Abstract
Background
To establish trajectories of cognitive and motor function, and to determine the sequence of change across individual tests in community-dwelling individuals aged 45–90 years.
Method
Between 1997 and 2016, we repeatedly assessed cognitive function with 5 tests in 9514 participants aged 45–90 years from the population-based Rotterdam Study. Between 1999 and 2016, we measured motor function with 3 tests in 8297 participants. All participants were free from dementia, stroke, and parkinsonism. We assessed overall and education-specific cognitive and motor trajectories using linear mixed models with age as time scale. Next, we determined the sequence of change across individual tests.
Results
The number of assessments per participant ranged between 1 and 6 (mean interval, years [SD]: 5.1 [1.4]) for cognitive function, and 1 and 4 (5.4 [1.4]) for motor function. Cognitive and motor trajectories declined linearly between ages 45 and 65 years, followed by steeper declines after ages 65–70 years. Lower educated participants had lower cognitive function at age 45 years (baseline), and declined faster on most cognitive, but not on motor tests than higher educated participants. Up to a 25-year age difference between the fastest and slowest declining test scores was observed.
Conclusions
On a population-level, cognitive and motor function decline similarly. Compared to higher educated individuals, lower educated individuals had lower cognitive function at baseline, and a faster rate of decline thereafter. These educational-effects were not seen for motor function. These findings benefit the understanding of the natural course of cognitive and motor function during aging, and highlight the role of education in the preservation of cognitive but not motor function.
Keywords: Brain aging, Cognitive aging, Epidemiology, Gait, Motor function
Understanding the natural course of cognitive and motor function during brain aging is pivotal to determine deviations in function that may signal early stages of clinical neurodegenerative diseases (1,2). Decline in both cognitive and motor function has been associated with an increased risk of dementia, Parkinson’s disease, and stroke (1–3). In addition, we recently showed that individuals in whom decline in motor function precedes decline in cognitive function are at an increased risk of dementia (3). Numerous studies have quantified the temporal relation of cognitive and motor function with advancing age (4–17), yet little is known about the sequence of individual cognitive and motor tests in a population free from neurodegenerative diseases and stroke.
Comparing trajectories of cognitive and motor tests in the general population reveals whether decline in motor function precedes decline in cognitive function. In addition, it identifies the specific individual tests that have the earliest signs of decline. Such findings could inform clinicians about which cognitive and motor tests are most sensitive to detect change in cognitive or motor function. These trajectories can also be used to signal vulnerable patient groups that deviate from their expected course based on several key characteristics, such as age, sex, educational level, or genes. These characteristics significantly influence cognitive function and the rate of cognitive decline, but their effects on motor function beyond gait speed are less understood (18,19).
Alike changes in brain structure, we hypothesize that change in cognitive and motor function accelerates with advancing age (20). To model this nonlinear change, we present trajectories of cognitive and motor function. In addition, we assess the effects of key determinants of cognitive and motor function, namely age, sex, education, and apolipoprotein E (APOE) genotype on these trajectories. Finally, we determine the sequence of change of individual cognitive and motor function tests.
Materials and Methods
Study Design
This study was embedded within the Rotterdam Study, a prospective population-based cohort designed to study the occurrence and determinants of age-related diseases in the general population (21). In 1989, all inhabitants aged 55 years and older from Ommoord, a well-defined district in Rotterdam, the Netherlands received an invitation to participate. This initial cohort comprised 7983 participants. In 2000, 3011 participants who had become 55 years of age or moved into the study district since the start of the study were additionally included in the cohort. In 2006, a further extension of the cohort was initiated in which 3932 participants aged 45 years and older participated. In total, the Rotterdam Study comprises 14 926 participants aged 45 years and older. The overall response rate across all 3 recruitment waves was 72%.
Standard Protocol Approvals, Registrations, and Patient Consents
The Rotterdam Study has been approved by the Medical Ethics Committee of the Erasmus MC (registration number MEC 02.1015) and by the Dutch Ministry of Health, Welfare and Sport (Population Screening Act WBO, license number 1071272-159521-PG). All participants provided written informed consent to participate in the study and to have their information obtained from treating physicians.
Study Population
Of a total of 14 926 participants, we excluded those with a history of dementia (n = 907), stroke (n = 846), Parkinson’s disease (n = 300), or parkinsonism (n = 20) at time of their first cognitive or motor assessment. Next, we excluded participants with insufficient data to determine whether they had a history of one or multiple of these diseases (n = 1800). Baseline and follow-up ascertainment methods for dementia, stroke, Parkinson’s disease, and parkinsonism have previously been described in detail (22). In addition, 5 participants were excluded because they did not provide informed consent to access medical records and hospital discharge letters during follow-up. From the remaining 11 048 participants, 1494 participants were excluded because they did not have data available on any cognitive or motor test. Finally, we excluded assessments from participants after they had reached age 90 years in order to minimize the influence of leverage points on the trajectories of cognitive and motor function. This resulted in an additional exclusion of 33 participants who did not have any cognitive or motor function assessment at all before the age of 90 years, leaving 9521 participants with at least one cognitive or motor assessment. During follow-up, we excluded assessments of participants after the age of 90 years (n = 1266) and of participants after a dementia, stroke, or Parkinson’s disease diagnosis (n = 3175). All of the included participants were thus free from neurodegenerative diseases and stroke at time of their test assessments. In total, 155 347 cognitive function assessments from 9514 participants and 62 545 motor function assessments from 8297 participants were available for analyses.
Assessment of Cognitive Function
Between 1997 and 2016, participants underwent cognitive assessments at the research center using a neuropsychological test battery every 3–5 years (6,21). This battery included the Word Fluency Test (23), Letter-Digit Substitution Test (24), and Stroop Test (Reading, Naming, and Interference subtask) (25). In 2002, the 15-Word Learning Test (Immediate recall, Delayed recall, and Recognition) was added to the test protocol (26). This test protocol was further expanded with the Design Organization Test in 2006 (27). Assessments of these cognitive tests have previously been validated and have a reasonable to good test–retest reliability (28–31).
Word Fluency Test
In the Word Fluency Test, participants were asked to mention as many animals as possible within 60 seconds, thereby measuring semantic fluency (23). The total number of correct answers was used as test score, with a maximum score of 30 in our study protocol.
Letter-Digit Substitution Test
The Letter-Digit Substitution Test is a modified version of the Symbol Digit Modalities Test for which participants were asked to write down as many numbers underneath the corresponding letters as possible in 60 seconds, following a key that shows correct combinations (24). This test captures both information processing speed and aspects of executive function. The total number of correct answers was used as test score with a maximum attainable score of 125.
