Abstract
Chronic inflammation (CI) in older adults is associated with reduced health span and life span. Interleukin-6 (IL-6) is one CI marker that is strongly associated with adverse health outcomes and mortality in aging. We have previously characterized a mouse model of frailty and chronic inflammatory pathway activation (IL-10tm/tm, IL-10 KO) that demonstrates the upregulation of numerous proinflammatory cytokines, including IL-6. We sought to identify a more specific role for IL-6 within the context of CI and aging and developed a mouse with targeted deletion of both IL-10 and IL-6 (IL-10tm/tm/IL-6tm/tm, DKO). Phenotypic characteristics, cytokine measurements, cardiac myocardial oxygen consumption, physical function, and survival were measured in DKO mice and compared to age- and gender-matched IL-10 KO and wild-type mice. Our findings demonstrate that selective knockdown of IL-6 in a frail mouse with CI resulted in the reversal of some of the CI-associated changes. We observed increased protective mitochondrial-associated lipid metabolites, decreased cardiac oxaloacetic acid, improved myocardial oxidative metabolism, and better short-term functional performance in DKO mice. However, the DKO mice also demonstrated higher mortality. This work shows the pleiotropic effects of IL-6 on aging and frailty.
Keywords: Interleukin-6, Interleukin-10, Knockout, Lysophosphatidylcholine, Mitochondria
Frail older adults exhibit chronic activation of inflammatory pathways, which is associated with functional decline, reduced health span, and increased risk for early mortality (1,2). Many cytokines are elevated in chronic inflammation (CI). Still, prior work suggests a more prominent role for a subset of these cytokines: interleukin-6 (IL-6), soluble tumor necrosis factor-α receptor-1 (TNFα-R1), C-reactive protein, interleukin-18 (IL-18), and interleukin-1β (IL-1β) (1,3,4). Chronic, low-grade elevation of IL-6 is independently associated with worse functional status, the progression of age-related diseases, and increased mortality in older adults (2,5). Given this known association between IL-6 and adverse outcomes, inhibiting IL-6 signaling using IL-6 receptor blockers has been proposed as a potential therapeutic intervention for frailty. Despite the availability of antibodies targeting IL-6, the clinical utility and outcomes of IL-6 inhibition in the context of aging and frailty remains unclear. Insufficient understanding of the complex biology that connects inflammation to late-life decline and the scarcity of animal models for the study of CI and aging have slowed further biological discovery and intervention development that may improve health and quality of life in older adults.
To study the biology linking CI, aging, and late-life functional decline, we utilized mice deficient for the anti-inflammatory cytokine interleukin-10 (IL-10). These mice have a propensity to develop age-associated elevation of serum proinflammatory cytokines, including IL-6 and TNF-α. Our prior findings in this mouse model include impaired mitochondrial energy production and abnormal ATP kinetics as well as differential expression of key mitochondrial genes (6–9). The IL-10tm/tm (IL-10 KO) mouse also has reduced fat mass, with significantly dysregulated adipokine levels with aging (10).
In order to ascertain the specific influence of IL-6 on CI and aging-related mitochondrial decline, we created a genetically modified “double knockout” mouse deficient in both IL-10 and IL-6 (IL-10tm/tm/IL-6tm/tm, DKO). This mouse develops CI but has no IL-6 expression. We hypothesized that targeted deletion of IL-6 in CI mice (IL-10tm/tm/IL-6tm/tm) would significantly improve measures of mitochondrial energetics, health, and life span as compared to age- and gender-matched IL-10 KO mice.
Method
Experimental Animals
IL-10 KO mice (7) and gender-matched C57BL/6 background mice were purchased from Jackson Laboratory (Bar Harbor, ME). Full methods of development of the DKO mice are detailed in Supplementary Methods.
Serum Cytokine Measurements
Serum TNFα-R1 was measured using 96-well plate-based Quantikine mouse solid-phase enzyme-linked immunosorbent assay kit per manufacturer’s instructions (R&D Systems, Minneapolis, MN). Sample cytokine concentrations were determined using curve fit models. Duplicate measurements were made for each sample, and the average value was used for statistical analysis.
