Abstract
Background and Aim
While dietary exposure to microplastics is increasingly recognized, it is unknown if ingested plastics remain within the digestive tract. We aimed to examine human colectomy specimens for microplastics and to report the characteristics as well as polymer composition of the particles.
Methods
Colectomy samples were obtained from 11 adults (mean age 45.7, six males) who were residents of Northeastern Peninsular Malaysia. Microplastics were identified following chemical digestion of specimens and subsequent filtration. The samples were then examined for characteristics (abundance, length, shape, and color) and composition of three common polymer types using stereo‐ and Fourier Transform InfraRed (FTIR) microscopes.
Results
Microplastics were detected in all 11 specimens with an average of 331 particles/individual specimen or 28.1 ± 15.4 particles/g tissue. Filaments or fibers accounted for 96.1% of particles, and 73.1% of all filaments were transparent. Out of 40 random filaments from 10 specimens (one had indeterminate spectra patterns), 90% were polycarbonate, 50% were polyamide, and 40% were polypropylene.
Conclusion
Our study suggests that microplastics are ubiquitously present in the human colon.
Keywords: cancer, colectomy, human, microplastic
Eleven human colectomy samples (mean age 45.7 years old, six males) were obtained due to clinical indications (nine colon cancer and two normal histology). All 11 specimens contained microplastics (average 331 particles per individual specimen or 28.1 ± 15.4 particles per g of tissue). Microplastics are ubiquitously present in the human colon.
Introduction
Since the 1950s, billions of tons of plastic waste have been indiscriminately disposed in the environment, ending up in remote locations and oceans. 1 , 2 Over time, some of these disposed plastics are degraded into microplastics (broadly defined as size <5 mm). 1 Microplastics pollution is a significant environmental issue in the Southeast Asia region. 2 , 3 For example, a recent study showed that microplastics were detected in abundance at the marine shores of Kuala Nerus and Kuantan, which are located on the east coast of Peninsular Malaysia. 4 There were also abundant microplastics found in local marine species; this included keystone species that are crucial in maintaining water quality. 5 Microplastics were also identified in cage‐cultured Asian sea bass. 6 Microplastics exist within the human food chain not only as a result of ingesting contaminated cetaceans and fish 7 ; microplastics are also disseminated through atmospheric transport from inland trash (e.g. landfills or indiscriminate waste), or even from everyday plastic materials (e.g. food packaging) and clothes. 8 , 9 , 10
There are increasing reports on potential human exposure to plastics in the food chain, 11 , 12 , 13 and a recently published study detected microplastics in eight human stool samples which was presumed to be due to ingestion of plastics from different sources. 14 Ingested microplastics have been shown to cause adverse bowel consequences in marine organisms. 15 For instance, in zebrafish, microplastics were found to cause inflammation and oxidative stress within gut tissue. 16 However, it is unknown if microplastics remain within the human digestive tract for prolonged periods after dietary exposure; and if so, the potential health impacts these microplastics may cause, including the possibility of malignancies. 17
Hence, in the present study, we aimed to examine human colectomy specimens for the presence of microplastics, particle characteristics, and polymer composition. These samples were readily available for research as colectomies are routinely performed in patients with colorectal malignancies.
Methods
Participants were recruited from Hospital Universiti Sains Malaysia (USM), a tertiary care center situated in the northeastern coastal region of Peninsular Malaysia (Fig. 1). Patients (≥18 years) who were scheduled for colectomy were recruited sequentially for this study. Clinical indications for colectomy included colorectal cancer and non‐cancer (e.g. bleeding arteriovenous malformation, colonic perforation, and trauma) diagnoses. Prior to colectomy, potential subjects were counseled, and informed consent was obtained. Colectomy was performed as per standard surgical practice. A portion of the colectomy specimen was then harvested for study analysis, the remaining sample was then sent for routine histopathological processing. Cautionary measures were taken during specimen harvesting by investigators to avoid possible contact with common plastics in the operation theater, which included sutures, mesh, and containers. All tissue samples were placed in formalin except for one tissue sample (stored on filter paper enclosed in a glass petri dish) during laboratory transport. The formalin and filter paper were thoroughly checked for microplastic contamination before use.
Figure 1.