Stroop Test
The Stroop Test consists of 3 different subtasks, that is, Reading, Naming, and Interference (25). In the Stroop Reading subtask, participants were asked to read the printed color names. For the Stroop Naming subtask, participants were asked to name the printed color blocks. In the Interference subtask, participants were asked to name the ink color of color names printed in incongruous ink colors (information processing on an interference subtask). The time taken to complete the subtask was used as the outcome for each subtask separately and was adjusted for failures, that is, total time plus for each failure the total time divided by the number of items, multiplied with 1.5 (32). Thus, a higher score indicates a worse performance. The Stroop Test assesses information processing speed and executive function.
Word Learning Test
The Word Learning Test comprises 3 subtasks: Immediate recall, Delayed recall, and Recognition (26). For Immediate recall, participants were 3 times visually presented with a sequence of 15 words and were subsequently asked to recall as many of these words as possible, measuring verbal learning. Free Delayed recall was tested approximately 10 minutes after visual presentation, evaluating retrieval from verbal memory. Recognition was tested by visually presenting the participants a sequence of 45 words, followed by correctly recognizing the 15 words presented during the Immediate recall while mixed with 30 other words. Outcome variables were the mean number of words of 3 trials immediately recalled (as a summary score for Immediate recall), after the delay of 10 minutes (as a score for free Delayed recall), and the mean number of correctly recognized words during the recognition trial (as a score for Recognition), with a maximum score of 15 per subtask.
Design Organization Test
The Design Organization Test consists of square black-and-white grids with visual patterns, of which participants were asked to reproduce as many designs as possible in 2 minutes using a numerical code key. It measures visuospatial abilities and is based on and highly correlated to WAIS-III block design (27), but is less dependent on motor skills. Test score on the Design Organization Test has a range from 0 to 56 points for each individual, with higher scores indicating better performance.
Assessment of Motor Function
Participants repeatedly underwent motor tests every 3–5 years at the research center between 1999 and 2016. This motor test battery included 2 tests to assess fine motor function and a quantitative gait assessment to assess gross motor function. From 1999 onwards, the Purdue Pegboard Test was implemented into the study protocol to assess manual dexterity. Assessment of fine motor function was further expanded in 2008 with the implementation of the Spiral Archimedes Test to assess manual precision. In 2009, a quantitative gait assessment using an electronic walkway at the research center was implemented in the core study protocol.
Purdue Pegboard Test
For the Purdue Pegboard Test, participants were asked to place as many as possible cylindrical metal pegs into one of the 25 holes in a pegboard in 30 seconds in 3 separate trials, using their left hand only, right hand only, and both hands simultaneously, measuring fine motor function (33). The test–retest reliability of assessments has been established previously. The outcome variable was the sum score of Purdue Pegboard Test score of these 3 trials, with a maximum of 75 points.
Archimedes Spiral Test
The Archimedes Spiral Test measures fine motor function by requiring participants to trace a picture of a spiral template that was printed on paper attached to an electronic drawing board (WACOM Graphire Wireless Pen Tablet, model CTE-630BT) (7). Participants were instructed to trace the spiral as accurately and as fast as possible using a special pen with their dominant hand, starting in the middle (Supplementary Figure 1). Automatic quantitative analyses were done using custom-made software written in MatLab (version 8.1; The Mathworks, Natick, MA), and processed and visually inspected by 2 trained physicians (S.L. and S.K.L.D.) for analyses (intraclass correlation coefficient [ICC] for interrater reliability for all test components >0.95). A smoothly drawn spiral would have a length of drawing about 56 cm (the length of the template) with little deviation from the template, a low variability in speed, and no crossings (Supplementary Figure 1). The mean amplitude in deviation from the template to spiral drawing (cm) was used as outcome, since it is sensitive to capture small differences in fine motor function (7). A higher deviation indicates worse performance.
Gait Assessment
Gait was evaluated using a 5.79 m long walkway (GAITRite Platinum; CIR systems, Sparta, NJ: 4.88m active area; 120-Hz sampling rate) (21). The reliability and validity of assessments obtained with this device have previously been established (34). The standardized gait protocol comprises 3 walking conditions: normal walk, turning, and tandem walk. In the normal walk, participants walked at their usual pace across the walkway. In turning, participants walked at their usual pace, turned halfway, and returned to the starting position. In the tandem walk, participants walked heel-to-toe on a line across the walkway. Based on the recorded footfalls, the walkway software calculated 30 parameters, including 25 from the normal walk, 2 from turning, and 3 from the tandem walk. In Supplementary Table 1, we provide descriptions of the 30 gait parameters.
To summarize these 30 gait parameters into several independent domains, we log-transformed skewed gait parameters to obtain a normal distribution, and subsequently standardized all continuous gait parameters. Next, we conducted a principal component analysis with Varimax rotation to derive gait domains, as previously described (35). This yielded 7 gait domains with an eigenvalue >1, which we labeled in accordance with the gait parameter that had the highest correlation coefficient with the corresponding domain: rhythm (step time), variability (standardized step length), phases (double support), pace (velocity), tandem (sum of step distance), turning (turning time), and base of support (stride width) (35). These gait domains are illustrated in Supplementary Figure 2. Higher values of the gait domains except “pace”, represent worse gait performance. Based on these 7 gait domains, the Purdue Pegboard Test, and the Archimedes Spiral Test, a total of 9 different facets of motor function were available for analysis.
Assessment of Study Population Characteristics
During home interviews, educational level was assessed and categorized as primary education (“primary”), lower/intermediate general education or lower vocational education (“lower”), intermediate vocational education or higher general education (“intermediate”), and higher vocational education or university (“higher”). Smoking and alcohol habits were assessed during the same home interviews. Participants were categorized as current, former, or never smokers. Alcohol habits were classified into any use or no use of alcohol. At the research center, height and weight were measured from which the body mass index (BMI; kg/m2) was computed. Blood pressure was measured twice in sitting position on the right arm using a random-zero sphygmomanometer, and the average of 2 measurements was used. In addition, non-fasting blood samples were collected and glucose levels were determined. In the initial subcohort, type 2 diabetes was defined as a random or post-load serum glucose concentration ≥11.1 mmol/L, or the use of drugs to lower blood glucose. In the first and second extension subcohorts, type 2 diabetes was defined as a fasting serum glucose concentration ≥7.0 mmol/L, a non-fasting serum glucose concentration ≥11.1 mmol/L (only if fasting serum was unavailable), or usage of blood glucose lowering drugs. APOE genotype was determined using polymerase chain reaction on coded DNA samples in the initial cohort and with a bi-allelic TaqMan assay in the 2 extensions (36,37). APOE ε4 carrier status was defined as carrier of one or 2 APOE ε4 alleles.