Carbon-11 Acetate and Dynamic Positron Emission Tomography/Computed Tomography
Myocardial oxidative metabolism was quantified using carbon-11 (C-11) acetate and dynamic positron emission tomography/computed tomography. Full details are listed in Supplementary Methods.
Lipid Metabolite Quantification by Liquid Chromatography-Tandem Mass Spectrometry
Serum lipid metabolites were measured using AbsoluteIDQ p180 kit (Biocrates Life Sciences AG, Innsbruck, Austria) following the manufacturers’ protocol. Detailed methods are included in Supplementary Methods.
Mitochondrial Metabolomics Analysis of Serum and Cardiac Tissue
Mitochondrial energetics of cardiac tissue and serum was assessed via measurement of glycolysis and tricarboxylic acid (TCA) cycle pathway intermediates. Metabolite measurement was performed at the Johns Hopkins Medical Institutions Metabolomics Facility using the protocol previously described by Nguyen and colleagues (11) and is fully detailed in Supplementary Methods.
Treadmill Testing
Mice were subjected to a 3-day exercise protocol using a treadmill with an electrical shock grid (Columbus Instruments, Columbus, OH). Full details of treadmill experiments are in Supplementary Methods.
Statistical Analysis
GraphPad Prism 8 (GraphPad Software, San Diego, CA) was used for statistical analysis. One-way ANOVA with Tukey’s post hoc test or Kruskal–Wallis nonparametric analysis with Dunnett’s post hoc test was used to determine differences among groups. When two groups were compared, unpaired Student’s t test was used. Two-way ANOVA was used to compare the differences in treadmill testing across all 3 days. p value < .05 was the threshold for significance.
Results
Characteristics of the IL-10tm/tm/IL-6tm/tm (DKO) Mouse Model
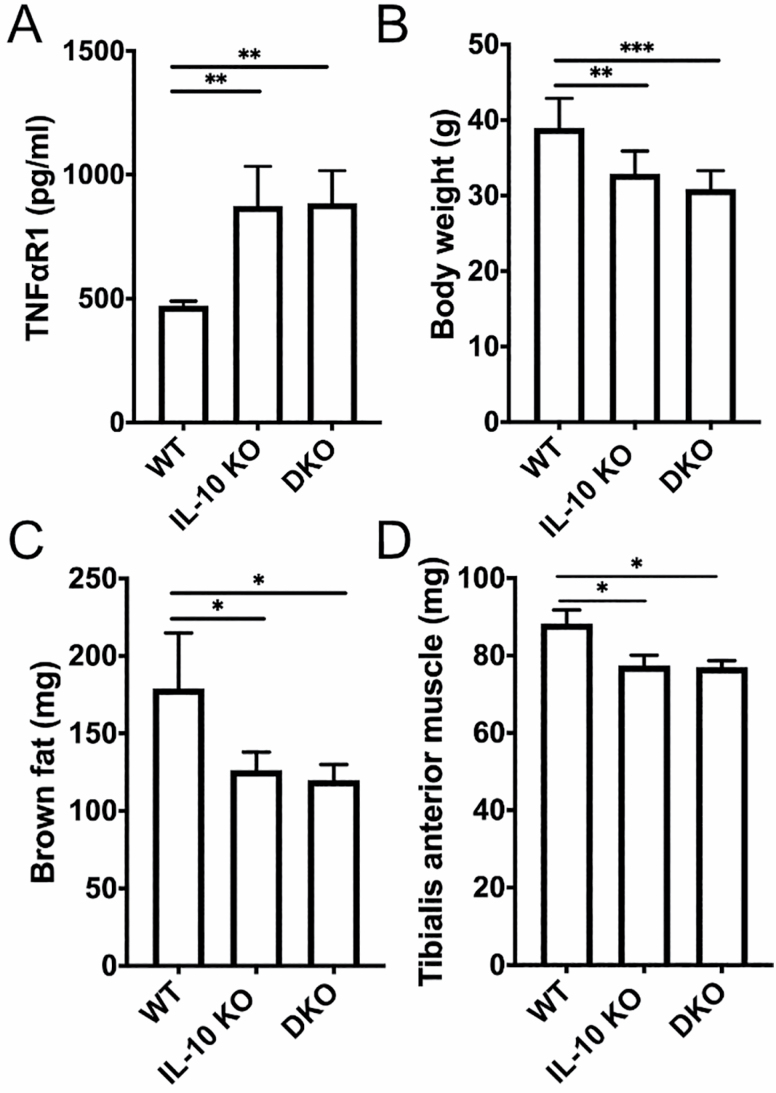
Prior work has shown chronic low-grade elevation of proinflammatory cytokines (IL-1β, IL-6, and TNF-α) in the IL-10 KO mouse (6). IL-10 KO mice also have reduced fat mass compared to wild-type (WT) mice, thought to be from lowered resting metabolic rate and dysregulated adipokine expression (10). We first confirmed that DKO mice have elevated levels of serum TNFα-R1 (Figure 1A). DKO mice demonstrated reduced body weight, brown fat mass, and tibialis anterior muscle mass at 23 months old compared to WT mice (Figure 1B–D). Despite the absence of IL-6, body weight, fat, and muscle mass measurements for DKO mice were similar to IL-10 KO mice.