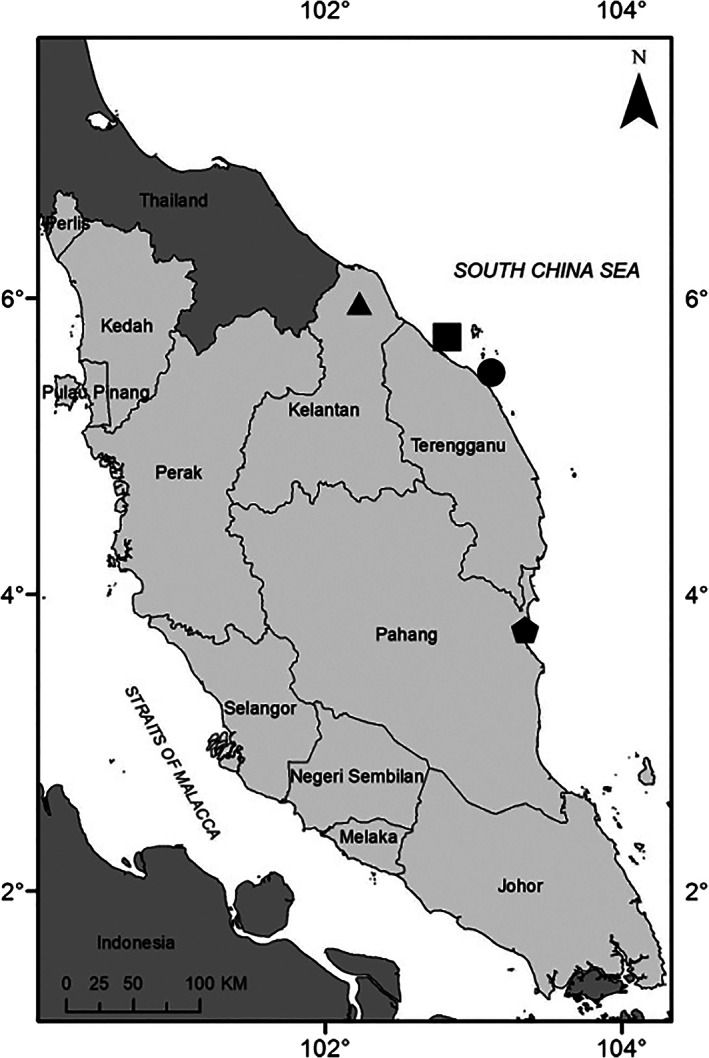
Map of Peninsular Malaysia. Sites of microplastics research in the pristine northeastern coast that include ( ) Kota Bharu (current study), (
) Kota Bharu (current study), ( ) Setiu wetlands, (
) Setiu wetlands, ( ) Kuala Nerus and (
) Kuala Nerus and ( ) Kuantan Port.
) Kuantan Port.
Sample preparation as well as techniques to quantify and identify polymers in microplastics were adapted from previously described methods which were used for marine organisms. 18 Weighed tissue were chemically digested with 10% potassium hydroxide at 60°C for 7–10 h. The digested samples were then diluted with deionized water to prevent clogging during filtration, filtered using 0.45 μm cellulose membrane paper (Whatman, Merck KGaA, Darmstadt, Germany) and subsequently dried in a glass desiccator with silica gel at room temperature over 2 days. Following this, using a stereo dissecting microscope with a 7:1 zoom ratio and a magnification of 0.8–5.6× (model SZX7, Olympus Corp., Tokyo, Japan), the abundance and characteristics (length, shape, and colors) of the microplastic particles that had the longest average length were determined. Micrographs of the microplastic particles were captured using microscope cameras (STEMI2000‐c, Zeiss, Oberkochen, Germany) and ScopePad‐500 (Yenway Microscopes, United Kingdom) at magnifications of 0.6‐5×. Using a scanning electron microscope with energy‐dispersive X‐ray (SEM/EDX) analysis (JOEL JSM‐6360LA, JEOL Ltd., Japan), the surface morphology and elemental composition (carbon counts) of the samples were also studied. Finally, the composition of three common polymers that is, polycarbonate (PC), polypropylene (PP), and polyamide (PA) in microplastics were identified for each specimen. These polymers were chosen not only because they are found abundantly in water and marine organisms but also because of their potential effects on human health. 19 , 20 To distinguish plastic from natural particles, especially smaller microplastics, a representative random subset of samples (10–30% of the total microplastic count) were subjected to a hot needle test which were then validated by micro‐FTIR spectroscopy. 4 , 21 , 22 , 23 , 24 , 25 In addition, to determine the presence of three common polymers that is, PA, PC, and PP, at least four representative filaments were chosen randomly from each specimen. In brief, plastic (but not natural) particles will melt when subjected to a hot needle test. A standalone micro‐Fourier Transform InfraRed (FTIR) microscope (model LUMOS, Bruker Optics Inc., MA, USA) in Attenuated Total Reflectance mode was used to identify the polymer types by recording the spectra in the mid‐IR range of 4000–400 cm−1 (60 scans per analysis). The recorded images and spectra were processed using the OPUS/IR Package Version 8.0 software, which identifies characteristic wavelengths and compares the recorded spectra against those from a reference library.
