Abstract
Cardiac implantable devices are commonly used for superior vena cava stenosis, but there have been few reports of electrode replacement in the stenosed superior vena cava. A 73-year-old man was diagnosed with second-degree type II atrioventricular block and a permanent dual-chamber, rate-modulated pacing pacemaker was implanted 10 years previously. Because of depletion of the pacemaker battery and an increase in the ventricular pacing threshold, replacement of the pacemaker and ventricular electrode was required. During the operation, we found that the patient had severe superior vena cava stenosis on angiography, and this caused obstruction when a common guidewire was used to pass through the superior vena cava. After attempting various methods, we successfully passed through the vascular stenosis with a super slide guidewire and a long sheath, and completed replacement of the pacemaker and ventricular electrode. We summarize the related literature of superior vena cava stenosis related to a cardiac implantable device, and discuss the replacement strategy of this complication and other treatment options.
Keywords: Superior vena cava stenosis, cardiac implantable device, arrhythmia, pacemaker electrode, guidewire, atrioventricular block
Introduction
Superior vena cava (SVC) stenosis is a complication after pacemaker implantation. The incidence of SVC is approximately 20% to 50%.1 This situation can make installing or replacing a cardiac implant more difficult. We report here an unusual case in which a 73-year-old man was diagnosed with second-degree type II atrioventricular block and was found to have severe SVC stenosis at the time of replacing the pacemaker. We successfully passed through the stenosis and completed replacement of the pacemaker and ventricular electrode by using a super slide guidewire and a long sheath. We discuss the strategy of replacing a pacemaker and electrode for SVC stenosis.
