Abstract
Background
Frailty is more prevalent among black versus white older Americans. We previously identified 37 metabolites associated with the vigor to frailty spectrum using the Scale of Aging Vigor in Epidemiology (SAVE) among older black men from the Health, Aging, and Body Composition (Health ABC) study. Here, we sought to develop a metabolite composite score based on the 37 SAVE-associated metabolites and determine whether the composite score predicts mortality and whether it attenuates the association between frailty and mortality among older black men.
Methods
Plasma metabolites were measured using liquid chromatography–mass spectrometry. Most of the 37 metabolites were organic acids/derivatives or lipids. Metabolites were ranked into tertiles: tertiles associated with more vigorous SAVE scores were scored 0, mid-tertiles were scored 1, and tertiles associated with frailer SAVE scores were scored 2. Composite scores were the sum of metabolite tertile scores. We examined mortality associations using Cox regression. Percent attenuation estimated the extent to which metabolites attenuated the association between frailty and mortality.
Results
One standard deviation frailer SAVE was associated with 30% higher mortality, adjusting for age and site (p = .0002); this association was attenuated by 56% after additionally adjusting for the metabolite composite score. In this model, one standard deviation higher metabolite composite score was associated with 46% higher mortality (p < .0001). Metabolite composite scores also predicted mortality (p = .045) in a validation sample of 120 older adults (40% men, 90% white).
Conclusion
These metabolites may provide a deeper characterization of the higher mortality that is associated with frailty among older adults.
Keywords: Metabolism, Biomarkers, Epidemiology
As the worldwide population ages (1), there will be more individuals affected by frailty than ever before. Frailty is a syndrome encapsulating decline across multiple physiologic systems that decreases reserve and resilience to stressors, ultimately increasing vulnerability to disease and death (2). Evidence suggests that metabolic changes are associated with a higher risk of frailty (3,4). Knowledge of specific metabolic alterations and pathways that characterize frailty and contribute to the frailty-associated vulnerability to death may help guide development of novel interventions to mitigate frailty and improve quality of life in the older adult population.
We previously identified 37 metabolites cross-sectionally associated with the spectrum of vigor to frailty (5). Most of these metabolites were classified as organic acids/derivatives or lipids/lipid-like molecules (eg, amino acids, glycerophospholipids, and sphingolipids). In this report, we developed a metabolite composite score using the 37 previously identified metabolites that were correlated with vigor to frailty. We sought to examine whether the metabolite composite score predicts mortality and whether it attenuates the higher mortality risk associated with frailer scores among 287 community-dwelling older black men from the Health, Aging, and Body Composition (Health ABC) study. We also validated the metabolite composite score against mortality among 120 black and white community-dwelling older adults from the Cardiovascular Health Study (CHS) All Stars study.
Methods
Cohorts
The Health ABC study was a prospective cohort of 3,075 men and women recruited from Pittsburgh, Pennsylvania and Memphis, Tennessee (6). Eligible individuals were aged 70–79 and self-reported no difficulty walking ¼ mile, climbing 10 steps, or with basic activities of daily living. Ineligibility included cancer treatment in the past 3 years or plans to move from the study area in the next 3 years. Institutional review boards from each site approved the study. All participants provided written informed consent. An ancillary study measured plasma metabolites in a subset of 319 black men at Year 2 (7).
The CHS All Stars study was examined as a replication cohort for the association between the metabolite composite score and mortality. The CHS All Stars study was an ancillary study of 1,862 men and women alive at Year 18 of the CHS (8), a population-based prospective cohort of 5,888 adults aged ≥65 years (9). Ineligibility included wheelchair bound, unable to participate in a clinic examination, undergoing active cancer treatment, or planning to move out of the study area in the next 3 years. Both studies were approved by the Human Research Protection Office at participating universities. All participants provided written informed consent. An ancillary study measured plasma metabolites in a subset of 120 men and women from the CHS All Stars study.