Statistical Analysis
We assessed trajectories of cognitive and motor function using linear mixed models with random intercepts and slopes. If models did not converge with both random intercepts and slopes, only a random intercept was used. Age of the participant at time of cognitive or motor function assessment was used as underlying time scale. To capture possible nonlinearity, we included natural cubic splines of age with 1, 2, or 3 knots, depending on model performance determined by a likelihood ratio test (p < .05). Knots were defined at the median, tertiles, or quartiles for, respectively, 1, 2, or 3 knots. We only reported p-values for each of the age intervals, since appropriate interpretation of effect estimates is hindered by the inclusion of natural cubic splines in the models. Skewed test outcomes (ie, Stroop Tests, Word Learning Test Recognition subtask, Archimedes Spiral Test, and gait domains “variability” and “tandem”) were natural log-transformed to reach an approximate normal distribution, and were back-transformed for visualization. In addition, we visualized trajectories of cognitive and motor function by sex, education, or both, using interaction terms on the additive scale between age and sex, age and educational level, and age with sex and educational level. Missing data on education level (1.1%) were imputed by chained equations with 5 iterations. We generated one imputed dataset based on age at baseline and sex. Furthermore, we assessed trajectories for APOE ε4 carriers and non-carriers separately by including an interaction term between age and APOE ε4 status. This analysis was limited to the participants with known APOE ε4 status (N participants = 8986 for cognitive tests and N participants = 7835 for motor tests).
Next, we repeated these analyses by standardizing the cognitive and motor test results to the test performance of the age of 45 years (study baseline) to investigate the temporal course of change across tests with aging. Skewed test outcomes were natural log-transformed before standardization. We depicted a threshold of decline in performance of 0.5 and 1.0 SD compared to the test score at age 45 years. We subsequently assessed the age at which the test score had reached a decline of 0.5 and 1.0 SD compared to the test result at age 45 years.
Data were analyzed with SPSS Statistics version 24.0 (IBM Corp., Armonk, NY) and R, CRAN version 3.4.3 “mice” and “nlme” packages (38,39).
Results
Characteristics of the study population at time of study entry are presented in Table 1. A total of 9514 participants contributed to the cognitive function assessments. The mean (SD) age at first cognitive assessment was 64.7 years (9.5 years) and 5442 (57.2%) of the participants were women. Of all participants, 2058 (21.6%) had a single cognitive assessment, 4362 (45.8%) had 2, 1174 (12.3%) had 3, and 1920 (20.2%) had at least 4 cognitive assessments. The mean interval between tests was 5.1 years (1.4 years). During follow-up up to January 1, 2016, 2977 of 9514 participants (31.3%) died.
Table 1.
Characteristics of the Study Populations
| Characteristic | Analysis of Cognitive Function (N = 9514) | Analysis of Motor Function (N = 8297) |
|---|---|---|
| Age at study entry, years, mean (SD) | 62.0 (7.9) | 60.9 (7.4) |
| Age at first assessment, years, mean (SD) | 64.7 (9.5) | 64.6 (10.0) |
| Sex, women, n (%) | 5442 (57.2) | 4737 (57.1) |
| Educational level, n (%) | ||
| Primary | 1160 (12.2) | 886 (10.7) |
| Lower | 3889 (40.9) | 3375 (40.7) |
| Intermediate | 2751 (28.9) | 2422 (29.2) |
| Higher | 1714 (18.0) | 1614 (19.5) |
| Number of assessmentsa, n (%) | ||
| 1 | 2058 (21.6) | 2136 (25.7) |
| 2 | 4362 (45.8) | 4192 (50.5) |
| 3 | 1174 (12.3) | 1091 (13.1) |
| ≥4 | 1920 (20.2) | 878 (10.6) |
| Median number of assessments (range) | 2 (1–6) | 2 (1–4) |
| Test assessment interval, years, mean (SD) | 5.1 (1.4) | 5.4 (1.4) |
| Body mass index, kg/m2, mean (SD) | 27.0 (4.1) | 27.1 (4.2) |
| Smoking, n (%) | ||
| Never | 2941 (30.9) | 2522 (30.4) |
| Past | 4558 (47.9) | 4063 (49.0) |
| Current | 1944 (20.4) | 1663 (20.0) |
| Alcohol use, n (%) | 7760 (81.6) | 6928 (83.5) |
| Systolic blood pressure, mm Hg, mean (SD) | 136 (20.8) | 136 (20.6) |
| Type 2 diabetes, n (%) | 865 (9.1) | 735 (8.9) |
| APOE ε4 carrier, n (%) | 2539 (26.7) | 2217 (26.7) |
Notes: APOE = apolipoprotein E; N = number of participants. Characteristics were measured at study entry except for age at first assessment. Missing values for all characteristics but educational level are not imputed and therefore numbers do not always sum up to 100%.
aGait was considered as one assessment, because virtually all participants (95%) with an available gait assessment had complete values for all underlying gait parameters. Therefore, the presented number of motor assessments is independent from the number of underlying available gait parameters that were used to compute 7 gait domains.
A total of 8297 participants contributed to the motor function assessments with a mean (SD) age at first assessment of 64.6 years (10.0 years), of whom 4737 (57.1%) were women (Table 1). Out of these participants, 2136 (25.7%) had a single motor function assessment, 4192 (50.5%) had 2, 1091 (13.1%) had 3, and 878 (10.6%) had 4 motor assessments with a mean (SD) test interval of 5.4 years (1.4 years). Out of 8297 participants, 1903 died (22.9%) during follow-up. The number of participants per cognitive and motor test is shown in Supplementary Table 2. Supplementary Table 3 shows the characteristics of the excluded participants. Overall, excluded participants were older at study entry, attained more often a lower level of education, and had a higher mean systolic blood pressure than included participants.
Trajectories of Cognitive Function
Performance on the cognitive tests declined with advancing age. Decline on cognitive tests was generally linear between ages 45 and 65 years, followed by a steeper, nonlinear decline. Men had higher scores on most cognitive tests and generally declined less fast than women (p = .003 for Letter-Digit Substitution Test, p = .02 for Word Learning Test: Immediate recall, p = 0.05 for Word Learning Test: Delayed recall). These differences between men and women disappeared after assessing the trajectories per educational level, suggesting that this sex difference was largely attributable to differences in the level of attained education between men and women. As such, results from here onwards are presented per educational level for men and women combined.