Case report
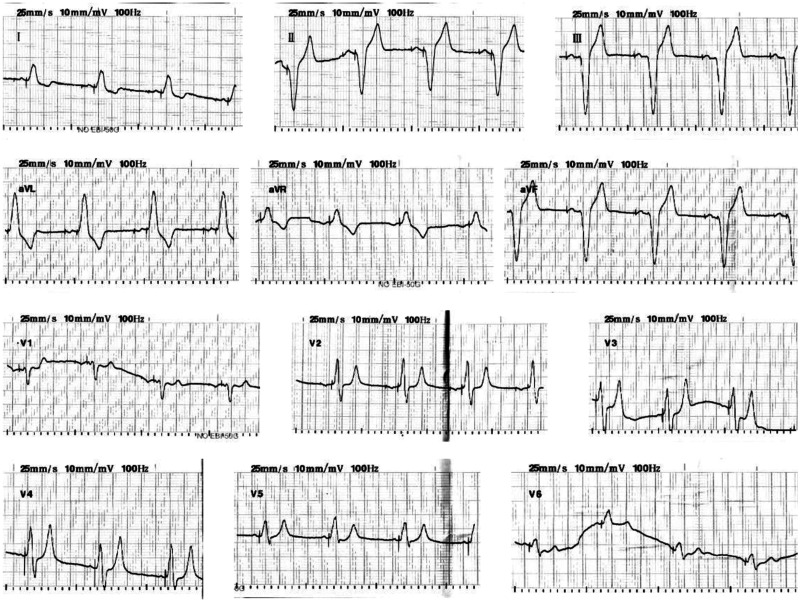
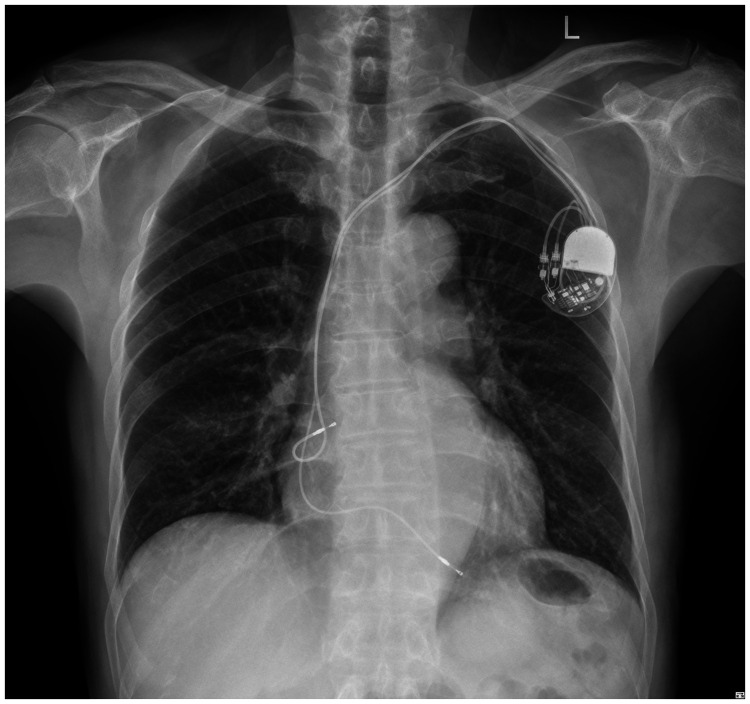
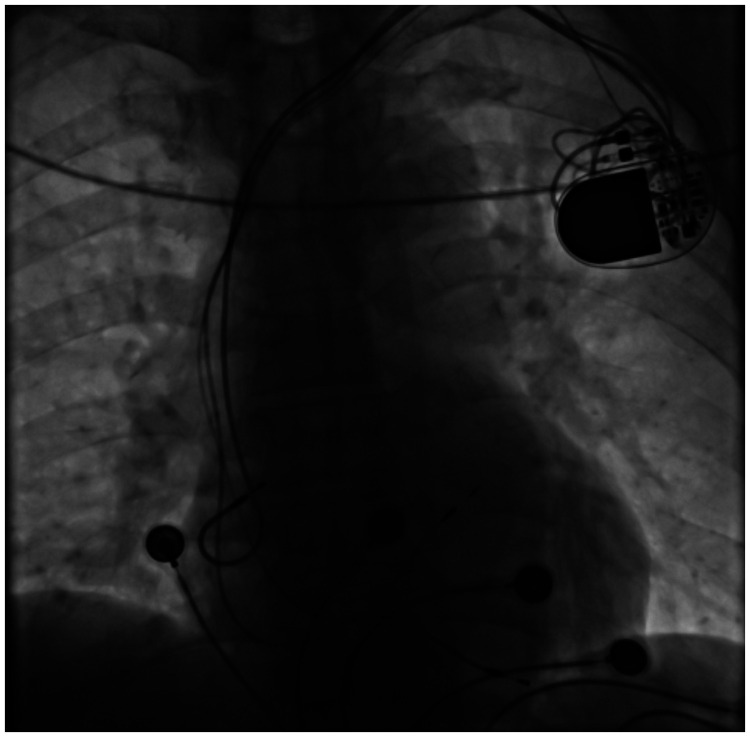
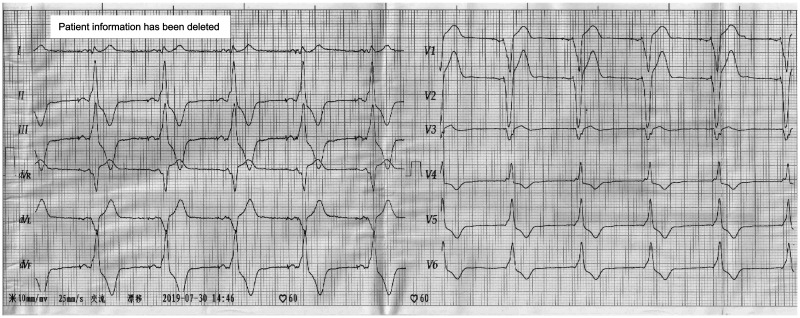
A 73-year-old man suffered from chest distress and chest pain 10 years previously, and was diagnosed with second-degree type II atrioventricular block and implanted with a permanent pacemaker. The patient visited the hospital for treatment because of chest tightness in the past week. An electrocardiogram showed that the patient’s heart rate depended on pacemaker pacing, the basic heart rate was 60 beats/minute, and a pacemaker pin was observed (Figure 1). A chest X-ray showed that there was no obvious dislocation of the pacemaker and electrode (Figure 2). A pacemaker program-controlled examination showed that the patient’s pacemaker battery was running out, accompanied by an increased pacing threshold of ventricular electrode (2.5 V) and dysfunction of ventricular perception. Therefore, the pacemaker and ventricular electrode needed to be replaced. After obtaining the patient’s consent and removing the contraindications, we performed a replacement operation. A formal ethical review by an ethics committee was not required because this was a case report. Written informed consent was obtained from the patient for publication of the case report and the accompanying images.
Figure 1.
An electrocardiogram shows that the patient’s heart rate depended on pacemaker pacing.
Figure 2.
A chest X-ray shows the position of the pacemaker and electrode.