Metabolites
Metabolites in the Health ABC study were measured in plasma collected at Year 2 after an overnight fast of ≥8 hours using liquid chromatography–mass spectrometry (LC-MS) methods (7). Samples had never been thawed and were stored at −80°C. Metabolite values were LC-MS peak areas. Among the 37 metabolites considered, 33 were measured in all 287 Health ABC participants. The remaining four metabolites were observed in at least 90% of participants; C54:10 triacylglycerol, C4-OH carnitine, cystathionine, and 5-hydroxytryptophan were missing for 1, 3, 7, and 24 participants, respectively. The low amount of missing values was assumed to be due to true values being below detectable limits (10) (ie, left-censored missing not at random) and were imputed with half the minimum recorded value for the respective metabolite (10,11). As a sensitivity analysis, we applied the Quantile Regression Imputation of Left-Censored data (11) and obtained identical results.
Metabolites were similarly measured by the same institute for the CHS All Stars study. Information on 32 of the metabolites was available. The five metabolites not detected in the overnight-fasting plasma samples from the CHS All Stars study were: glucoronate, cystathionine, homogentisate, C54:10 triacylglycerol, and C44:13 phosphatidylethanolamine plasmalogen. Among the 32 metabolites available, 30 were measured in all 120 CHS All Stars. The remaining two metabolites, glycodeoxycholate and inosine, were only missing in 1 and 7 participants, respectively. Half the minimum recorded value for the respective metabolite was used to replace the low amount of missing values (10). Metabolites for both cohorts were log-transformed and standardized using cohort-specific means and standard deviations (SDs).
Metabolite Composite Score
The metabolite composite score was developed in the Health ABC study similarly to the Physiologic Index of Comorbidity (12) and Scale of Aging Vigor in Epidemiology (SAVE) (13). We ranked metabolites into tertiles (Supplementary Table S1). The “best” metabolite tertile was defined as the tertile associated with more vigorous SAVE scores. Similarly, the “worst” metabolite tertile was the tertile associated with frailer SAVE scores. Associations between all 37 metabolites and SAVE scores were monotonic. The best, middle, and worst tertile for a metabolite was scored 0, 1, and 2, respectively (Supplementary Table S1). The metabolite composite score was then calculated as the sum of tertile scores for the 37 metabolites, ranging from 0 (best) to 74 (worst); 0 meant measurements for all metabolites fell into the “best” tertile and 74 meant measurements for all metabolites fell into the “worst” tertile for an individual. The metabolite composite score was validated against all-cause mortality in 120 participants from the CHS All Stars study. The composite score was calculated in the same way, using the Health ABC-specific tertile cutoffs and scoring for each metabolite.
To determine whether results were based on a subset of metabolites, we calculated separate metabolite subscores by taxonomy superclass according to the Human Metabolome Database (Supplementary Table S2) (14). Five metabolite subscores were calculated for 14 metabolites classified as organic acids/derivatives; 12 metabolites classified as lipids/lipid-like molecules; 4 metabolites classified as organoheterocyclic compounds; 3 metabolites classified as benzenoids; and the remaining four metabolites classified as organic nitrogen compounds, organic oxygen compounds, and nucleosides/nucleotides/analogs. Metabolite subscores were calculated identically to the total score, but with a subset of the 37 metabolites.
Scale of Aging Vigor in Epidemiology
The Scale of Aging Vigor in Epidemiology (SAVE) measured the spectrum of vigor to frailty (13), calculated using weight change, physical activity, grip strength, gait speed, and energy level assessed at Year 2 of the Health ABC study; Supplementary Table S3 includes cutoffs and scoring for the five SAVE items. Weight change was the difference between Years 1 and 2. Physical activity was the kilocalories/kilogram/week sum of self-reported time spent doing major chores, walking, climbing stairs, and working/volunteering/caregiving. Grip strength was the maximum of two trials on the right hand using a hand-held dynamometer. Gait speed was average speed over 20 m. Participants self-reported usual energy in the past month, ranging from 0 (no energy) to 10 (most energy ever had). The five SAVE items were ranked into tertiles using information from all Health ABC men (5). The best, middle, and worst tertile for an item was scored 0, 1, and 2, respectively. SAVE scores were the sum of tertile scores for the five items, ranging from 0 (most vigorous) to 10 (most frail). SAVE tertiles were determined using information from all Health ABC participants. Among 319 Health ABC black men with metabolites measured, 287 (90%) had information on the SAVE.