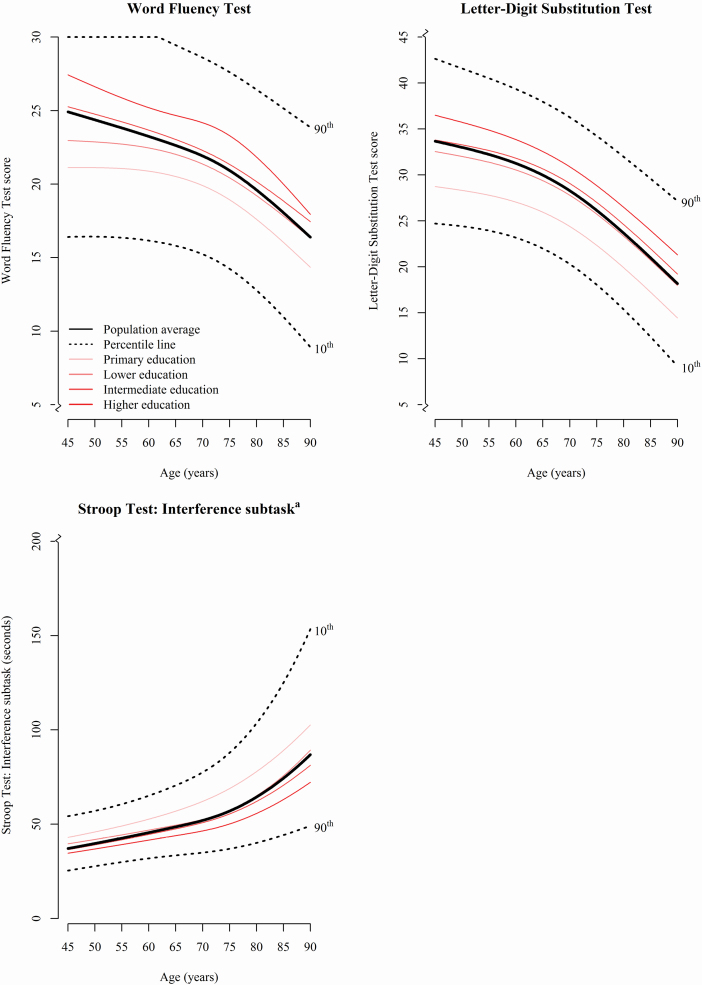
For each higher level of attained education, participants showed better performance on all cognitive tests at age 45 years (Figure 1 and Supplementary Figure 3). Differences in trajectories of cognitive function between participants with “primary” educational level and participants with other educational levels became larger with advancing age, albeit not statistically significant. Furthermore, participants with “higher” education declined slower than those with “primary” education over time on the Interference subtask of the Stroop Test (p = .002, Figure 1) and the Word Learning Test Recognition subtask (p = .017, Supplementary Figure 3). However, they declined faster than participants with “primary” education on the Word Fluency Test (p = .048, Figure 1) and the Word Learning Test Delayed recall subtask (p = .007, Supplementary Figure 3).
Figure 1.
Trajectories of cognitive function. The thick black continuous line reflects the trajectory of performance for the total study population based on the results of the linear mixed model; the black dotted lines represent the 10th and 90th percentile curves. Furthermore, test performance was visualized per educational level in red. Only cognitive tests most commonly studied in studies of cognitive aging are presented in this Figure. The remaining cognitive tests are shown in Supplementary Figure 3. aHigher scores indicate worse performance.
Regarding APOE ε4 carrier status, carriers declined faster on all cognitive tests than non-carriers (p for interaction between age and APOE ε4 carrier status <.005), except on the Design Organization Test that showed similar trajectories for carriers and non-carriers (Supplementary Figure 4).
Trajectories of Motor Function
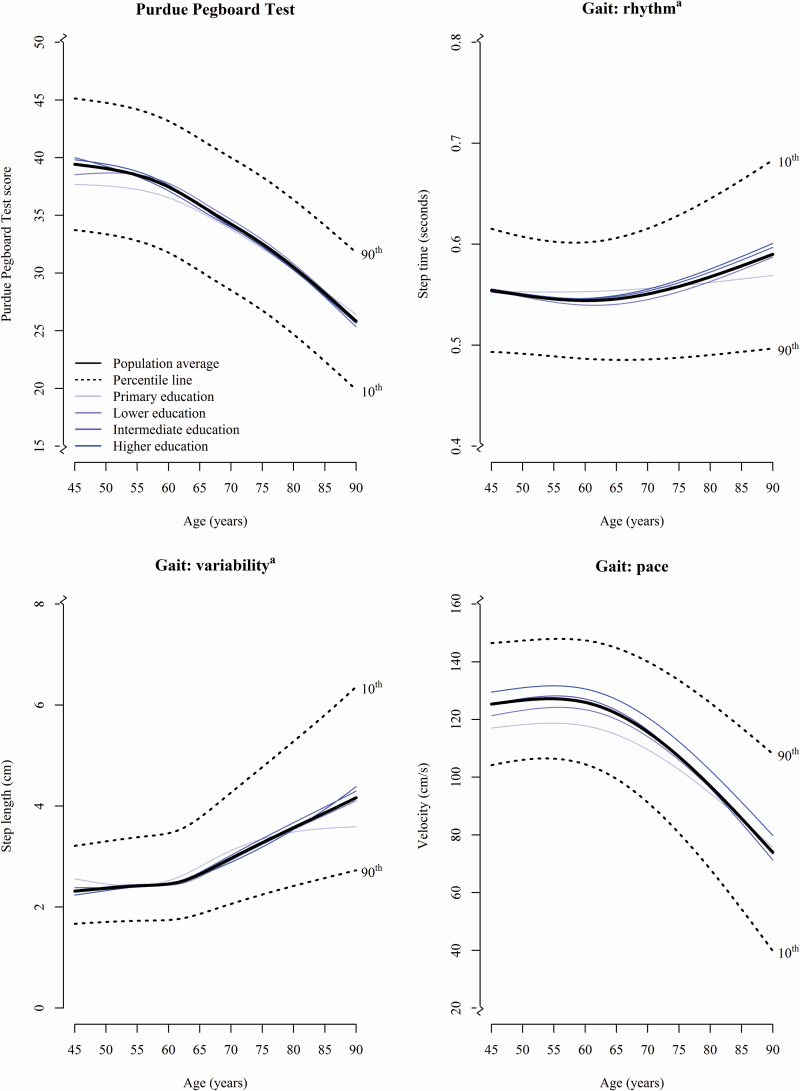
Trajectories of decline in motor function varied across different motor tests (Figure 2 and Supplementary Figure 3) with the gait domain “phases” and the Purdue Pegboard Test declining first at the age of 56 and 60 years, respectively. Performance on the gait domains “rhythm,” “tandem,” and “base of support” remained largely stable over time. Significant differences between men and women were only found for trajectories of the domain “tandem” and “phases,” with women performing increasingly worse over age than men (p = .005 for “tandem” and p < .001 for “phases”).
Figure 2.