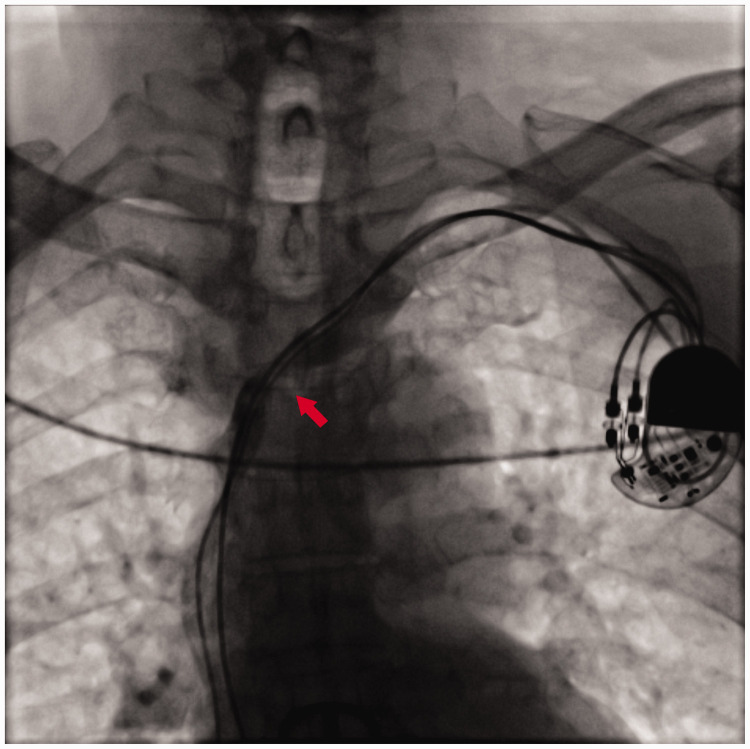
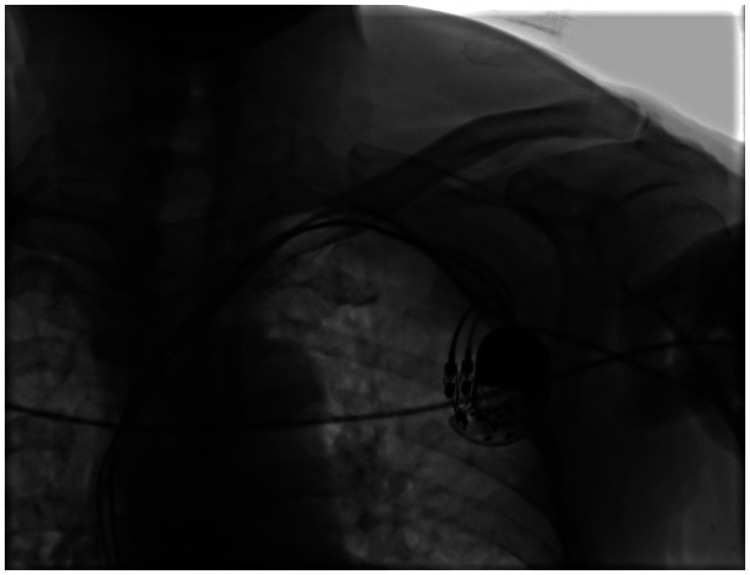
Under local anesthesia, we located the middle line of the left clavicle, and made a transverse incision of approximately 4 cm at approximately 2 cm under the clavicle as the center point. We separated subcutaneous tissue layer by layer to the deep fascia to separate the original pacemaker. After successful puncture of the axillary vein, the patient was implanted with a common sheath (SafeSheath, length: 13 cm; Medtronic, Minneapolis, MN, USA). During venography of the SVC, severe stenosis was observed at the level of the third thoracic vertebra (T3), and the guidewire was difficult to pass through (Figure 3). After replacing this guidewire with a 0.035 × 180-cm super slide guidewire (RF*GA35183M, Lot 44A-B-C; Terumo, Hanoi City, Vietnam), it passed through the narrow part smoothly. We then used a long sheath (Medtronic SafeSheath Long, length: 25 cm) and replaced the super slide guidewire with a 0.035 × 260-cm EMERALD™ Guidewire (Amplatz Type Super Stiff Straight Tip; Cordis, Miami, FL, USA) to strengthen the support, and the long sheath passed through the stenosis smoothly. We successfully delivered the head end of an active fixation electrode (Medtronic pacemaker electrode, 3830-69) to the right ventricular septum along the long sheath (Figure 4). During left bundle branch pacing, an electrocardiogram on the body’s surface showed that the QRS wave became narrower and the QS wave in lead V1 showed notches. After the electrode was fixed, the pacing threshold of the ventricular electrode was 0.6 V, the impedance was 800 Ω, and the R wave height was 15.0 mV. We then released the original pacemaker electrode and detected the original atrial electrode. The pacing threshold of the atrial electrode was 0.42 V, the impedance was 620 Ω, and the P wave height was 3.0 mV. The tail end of the electrode was closely connected with the new pacing pulse generator (Medtronic pacemaker, ADDRL1) and was placed in a subcutaneous pouch washed by gentamicin. After ligation with silica gel, the original ventricular electrode was fixed at the back of the pacemaker, and subcutaneous tissue and the skin were sutured layer by layer (Figure 5). After disinfection, the wound was compressed with a bandage and the patient was transferred to the intensive care unit for monitoring. An electrocardiogram of the patient postoperatively is shown in Figure 6.
