Abstract
Background
Muscle metrics derived from computed tomography (CT) are associated with adverse health events in older persons, but obtaining these metrics using current methods is not practical for large datasets. We developed a fully automated method for muscle measurement on CT images. This study aimed to determine the relationship between muscle measurements on CT with survival in a large multicenter trial of older adults.
Method
The relationship between baseline paraspinous skeletal muscle area (SMA) and skeletal muscle density (SMD) and survival over 6 years was determined in 6,803 men and 4,558 women (baseline age: 60–69 years) in the National Lung Screening Trial (NLST). The automated machine learning pipeline selected appropriate CT series, chose a single image at T12, and segmented left paraspinous muscle, recording cross-sectional area and density. Associations between SMA and SMD with all-cause mortality were determined using sex-stratified Cox proportional hazards models, adjusted for age, race, height, weight, pack-years of smoking, and presence of diabetes, chronic lung disease, cardiovascular disease, and cancer at enrollment.
Results
After a mean 6.44 ± 1.06 years of follow-up, 635 (9.33%) men and 265 (5.81%) women died. In men, higher SMA and SMD were associated with a lower risk of all-cause mortality, in fully adjusted models. A one-unit standard deviation increase was associated with a hazard ratio (HR) = 0.85 (95% confidence interval [CI] = 0.79, 0.91; p < .001) for SMA and HR = 0.91 (95% CI = 0.84, 0.98; p = .012) for SMD. In women, the associations did not reach significance.
Conclusion
Higher paraspinous SMA and SMD, automatically derived from CT exams, were associated with better survival in a large multicenter cohort of community-dwelling older men.
Keywords: Sarcopenia, Myosteatosis, Mortality, Computed tomography, Machine learning
Maintenance of skeletal muscle quantity and quality is an essential component of physical health of older adults (1,2). For over two decades, noninvasive measurement of muscle mass in large observational studies was performed using dual x-ray absorptiometry (DXA) (3–12). DXA-derived appendicular lean mass has been associated with various biomarkers of aging in the Health Aging and Body Composition Study (5,7), Osteoporotic Fractures in Men Study (8,9), and National Health and Nutrition Examination Surveys (NHANES) (10–12). However, recent data from the Sarcopenia Definitions and Outcomes Consortium (13) brought the value of DXA measurements of muscle in older adults into question, concluding that these were poor predictors of mobility disability.
In contrast to DXA, the use of computed tomography (CT) for measuring muscle has become more common in studies of older adults (14–26). However, most of the studies evaluating CT-derived muscle metrics and mortality in older adults have been convenience samples in hospitalized patients with higher comorbidities (14–26). In a recent pragmatic evaluation of community-dwelling Medicare recipients, Lenchik et al. (27) reported that higher CT-derived skeletal muscle index (SMI) and skeletal muscle density (SMD) were associated with improved overall survival even after adjusting for multiple comorbidities. However, this study and most others (26,27) used manual or semi-automated approaches for segmenting muscles on CT images, a process that is time-consuming and unrealistic for large research datasets and clinical practice.
Automated segmentation of medical images has benefited greatly from machine learning algorithms, promising to transform large-scale research and eventually improve patient care. In a recent systematic review, Lenchik et al. (28) reported on 408 studies of automated segmentation of CT or MR images. Most of these studies involved segmentations of the heart on CT images or the brain on MR images (28). Only a handful of studies used automated segmentation of skeletal muscle (28). More importantly, while some centers have already implemented automated pipelines for neuroimaging, such pipelines for musculoskeletal imaging have not previously been reported.
We developed the first automated pipeline for skeletal muscle measurement based on open-source machine learning methods (29,30). Our pipeline selects the appropriate chest CT series, chooses a single CT image at the level of T12 vertebra, and segments the left paraspinous muscle, recording the muscle cross-sectional area (a measure of muscle mass) and muscle density (a measure of myosteatosis).
The purpose of our study was to determine whether the automated measurement of paraspinous skeletal muscle area (SMA) and SMD on chest CT examinations can be used to predict survival in a large cohort of community-dwelling older adults evaluated at 33 different medical centers across the United States followed for over 6 years. We hypothesized that higher paraspinous SMA and SMD would be associated with improved survival.
Method
This retrospective cohort study was conducted on data from the National Lung Screening Trial (NLST). NLST is one of the largest datasets from which CT images and mortality data are publically available from the National Cancer Institute (31,32). The NLST enrolled 53,454 participants, aged 55–74 years, at 33 medical centers in the United States, from August 2002 to April 2004 (31). Subjects were randomly assigned to a chest CT scan arm (n = 26,722) or chest x-ray arm (n = 26,732) (31). Cancers and deaths were recorded through December 31, 2009 (31). Although lung cancer was the leading cause of death in the chest x-ray arm (n = 503), the reduction in lung cancer deaths in the CT arm (n = 427) resulted in cardiovascular disease becoming the leading cause of death for the CT arm (n = 486) and the entire study (n = 956) (32). For the current study, a subgroup of older participants (age 60–69 years at enrollment) in the CT arm was examined to increase the prevalence of low SMA and SMD. The oldest NLST subgroup (baseline age, 70 years and older) was used to develop and train the automated CT segmentation algorithm and was excluded. CT scans were acquired using scanners from all four major manufacturers (General Electric [n = 6,281], Siemens [n = 3,336], Philips [n = 1,115], Toshiba (n = 643]) using an unenhanced, ungated, low-dose CT protocol. CT acquisition parameters were: 120 kVp, 40–80 mAs (depending on patient size), 1.0–2.5 mm slice thickness, and soft tissue reconstruction algorithm.
Automated Muscle Measurements
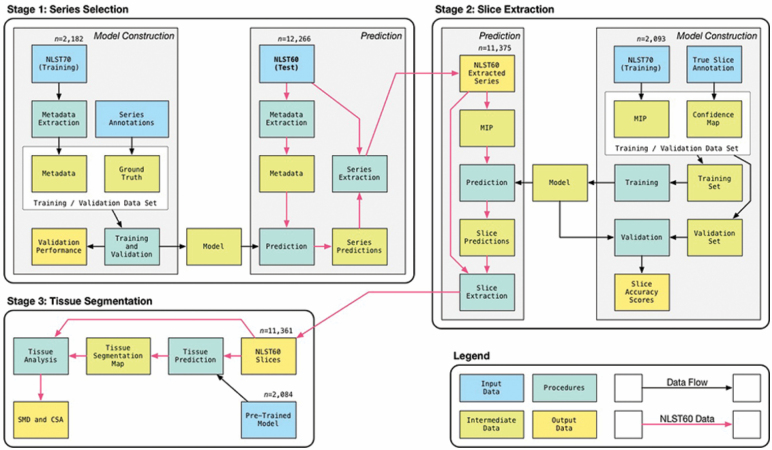
Using open-source machine learning tools, we developed a fully automated pipeline for obtaining muscle measurements on chest CT exams. The pipeline uses three sequential processing stages (Figure 1). The first stage uses a random forest classifier to analyze metadata from the raw DICOM files to select and extract the appropriate CT series. The second stage uses a convolutional neural network to localize and extract the CT slices at the level of the T12 vertebra. The third stage applies another convolutional neural network to the CT image extracted during the second stage to segment the left paraspinous muscle and record SMA and SMD. The automated pipeline was developed, trained, and tested on a separate set of CT examinations from the current study. The training set comprised of 2,084 exams from an older subgroup of NLST (70 years and older at enrollment). Further details on the automated CT analysis methods are provided in the Supplementary Appendix.