Trajectories of motor function. The thick black continuous line reflects the trajectory of performance for the total study population based on the results of the linear mixed model; the black dotted lines represent the 10th and 90th percentile curves. Furthermore, test performance was visualized per educational level in blue. Only gait domains most strongly related to are presented in this figure. The Archimedes Spiral Test and remaining gait domains are shown in Supplementary Figure 3. aHigher scores indicate worse performance.
In contrast to the effects of education on cognitive function, motor function trajectories were not associated with educational level (Figure 2 and Supplementary Figure 3), but those with a “primary” educational level performed better over time on the Purdue Pegboard Test than participants with other educational levels (p < .016, Figure 2). In addition, they decreased less fast on the gait domains “rhythm,” “phases,” and “turning” than participants with higher education levels (p for all tests <.039, Figure 2 and Supplementary Figure 3).
APOE ε4 carriers performed worse with advancing age than non-carriers on the Purdue Pegboard Test and on the gait parameters “phases,” and “turning” (p for all tests <.034, Supplementary Figure 4).
Sequence of Change in Cognitive and Motor Function
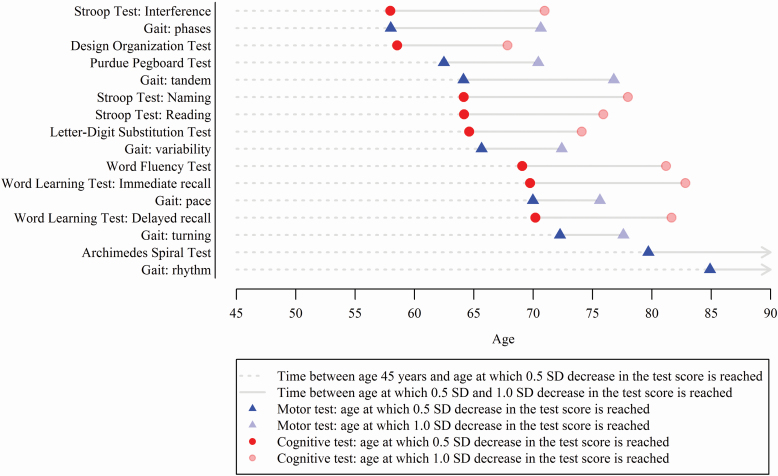
Before the age of 75 years, most cognitive and motor test scores had reached a decline of 0.5 SD in standardized test score compared to test scores at age 45 years (Figure 3). Considering both cognitive and motor tests, the decline of 0.5 SD was first reached for the Stroop Test Interference subtask at the age of 58 years. This was followed by the Design Organization Test at the age of 59 years and the Stroop Test Naming subtask at the age of 64 years. Of all motor tests, the gait domain “phases” showed the fastest decline, reaching a 0.5 SD decrease in test score at the age of 58 years. Across all tests, the average time between the age of 45 years and the age at which 0.5 SD decrease in test score was reached, was shorter for cognitive tests than for motor tests (20.0 vs 24.7 years, respectively, p = .039). By contrast, the time between 0.5 SD and 1.0 SD decrease in test scores was longer for cognitive compared to motor tests (11.2 years vs 8.9 years, respectively, p < .001).
Figure 3.
Sequence of decline of cognitive and motor function. Decline was defined as reaching an average population test score of 0.5 or 1.0 SD below the population mean of the test score at age 45 years. The circle or triangle is displayed at the age at which 0.5 SD (opaque) or 1.0 SD (transparent) lower score was reached with cognitive tests depicted in red circles and motor tests in blue triangles. The dotted line represents time between mean population test score at age 45 years and the age at which 0.5 SD decrease in that test score is reached. The continuous line denotes time between the age at which 0.5 SD decrease in the test score was reached and the age at which 1.0 SD in the test score was reached. The Word Learning Test Recognition subtask and the gait domains “tandem” and “base of support” did not reach a score of 0.5 SD lower at a certain age than the score at age 45 years and are therefore not shown. This sequence of decline was estimated based on the total study population. Note that not all participants had all cognitive and motor tests completed.
Discussion
In this population-based study, we showed that both cognitive and motor function generally decline linearly between the ages of 45 and 65 years, followed by a steeper decline after the age of 65–70 years. Test scores for cognitive and motor function declined similarly, with high variation in the rate of decline across age for individual tests. Importantly, whereas a higher level of education was associated with higher cognitive function, there was no association between level of education and function on the majority of the motor tests.
Various studies have reported changes in cognitive function with aging, but evidence on the temporal relation between change in cognitive compared to motor function is limited. Most evidence comes from memory clinics (11), or from studies that solely rely on gait speed to assess motor function (11,19,40–44). These studies have closely linked global cognitive function to gait speed. As yet, no studies have investigated differences in performance on specific cognitive tests nor studied other facets of motor function, such as fine motor skill. These knowledge gaps remain unaddressed since prior studies found that decline of cognitive and motor function may vary, or that one may predate the other (12,45–47). Most of these studies were conducted in older participants (aged 70 years and older), with a limited sample size (varying between 488 and 2276 participants), or with relatively short follow-up (ranging from 5 to 7 years). The current study is able to extend these findings by leveraging a detailed set of cognitive and motor tests among a broader age range (ages 45–90 years) in a larger, population-based sample (N ≥ 8297) with up to 6 repeated assessments during a maximum follow-up of 19.4 years.
We did not find distinct patterns of an overall decline in cognitive function preceding motor function or vice versa, yet we observed large variability in test-specific decline. For instance, tendency to shuffle (“phases” gait domain) and fine motor function generally started to show initial signs of decline up to 25 years earlier than widely used cognitive (screening) tests, such as the Word Learning Tests Delayed recall and Recognition (11,40–42,48). These findings may be explained by accelerating changes in brain structure during aging, with loss of white matter preceding loss of gray matter (20,49). We indeed observed the earliest changes in cognitive and motor domains that depend on white matter integrity, including information processing speed, executive function, and the gait domain “phases” (20,50–52). In contrast, cognitive and motor domains related to alterations in gray matter volume (ie, memory and the gait domain “base of support”) showed a later decline in function than those related to white matter integrity (20,51–53).
Variability in test-specific decline may also be explained by diseases and common comorbidities in these older adults, such as cardiovascular diseases, depression, respiratory diseases, cancer, or impairments in sensory organs (54–57). These may differentially influence cognitive compared to motor function in some individuals. As an example, presence of peripheral artery disease or arthrosis limits walking speed, but does not directly influences executive functioning as assessed by the Stroop Task (58). The contribution of these potentially modifiable determinants to sequence of test-specific decline and the shape of these trajectories was beyond the scope of the present study, and warrants further investigation using more advanced statistical models.