Figure 1.
Physical and biochemical characterization of DKO mice. Serum soluble TNFα-R1 in WT, IL-10 KO, and DKO mice at 18–23 months old (A). Comparison of average weight (B), brown fat (C), and tibialis anterior muscle (D) mass for WT, IL-10 KO, and DKO mice. DKO = IL-10tm/tm/IL-6tm/tm; IL-10 KO = IL-10tm/tm; TNFα-R1 = tumor necrosis factor-α receptor 1; WT = wild-type, C57BL/6. *p < .05, **p < .001, ***p < .0001. N = 5–9 mice per group.
DKO Mice Have Altered Serum Lipidomics Compared to IL-10 KO and WT Mice
Given the reduced brown fat mass in aged DKO mice, we examined lipidomic profiles to determine if there were also associated changes in lipid metabolism with IL-6 knockout in the setting of CI. Targeted serum lipidomic analysis of glycerophospholipids and sphingolipids was performed in old WT, IL-10 KO, and DKO mice using liquid chromatography-mass spectrometry. Table 1 demonstrates serum lipid metabolites in DKO mice compared to WT and IL-10 KO mice. Specifically, we observed an increase in serum levels of a subset of lysophosphatidylcholine (LPC) compounds (LPC a C16:1, 18:1, 18:2, 20:3, and 20:4) in DKO mice compared to IL-10 KO mice.
Table 1.
Comparison of Serum Lipid Metabolites Among DKO Mice and WT, IL-10 KO Mice
| Lipid Metabolites | WT | IL-10 KO | DKO |
|---|---|---|---|
| Concentration (μM, mean) | |||
| LPC a C16:0* | 337 | 218 | 314 |
| LPC a C16:1* | 7.95 | 3.71 | 7.16 |
| LPC a C18:1* | 63.7 | 34.7 | 56.5 |
| LPC a C20:3* | 10.6 | 4.12 | 7.92 |
| PC aa C30:0# | 0.58 | 0.56 | 0.66 |
| PC aa C32:3 | 0.09 | 0.11 | 0.12 |
| PC aa C34:3* ,# | 13.2 | 10.3 | 20.1 |
| PC aa C34:4* ,# | 0.49 | 0.40 | 0.77 |
| PC aa C36:5* ,# | 6.57 | 4.84 | 10.1 |
| PC aa C36:6 # | 0.34 | 0.36 | 0.46 |
| PC aa C38:6* | 94.3 | 74.0 | 120 |
| PC aa C40:1* | 0.31 | 0.38 | 0.35 |
| PC aa C42:0* ,# | 0.27 | 0.25 | 0.34 |
| PC aa C42:1 | 0.14 | 0.14 | 0.16 |
| PC aa C42:2* | 0.23 | 0.24 | 0.29 |
| PC aa C42:5# | 0.29 | 0.31 | 0.36 |
| PC ae C34:2# | 3.94 | 4.87 | 5.30 |
| PC ae C38:0* | 2.60 | 1.81 | 3.28 |
| PC ae C38:2# | 7.37 | 4.65 | 3.90 |
| PC ae C42:1*,# | 0.49 | 0.48 | 0.73 |
| PC ae C44:4 | 0.25 | 0.28 | 0.28 |
| SM (OH) C14:1# | 1.56 | 1.06 | 0.78 |
| SM (OH) C22:2# | 2.56 | 1.85 | 1.43 |
| SM C20:2*,# | 0.33 | 0.29 | 0.51 |
Notes: DKO = IL-10tm/tm/IL-6tm/tm; IL-10 KO = IL-10tm/tm; LPC a = lysophosphatidylcholine acyl; PC aa = phosphatidylcholine diacyl; SM (OH) = hydroxysphingomyelin; SM = sphingomyelin; WT = wild-type, C57BL/6. 1-sided unpaired t-test. Mice 18–23 months old.