Figure 1.
Diagram of the automated machine learning pipeline shows three stages used to derive muscle metrics from computed tomography (CT) images: (a) series selection, (b) slice extraction, and (c) tissue segmentation.
Mortality
Mortality was provided by the National Cancer Institute. For participants confirmed as deceased, the length of follow-up was determined from the date of the initial study visit to date of death. For living participants, the length of follow-up was determined from the date of the initial study visit to December 31, 2009. More recent mortality data were not available from the National Cancer Institute. All covariates used in the current study were obtained from the National Cancer Institute.
Statistical Analysis
For participant characteristics and skeletal muscle measures, summary statistics by sex were determined as counts and percentages for categorical variables and as means and standard deviations for continuous variables. For non-normally distributed continuous variables, medians, first quartiles, and third quartiles were additionally determined. Two predictor variables of interest were considered: (a) baseline paraspinous SMA and (b) baseline paraspinous SMD. Unadjusted Cox proportional hazards models were used to evaluate the association between mortality and each characteristic.
The interactions between sex and CT-derived muscle metrics were determined. While interactions between sex and skeletal muscle index were significant (p = .017–.045), those between sex and SMA or SMD were not (p = .2–.3). However, because many prior clinical and experimental studies have shown sex differences in muscle metabolism and its effect on longevity, all of our analyses were stratified by sex (16,33–37).
The sex-specific standardized muscle measures were evaluated for relative importance. Kaplan–Meier survival curves were constructed to examine patterns of mortality for baseline SMA and SMD. The survival curves for each muscle measure were stratified as follows: standardized muscle measure ≤−1, between −1 and 1, and >1. To examine how the survival curves changed by considering the two muscle measures together, the survival curves were stratified into three categories: either standardized muscle measure ≤−1, both between −1 and 1, and either >1. The log-rank test was used to compare survival curves. Pairwise comparisons for the log-rank test were performed based on Tukey’s studentized range test.
Cox proportional hazards models were used to examine the relationships between muscle metrics and all-cause mortality. Five models were used: (a) unadjusted; (b) adjusted for age, race, and pack-years of smoking; (c) adjusted for Model 2 plus height (3A) or weight (3B); (d) adjusted for Model 3 plus presence at enrollment of type 2 diabetes (T2D), chronic lung disease, cardiovascular disease, and cancer (4A adjusted for height, 4B adjusted for weight); and (e) adjusted for Model 4 plus the other muscle metric (ie, SMA or SMD) of interest (5A adjusted for height, 5B adjusted for weight).
To check the potential nonlinearity association between the muscle metrics and mortality, the square term and cubic term of muscle metrics were included in Models 4A and 4B. If the polynomial term was not significant, it was removed from the model.
Analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC). Statistical significance was set at p < .025 after Bonferroni correction for two primary muscle measures (ie, SMA and SMD).
Results
The NLST cohort of 6,803 men and 4,558 women was followed for 6.44 ± 1.06 years. Tables 1 and 2 present demographic characteristics, muscle metrics, and survival data in men and women. In men and women, compared to the survivor group, the deceased group had a significantly higher pack-years of smoking, T2D, chronic lung disease, and cardiovascular disease.
Table 1.
Participant Characteristics at Baseline in 6,803 Men, Aged 60–69 Years, From the CT Arm of the National Lung Screening Trial
| Characteristic | All (n = 6,803) | Survivors (n = 6,168) | Deceased (n = 635) | HR (95% CI) | p-Value |
|---|---|---|---|---|---|
| Age ± SD (y) | 63.7 ± 2.8 | 63.6 ± 2.8 | 64.4 ± 2.9 | 1.10 (1.07, 1.13) | <.001 |
| BMI ± SD (kg/m2) | 27.9 ± 4.5 | 27.9 ± 4.3 | 28.0 ± 5.4 | 1.00 (0.99, 1.02) | .711 |
| Caucasian | 6,237 (92.0) | 5,661 (92.1) | 576 (91.0) | 0.87 (0.66, 1.14) | .309 |
| Pack-years of smoking | 61.7 ± 26.8 53 (43, 75)a | 61.1 ± 26.6 53 (42, 74)a | 67.6 ± 28.2 60 (46, 83)a | 1.01 (1.01, 1.01) | <.001 |
| Type 2 diabetes | 812 (12.0) | 699 (11.4) | 113 (17.8) | 1.64 (1.34, 2.02) | <.001 |
| Chronic lung disease | 1,656 (24.4) | 1,454 (23.6) | 202 (31.8) | 1.48 (1.25, 1.75) | <.001 |
| Cardiovascular disease | 3,274 (48.1) | 2,924 (47.4) | 350 (55.1) | 1.35 (1.15, 1.57) | .002 |
| Cancer | 134 (2.0) | 119 (1.9) | 15 (2.4) | 1.20 (0.72, 2.01) | .477 |
| Skeletal muscle area (cm2) | 16.0 ± 3.50 | 16.1 ± 3.48 | 15.3 ± 3.61 | 0.82 (0.76, 0.88)b | <.001 |
| Skeletal muscle density (HU) | 49.1 ± 7.0 | 49.2 ± 6.9 | 48.4 ± 7.6 | 0.90 (0.84, 0.98)b | .010 |
Notes: BMI = body mass index; CI = confidence interval; CT = computed tomography; HR = hazard ratio; HU = Hounsfield units. Mean ± SD for continuous variables; n (%) for categorical variables. p-values are calculated using the unadjusted Cox proportional hazards model.
aMedian (first quartile, third quartile).
bHR per SD.
Table 2.