As expected, we found that participants with a higher educational level had higher baseline scores (scores at age 45 years) for cognitive tests than participants with a lower educational level. Regarding the rate of change in cognitive function, we found that participants with a “primary” educational level declined faster on most tests than higher educated participants. The declines over time were largely similar among “lower,” “intermediate,” and “higher” educated participants. This implies that higher educated individuals are generally older when they reach the same cognitive test performance than lower educated individuals. As an example, comparing performance between lower and higher educated participants on the Word Learning Test Delayed subtask score, reveals that at age 45 years, the lowest educated individuals remembered on average 8 of the 15 originally presented words after 10 minutes. The highest educated individuals, however, attained this same score when they were on average 73 years. Yet, no association was found between educational level and motor function for the majority of the motor tests. These findings support emerging evidence that cognitive reserve, operationalized by for example educational attainment, could have long-lasting compensatory effects on cognitive but not on motor function, with the potential to postpone cognitive decline and thereby the clinical diagnosis of dementia (59–61).
Limitations and Strengths
This study has several limitations. First, given that participants underwent most cognitive tests at the research center, we cannot exclude that selection bias may have influenced our results, with those who are considered less healthy being less likely to participate. Therefore, the presented test scores on cognitive and motor function may be an overestimation of the true performance in the general population, especially for those at older ages (62). Second, repetitive administering of cognitive tests can lead to learning effects, which could have led to overestimating performance with increasing age. However, these effects are expected to be limited, since the median test interval was 5.1 years for cognitive assessments and 5.4 years for motor assessments. Third, in the early nineties, the completed level of education was determined by several factors including sex and social economic status. As such, educational attainment in this study may not be a proper proxy for cognitive reserve in women. Lastly, we estimated trajectories of cognitive and motor function on a population level, yet deviations from this pattern on an individual level may signal an under recognized group at high risk for neurodegenerative diseases and stroke. Strengths of this study include the large sample size and the repeated and simultaneous assessments of cognitive and motor function in a single, community-dwelling population.
Conclusions
In this study, we present trajectories of decline of both cognitive and motor functioning among individuals aged 45–90 years in the general population. Such data are essential to understand the natural course of cognitive and motor function during aging. Cognitive and motor function decline similarly during aging, characterized by a linear decline between the ages of 45 and 65, and a steeper decline thereafter. Higher educational attainment was related to higher cognitive function at baseline and to a slower rate of subsequent decline, but it did not affect motor function. In the sequence of decline across individual tests, up to a 25-year age difference between the fastest and slowest declining test scores was observed.
Supplementary Material
Acknowledgments
We acknowledge the dedication, commitment, and contribution of inhabitants, general practitioners, and pharmacists of the Ommoord district who took part in the Rotterdam Study. We acknowledge Frank van Rooij as data manager, and Jolande Verkroost-van Heemst for her contribution to the collection of the data.
Funding
This work was supported by the Erasmus Medical Center and Erasmus University Rotterdam; Netherlands Organization for the Health Research and Development (ZonMw); the Research Institute for Diseases in the Elderly (RIDE); the Ministry of Education, Culture and Science; the Ministry for Health, Welfare and Sports; the European Commission (DG XII); and the Municipality of Rotterdam. Further support was obtained from the Dutch Cancer Society (NKI-20157737) and the Netherlands Consortium for Healthy Ageing and the Dutch Heart Foundation (2012T008). This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (project: ORACLE, grant agreement no: 678543). The funding sources had no involvement in study design, collection, analysis and interpretation of data, writing the report, and in the decision to submit the article for publication.
Conflict of Interest
None declared.
References
- 1. Darweesh SKL, Wolters FJ, Postuma RB, et al. Association between poor cognitive functioning and risk of incident parkinsonism: the Rotterdam Study. JAMA Neurol. 2017;74:1431–1438. doi: 10.1001/jamaneurol.2017.2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ferrucci L, Guralnik JM, Salive ME, et al. Cognitive impairment and risk of stroke in the older population. J Am Geriatr Soc. 1996;44:237–241. doi: 10.1111/j.1532-5415.1996.tb00908.x [DOI] [PubMed] [Google Scholar]
- 3. Darweesh SKL, Licher S, Wolters FJ, Koudstaal PJ, Ikram MK, Ikram MA. Quantitative gait, cognitive decline, and incident dementia: The Rotterdam Study. Alzheimers Dement. 2019;15:1264–1273. doi: 10.1016/j.jalz.2019.03.013 [DOI] [PubMed] [Google Scholar]
- 4. Darweesh SK, Verlinden VJ, Stricker BH, Hofman A, Koudstaal PJ, Ikram MA. Trajectories of prediagnostic functioning in Parkinson’s disease. Brain. 2017;140:429–441. doi: 10.1093/brain/aww291 [DOI] [PubMed] [Google Scholar]
- 5. Licher S, Leening MJG, Yilmaz P, et al. Development and validation of a dementia risk prediction model in the general population: an analysis of three longitudinal studies. Am J Psychiatry. 2018;176:654–551 . doi: 10.1176/appi.ajp.2018.18050566 [DOI] [PubMed] [Google Scholar]
- 6. Hoogendam YY, Hofman A, van der Geest JN, van der Lugt A, Ikram MA. Patterns of cognitive function in aging: the Rotterdam Study. Eur J Epidemiol. 2014;29:133–140. doi: 10.1007/s10654-014-9885-4 [DOI] [PubMed] [Google Scholar]