Based on available information in the CHS All Stars study, we calculated a modified version of the SAVE; Supplementary Table S4 includes cutoffs and scoring for the five items used to calculate the modified SAVE. Weight change in the past year was based on self-report with the following possible responses: lost >10 lbs, gained >10 lbs, both lost and gained >10 lbs, or little/no change. Physical activity in kilocalories/kilogram/week was estimated from the Minnesota Leisure Time Activity Questionnaire. Grip strength was the average of three trials on the dominant hand using a hand-held dynamometer. Gait speed was average speed over 15 feet or 3 m if a 15-feet space was unavailable. Participants self-reported usual energy in the past month, ranging from 0 (no energy) to 10 (most energy ever had). Among the 120 CHS All Stars, 116 had information to calculate the modified SAVE.
Mortality
Participants in both cohorts were contacted biannually. Deaths were identified by obituaries and proxy interviews during follow-up. At the time of analysis, those alive were censored at their last interview date. Median follow-up after Year 2 was 10.3 years, and 218 (76%) died among 287 Health ABC black men; top causes of death were cancer (31%), cardiovascular disease (26%), stroke (10%), and dementia (10%). Median follow-up was 7.4 years and 69 (58%) died among 120 CHS All Stars; top causes of death were cardiovascular disease (20%), cancer (19%), dementia (17%), and infections (10%).
Participant Characteristics
Participants self-reported age, race, highest level of education, and smoking habits. Body mass index was calculated from height and weight measured during the visit. History or presence of cardiovascular disease, hypertension, diabetes, cancer, osteoarthritis, and pulmonary disease was based on self-reported physician diagnosis or taking medication. Health ABC participants brought all prescription medications used in the last 2 weeks to their Year 2 visit.
Statistical Analysis
Mean and SD and frequency and percent described differences in continuous and categorical measures, respectively, by SAVE tertiles. Differences were tested using analysis of variance for continuous measures and chi-square tests for categorical measures. Pairwise comparisons were made when overall differences were observed. Associations between SAVE scores and mortality were estimated using Cox proportional hazards regression, adjusting for age and study site. We examined the percent attenuation in the association between SAVE scores and mortality after additionally adjusting for a single metabolite (Model 1), all 37 metabolites (Model 2), a subset of metabolites after backward stepwise elimination (Model 3), the metabolite composite score (Model 4), more commonly measured risk factors (Model 5), or both the metabolite composite score and more commonly measured risk factors (Model 6). Analyses were performed in SAS 9.4. As a sensitivity analysis, we reran mortality analyses using Aalen additive hazards models (15) in R and found similar attenuations (data not shown).
Results
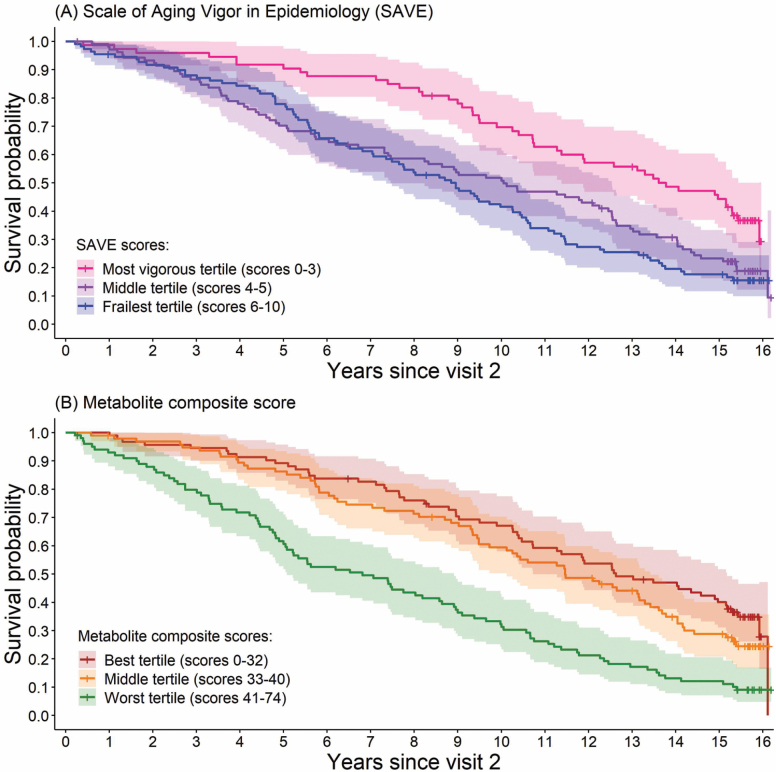
The Health ABC black men were 75 years old, on average. Frailer participants were older, taking more prescription medications, and more likely to have cardiovascular disease, diabetes, and pulmonary disease (Table 1). The most vigorous, average, and frailest Health ABC men had a median survival (95% confidence interval [CI]) of 13.8 (11.3, 15.3), 10.1 (7.4, 12.5), and 8.8 (7.1, 10.3) years, respectively (Figure 1A); the most vigorous tertile was significantly different from the middle and frailest tertiles (p = .002 and p < .0001, respectively), though the frailest tertile was not significantly different from the middle tertile (p = .34). Those in the best, middle, and worst tertile of the metabolite composite score had a median survival (95% CI) of 12.6 (10.7, 15.0), 11.5 (9.5, 13.3), and 6.8 (5.0, 8.6), years, respectively (Figure 1B); the best and middle tertiles of the metabolite composite score were significantly different from the worst tertile (p < .0001), though the best versus middle tertiles were not significantly different (p = .28). One standard deviation higher metabolite composite score was associated with 52% higher mortality among Health ABC men, while adjusting for age and study site (95% CI = 1.33, 1.75; p < .0001).