Participant Characteristics at Baseline in 4,558 Women, Aged 60–69 Years, From the CT Arm of the National Lung Screening Trial
| Characteristic | All (n = 4,558) | Survivors (n = 4,293) | Deceased (n = 265) | HR (95% CI) | p-Value |
|---|---|---|---|---|---|
| Age ± SD (y) | 63.6 ± 2.8 | 63.5 ± 2.8 | 64.2 ± 2.7 | 1.08 (1.03, 1.13) | <.001 |
| BMI ± SD (kg/m2) | 27.2 ± 5.4 | 27.2 ± 5.3 | 27.3 ± 6.9 | 1.00 (0.98, 1.03) | .703 |
| Caucasian | 4,193 (92.2) | 3,956 (92.3) | 237 (89.4) | 0.69 (0.47, 1.03) | .066 |
| Pack-years of smoking | 52.7 ± 21.0 46 (39, 62)a | 52.4 ± 20.9 45 (39, 62)a | 57.2 ± 22.1 50 (43, 68)a | 1.01 (1.00, 1.01) | <.001 |
| Type 2 diabetes | 373 (8.2) | 338 (7.9) | 35 (13.3) | 1.79 (1.26, 2.56) | .001 |
| Chronic lung disease | 1,400 (30.7) | 1,298 (30.2) | 102 (38.5) | 1.43 (1.12, 1.84) | .004 |
| Cardiovascular disease | 1,929 (42.3) | 1,802 (42.0) | 127 (47.9) | 1.28 (1.01, 1.63) | .046 |
| Cancer | 364 (8.0) | 333 (7.8) | 31 (11.7) | 1.54 (1.06, 2.24) | .024 |
| Skeletal muscle area (cm2) | 11.9 ± 2.50 | 11.9 ± 2.49 | 11.8 ± 2.70 | 0.97 (0.86, 1.09)b | .564 |
| Skeletal muscle density (HU) | 42.7 ± 7.3 | 42.7 ± 7.3 | 42.6 ± 8.0 | 0.99 (0.88, 1.12)b | .843 |
Notes: BMI = body mass index; CI = confidence interval; CT = computed tomography; HR = hazard ratio; HU = Hounsfield units. Mean ± SD for continuous variables; n (%) for categorical variables. p-values are calculated using the unadjusted Cox proportional hazards model.
aMedian (first quartile, third quartile).
bHR per SD.
Relationships between CT-derived baseline paraspinous SMA and all-cause mortality in men and women are given in Table 3. In men, increased SMA was associated with decreased all-cause mortality in all models. Importantly, adjusting for SMD did not reduce the magnitude of the association, indicating that SMA and SMD are independent predictors of mortality. Each standard deviation (3.5 cm2) increase in SMA was associated with a 17% decrease in mortality. In women, the association between SMA and mortality was not significant.
Table 3.
Association Between CT-Derived Baseline Paraspinous Skeletal Muscle Area and All-Cause Mortality in 6,803 Men and 4,558 Women, Aged 60–69 Years, From the CT Arm of the National Lung Screening Trial
| Skeletal Muscle Area | HR per SD (95% CI) | p-Value | |
|---|---|---|---|
| Men (SD = 3.50 cm2) | |||
| Model 1 | Unadjusted | 0.82 (0.76, 0.88) | <.001 |
| Model 2 | Adjusted for age, race, pack-years of smoking | 0.85 (0.79, 0.92) | <.001 |
| Model 3A | Adjusted for Model 2 and height | 0.85 (0.78, 0.91) | <.001 |
| Model 3B | Adjusted for Model 2 and weight | 0.85 (0.79, 0.91) | <.001 |
| Model 4A | Adjusted for Model 3A and type 2 diabetes, chronic lung disease, cardiovascular disease, and cancer | 0.85 (0.79, 0.91) | <.001 |
| Model 4B | Adjusted for Model 3B and type 2 diabetes, chronic lung disease, cardiovascular disease, and cancer | 0.85 (0.79, 0.92) | <.001 |
| Model 5A | Adjusted for Model 4A and skeletal muscle density | 0.83 (0.77, 0.90) | <.001 |
| Model 5B | Adjusted for 4B and skeletal muscle density | 0.83 (0.77, 0.90) | <.001 |
| Women (SD = 2.50 cm2) | |||
| Model 1 | Unadjusted | 0.97 (0.86, 1.09) | .564 |
| Model 2 | Adjusted for age, race, pack-years of smoking | 0.97 (0.86, 1.09) | .620 |
| Model 3A | Adjusted for Model 2 and height | 0.98 (0.87, 1.11) | .781 |
| Model 3B | Adjusted for Model 2 and weight | 0.97 (0.86, 1.10) | .680 |
| Model 4A | Adjusted for Model 3A and type 2 diabetes, chronic lung disease, cardiovascular disease, and cancer | 0.97 (0.85, 1.09) | .560 |
| Model 4B | Adjusted for Model 3B and presence of type 2 diabetes, chronic lung disease, cardiovascular disease, and cancer | 0.97 (0.86, 1.09) | .595 |
| Model 5A | Adjusted for Model 4A and skeletal muscle density | 0.96 (0.85, 1.09) | .541 |
| Model 5B | Adjusted for Model 4B and skeletal muscle density | 0.97 (0.85, 1.09) | .583 |
Note: CI = confidence interval; CT = computed tomography; SD = standard deviation; HR = hazard ratio; BMI = body mass index. p-values are calculated using the Cox proportional hazards model.
Relationships between baseline paraspinous SMD and all-cause mortality in men and women are given in Table 4. In men, increased SMD was associated with decreased all-cause mortality in all models. Adjusting for SMA did not reduce the magnitude of the association, indicating that SMA and SMD are independent predictors of mortality. Each standard deviation (6.99 HU) increase in SMD was associated with an 11% decrease in mortality. In women, the association between SMD and mortality was not significant.
Table 4.
Association Between CT-Derived Baseline Paraspinous Skeletal Muscle Density and All-Cause Mortality in 6,803 Men and 4,558 Women, Aged 60–69 Years, From the CT Arm of the National Lung Screening Trial
| Skeletal Muscle Density | HR per SD (95% CI) | p-Value | |
|---|---|---|---|
| Men (SD = 6.99 HU) | |||
| Model 1 | Unadjusted | 0.90 (0.84, 0.98) | .010 |
| Model 2 | Adjusted for age, race, pack-years of smoking | 0.91 (0.85, 0.99) | .023 |
| Model 3A | Adjusted for Model 2 and height | 0.91 (0.84, 0.98) | .018 |
| Model 3B | Adjusted for Model 2 and weight | 0.90 (0.83, 0.98) | .011 |
| Model 4A | Adjusted for Model 3A and type 2 diabetes, chronic lung disease, cardiovascular disease, and cancer | 0.91 (0.84, 0.98) | .012 |
| Model 4B | Adjusted for Model 3B and type 2 diabetes, chronic lung disease, cardiovascular disease, and cancer | 0.91 (0.84, 0.98) | .017 |
| Model 5A | Adjusted for Model 4A and skeletal muscle area | 0.89 (0.83, 0.96) | .002 |
| Model 5B | Adjusted for 4B and skeletal muscle area | 0.89 (0.82, 0.96) | .002 |
| Women (SD = 7.31 HU) | |||
| Model 1 | Unadjusted | 0.99 (0.88, 1.12) | .843 |
| Model 2 | Adjusted for age, race, pack-years of smoking | 1.00 (0.88, 1.13) | .962 |
| Model 3A | Adjusted for Model 2 and height | 1.00 (0.89, 1.13) | .997 |
| Model 3B | Adjusted for Model 2 and weight | 1.00 (0.89, 1.14) | .964 |
| Model 4A | Adjusted for Model 3A and type 2 diabetes, chronic lung disease, cardiovascular disease, and cancer | 0.98 (0.87, 1.11) | .754 |
| Model 4B | Adjusted for Model 3B and presence of type 2 diabetes, chronic lung disease, cardiovascular disease, and cancer | 0.99 (0.88, 1.12) | .910 |
| Model 5A | Adjusted for Model 4A and skeletal muscle area | 0.98 (0.87, 1.10) | .714 |
| Model 5B | Adjusted for Model 4B and skeletal muscle area | 0.99 (0.87, 1.12) | .857 |
Note: CI = confidence interval; CT = computed tomography; SD = standard deviation; HR = hazard ratio; HU = Hounsfield units; BMI = body mass index. p-Values are calculated using the Cox proportional hazards model.
