CDC/ERS/IDSA clinical practice guideline. Am J Respir Crit Care Med. 2019;200:e93-e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. World Health Organization. WHO best-practice statement on the off-label use of bedaquiline and delamanid for the treatment of multidrug-resistant tuberculosis. Published online 2017. Accessed May 18, 2020 https://apps.who.int/iris/bitstream/handle/10665/258941/WHO-HTM-TB-2017.20-eng.pdf
- 90. Sotgiu G, Tiberi S, Centis R, et al. Applicability of the shorter “Bangladesh regimen” in high multidrug-resistant tuberculosis settings. Int J Infect Dis. 2017;56:190-193. [DOI] [PubMed] [Google Scholar]
- 91. Nunn AJ, Phillips PPJ, Meredith SK. A trial of a shorter regimen for rifampin-resistant tuberculosis. New Engl J Med. 2019;380:1201-1213. [DOI] [PubMed] [Google Scholar]
- 92. World Health Organization. Rapid communication: key changes to the treatment of drug-resistant tuberculosis. Published online December 2019. Accessed June 18, 2020 https://www.who.int/tb/publications/2019/WHO_RapidCommunicationMDR_TB2019.pdf?ua=1
- 93. Nahid P, Dorman SE, Alipanah N, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. 2016;63:e147-e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Huaman M, Brawley R, Ashkin D. Multidrug-resistant tuberculosis in transplant recipients: case report and review of the literature. Transpl Infect Dis. 2017;19: e12672. doi: 10.1111/tid.12672 [DOI] [PubMed] [Google Scholar]
- 95. Nelson CA, Zunt JR. Tuberculosis of the central nervous system in immunocompromised patients: HIV infection and solid organ transplant recipients. Clin Infect Dis. 2011;53:915-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Saukkonen JJ, Cohn DL, Jasmer RM, et al. An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med. 2006;174:935-952. [DOI] [PubMed] [Google Scholar]
- 97. Sun H-Y, Munoz P, Torre-Cisneros J, et al. Mycobacterium tuberculosis—associated immune reconstitution syndrome in solid-organ transplant recipients. Transplant J. 2013;95:1173-1181. [DOI] [PubMed] [Google Scholar]