Table 1.
Characteristics of 287 Health ABC Black Men by Tertiles of the Scale of Aging Vigor in Epidemiology (SAVE)
| Mean (SD) or Frequency (%) | SAVE Tertiles | Overall p Value, Pairwise Comparisons | ||
|---|---|---|---|---|
| Vigorous (T1) n = 73 | Average (T2) n = 105 | Frail (T3) n = 109 | ||
| SAVE scores | 2.4 (0.7) Range: 0–3 | 4.5 (0.5) Range: 4–5 | 7.0 (1.1) Range: 6–10 | — |
| Age | 74 (3) | 75 (3) | 75 (3) | .006, T1 < T2, T3 |
| Pittsburgh site | 34 (47%) | 56 (53%) | 63 (58%) | .33 |
| More than high school education | 28 (38%) | 24 (23%) | 28 (26%) | .06 |
| Current smoker at baseline | 9 (12%) | 22 (21%) | 21 (19%) | .31 |
| Body mass index (kg/m2) | 27 (4) | 27 (4) | 27 (5) | .82 |
| Prevalent disease at baseline | ||||
| Cardiovascular disease | 11 (15%) | 36 (34%) | 39 (36%) | .006, T1 < T2, T3 |
| Hypertension | 34 (47%) | 65 (62%) | 67 (61%) | .08 |
| Diabetes | 8 (11%) | 18 (17%) | 37 (34%) | .0004, T1, T2 < T3 |
| Cancer | 10 (14%) | 11 (10%) | 11 (10%) | .72 |
| Osteoarthritis | 2 (3%) | 9 (9%) | 11 (10%) | .17 |
| Pulmonary disease | 7 (10%) | 8 (8%) | 21 (19%) | .02, T2 < T3 |
| Total number of prescription medications | 2.2 (2) | 3.0 (3) | 4.0 (4) | .0003, T1, T2 < T3 |
| Deaths | 46 (63%) | 82 (78%) | 90 (83%) | — |
| Death rate per 100 person-years | 5.3 (4.0, 7.0) | 8.5 (6.8, 10.5) | 9.5 (7.7, 11.7) | — |
Figure 1.
Kaplan–Meier survival curves with 95% confidence intervals (A) by tertiles of the Scale of Aging Vigor in Epidemiology (SAVE) and (B) by tertiles of the metabolite composite score among 287 Health ABC black men.