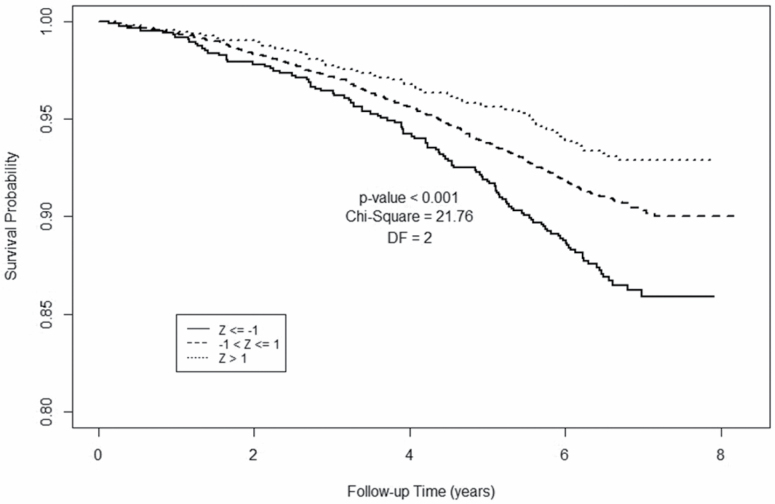
Figure 2 shows Kaplan–Meier survival analyses for paraspinous SMA in men. Compared to men at least 1 SD below the cohort median SMA, men with higher SMA had more favorable survival at each time point. Pairwise comparison for the log-rank test based on Tukey’s studentized range test shows that the survival probability for Z > 1 is significantly different from Z ≤ −1 (p < .001, χ 2 = 20.72, df = 1) and −1 < Z ≤ 1 is also significantly different from Z ≤ −1 (p = .039, χ 2 = 5.97, df = 1). However, the survival probability for −1 < Z ≤ 1 is not statistically different from Z > 1 (p = .65, χ 2 = 0.78, df = 1).
Figure 2.
Kaplan–Meier plot for 6,803 men, aged 60–69 years, from the National Lung Screening Trial, based on computed tomography (CT)-derived skeletal muscle area. Survival is compared among three groups stratified by baseline skeletal muscle area: Z ≤ −1; Z > −1 and Z ≤ 1; Z > 1.
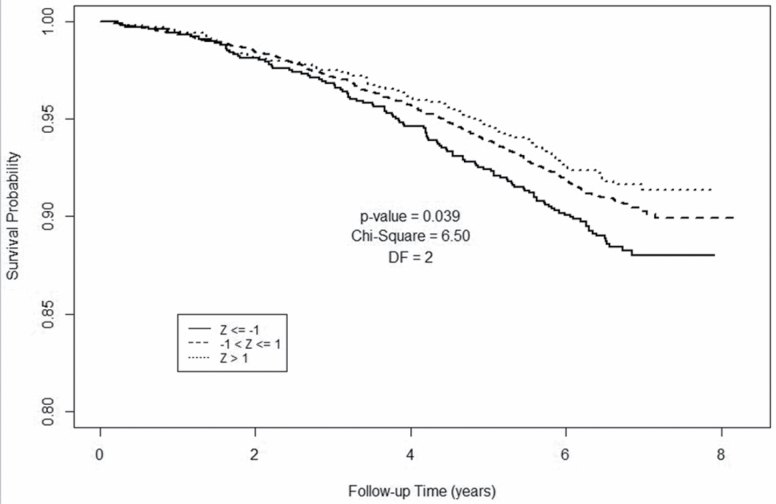
Figure 3 shows Kaplan–Meier survival analyses for paraspinous SMD in men. Compared to men at least 1 SD below the cohort median SMD, men with higher SMD had more favorable survival at each time point. Pairwise comparison for the log-rank test based on Tukey’s studentized range test shows that the survival probability for Z > 1 is significantly different from Z ≤ −1 (p = .037, χ 2 = 6.04, df = 1). However, the survival probability for −1 < Z ≤ 1 is not statistically different from Z > 1 (p = .94, χ 2 = 0.11, df = 1) or Z ≤ −1 (p = .29, χ 2 = 2.25, df = 1).
Figure 3.
Kaplan–Meier plot for 6,803 men, aged 60–69 years, from the National Lung Screening Trial, based on computed tomography (CT)-derived skeletal muscle density. Survival is compared among three groups stratified by baseline skeletal muscle density: Z ≤ −1; Z > −1 and Z ≤ 1; Z > 1.
Figure 4 shows the combined effect of SMA and SMD on overall survival. At each time point, men with higher SMA or SMD had more favorable survival, while men with lower SMA or SMD had less favorable survival. Pairwise comparison for the log-rank test based on Tukey’s studentized range test shows that the survival probability for Z > 1 is significantly different from Z ≤ −1 (p < .001, χ 2 = 23.28, df = 1). However, the survival probability for −1 < Z ≤ 1 is not statistically different from Z > 1 (p = .086, χ 2 = 4.50, df = 1) or Z ≤ −1 (p = .14, χ 2 = 3.56, df = 1).
Figure 4.
Kaplan–Meier plot for 6,803 men, aged 60–69 years, from the National Lung Screening Trial, based on both skeletal muscle area and skeletal muscle density. Survival is compared among three groups stratified by baseline skeletal muscle area (SMA) and skeletal muscle density (SMD): either SMA or SMD measure Z ≤ −1; both SMA and SMD Z > −1 and Z ≤ 1; and either SMA or SMD Z > 1.
Discussion
The novel finding in this cohort of 11,361 older adults, with over 6 years of follow-up and CT imaging at 33 different sites, is that higher baseline paraspinous SMA and higher baseline SMD were associated with better survival in men, but not women. The association of CT-derived muscle metrics with survival persisted after adjusting for age, race, smoking, cancer history, and other comorbidities. Importantly, after adjusting for SMD (a measure of myosteatosis), SMA (a measure of muscle mass) remained predictive of mortality. Similarly, after adjusting for SMA, SMD remained predictive of survival. This indicates that muscle mass and myosteatosis contribute independently to survival in older adults.