Figure 3.
Angiography shows stenosis at the T3 level of the superior vena cava (arrowhead).
Figure 4.
After the ventricular electrode passed through the SVC stenosis, the long sheath was withdrawn.
Figure 5.
Successful replacement of the pacemaker and ventricular electrode.
Figure 6.
The patient’s postoperative electrocardiogram shows a pacing rhythm.
The patient’s chest tightness was relieved and the postoperative wound healed well. There was no SVC syndrome during follow-up.
Discussion
SVC stenosis is not uncommon after cardiac implantation, but there have been few reports of patients requiring electrode replacement.2–4 The causes of venous stenosis associated with cardiac implantable devices may be related to the number of leads, lead materials, lead diameter, and anticoagulant therapy, but these associations are still controversial.1,5–7 Stenosis of the SVC can cause clinical manifestations of SVC syndrome, such as progressive dyspnea, headache, facial and upper limb edema, superficial subcutaneous collateral circulation formation, and jugular vein distension.
We report a case of pacemaker and electrode replacement for SVC stenosis in an older male patient. This patient had second-degree type II atrioventricular block. The pacemaker and the ventricular electrode needed to be replaced because of depletion of the battery and an increase in the ventricular pacing threshold. The patient did not have obvious symptoms of SVC syndrome, but we found that he had severe SVC stenosis when we performed intraoperative SVC angiography. A common guidewire could not pass through the SVC. After changing the guidewire to a different size and hardness, we still could not pass through the stenosis. On the basis of the patient’s condition, age, and on the basis of the cost and the existing equipment and technology in our hospital, we completed replacement of the pacemaker and electrode in a simple, safe, and effective manner using a super slide guidewire and long sheath. We discuss the pacemaker and electrode replacement strategy for SVC stenosis below.
For patients with SVC stenosis who need to have a cardiac device installed or replaced, epicardial pacing through thoracotomy is a traditional method to treat them. However, thoracotomy is traumatic and has a high risk, accidents with anesthesia, and expensive operation costs. In our case, the patient was unable to receive thoracotomy because of his age, the high risk of surgery, and financial difficulties.
Video-assisted thoracoscopy is a minimally invasive and mature surgical method. Stoker et al.8 successfully implanted a left ventricular epicardial lead and intrathoracic tunnel into a right cardiac resynchronization therapy defibrillator device under the guidance of video-assisted thoracoscopy. However, our hospital is unable to provide this technical support, and in terms of simplicity, effectiveness, and minimal invasiveness, medical intervention appears to be a better option.
In our case, the main challenge of using medical interventional therapy in replacement of pacemaker electrodes for SVC stenosis is that the guidewire was difficult to pass through the stenosis, and the electrode was even more difficult to pass through. Directly using a common guidewire to pass through stenosis is difficult. Forced passage may prolong the operation time and increase the risk of the operation. This may further damage venous blood vessels, increase the risk of thromboembolism, and increase the degree of stenosis of the lumen, and even lead to uncontrollable bleeding due to puncture of venous blood vessels.
Venous stenosis angioplasty is performed to expand a stenosed lumen during installation or replacement of a cardiac implant.9 The femoral vein,10–12 iliac vein,13,14 and other vascular channels can be chosen to directly avoid the stenosis or absence of the SVC to complete the operation. However, angioplasty is difficult and risky. There are many disadvantages in the femoral vein and iliac vein approach,15 such as being far from the heart, having a large effect on the daily activities of patients, and increasing the wear of electrodes. Therefore, these methods are not the best choices for patients.
In addition to the above-mentioned methods, use of “special instruments” to install cardiac devices in patients with SVC stenosis has been reported. Zhang et al.2 described successful implantation of a cardiac resynchronization defibrillator with a long Swartz introducer through severe stenosis of the SVC. Sun et al.3 successfully completed cardiac resynchronization treatment by using a super slide guidewire and long guiding to pass through multiple occlusions of the SVC system. Xu et al.4 reported the use of a super smooth hydrophilic guidewire and long artery sheath through SVC stenosis and successful installation of an implanted permanent pacemaker. These methods are simple and effective, and no postoperative complications have been reported.