*indicates p < .05 between IL-10 KO and DKO comparisons.
#indicates p < .05 between WT and DKO comparisons.
DKO Mice Have Higher Cardiac Mitochondrial Oxidative Metabolism Compared to IL-10 KO Mice
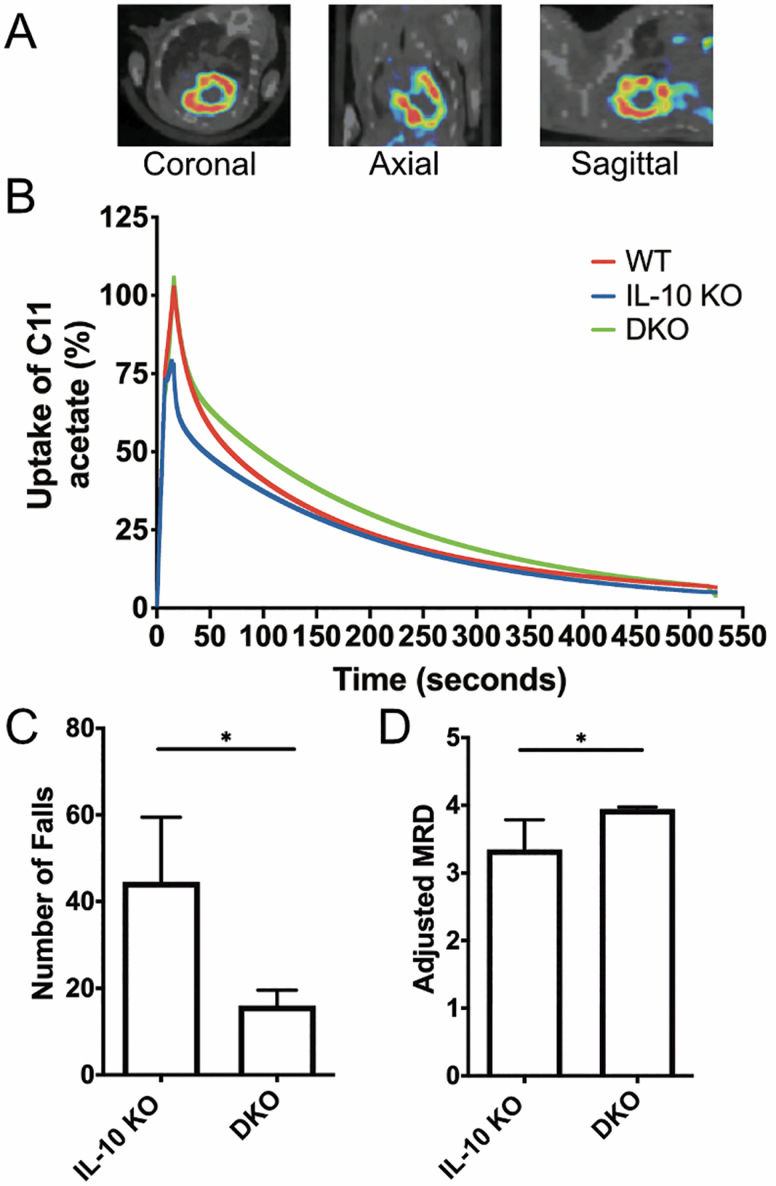
Given the observed impairment in mitochondrial energetics and ATP kinetics in the chronically inflamed IL-10 KO mice, we next studied myocardial oxidative phosphorylation in our mouse cohorts using carbon-11 (C-11) acetate uptake and clearance with dynamic positron emission tomography/computed tomography. The two-carbon fatty acid C-11 acetate is avidly taken up by the myocardium (Figure 2A), esterifies and enters the TCA cycle where the labeled C-1 carbon egresses as carbon dioxide (CO2) (12). The TCA cycle is closely coupled to cardiac oxidative phosphorylation, and quantifying C-11 acetate uptake is a noninvasive method of measuring myocardial oxygen consumption (13).