Our results on the relationship between CT-derived muscle mass and all-cause mortality are similar to another study of community-dwelling adults, the Diabetes Heart Study (DHS). In two separate cohorts of the DHS (ie, African American and European American), CT measurements of skeletal muscle index (SMI) were performed on paraspinous and psoas muscles. In African Americans with T2D with over 7 years of follow-up, Murea et al. (34) reported an association between baseline paraspinous SMI (hazard ratio [HR] =0.64; p = .004) and psoas SMI (HR = 0.61; p = .004) with all-cause mortality in men, but not women. Similar sex differences were observed in our study. In European Americans with T2D with over 11 years of follow-up, Tucker et al. (38) reported an association between psoas SMI (HR = 0.82; p = .008) and all-cause mortality, but not paraspinous SMI. In that study, the results did not differ significantly between men and women. Unlike our multicenter study, both DHS cohorts were enrolled at a single-center, had slightly younger participants (DHS-AA median age: 56 years; DHS-EA median age: 63 years), had a longer follow-up period, and used a manual approach to measuring muscle (ie, using clinical PACS software at the L4 level). Future longitudinal studies will be needed to confirm the relationship between CT-derived muscle mass and survival in older adults.
Our results on the relationship between CT-derived SMD (a surrogate of myosteatosis) and all-cause mortality compare favorably with the DHS-EA and the Framingham Heart Study. In 839 European Americans with T2D in DHS-EA (38), paraspinous SMD (HR = 0.85, p = .003) and psoas SMD (HR = 0.81, p < .001) were associated with all-cause mortality. However, in 570 African Americans with T2D in DHS-AA, SMD was not associated with mortality (34). In the Framingham Heart Study, Shah et al. (39) reported on the association of CT-derived metrics and health outcomes in 2,924 participants (median age, 50 years). Their principal component analysis of 11 different CT measurements showed that SMD was associated with mortality (39). Although the inconsistency among studies of CT-derived SMD and mortality may be explained in part by different study populations and measurement methods, the underlying mechanisms of how increased fatty infiltration of muscle contributes to decreased survival is an area of active research (35,40).
Unlike our study that evaluated axial (ie, paraspinous) muscle metrics, most other large studies in community-dwelling adults have evaluated appendicular muscle metrics, typically in the thigh or calf. These CT-derived muscle metrics have been associated with all-cause mortality in the Age, Gene/Environment Susceptibility (AGES)-Reykjavik Study (41), Health Aging and Body Composition Study (42), Osteoporotic Fractures in Men Study (43), and Tobago Health study (44). In the Age, Gene/Environment Susceptibility (AGES)-Reykjavik Study, Reinders et al. (41) reported on the association of muscle metrics and mortality in 4,824 participants (median age, 76 years) followed for over 8 years. CT-derived SMA and SMD of the thigh were associated with mortality in men and women. In a longitudinal analysis of thigh CT muscle measurements obtained 5 years apart in Health Aging and Body Composition Study, Santansato et al. (42) reported an association between the loss of muscle mass and gain of intermuscular adipose tissue with increased all-cause mortality. In Osteoporotic Fractures in Men Study, Miljkovic et al. (43) reported an association SMD of the calf and mortality in 1,063 men (median age, 77 years) followed for over 7 years. Similarly, in the Tobago Health study, Zhao et al. (44) reported an association of calf SMD and mortality in 1,652 men (median age, 57 years) followed for over 5 years. In sum, these four studies using appendicular muscle metrics appear to support our current study using axial muscle metrics. This is significant because calf and thigh muscle metrics typically serve as surrogates of lower extremity muscle function, while axial metrics are more indicative of global muscle function (45). Improved survival in older adults appears to depend on the preservation of lower extremity as well as truncal muscle groups.
Our results confirm the sex-specific differences in the association of CT-derived muscle metrics and mortality in several prior studies. In DHS-AA, the association between SMI and mortality was seen in men, but not women (34). Similarly, in a study of orthopedic trauma patients, Touban et al. (16) reported on the association between CT-derived psoas SMA and all-cause mortality in 558 older adults (median age, 77 years) followed for 1 year. In men, increased psoas SMA was associated with decreased all-cause mortality (HR = 0.89; confidence interval [CI] = 0.74, 0.96; p < .002), but in women the association was not significant (p = .103) (16). In a study of hospitalized geriatric patients, Perkisas et al. (33) reported an association between CT-derived intermuscular adipose tissue of the thigh and mortality in men, but not women. The mechanisms underlying the male-specific association between CT-derived muscle metrics and mortality are not well established, but research points to sex differences between hormonal actions, muscle fiber composition, and mitochondrial function that may impact survival (35). For example, in the Framingham Heart Study, sarcopenia appeared to reflect a withdrawal of anabolic stimuli (eg, growth hormone) in men, but an increase in catabolic stimuli (eg, IL-6) in women (46). Besides these differences, men and women may also differ in their physical activity profiles, dietary habits, psychological health, and social behaviors.
Most prior CT studies of muscle have used abdominal CTs and measured various muscles or muscle groups at the level of L3 or L4 (26). Studies using chest CTs are far less common. Our study results at the level of T12 are consistent with a much smaller study of 274 hip fracture patients followed for over 8 years, where lower paraspinous SMI and SMD were associated with increased all-cause mortality (47).
Studies in community-dwelling older adults have shown that strength and physical performance decline more rapidly than does CT-derived muscle area (48). Weakness and physical performance have consistently been associated with increased risk of mortality, mobility limitations, disability, falls, fractures, and hospitalizations in older adults (49–56). Our evidence and the results of other studies support a relation between CT-derived muscle metrics and mortality in older adults. Taken together, these results raise an important question. What measures should be included in research studies of older adults: (a) CT-derived muscle size and density and/or (b) gait speed and grip strength? To a large extent, the answer depends on feasibility. With our automated approach to measuring muscle size and density on CT images, processing CT scans obtained for other purposes to derive these measures is relatively simple and inexpensive.
Future use of CT in studies of older adults will depend on how quickly such automated approaches to image processing become available. In a recent systematic review, Amini et al. (26) reported on 388 studies that measured muscle mass or myosteatosis using CT. Only 6 of 388 (1.5%) studies used automated methods for muscle segmentation, and none had used an end-to-end pipeline for selecting the correct series, extracting the correct CT slice, and performing muscle measurements (26). This is the first study of such an automated pipeline for skeletal muscle measurements. The automation allowed us to conduct the largest multicenter study to date, evaluating the relationship between CT-derived muscle metrics and mortality in community-dwelling older adults.
Clinical Relevance
The clinical utility of CT compared to DXA for evaluating muscle mass in older adults warrants mention. In an analysis of 5,934 community-dwelling men (65 years and older) from Osteoporotic Fractures in Men Study, three widely used DXA definitions of sarcopenia were evaluated. Importantly, very small improvements in the C-statistic for predicting mortality using DXA were reported, compared to a reference model using age alone (57). In contrast, a study of 1,326 cancer surgery patients showed that CT-derived muscle mass predicted 1-year mortality (C-statistic = 0.70) better than the modified Frailty Index (C-statistic = 0.55) and the Eastern Cooperative Oncology Group performance score (C-statistic = 0.57) (58). These results, together with more recent data from Sarcopenia Definitions and Outcomes Consortium (13) and the results of our current study, suggest that CT may be preferable to DXA for the evaluation of muscle health in older adults.