In our case, we used an operative method similar to that described above. We decided to use a super slide guidewire to reduce friction between the guidewire and the stenosed blood vessel after many attempts of a common guidewire still being unable to pass through severe stenosis of the SVC. At the same time, we used a long sheath to provide a smooth channel for the pacing ventricular electrode to pass through the stenosis and to the right ventricular septum. Finally, replacement of the pacemaker electrode was successfully completed. There was no SVC syndrome during follow-up.
The success of the operation and satisfactory postoperative follow-up results, as well as related literature, indicate that our method of replacing a pacemaker and electrode with a super slide guidewire and long sheath in severe stenosis of the SVC is safe and feasible.
Conclusion
The incidence of SVC stenosis after cardiac implantation is not low and preoperative examination of the SVC is particularly important. Installing or replacing a heart implantation device for patients with SVC stenosis by using a super slide guidewire and long sheath is a simple and effective method. Furthermore, SVC angioplasty, using either the femoral or iliac vein approach, and epicardial pacing under video-assisted thoracoscopy are also feasible.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This work was funded by a grant from the Special Health Project of Yuexiu District in 2018, Bureau of Science, Technology, Industry and Informatization, Yuexiu District, Guangzhou (No. 2018-WS-003).
References
- 1.Abu-El-Haija B, Bhave PD, Campbell DN, et al. Venous stenosis after transvenous lead placement: a study of outcomes and risk factors in 212 consecutive patients. J Am Heart Assoc 2015; 4: e001878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang C, Gao Y, Zheng J, et al. A case of cardiac resynchronization treatment of defibrillator by implantation of severe stenosis of superior vena cava. Chinese Journal of Cardiac Arrhythmias 2014; 18: 233–234. [Google Scholar]
- 3.Sun G, Shen F. Cardiac resynchronization for multiple occlusion of superior vena cava. Chinese Journal of Cardiac Arrhythmias 2013; 17: 467–468. [Google Scholar]
- 4.Xu S, Feng G, Chen Z. Application of long artery sheath in pacemaker installation for patients with superior vena cava stenosis. Journal of Clinical Medicine in Practice 2011; 15: 60–61. [Google Scholar]
- 5.Vidyasagar R, Sudarshan, Singh RN, et al. Upper extremity deep venous thrombosis and stenosis after implantation of pacemakers and defibrillators; A prospective study. Rom J Intern Med 2017; 55: 139–144. [DOI] [PubMed] [Google Scholar]
- 6.Cacko A, Kozyra-Pydyś E, Gawałko M, et al. Predictors of venous stenosis or occlusion following first transvenous cardiac device implantation: prospective observational study. J Vasc Access 2019; 20: 495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ze F, Li X, Guo J. Vein stenosis related to cardiac implantable device. Chinese Journal of Cardiac Pacing and Electrophysiology 2014; 28: 197–201. [Google Scholar]
- 8.Stoker T, Klinkenberg TJ, Maass AH, et al. Video-assisted implantation of a left ventricular lead and intrathoracic tunneling to a right-sided CRT-D device. Innovations (Phila) 2011; 6: 341–343. [DOI] [PubMed] [Google Scholar]
- 9.Pecha S, Burger H, Castro L, et al. The bridge occlusion balloon for venous angioplasty in superior vena cava occlusion. Braz J Cardiovasc Surg 2019; 34: 368–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tereno Valente B, Conceição JM, Nogueira Da Silva M, et al. Femoral approach: an exceptional alternative for permanent pacemaker implantation. Rev Port Cardiol 2014; 33: 311.e1–5. [DOI] [PubMed] [Google Scholar]
- 11.Chow DH, Choy CC, Chan NY. Idiopathic left innominate vein stenosis during pacemaker implantation with venoplasty in a retrograde approach. HeartRhythm Case Rep 2016; 2: 310–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodrigues P, Reis H, Lagarto V, et al. Permanent pacemaker implantation using a femoral approach. Rev Port Cardiol 2014; 33: 733.e1–6. [DOI] [PubMed] [Google Scholar]
- 13.Wiliński J, Jastrzębski M, Czarnecka D. Permanent pacemaker implantation via iliac vein approach in a patient with no venous access to the superior vena cava. Kardiol Pol 2015; 73: 573. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi T, Miyamoto T, Yamauchi Y, et al. A case report of successful permanent pacemaker implantation via the iliac vein. J Arrhythm 2016; 32: 151–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arenja N, Knecht S, Schaer B, et al. Comparison of different approaches to atrioventricular junction ablation and pacemaker implantation in patients with atrial fibrillation. Pacing Clin Electrophysiol 2014; 37: 1686–1693. [DOI] [PubMed] [Google Scholar]