Figure 2.
Dynamic physical measures of the DKO mouse. Comparison of cardiac uptake of C-11 acetate among DKO, IL-10 KO, and WT mice using PET-CT imaging studies with representative fused C-11 acetate myocardial PET (in color-scale) and CT (in gray-scale) images in (from left to right) coronal, axial, and sagittal cross-sections (A). Time-activity curves of C-11 uptake with PET/CT in old female DKO mice compared to age- and gender-matched IL-10 KO and WT mice (B). Comparison of number of falls off the treadmill (C) and adjusted running capacity (ln(MRD/weight)) (D) on the first day of testing between DKO and IL-10 KO groups. Old DKO mice were compared to age- and gender-matched IL-10 KO and WT mice. DKO = IL-10tm/tm/IL-6tm/tm; IL-10 KO: IL-10tm/tm; MRD = maximum running distance (meters); PET/CT = positron emission tomography/computed tomography; WT = wild-type, C57BL/6. *p < .05. Mice 18–23 months old. N = 4–8 mice per group.
Compared to WT mice, IL-10 KO mice had a significantly lower uptake of C-11 acetate and a faster rate of substrate utilization (p < .001), suggesting impaired myocardial oxidative phosphorylation (Figure 2B). DKO mice demonstrated normalization of C-11 acetate uptake and clearance, with rates similar to WT mice (Figure 2B).
Characterization of Glycolysis and TCA Cycle Intermediates in Heart and Serum of IL-10 KO, DKO, and WT Mice
Given the improved myocardial oxidative phosphorylation measures in DKO mice compared to IL-10 KO mice, we next measured TCA and glycolysis pathway intermediates in serum and cardiac tissue to examine changes in mitochondrial energetics of our mouse cohorts. DKO mouse hearts had significantly decreased oxaloacetic acid compared to IL-10 KO mice (Supplementary Table 1). Serum succinate levels were significantly reduced in both IL-10 KO and DKO mice compared to WT (Supplementary Table 1).
DKO Mice Exhibit Improved Short-Term Physical Function Compared to IL-10 KO Mice
Chronic inflammation has been linked to impaired physical function; IL-10 KO mice demonstrate muscle weakness, and frail humans with elevated inflammatory markers show slowed gait speed (7,14). Therefore, we examined if IL-6 knockdown in the setting of CI is sufficient to improve physical function. We assessed short-term running capacity by measuring performance on a treadmill over three consecutive days. Treadmill running was chosen as a stressor in part because of its use in humans for “stress tests” and because it stresses several integrative systems at once, including cardiovascular, skeletal muscle, and pulmonary.
On the first day of testing, DKO mice had fewer falls off of the treadmill and a higher adjusted maximum running distance compared to IL-10 KO mice (Figure 2C and D). On Days 2 and 3, however, there were no significant differences among the groups (Supplementary Figure 1). Our results show that the improved short-term physical performance observed in DKO mice did not persist after two additional days of exercise testing.
Knockdown of IL-6 in CI Increased Mortality
While CI remains a strong predictor of all-cause and disease-specific mortality, it is not known whether higher IL-6 expression drives this observation. We compared mortality rates among the three groups to determine whether IL-6 knockout improved survival in CI (Supplementary Figure 2). DKO mice had fourfold higher mortality compared to old WT and IL-10 KO mice (p < .05) at 23 months old. The mortality rate in IL-10 KO mice was higher than the WT group, though not statistically significant. Necropsy showed no distinct macropathology differences among the three groups.
Discussion
This study shows that knockdown of IL-6 in a mouse model of CI leads to increased mitochondrial-associated lipid metabolites, improved myocardial oxidative metabolism, short-term improvements in physical function (higher maximum running distance and fewer falls), but higher mortality. The role of IL-6 in exercise-associated muscle recovery may have contributed to our physical function findings in the DKO mice. Prior research suggested that during exercise, IL-6 is released by skeletal muscle fibers, peaking immediately at the end of exercise before slowly declining (15). Both pro- and anti-inflammatory cytokines contribute to tissue healing in exercise (16); therefore, selective knockdown of cytokines in CI may impair vital repair processes.