The clinical utility of chest rather than abdominopelvic CT exams for analysis of muscle health also merits mention. The lung cancer screening recommendations from the United States Preventive Services Task Force (USPSTF) and Centers for Medicare and Medicaid Services (CMS) were developed based on the NLST data (59). Since then, increasing numbers of smokers, aged 55 years and older, are undergoing screenings using low-dose chest CT scans. To increase the cost-effectiveness of these screenings, some investigators have proposed opportunistic measurements of coronary artery calcium (60), chronic obstructive pulmonary disease (61), and bone mineral density (62). Cressman et al. (63) reported that the cost-effectiveness of lung cancer screening was predominantly driven by non-lung cancer outcomes. Similarly, there is potential for muscle screening to be performed on the same chest CTs that are used for lung cancer screening. As the number of chest CTs performed in older adults increases, such evaluation of muscle metrics may help improve the cost-effectiveness of lung screening programs while at the same time providing valuable prognostic information about overall survival.
Strengths and Limitations
Our study has several limitations. This was a retrospective cohort study that may introduce bias concerning the causal chain between muscle metrics and mortality. The NLST did not collect physical function measurements or lean mass measurements using DXA.
Our study also has several important strengths. This is the largest multicenter study using CT-derived measures of skeletal muscles in community-dwelling older adults. The CT examinations were acquired at 33 medical centers, using CT scanners from four different manufacturers. Unlike most prior studies, we measured muscle attenuation on chest CTs at T12 rather than abdominopelvic CTs. This has broad generalizability since the T12 level is included in the field of view of both chest CTs and abdominopelvic CTs.
Perhaps the greatest strength of our study is that all 11,361 chest CT examinations were evaluated using an open-source, fully automated, image processing pipeline. For researchers, the value of such a pipeline cannot be overstated. In most prior studies using CT to evaluate skeletal muscles, the correct CT series, the correct CT image, and the correct muscle group were chosen individually by the researcher in a very time-consuming process. After that, even more time was spent either on manual or semi-automated segmentation of various muscles to obtain the desired muscle metrics. With the increasing size and complexity of multicenter studies, the burden of such image analysis becomes overwhelming, which motivated us to develop an automated solution. Our results indicate that such an automated pipeline can easily be applied to a multicenter study of older adults with a heterogeneous set of CT images acquired on various scanners. Soon, our pipeline will be adapted to evaluate other muscle groups, including commonly measured total abdominal muscles at the level of L3, as well as other tissues, including visceral, subcutaneous, and intermuscular adipose tissue.
Conclusion
This is the first large study of community-dwelling older adults where CT-derived muscle mass and muscle density showed a robust association with all-cause mortality. Our study confirms that CT-derived muscle metrics are useful prognostic biomarkers in older adults. In the future, a better understanding of the relationships between these muscle metrics and other health outcomes should help target interventions to improve the health of older adults.
Supplementary Material
Funding
This work was supported by National Institutes of Health (NIH) Grants: P30 AG021332, UL1TR001420, K25 AG058804.
Conflict of Interest
None reported.
References
- 1. Fanning J, Rejeski WJ, Chen SH, et al. ; LIFE Study Investigators A case for promoting movement medicine: preventing disability in the life randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2019;74(11):1821–1827. doi: 10.1093/gerona/glz050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Santanasto AJ, Marron MM, Boudreau RM, et al. Prevalence, incidence and risk factors for overall, physical and cognitive independence among those from exceptionally long-lived families: the long life family study. J Gerontol A Biol Sci Med Sci. 2020;75:899–905. doi: 10.1093/gerona/glz124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boutin RD, Yao L, Canter RJ, Lenchik L. Sarcopenia: current concepts and imaging implications. AJR Am J Roentgenol. 2015;205(3):W255–W266. doi: 10.2214/AJR.15.14635 [DOI] [PubMed] [Google Scholar]
- 4. Lenchik L, Boutin RD. Sarcopenia: beyond muscle atrophy and into the new frontiers of opportunistic imaging, precision medicine, and machine learning. Semin Musculoskelet Radiol. 2018;22(3):307–322. doi: 10.1055/s-0038-1641573 [DOI] [PubMed] [Google Scholar]
- 5. Murphy RA, Ip EH, Zhang Q, et al. ; Health, Aging, and Body Composition Study Transition to sarcopenia and determinants of transitions in older adults: a population-based study. J Gerontol A Biol Sci Med Sci. 2014;69(6):751–758. doi: 10.1093/gerona/glt131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Murphy RA, Patel KV, Kritchevsky SB, et al. Weight change, body composition, and risk of mobility disability and mortality in older adults: a population-based cohort study. J Am Geriatr Soc. 2014;62(8):1476–1483. doi: 10.1111/jgs.12954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Newman AB, Kupelian V, Visser M, et al. Strength, but not muscle mass, is associated with mortality in the Health, Aging and Body Composition Study Cohort. J Gerontol A Biol Sci Med Sci. 2006;61(1):72–77. doi: 10.1093/gerona/61.1.72 [DOI] [PubMed] [Google Scholar]
- 8. Buehring B, Hansen KE, Lewis BL, et al. ; Osteoporotic Fractures in Men (MrOS) Study Research Group Dysmobility syndrome independently increases fracture risk in the osteoporotic fractures in men (MrOS) prospective cohort study. J Bone Miner Res. 2018;33(9):1622–1629. doi: 10.1002/jbmr.3455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chalhoub D, Boudreau R, Greenspan S, et al. ; Osteoporotic Fractures in Men (MrOS) Study Research Group Associations between lean mass, muscle strength and power, and skeletal size, density and strength in older men. J Bone Miner Res. 2018;33(9):1612–1621. doi: 10.1002/jbmr.3458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Navaneethan SD, Kirwan JP, Arrigain S, Schold JD. Adiposity measures, lean body mass, physical activity and mortality: NHANES 1999-2004. BMC Nephrol. 2014;15:108. doi: 10.1186/1471-2369-15-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheung CL, Lam KS, Cheung BM. Evaluation of cutpoints for low lean mass and slow gait speed in predicting death in the national health and nutrition examination survey 1999–2004. J Gerontol A Biol Sci Med Sci. 2016;71(1):90–95. doi: 10.1093/gerona/glv112 [DOI] [PubMed] [Google Scholar]