One standard deviation frailer SAVE score was associated with 30% higher mortality among the Health ABC black men, while adjusting for age and study site (p = .0002; Table 2). Supplementary Table S5 includes information on how the association between frailer SAVE scores and mortality was attenuated after further adjustment for metabolite(s), the metabolite composite score, or more commonly measured risk factors, in addition to age and study site. Adjusting for a single metabolite resulted in small attenuations (≤16%; Model 1). Forcing all 37 metabolites into the same model attenuated the association between frailer SAVE scores and mortality by 33% (Model 2). Applying a backward stepwise selection approach (p < .10 criterion) to the model containing all 37 metabolites while forcing SAVE scores, age, and study site, left 10 metabolites in the model (Model 3), which attenuated the association between frailer SAVE scores and mortality by 26%. When adjusting for the metabolite composite score, the association between SAVE scores and mortality was attenuated by 56% (Model 4). For comparison, adjusting for more commonly measured risk factors (education, smoking status, body mass index, and certain chronic conditions) attenuated the association between SAVE scores and mortality by 12% (Model 5). Adjusting for the metabolite composite score and more commonly measured risk factors resulted in the greatest amount of attenuation (63%) in the association between SAVE scores and mortality (Model 6), driven mostly by the metabolite composite score. The SAVE and more commonly measured risk factors minimally attenuated the association between the metabolite composite score and mortality; in this model, one standard deviation higher metabolite composite score was associated with 46% higher mortality (p < .0001; Model 6, Supplementary Table S5).
Table 2.
Mortality Hazard Ratio Per Standard Deviation Frailer Scale of Aging Vigor in Epidemiology (SAVE) Score and Hazard Ratio Per Standard Deviation Higher Metabolite Composite Score and Subscores Adjusting for Age and Study Site Among 287 Health ABC Black Men.
| Model | Mortality Hazard Ratio Per Standard Deviation (95% Confidence Interval), p Value | Percent Attenuationa |
|---|---|---|
| Model 1 | ||
| SAVE | 1.30 (1.14, 1.50), p = .0002 | Reference |
| Model 2 | ||
| SAVE | 1.12 (0.96, 1.31), p = .14 | 56% |
| Total metabolite composite score | 1.46 (1.25, 1.69), p < .0001 | — |
| Model 3 | ||
| SAVE | 1.19 (1.03, 1.38), p = .02 | 35% |
| Subscore of 14 organic acids/derivatives | 1.35 (1.17, 1.56), p < .0001 | — |
| Model 4 | ||
| SAVE | 1.23 (1.06, 1.42), p = .007 | 24% |
| Subscore of 12 lipids/lipid-like molecules | 1.25 (1.09, 1.44), p = .002 | — |
| Model 5 | ||
| SAVE | 1.26 (1.09, 1.46), p = .001 | 12% |
| Subscore of 4 organoheterocyclic compounds | 1.16 (1.01, 1.33), p = .03 | — |
| Model 6 | ||
| SAVE | 1.28 (1.11, 1.48), p = .0005 | 6% |
| Subscore of 3 benzenoids | 1.10 (0.96, 1.26), p = .19 | — |
| Model 7 | ||
| SAVE | 1.29 (1.12, 1.48), p = .0005 | 5% |
| Subscore of 4 remaining metabolitesb | 1.07 (0.93, 1.23), p = .33 | — |
| Model 8 | ||
| SAVE | 1.14 (0.98, 1.33), p = .08 | 50% |
| Subscore of 14 organic acids/derivatives | 1.31 (1.13, 1.52), p = .0003 | — |
| Subscore of 12 lipids/lipid-like molecules | 1.20 (1.05, 1.38), p = .009 |
.
bTwo organic nitrogen compounds, one organic oxygen compounds, and one nucleosides, nucleotides, and analogs.
We examined whether a specific chemical taxonomy superclass was driving the 56% attenuation in the association between frailer SAVE scores and mortality after adjusting for the metabolite composite score (Table 2). Adjusting for the metabolite subscore based on the 14 metabolites classified as organic acids/derivatives or the 12 metabolites classified as lipids/lipid-like molecules resulted in attenuations of 35% and 24%, respectively, in the age- and site-adjusted association between frailer SAVE scores and mortality. Adjusting for the other metabolite subscores minimally attenuated (≤12%) the association between SAVE scores and mortality. When adjusting for both the metabolite subscores containing organic acids/derivatives and lipids/lipid-like molecules (ie, 26 of the 37 metabolites), the association between frailer SAVE scores and mortality was attenuated by 50%, which accounted for almost the full amount of attenuation observed when adjusting for the total metabolite composite score.