- 7. Hoogendam YY, van der Lijn F, Vernooij MW, et al. Older age relates to worsening of fine motor skills: a population-based study of middle-aged and elderly persons. Front Aging Neurosci. 2014;6:259. doi: 10.3389/fnagi.2014.00259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Singh-Manoux A, Kivimaki M, Glymour MM, et al. Timing of onset of cognitive decline: results from Whitehall II prospective cohort study. Br Med J. 2012;344:d7622. doi: 10.1136/bmj.d7622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cooper R, Hardy R, Aihie Sayer A, et al. ; HALCyon Study Team Age and gender differences in physical capability levels from mid-life onwards: the harmonisation and meta-analysis of data from eight UK cohort studies. PLoS One. 2011;6:e27899. doi: 10.1371/journal.pone.0027899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Downer B, Chen NW, Raji M, Markides KS. A longitudinal study of cognitive trajectories in Mexican Americans age 75 and older. Int J Geriatr Psychiatry. 2017;32:1122–1130. doi: 10.1002/gps.4575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mielke MM, Roberts RO, Savica R, et al. Assessing the temporal relationship between cognition and gait: slow gait predicts cognitive decline in the Mayo Clinic Study of Aging. J Gerontol A Biol Sci Med Sci. 2013;68:929–937. doi: 10.1093/gerona/gls256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Watson NL, Rosano C, Boudreau RM, et al. ; Health ABC Study Executive function, memory, and gait speed decline in well-functioning older adults. J Gerontol A Biol Sci Med Sci. 2010;65:1093–1100. doi: 10.1093/gerona/glq111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gale CR, Allerhand M, Sayer AA, Cooper C, Deary IJ. The dynamic relationship between cognitive function and walking speed: the English Longitudinal Study of Ageing. Age (Dordr). 2014;36:9682. doi: 10.1007/s11357-014-9682-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Krall JR, Carlson MC, Fried LP, Xue QL. Examining the dynamic, bidirectional associations between cognitive and physical functioning in older adults. Am J Epidemiol. 2014;180:838–846. doi: 10.1093/aje/kwu198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Atkinson HH, Rapp SR, Williamson JD, et al. The relationship between cognitive function and physical performance in older women: results from the Women’s Health Initiative Memory Study. J Gerontol A Biol Sci Med Sci. 2010;65:300–306. doi: 10.1093/gerona/glp149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Clouston SA, Brewster P, Kuh D, et al. The dynamic relationship between physical function and cognition in longitudinal aging cohorts. Epidemiol Rev. 2013;35:33–50. doi: 10.1093/epirev/mxs004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Montero-Odasso M, Verghese J, Beauchet O, Hausdorff JM. Gait and cognition: a complementary approach to understanding brain function and the risk of falling. J Am Geriatr Soc. 2012;60:2127–2136. doi: 10.1111/j.1532-5415.2012.04209.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Elbaz A, Vicente-Vytopilova P, Tavernier B, et al. Motor function in the elderly: evidence for the reserve hypothesis. Neurology. 2013;81:417–426. doi: 10.1212/WNL.0b013e31829d8761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Atkinson HH, Rosano C, Simonsick EM, et al. ; Health ABC Study Cognitive function, gait speed decline, and comorbidities: The Health, Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2007;62:844–850. doi: 10.1093/gerona/62.8.844 [DOI] [PubMed] [Google Scholar]
- 20. Vinke EJ, de Groot M, Venkatraghavan V, et al. Trajectories of imaging markers in brain aging: the Rotterdam Study. Neurobiol Aging. 2018;71:32–40. doi: 10.1016/j.neurobiolaging.2018.07.001 [DOI] [PubMed] [Google Scholar]
- 21. Ikram MA, Brusselle GGO, Murad SD, et al. The Rotterdam Study: 2018 update on objectives, design and main results. Eur J Epidemiol. 2017;32:807–850. doi: 10.1007/s10654-017-0321-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Licher S, Darweesh SKL, Wolters FJ, et al. Lifetime risk of common neurological diseases in the elderly population. J Neurol Neurosurg Psychiatry. 2019;90:148–156. doi: 10.1136/jnnp-2018-318650 [DOI] [PubMed] [Google Scholar]
- 23. Welsh KA, Butters N, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology. 1994;44:609–614. doi: 10.1212/wnl.44.4.609 [DOI] [PubMed] [Google Scholar]
- 24. Smith A. The symbol-digit modalities test: a neuropsychologic test of learning and other cerebral disorders. In: Helmuth J, ed. Learning Disorders. Seattle: Special Child Publications; 1968:83–91. [Google Scholar]
- 25. Stroop JR. Studies of interference in serial verbal reactions. J Experimen Psychol. 1935;18:643–662. doi: 10.1037/h0054651 [DOI] [Google Scholar]
- 26. Brand N, Jolles J. Learning and retrieval rate of words presented auditorily and visually. J Gen Psychol. 1985;112:201–210. doi: 10.1080/00221309.1985.9711004 [DOI] [PubMed] [Google Scholar]
- 27. Killgore WD, Glahn DC, Casasanto DJ. Development and validation of the Design Organization Test (DOT): a rapid screening instrument for assessing visuospatial ability. J Clin Exp Neuropsychol. 2005;27:449–459. doi: 10.1080/13803390490520436 [DOI] [PubMed] [Google Scholar]
- 28. van der Elst W, van Boxtel MP, van Breukelen GJ, Jolles J. The Letter Digit Substitution Test: normative data for 1,858 healthy participants aged 24-81 from the Maastricht Aging Study (MAAS): influence of age, education, and sex. J Clin Exp Neuropsychol. 2006;28:998–1009. doi: 10.1080/13803390591004428 [DOI] [PubMed] [Google Scholar]
- 29. Franzen MD, Tishelman AC, Sharp BH, Friedman AG. An investigation of the test-retest reliability of the Stroop Color-Word Test across two intervals. Arch Clin Neuropsychol. 1987;2:265–272. [PubMed] [Google Scholar]
- 30. Lezak MD, Howieson DB, Loring DW, Fisher JS Neuropsychological Assessment. Oxford University Press; 2004. [Google Scholar]
- 31. Van der Elst W, van Boxtel MP, van Breukelen GJ, Jolles J. Rey’s Verbal Learning Test: normative data for 1855 healthy participants aged 24-81 years and the influence of age, sex, education, and mode of presentation. J Int Neuropsychol Soc. 2005;11:290–302. doi: 10.1017/S1355617705050344 [DOI] [PubMed] [Google Scholar]
- 32. MacKinnon DP, Geiselman RE, Woodward JA. The effects of effort on stroop interference. Acta Psychol (Amst). 1985;58:225–235. doi: 10.1016/0001-6918(85)90022-8 [DOI] [PubMed] [Google Scholar]
- 33. Desrosiers J, Hébert R, Bravo G, Dutil E. The Purdue Pegboard Test: normative data for people aged 60 and over. Disabil Rehabil. 1995;17:217–224. doi: 10.3109/09638289509166638 [DOI] [PubMed] [Google Scholar]
- 34. Verlinden VJ, van der Geest JN, Hofman A, Ikram MA. Cognition and gait show a distinct pattern of association in the general population. Alzheimers Dement. 2014;10:328–335. doi: 10.1016/j.jalz.2013.03.009 [DOI] [PubMed] [Google Scholar]