We saw increases in mitochondrial-associated lipid metabolites, specifically LPC a C16:1, 18:1, and 20:3, in DKO mice compared to IL-10 KO mice. Lysophosphatidylcholine compounds function as intracellular signaling molecules and regulate acute and chronic inflammation (17). Studies show associations between higher plasma levels of palmitoleic acid (LPC 16:1), oleic acid (LPC 18:1), and eicosatrienoic acid (LPC 20:3) and increased skeletal muscle mitochondrial oxidative capacity (18). Additionally, older adults demonstrate decreased levels of LPC metabolites, which based on our findings, may be regulated by IL-6 (18,19). Lysophosphatidylcholine compounds are proposed to improve mitochondrial oxidative energetics by changing the fatty acid composition of cardiolipin, a key inner mitochondrial membrane component and also show dysregulation in older rats undergoing calorie restriction and exercise (18,20). However, despite these changes in LPC compounds between IL-10 and DKO mice, overall, the lipidomics profile in chronically inflamed mice in the presence or absence of IL-6 were similar.
Our study sheds light on the diverse roles of IL-6 in aging and CI. Chronic inflammation in the presence of IL-6 was associated with dysregulated lipid metabolism and worse cardiac mitochondrial oxygen uptake. Both abnormalities were reversed with the additional deletion of IL-6. Furthermore, we observed early gains in physical function in the chronically inflamed mice in the absence of IL-6. On the other hand, that early gain was not sustained, and we observed a dramatic increase in mortality in the aged DKO mice.
Targeted therapies that block IL-6 signaling may lead to unintended consequences by dysregulating essential repair functions that require IL-6. This work is a first step in understanding the effects of individual cytokines in CI that is seen in aging and frailty and demonstrates the pleiotropic effects of IL-6 on these processes. This novel animal model will enable us and others in the future to probe questions related to the specific impact of IL-6 on adverse health outcomes and chronic disease states in an in vivo model.
Supplementary Material
Funding
This work was supported by the National Institute on Aging at the National Institutes of Health (R01-AG046441) and Johns Hopkins University Older Americans Independence Center (National Institute on Aging P30-AG021334) to P.M.A.; Beijing Natural Science Foundation (7202059) to L.M.; Translational Aging Research Training Program, National Institute on Aging (T32AG058527) to L.S.N.; Intramural Research Program of the National Institute on Aging at the National Institutes of Health to R.M.; and the Shared Instrument Grant-S10 (Grant Number 1S10OD025226-01) funded by the National Institutes of Health to A.L.
Conflict of Interest
None declared.
Author Contributions
Study concept and design: P.M.A. and J.D.W.; analysis and interpretation of data, and drafting of the manuscript: L.M., L.S.N., J.D.W., and P.M.A.; animal breeding and mortality observation: J.L.; treadmill test: L.M. and H.Y.; carbon-11 acetate and dynamic PET: B.M.W.T., T.-S.L., S.L., R.M.-R., and Y.W.; ELISA assay: H.Y.; metabolomics and lipidomics: J.H., R.W., M.K., and R.M.; glycolysis and Krebs cycle measurement: A.L., T.N., and J.T.; critical revision of the manuscript for important intellectual content: all authors. All authors read and approved the final manuscript.