As potential airborne contamination was a significant concern, the following preventive steps were rigorously undertaken during laboratory experiments: (i) cotton lab gowns and latex gloves were worn during laboratory work, (ii) all liquid reagents and media were filtered before use, (iii) when not in use, samples were covered with a lid or aluminum foil, (iv) each test apparatus would be cleaned with distilled or deionized water before the experiment, (v) sorting of samples were performed inside contamination chambers, and (vi) use of plastic‐containing apparatus was kept to a bare minimum. In addition, during microscopic analysis, dampened and exposed filter papers adjacent to the sample dishes as well as systematic blanks were used for quality control.
All data were entered into and analyzed using SPSS version 22 (SPSS Inc., Chicago, IL, USA). Numerical data were presented as mean and SD unless otherwise stated.
Approval was obtained from the Human Research Ethics Committee of Universiti Sains Malaysia (USM) (reference: USM/JEPeM/19010050).
Results
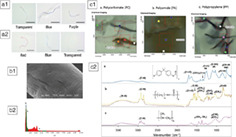
Samples were obtained from 11 participants (mean age 45.7 ± 17.9 years, age range 34–88 years, six males); nine subjects had colorectal cancer while two had a normal colon (Table 1). All systematic blanks were negative. The mean weight of colectomy samples was 13.4 ± 4.5 g. All samples had evidence of microplastics with an average count of 331 per individual or 28.1 ± 15.4 particles per g of colon tissue. The filament form accounted for 96.1% of all samples. The filamentous particles came in different colors including transparent, black, red, green, blue, brown, purple, and yellow (Fig. 2a). Overall, transparent filaments accounted for 73.1% of all colors. The longest dimension of particles ranged from 0.8 to 1.6 mm with an average of 1.1 ± 0.3 mm. Figure 2b shows the typical morphology and the high carbon counts of polymeric microplastics observed under SEM/EDX. Polymers were identified in a subset of samples, except for one sample because of an indeterminate spectra pattern. Of the subset of 40 randomly chosen filaments from 10 specimens (eight cancer and two normal subjects), 90% were PCs, 50% were PAs 50%, and 40% were PPs (Fig. 2c).
Table 1.
Characteristics of study participants
| Age (years) | Sex | Colectomy diagnosis | Weight of sample (g) | Microplastic abundance | Transparent filaments abundance | % of transparent filaments | Polymer content |
|---|---|---|---|---|---|---|---|
| 34 | Female | Moderately differentiated adenocarcinoma | 17.0 | 35 | 20 | 57.1 | Polycarbonate |
| 88 | Female | Moderately differentiated adenocarcinoma | 17.3 | 214 | 115 | 54.0 | Polycarbonate, polypropylene |
| 35 | Male | Poorly differentiated adenocarcinoma with mucinous and signet‐ring component | 15.8 | 450 | 407 | 91.5 | Polycarbonate, polyamide |
| 63 | Female | Moderately differentiated adenocarcinoma | 15.9 | 563 | 528 | 95.1 | Polycarbonate, polyamide |
| 63 | Female | Moderately differentiated adenocarcinoma | 4.7 | 202 | 104 | 55.0 | Polycarbonate |
| 34 | Male | Moderately differentiated adenocarcinoma | 14.8 | 615 | 285 | 66.3 | Polycarbonate, polyamide |
| 48 | Female | Poorly differentiated neuroendocrine carcinoma | 6.4 | 331 | 192 | 58.0 | Not available |
| 41 | Male | Mucinous adenocarcinoma with carcinomatous peritonei | 11.0 | 375 | 317 | 84.5 | Polycarbonate, polyamide |
| 36 | Male | Signet ring carcinoma | 17.0 | 145 | 87 | 60.0 | Polyamide, polypropylene |
| 67 | Male | Bleeding arteriovenous malformation | 16.7 | 430 | 385 | 93.0 | Polypropylene |
| 34 | Male | Normal perforated colon with background history of inflammatory bowel disease | 10.7 | 278 | 250 | 89.9 | Polycarbonate, polypropylene |
Figure 2.