Replication Cohort
The CHS All Stars were 85 years old on average, 62 (52%) were white women, 10 (8%) were black women, 46 (38%) were white men, and 2 (2%) were black men. The metabolite composite score significantly predicted mortality among the CHS All Stars; one standard deviation higher metabolite composite score was associated with 30% higher mortality, adjusting for age and gender (95% CI = 1.01, 1.67; p = .045). Since the metabolite composite score was developed among Health ABC black men, we also examined its association with mortality stratified by gender among the CHS All Stars. One standard deviation higher metabolite composite score was associated with 52% higher mortality among the 48 men, while adjusting for age (95% CI = 1.04, 2.22; p = .03); the association was not statistically significant among the 72 women (1.11 (0.78, 1.58); p = .57; and p value for interaction = 0.31).
The modified SAVE was skewed toward frailer scores in the CHS All Stars study (Supplementary Figure S1), and the mortality hazard ratio per standard deviation frailer score was 1.21 (0.96, 1.54); p = .11, adjusting for age and gender. The association between the modified SAVE and mortality in the CHS All Stars study changed very little after additionally adjusting for the metabolite composite score [1.20 (0.95, 1.52); p = .12]; in this same model, the metabolite composite score remained significantly associated with mortality [1.32 (1.02, 1.70); p = .04].
Discussion
We developed a novel metabolite composite score using information from 37 metabolites associated with the spectrum of vigor to frailty among older black men and found that the metabolite composite score attenuated the association between frailer SAVE scores and mortality by 56%. The metabolite composite score also significantly predicted mortality independent of age, study site, the SAVE, and more commonly measured risk factors, such as education, smoking status, body mass index, and chronic diseases. We also found that the metabolite composite score significantly predicted mortality in a replication sample of older adults.
Among the 37 metabolites included in our composite score, 70% were classified as organic acids/derivatives (eg, amino acids) or lipids/lipid-like molecules (eg, glycerophospholipids, sphingolipids). This subset of metabolites attenuated the higher mortality risk associated with frailty most prominently. Lower levels of most (9 of 12) lipids/lipid-like molecules included in our composite score were associated with frailer SAVE scores. This is congruent with prior studies of lipids among older adults (16). For example, one study reported low-density lipoprotein cholesterol in the lowest quartile (≤97.8 mg/dL) was associated with 90% higher mortality when compared with levels in the highest quartile (>144.0 mg/dL) (16). Low-density lipoprotein cholesterol appears to decline with age among older adults (17–19), decreasing by an average of 19 mg/dL over 15 years in a prospective cohort of community-dwelling Finnish adults aged ≥70 not taking lipid-lowering medications (19). Predictors of decline in lipids among older adults include male gender, older age, higher baseline white blood cell count, weight loss during follow-up (20), and potentially an increase in cytokines (21). Thus, acquired low levels of cholesterol have been suggested as a surrogate marker for frailty (16) and thought to be a result of chronic conditions and immune dysregulation (21).
In addition to certain lipids, we observed an inverse association between SAVE scores and amino acids involved in our composite score (ie, methionine, tyrosine, asparagine, leucine, histidine, and tryptophan). Metabolic pathways involving metabolism of lipids and amino acids are located in the mitochondrial matrix (22). Mitochondria are especially vulnerable to oxidative stress that can then cause damage to lipids and proteins (22). A previous report noted higher levels of oxidative stress among frailer older adults (23). Oxidative stress may be one common factor contributing to lower plasma amino acid and lipid levels among frailer Health ABC black men.
We also calculated the metabolite composite score in 120 participants from the CHS All Stars study, which included black and white men and women who were about 12 years older, on average, when compared with the 287 Health ABC black men. Even with these demographic differences, we still found a significant association between the metabolite composite score and mortality among the CHS All Stars. We also observed a similar pattern in the association between SAVE scores and mortality in the CHS All Stars, though the SAVE scores in this cohort were skewed toward the frailer end of the distribution.
Frailty is defined as a dynamic state at which physiologic reserves are so reduced that an individual cannot tolerate additional challenges, causing an increased vulnerability to disease, disability, and death (24). Dysregulated immune, endocrine, stress, and energy are thought to be involved in the pathophysiology of frailty (25). Metabolomics has the potential to identify new biomarkers and metabolic pathways for therapeutic targets to improve physiologic reserve and mitigate the associated vulnerabilities. To inform interventions aimed at alleviating symptoms of frailty, improving quality of life, and reducing frailty-associated mortality, more research is needed to determine the underlying mechanisms causing differences in metabolite profiles during a frail state and an understanding of how these metabolic differences can contribute to the frailty-associated higher risk of death.