References
- 1. Varadhan R, Yao W, Matteini A, et al. Simple biologically informed inflammatory index of two serum cytokines predicts 10 year all-cause mortality in older adults. J Gerontol A Biol Sci Med Sci. 2014;69(2):165–173. doi: 10.1093/gerona/glt023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ferrucci L, Harris TB, Guralnik JM, et al. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47(6):639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x [DOI] [PubMed] [Google Scholar]
- 3. Walker KA, Walston J, Gottesman RF, Kucharska-Newton A, Palta P, Windham BG. Midlife systemic inflammation is associated with frailty in later life: the ARIC study. J Gerontol A Biol Sci Med Sci. 2019;74(3):343–349. doi: 10.1093/gerona/gly045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Soysal P, Stubbs B, Lucato P, et al. Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res Rev. 2016;31:1–8. doi: 10.1016/j.arr.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 5. De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflamm-ageing and lifelong antigenic load as major determinants of ageing rate and longevity. FEBS Lett. 2005;579(10):2035–2039. doi: 10.1016/j.febslet.2005.02.055 [DOI] [PubMed] [Google Scholar]
- 6. Ko F, Yu Q, Xue QL, et al. Inflammation and mortality in a frail mouse model. Age (Dordr). 2012;34(3):705–715. doi: 10.1007/s11357-011-9269-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walston J, Fedarko N, Yang H, et al. The physical and biological characterization of a frail mouse model. J Gerontol A Biol Sci Med Sci. 2008;63(4):391–398. doi: 10.1093/gerona/63.4.391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deng B, Wehling-Henricks M, Villalta SA, Wang Y, Tidball JG. IL-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration. J Immunol. 2012;189(7):3669–3680. doi: 10.4049/jimmunol.1103180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Akki A, Yang H, Gupta A, et al. Skeletal muscle ATP kinetics are impaired in frail mice. Age (Dordr). 2014;36(1):21–30. doi: 10.1007/s11357-013-9540-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Westbrook RM, Yang HL, Langdon JM, et al. Aged interleukin-10tm1Cgn chronically inflamed mice have substantially reduced fat mass, metabolic rate, and adipokines. PLoS One. 2017;12(12):e0186811. doi: 10.1371/journal.pone.0186811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nguyen T, Kirsch BJ, Asaka R, et al. Uncovering the role of N-acetyl-aspartyl-glutamate as a glutamate reservoir in cancer. Cell Rep. 2019;27(2):491–501.e6. doi: 10.1016/j.celrep.2019.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van den Hoff J, Burchert W, Börner AR, et al. [1-(11)C]Acetate as a quantitative perfusion tracer in myocardial PET. J Nucl Med. 2001;42(8):1174–1182. http://www.ncbi.nlm.nih.gov/pubmed/11483676. Accessed April 3, 2018. [PubMed] [Google Scholar]
- 13. Peterson LR, Gropler RJ. Radionuclide imaging of myocardial metabolism. Circ Cardiovasc Imaging. 2010;3(2):211–222. doi: 10.1161/CIRCIMAGING.109.860593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baptista G, Dupuy AM, Jaussent A, et al. Low-grade chronic inflammation and superoxide anion production by NADPH oxidase are the main determinants of physical frailty in older adults. Free Radic Res. 2012;46(9):1108–1114. doi: 10.3109/10715762.2012.692784 [DOI] [PubMed] [Google Scholar]
- 15. Keller P, Keller C, Carey AL, et al. Interleukin-6 production by contracting human skeletal muscle: autocrine regulation by IL-6. Biochem Biophys Res Commun. 2003;310(2):550–554. doi: 10.1016/j.bbrc.2003.09.048 [DOI] [PubMed] [Google Scholar]
- 16. Townsend JR, Hoffman JR, Fragala MS, et al. TNF-α and TNFR1 responses to recovery therapies following acute resistance exercise. Front Physiol. 2015;6:48. doi: 10.3389/fphys.2015.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schmitz G, Ruebsaamen K. Metabolism and atherogenic disease association of lysophosphatidylcholine. Atherosclerosis. 2010;208(1):10–18. doi: 10.1016/j.atherosclerosis.2009.05.029 [DOI] [PubMed] [Google Scholar]
- 18. Semba RD, Zhang P, Adelnia F, et al. Low plasma lysophosphatidylcholines are associated with impaired mitochondrial oxidative capacity in adults in the Baltimore Longitudinal Study of Aging. Aging Cell. 2019;18:e12915. doi: 10.1111/acel.12915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gonzalez-Freire M, Moaddel R, Sun K, et al. Targeted metabolomics shows low plasma lysophosphatidylcholine 18:2 predicts greater decline of gait speed in older adults: the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci. 2019;74(1):62–67. doi: 10.1093/gerona/gly100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oki K, Arias EB, Kanzaki M, Cartee GD. Effects of acute exercise combined with calorie restriction initiated late-in-life on insulin signaling, lipids, and glucose uptake in skeletal muscle from old rats. J Gerontol A Biol Sci Med Sci. 2020;75(2):207–217. doi: 10.1093/gerona/gly222 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.