- 12. Looker AC. Dysmobility syndrome and mortality risk in US men and women age 50 years and older. Osteoporos Int. 2015;26(1):93–102. doi: 10.1007/s00198-014-2904-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cawthon PM, Travison TG, Manini TM, et al. Establishing the link between lean mass and grip strength cut points with mobility disability and other health outcomes: proceedings of the sarcopenia definition and outcomes consortium conference. J Gerontol A Biol Sci Med Sci. 2019. doi: 10.1093/gerona/glz081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jones K, Gordon-Weeks A, Coleman C, Silva M. Radiologically determined sarcopenia predicts morbidity and mortality following abdominal surgery: a systematic review and meta-analysis. World J Surg. 2017;41:2266–2279. doi: 10.1007/s00268-017-3999-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lopez PD, Nepal P, Akinlonu A, et al. Low skeletal muscle mass independently predicts mortality in patients with chronic heart failure after an acute hospitalization. Cardiology. 2019;142(1):28–36. doi: 10.1159/000496460 [DOI] [PubMed] [Google Scholar]
- 16. Touban BM, Pavlesen S, Smoak JB, et al. Decreased lean psoas cross-sectional area is associated with increased 1-year all-cause mortality in male elderly orthopaedic trauma patients. J Orthop Trauma. 2019;33(1):e1–e7. doi: 10.1097/BOT.0000000000001331 [DOI] [PubMed] [Google Scholar]
- 17. Kaplan SJ, Pham TN, Arbabi S, et al. Association of radiologic indicators of frailty with 1-year mortality in older trauma patients: opportunistic screening for sarcopenia and osteopenia. JAMA Surg. 2017;152(2):e164604. doi: 10.1001/jamasurg.2016.4604 [DOI] [PubMed] [Google Scholar]
- 18. Paknikar R, Friedman J, Cron D, et al. Psoas muscle size as a frailty measure for open and transcatheter aortic valve replacement. J Thorac Cardiovasc Surg. 2016;151(3):745–751. doi: 10.1016/j.jtcvs.2015.11.022 [DOI] [PubMed] [Google Scholar]
- 19. Leeper CM, Lin E, Hoffman M, et al. Computed tomography abbreviated assessment of sarcopenia following trauma: the CAAST measurement predicts 6-month mortality in older adult trauma patients. J Trauma Acute Care Surg. 2016;80(5):805–811. doi: 10.1097/TA.0000000000000989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oakland K, Nadler R, Cresswell L, Jackson D, Coughlin PA. Systematic review and meta-analysis of the association between frailty and outcome in surgical patients. Ann R Coll Surg Engl. 2016;98(2):80–85. doi: 10.1308/rcsann.2016.0048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moisey LL, Mourtzakis M, Cotton BA, et al. ; Nutrition and Rehabilitation Investigators Consortium (NUTRIC) Skeletal muscle predicts ventilator-free days, ICU-free days, and mortality in elderly ICU patients. Crit Care. 2013;17(5):R206. doi: 10.1186/cc12901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Locke JE, Carr JJ, Nair S, et al. Abdominal lean muscle is associated with lower mortality among kidney waitlist candidates. Clin Transplant. 2017;31(3). doi: 10.1111/ctr.12911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Deren ME, Babu J, Cohen EM, Machan J, Born CT, Hayda R. Increased Mortality in elderly patients with sarcopenia and acetabular fractures. J Bone Joint Surg Am. 2017;99(3):200–206. doi: 10.2106/JBJS.16.00734 [DOI] [PubMed] [Google Scholar]
- 24. Shibahashi K, Sugiyama K, Kashiura M, Hamabe Y. Decreasing skeletal muscle as a risk factor for mortality in elderly patients with sepsis: a retrospective cohort study. J Intensive Care. 2017;5:8. doi: 10.1186/s40560-016-0205-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Vugt JL, Levolger S, de Bruin RW, van Rosmalen J, Metselaar HJ, IJzermans JN. systematic review and meta-analysis of the impact of computed tomography-assessed skeletal muscle mass on outcome in patients awaiting or undergoing liver transplantation. Am J Transplant. 2016;16(8):2277–2292. doi: 10.1111/ajt.13732 [DOI] [PubMed] [Google Scholar]
- 26. Amini B, Boyle SP, Boutin RD, Lenchik L. Approaches to assessment of muscle mass and myosteatosis on computed tomography: a systematic review. J Gerontol A Biol Sci Med Sci. 2019;74(10):1671–1678. doi: 10.1093/gerona/glz034. PMID: 30726878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lenchik L, Lenoir KM, Tan J, et al. Opportunistic measurement of skeletal muscle size and muscle attenuation on computed tomography predicts 1-year mortality in medicare patients. J Gerontol A Biol Sci Med Sci. 2019;74(7):1063–1069. doi: 10.1093/gerona/gly183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lenchik L, Heacock L, Weaver AA, et al. Automated segmentation of tissues using CT and MRI: a systematic review. Acad Radiol. 2019;26:1695–1706. doi: 10.1016/j.acra.2019.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barnard R, Tan J, Roller B, et al. Machine learning for automatic paraspinous muscle area and attenuation measures on low-dose chest CT scans. Acad Radiol. 2019;26(12):1686–1694. doi: 10.1016/j.acra.2019.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kanavati F, Islam S, Aboagye EO, Rockall A. Automatic L3 slice detection in 3D CT images using fully-convolutional networks. arXiv Preprint. 2018arXiv:1811:09244. [Google Scholar]
- 31. National Lung Screening Trial Research Team, Aberle DR, Berg CD, et al. The National Lung Screening Trial: overview and Study Design. Radiology 2011;258:243–253. doi: 10.1148/radiol.10091808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Perkisas S, Lamers S, Degerickx R, et al. The relation between mortality, intramuscular adipose tissue and sarcopenia in hospitalized geriatric patients. Eur Geriatr Med. 2018;9(6):801–807. doi: 10.1007/s41999-018-0110-y [DOI] [PubMed] [Google Scholar]
- 34. Murea M, Lenchik L, Register TC, et al. Psoas and paraspinous muscle index as a predictor of mortality in African American men with type 2 diabetes mellitus. J Diabetes Complications. 2018;32(6):558–564. doi: 10.1016/j.jdiacomp.2018.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rosa-Caldwell ME, Greene NP. Muscle metabolism and atrophy: let’s talk about sex. Biol Sex Differ. 2019;10(1):43. doi: 10.1186/s13293-019-0257-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tay L, Ding YY, Leung BP, et al. Sex-specific differences in risk factors for sarcopenia amongst community-dwelling older adults. Age (Dordr). 2015;37(6):121. doi: 10.1007/s11357-015-9860-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yoon HE, Nam Y, Kang E, et al. Gender-Specific associations between low skeletal muscle mass and albuminuria in the middle-aged and elderly population. Int J Med Sci. 2017;14(11):1054–1064. doi: 10.7150/ijms.20286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tucker BM, Hsu FC, Register TC, et al. Psoas and paraspinous muscle measurements on computed tomography predict mortality in European Americans with type 2 diabetes mellitus. J Frailty Aging. 2019;8(2):72–78. doi: 10.14283/jfa.2019.5 [DOI] [PubMed] [Google Scholar]