- 35. Verlinden VJ, van der Geest JN, Hoogendam YY, Hofman A, Breteler MM, Ikram MA. Gait patterns in a community-dwelling population aged 50 years and older. Gait Posture. 2013;37:500–505. doi: 10.1016/j.gaitpost.2012.09.005 [DOI] [PubMed] [Google Scholar]
- 36. Wingbermühle R, Wen KX, Wolters FJ, Ikram MA, Bos D. Smoking, APOE genotype, and cognitive decline: the Rotterdam Study. J Alzheimers Dis. 2017;57:1191–1195. doi: 10.3233/JAD-170063 [DOI] [PubMed] [Google Scholar]
- 37. Slooter AJ, Cruts M, Kalmijn S, et al. Risk estimates of dementia by apolipoprotein E genotypes from a population-based incidence study: the Rotterdam Study. Arch Neurol. 1998;55:964–968. doi: 10.1001/archneur.55.7.964 [DOI] [PubMed] [Google Scholar]
- 38. Pinheiro J, Bates D, DebRoy S, Sarkar D; R Development Core Team nlme: linear and nonlinear mixed effects models. R package version 3.1-108. 2013. https://CRAN.R-project.org/package=nlme [Google Scholar]
- 39. van Buuren S, Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67. [Google Scholar]
- 40. Camicioli R, Howieson D, Oken B, Sexton G, Kaye J. Motor slowing precedes cognitive impairment in the oldest old. Neurology. 1998;50:1496–1498. doi: 10.1212/wnl.50.5.1496 [DOI] [PubMed] [Google Scholar]
- 41. Deshpande N, Metter EJ, Bandinelli S, Guralnik J, Ferrucci L. Gait speed under varied challenges and cognitive decline in older persons: a prospective study. Age Ageing. 2009;38:509–514. doi: 10.1093/ageing/afp093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Buracchio T, Dodge HH, Howieson D, Wasserman D, Kaye J. The trajectory of gait speed preceding mild cognitive impairment. Arch Neurol. 2010;67:980–986. doi: 10.1001/archneurol.2010.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Morris R, Lord S, Lawson RA, et al. Gait rather than cognition predicts decline in specific cognitive domains in early Parkinson’s disease. J Gerontol A Biol Sci Med Sci. 2017;72:1656–1662. doi: 10.1093/gerona/glx071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Callisaya ML, Blizzard CL, Wood AG, Thrift AG, Wardill T, Srikanth VK. Longitudinal relationships between cognitive decline and gait slowing: the Tasmanian Study of Cognition and Gait. J Gerontol A Biol Sci Med Sci. 2015;70:1226–1232. doi: 10.1093/gerona/glv066 [DOI] [PubMed] [Google Scholar]
- 45. Tabbarah M, Crimmins EM, Seeman TE. The relationship between cognitive and physical performance: MacArthur Studies of Successful Aging. J Gerontol A Biol Sci Med Sci. 2002;57:M228–M235. doi: 10.1093/gerona/57.4.m228 [DOI] [PubMed] [Google Scholar]
- 46. Soumaré A, Tavernier B, Alpérovitch A, Tzourio C, Elbaz A. A cross-sectional and longitudinal study of the relationship between walking speed and cognitive function in community-dwelling elderly people. J Gerontol A Biol Sci Med Sci. 2009;64:1058–1065. doi: 10.1093/gerona/glp077 [DOI] [PubMed] [Google Scholar]
- 47. Inzitari M, Newman AB, Yaffe K, et al. Gait speed predicts decline in attention and psychomotor speed in older adults: the Health Aging and Body Composition Study. Neuroepidemiology. 2007;29:156–162. doi: 10.1159/000111577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Verghese J, Wang C, Lipton RB, Holtzer R, Xue X. Quantitative gait dysfunction and risk of cognitive decline and dementia. J Neurol Neurosurg Psychiatry. 2007;78:929–935. doi: 10.1136/jnnp.2006.106914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ikram MA, Vrooman HA, Vernooij MW, et al. Brain tissue volumes in the general elderly population. The Rotterdam Scan Study. Neurobiol Aging. 2008;29:882–890. doi: 10.1016/j.neurobiolaging.2006.12.012 [DOI] [PubMed] [Google Scholar]
- 50. Cremers LG, de Groot M, Hofman A, et al. Altered tract-specific white matter microstructure is related to poorer cognitive performance: The Rotterdam Study. Neurobiol Aging. 2016;39:108–117. doi: 10.1016/j.neurobiolaging.2015.11.021 [DOI] [PubMed] [Google Scholar]
- 51. Ikram MA, Vrooman HA, Vernooij MW, et al. Brain tissue volumes in relation to cognitive function and risk of dementia. Neurobiol Aging. 2010;31:378–386. doi: 10.1016/j.neurobiolaging.2008.04.008 [DOI] [PubMed] [Google Scholar]
- 52. Rosano C, Aizenstein H, Brach J, Longenberger A, Studenski S, Newman AB. Special article: gait measures indicate underlying focal gray matter atrophy in the brain of older adults. J Gerontol A Biol Sci Med Sci. 2008;63:1380–1388. doi: 10.1093/gerona/63.12.1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Verlinden VJ, de Groot M, Cremers LG, et al. Tract-specific white matter microstructure and gait in humans. Neurobiol Aging. 2016;43:164–173. doi: 10.1016/j.neurobiolaging.2016.04.005 [DOI] [PubMed] [Google Scholar]
- 54. Alattar AA, Bergstrom J, Laughlin GA, et al. Hearing impairment and cognitive decline in older, community-dwelling adults. J Gerontol A Biol Sci Med Sci. 2019;75:567–573. doi: 10.1093/gerona/glz035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schubert CR, Cruickshanks KJ, Fischer ME, et al. Sensorineural impairments, cardiovascular risk factors, and 10-year incidence of cognitive impairment and decline in midlife: the Beaver Dam Offspring Study. J Gerontol A Biol Sci Med Sci. 2019;74:1786–1792. doi: 10.1093/gerona/glz011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. van der Willik KD, Schagen SB, Ikram MA. Cancer and dementia: two sides of the same coin? Eur J Clin Invest. 2018;48:e13019. doi: 10.1111/eci.13019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Federman AD, Wolf MS, Sheng T, O’Conor R, Martynenko M, Wisnivesky J. Diminished cognitive function among chronic obstructive pulmonary disease patients during periods of acute illness exacerbation. J Gerontol A Biol Sci Med Sci. 2016;71:279–280. doi: 10.1093/gerona/glv200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Scherer SA, Bainbridge JS, Hiatt WR, Regensteiner JG. Gait characteristics of patients with claudication. Arch Phys Med Rehabil. 1998;79:529–531. doi: 10.1016/s0003-9993(98)90067-3 [DOI] [PubMed] [Google Scholar]
- 59. Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer’s disease. J Am Med Assoc. 1994;271:1004–1010. [PubMed] [Google Scholar]
- 60. Valenzuela MJ, Sachdev P. Brain reserve and cognitive decline: a non-parametric systematic review. Psychol Med. 2006;36:1065–1073. doi: 10.1017/S0033291706007744 [DOI] [PubMed] [Google Scholar]
- 61. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390:2673–2734. doi: 10.1016/S0140-6736(17)31363-6 [DOI] [PubMed] [Google Scholar]
- 62. Euser SM, Schram MT, Hofman A, Westendorp RG, Breteler MM. Measuring cognitive function with age: the influence of selection by health and survival. Epidemiology. 2008;19:440–447. doi: 10.1097/EDE.0b013e31816a1d31 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.