A potential limitation of the current study was the unit-less LC-MS peak areas used for metabolite values, which do not provide information on whether values were outside a healthy range. Another potential limitation was lack of a measure of clinically manifested frailty. Instead, we only had a measure of vigor to frailty relative to all Health ABC participants, that is, a relatively healthy older adult cohort, recruited to be nondisabled. It may be that a Health ABC participant with a frail SAVE score may not actually appear frail when compared with a more general older adult population. Last, we were limited by the small number of CHS All Stars, which did not provide enough power to examine gender differences in the association between the metabolite composite score and mortality. There were several strengths of this study, including the well-characterized cohort with detailed mortality information available on every participant, plasma samples collected after overnight-fasting, information on known metabolites among a unique sample of older black men, and replicating the association between the metabolite composite score and mortality in an independent sample of older adults.
In this study, we developed a composite score of 37 metabolites that appeared to be a meaningful marker of the frailty-associated higher mortality among older black men from the Health ABC study. We validated the metabolite composite score against mortality among older adults from the CHS All Stars study. Future work needs to determine temporality between metabolite composite scores and vigor to frailty to better inform points of intervention that can have the largest and most sustainable positive impact among older adults. The identified set of plasma metabolites, especially those classified as organic acids/derivatives or lipids/lipid-like molecules appeared to provide a deeper characterization of frailty that attenuated a substantial portion of the frailty-associated higher vulnerability to death.
Supplementary Material
Funding
This work was supported by National Institute on Aging (NIA) contracts N01-AG-6–2101, N01-AG-6–2103, and N01-AG-6–2106, NIA grant (R01-AG028050), and National Institute of Nursing Research grant R01-NR012459. This work was also supported in part by the Intramural Research Program of the NIA. M.M.M. was supported by the Epidemiology of Aging training grant at the University of Pittsburgh: NIA (T32-AG00018129) and is now supported by the Cardiovascular Epidemiology training grant at the University of Pittsburgh: National Heart, Lung, and Blood Institute (T32-HL08382512). R.A.M. is supported through the Canadian Cancer Society (704735) and the Michael Smith Foundation for Health Research (17644).
Author Contributions
T.B.H. and A.B.N. were involved in the Health ABC study concept and design; S.C.M. and T.B.H. were involved in the metabolomics ancillary study; M.M.M. was involved in the data analysis; M.M.M. and A.B.N. were involved in manuscript writing; all authors were involved in the interpretation of data and manuscript critical review.
Conflict of Interest
The authors have no conflicts of interest to report.
References
- 1. He W, Goodkind D, Kowal PR. An Aging World: 2015. Washington, DC: United States Census Bureau; 2016. [Google Scholar]
- 2. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- 3. Barzilai N, Huffman DM, Muzumdar RH, Bartke A. The critical role of metabolic pathways in aging. Diabetes. 2012;61:1315–1322. doi: 10.2337/db11-1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. López-Otín C, Galluzzi L, Freije JMP, Madeo F, Kroemer G. Metabolic control of longevity. Cell. 2016;166:802–821. doi: 10.1016/j.cell.2016.07.031 [DOI] [PubMed] [Google Scholar]
- 5. Marron MM, Harris TB, Boudreau RM, et al. Metabolites associated with vigor to frailty among community-dwelling older black men. Metabolites. 2019;9:83. doi: 10.3390/metabo9050083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Newman AB, Haggerty CL, Goodpaster B, et al. ; Health Aging and Body Composition Research Group Strength and muscle quality in a well-functioning cohort of older adults: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2003;51:323–330. doi: 10.1046/j.1532-5415.2003.51105.x [DOI] [PubMed] [Google Scholar]