- 39. Shah RV, Yeri AS, Murthy VL, et al. Association of multiorgan computed tomographic phenomap with adverse cardiovascular health outcomes: the Framingham Heart Study. JAMA Cardiol. 2017;2(11):1236–1246. doi: 10.1001/jamacardio.2017.3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hamrick MW, McGee-Lawrence ME, Frechette DM. Fatty infiltration of skeletal muscle: mechanisms and comparisons with bone marrow adiposity. Front Endocrinol (Lausanne). 2016;7:69. doi: 10.3389/fendo.2016.00069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Reinders I, Murphy RA, Brouwer IA, et al. ; Age, Gene/Environment Susceptibility (AGES)-Reykjavik Study Muscle quality and myosteatosis: novel associations with mortality risk: the age, gene/environment susceptibility (AGES)-Reykjavik study. Am J Epidemiol. 2016;183(1):53–60. doi: 10.1093/aje/kwv153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Santanasto AJ, Goodpaster BH, Kritchevsky SB, et al. Body composition remodeling and mortality: the Health Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2017;72(4):513–519. doi: 10.1093/gerona/glw163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Miljkovic I, Kuipers AL, Cauley JA, et al. ; Osteoporotic Fractures in Men Study Group Greater skeletal muscle fat infiltration is associated with higher all-cause and cardiovascular mortality in older men. J Gerontol A Biol Sci Med Sci. 2015;70(9):1133–1140. doi: 10.1093/gerona/glv027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhao Q, Zmuda JM, Kuipers AL, et al. Greater skeletal muscle fat infiltration is associated with higher all-cause mortality among men of African ancestry. Age Ageing. 2016;45(4):529–534. doi: 10.1093/ageing/afw062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. MacDonald AJ, Miller J, Ramage MI, et al. Cross sectional imaging of truncal and quadriceps muscles relates to different functional outcomes in cancer. Clin Nutr. 2019;38(6):2875–2880. doi: 10.1016/j.clnu.2018.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Payette H, Roubenoff R, Jacques PF, et al. Insulin-like growth factor-1 and interleukin 6 predict sarcopenia in very old community-living men and women: the Framingham Heart Study. J Am Geriatr Soc. 2003;51(9):1237–1243. doi: 10.1046/j.1532-5415.2003.51407.x [DOI] [PubMed] [Google Scholar]
- 47. Boutin RD, Bamrungchart S, Bateni CP, et al. CT of patients with hip fracture: muscle size and attenuation help predict mortality. AJR Am J Roentgenol. 2017;208(6):W208–W215. doi: 10.2214/AJR.16.17226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the Health, Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2006;61(10):1059–1064. doi: 10.1093/gerona/61.10.1059 [DOI] [PubMed] [Google Scholar]
- 49. Gill TM, Murphy TE, Barry LC, Allore HG. Risk factors for disability subtypes in older persons. J Am Geriatr Soc. 2009;57(10):1850–1855. doi: 10.1111/j.1532-5415.2009.02443.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Taş U, Verhagen AP, Bierma-Zeinstra SM, et al. Incidence and risk factors of disability in the elderly: the Rotterdam Study. Prev Med. 2007;44(3):272–278. doi: 10.1016/j.ypmed.2006.11.007 [DOI] [PubMed] [Google Scholar]
- 51. Visser M, Deeg DJ, Lips P, Harris TB, Bouter LM. Skeletal muscle mass and muscle strength in relation to lower-extremity performance in older men and women. J Am Geriatr Soc. 2000;48(4):381–386. doi: 10.1111/j.1532-5415.2000.tb04694.x [DOI] [PubMed] [Google Scholar]
- 52. Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60(3):324–333. doi: 10.1093/gerona/60.3.324 [DOI] [PubMed] [Google Scholar]
- 53. Chan BK, Marshall LM, Winters KM, Faulkner KA, Schwartz AV, Orwoll ES. Incident fall risk and physical activity and physical performance among older men: the Osteoporotic Fractures in Men Study. Am J Epidemiol. 2007;165(6):696–703. doi: 10.1093/aje/kwk050 [DOI] [PubMed] [Google Scholar]
- 54. Cawthon PM, Fullman RL, Marshall L, et al. ; Osteoporotic Fractures in Men (MrOS) Research Group Physical performance and risk of hip fractures in older men. J Bone Miner Res. 2008;23(7):1037–1044. doi: 10.1359/jbmr.080227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cawthon PM, Fox KM, Gandra SR, et al. ; Health, Aging and Body Composition Study Do muscle mass, muscle density, strength, and physical function similarly influence risk of hospitalization in older adults? J Am Geriatr Soc. 2009;57(8):1411–1419. doi: 10.1111/j.1532-5415.2009.02366.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Penninx BW, Ferrucci L, Leveille SG, Rantanen T, Pahor M, Guralnik JM. Lower extremity performance in nondisabled older persons as a predictor of subsequent hospitalization. J Gerontol A Biol Sci Med Sci. 2000;55(11):M691–M697. doi: 10.1093/gerona/55.11.m691 [DOI] [PubMed] [Google Scholar]
- 57. Cawthon PM, Blackwell TL, Cauley J, et al. Evaluation of the usefulness of consensus definitions of sarcopenia in older men: results from the observational osteoporotic fractures in men cohort study. J Am Geriatr Soc. 2015;63(11):2247–2259. doi: 10.1111/jgs.13788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Buettner S, Wagner D, Kim Y, et al. Inclusion of sarcopenia outperforms the modified frailty index in predicting 1-year mortality among 1,326 patients undergoing gastrointestinal surgery for a malignant indication. J Am Coll Surg. 2016;222(4):397–407.e2. doi: 10.1016/j.jamcollsurg.2015.12.020 [DOI] [PubMed] [Google Scholar]
- 59. Moyer VA; U.S. Preventive Services Task Force screening for lung cancer: U.S. preventive services task force recommendation statement. Ann Intern Med. 2014;160:330–338. doi: 10.7326/M13-2771 [DOI] [PubMed] [Google Scholar]
- 60. Chiles C, Duan F, Gladish GW, et al. ; NLST Study Team Association of coronary artery calcification and mortality in the national lung screening trial: a comparison of three scoring methods. Radiology. 2015;276:82–90. doi: 10.1148/radiol.15142062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mets OM, Buckens CF, Zanen P, et al. Identification of chronic obstructive pulmonary disease in lung cancer screening computed tomographic scans. JAMA. 2011;306:1775–1781. doi: 10.1001/jama.2011.1531 [DOI] [PubMed] [Google Scholar]
- 62. Buckens CF, van der Graaf Y, Verkooijen HM, et al. Osteoporosis markers on low-dose lung cancer screening chest computed tomography scans predict all-cause mortality. Eur Radiol. 2015;25(1):132–139. doi: 10.1007/s00330-014-3361-0 [DOI] [PubMed] [Google Scholar]
- 63. Cressman S, Peacock SJ, Tammemägi MC, et al. The cost-effectiveness of high-risk lung cancer screening and drivers of program efficiency. J Thorac Oncol. 2017;12(8):1210–1222. doi: 10.1016/j.jtho.2017.04.021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.