- 7. Murphy RA, Moore SC, Playdon M, et al. ; Health ABC Study Metabolites associated with lean mass and adiposity in older black men. J Gerontol A Biol Sci Med Sci. 2017;72:1352–1359. doi: 10.1093/gerona/glw245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Newman AB, Arnold AM, Sachs MC, et al. Long-term function in an older cohort—the Cardiovascular Health Study All Stars Study. J Am Geriatr Soc. 2009;57:432–440. doi: 10.1111/j.1532-5415.2008.02152.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fried LP, Borhani NO, Enright P, et al. The cardiovascular health study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w [DOI] [PubMed] [Google Scholar]
- 10. Xia J, Psychogios N, Young N, Wishart DS. MetaboAnalyst: a web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 2009;37(Web Server issue):W652–W660. doi: 10.1093/nar/gkp356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wei R, Wang J, Su M, et al. Missing value imputation approach for mass spectrometry-based metabolomics data. Sci Rep. 2018;8:663. doi: 10.1038/s41598-017-19120-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Newman AB, Boudreau RM, Naydeck BL, Fried LF, Harris TB. A physiologic index of comorbidity: relationship to mortality and disability. J Gerontol A Biol Sci Med Sci. 2008;63:603–609. doi: 10.1093/gerona/63.6.603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sanders JL, Boudreau RM, Fried LP, Walston JD, Harris TB, Newman AB. Measurement of organ structure and function enhances understanding of the physiological basis of frailty: the Cardiovascular Health Study. J Am Geriatr Soc. 2011;59:1581–1588. doi: 10.1111/j.1532-5415.2011.03557.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wishart DS, Tzur D, Knox C, et al. HMDB: the human metabolome database. Nucleic Acids Res. 2007;35(Database issue):D521–D526. doi: 10.1093/nar/gkl923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aalen O. A Model for Nonparametric Regression Analysis of Counting Processes. Mathematical Statistics and Probability Theory. New York, NY: Springer; 1980:1–25. [Google Scholar]
- 16. Schupf N, Costa R, Luchsinger J, Tang MX, Lee JH, Mayeux R. Relationship between plasma lipids and all-cause mortality in nondemented elderly. J Am Geriatr Soc. 2005;53:219–226. doi: 10.1111/j.1532-5415.2005.53106.x [DOI] [PubMed] [Google Scholar]
- 17. Upmeier E, Lavonius S, Heinonen P, et al. Longitudinal changes in serum lipids in older people the Turku elderly study 1991–2006. Age Ageing. 2011;40:280–283. doi: 10.1093/ageing/afq180 [DOI] [PubMed] [Google Scholar]
- 18. Abbott RD, Yano K, Hakim AA, et al. Changes in total and high-density lipoprotein cholesterol over 10- and 20-year periods (the Honolulu Heart Program). Am J Cardiol. 1998;82:172–178. doi: 10.1016/s0002-9149(98)00310-5 [DOI] [PubMed] [Google Scholar]
- 19. Ferrara A, Barrett-Connor E, Shan J. Total LDL, and HDL cholesterol decrease with age in older men and women. The Rancho Bernardo Study 1984–1994. Circulation. 1997;96:37–43. doi: 10.1161/01.cir.96.1.37 [DOI] [PubMed] [Google Scholar]
- 20. Manolio TA, Cushman M, Gottdiener JS, Dobs A, Kuller LH, Kronmal RA; CHS Collaborative Research Group Predictors of falling cholesterol levels in older adults: the Cardiovascular Health Study. Ann Epidemiol. 2004;14:325–331. doi: 10.1016/j.annepidem.2003.09.006 [DOI] [PubMed] [Google Scholar]
- 21. Ettinger WH Jr, Harris T, Verdery RB, Tracy R, Kouba E. Evidence for inflammation as a cause of hypocholesterolemia in older people. J Am Geriatr Soc. 1995;43:264–266. doi: 10.1111/j.1532-5415.1995.tb07334.x [DOI] [PubMed] [Google Scholar]
- 22. McCance KL, Huether SE. Pathophysiology: The Biologic Basis of Disease in Adults and Children. 7th ed.St. Louis, MO: Elsevier Mosby; 2014. [Google Scholar]
- 23. Inglés M, Gambini J, Carnicero JA, et al. Oxidative stress is related to frailty, not to age or sex, in a geriatric population: lipid and protein oxidation as biomarkers of frailty. J Am Geriatr Soc. 2014;62:1324–1328. doi: 10.1111/jgs.12876 [DOI] [PubMed] [Google Scholar]
- 24. Taffett GE. Physiology of Aging. Geriatric Medicine. New York, NY: Springer; 2003:27–35. [Google Scholar]
- 25. Kane AE, Sinclair DA. Frailty biomarkers in humans and rodents: Current approaches and future advances. Mech Ageing Dev. 2019;180:117–128. doi: 10.1016/j.mad.2019.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.