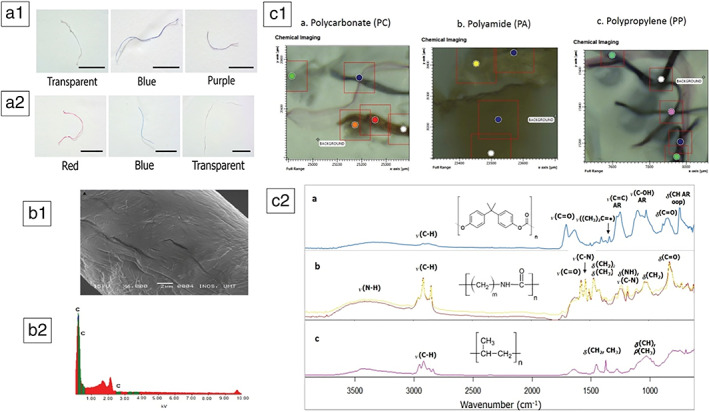
Filament form in various colors detected in (A1) normal colon and (A2) colon cancer; (B1) SEM/DX image of morphology and (B2) carbon counts of a representative plastic sample confirming its polymeric nature. (C1) chemical imaging using micro‐FTIR of the three polymers in filament particles and (C2) respective spectroscopy wavelengths.
Discussion
Our study detected microplastics in colectomy specimens; this indicates the ubiquitous nature of microplastics in our digestive tract and corroborates recent findings from a study on human stool samples. 14 On average, we detected 331 particles per individual specimen or 28 particles per g of colon tissue. Our study provides evidence supporting the ingestion (and or inhalation) of plastics by humans. 7 , 11 As ours is the first human study using colectomy samples, there are no similar data for comparison; however, recent estimates of human exposure to microplastics might provide some clues. 11 , 12 , 26 For instance, based on 15% of an American citizen's caloric intake, Cox et al. have estimated an annual microplastics ingestion of 39 000 to 52 000 particles, this estimate increases from 74 000 to 121 000 when the inhalation route is included. 11 Zhang et al. have also estimated human microplastics burden through the use of table salt and drinking water as well as inhaled air of (0–7.3) × 104, (0–4.7) × 103 and (0–3.0) × 107 items per person per year, respectively. 26 In a prospective study of 2000 individuals from Iran, there were 650 microplastics or an average of 0.33 particles per individual which were identified from saliva samples. 12 Moreover, existing literature reveals that the abundance of microplastics in the digestive tract seems to vary across different marine organisms; for example, one study states that there were 5.5 particles per marine mammal 27 while another study found 2 particles per fish. 28 In human stool, however, there was a median of 20 pieces of microplastic per 10 g of stool. 14
Subjects included in our study were long‐time coastal residents where seafood and fish sauce are the dietary norm. 29 , 30 Previous studies have shown that even at pristine shores, microplastics could be found in abundance 4 , 31 which was probably associated with the use of fishing nets, plastic fishing gear, food packaging, and also the likelihood of airborne particles. 5 , 32 Subsequent ingestion of these microplastics by zooplankton and other marine organisms for example, Scapharca cornea would then introduce plastics into the food web of nearby residents. 5 , 32 Therefore, the plastic particles found in the colonic samples of our subjects could be explained by exposure to microplastics contained in contaminated seafood or similarly sourced foods. However, it is important to note that even though bivalve consumption may be a major exposure pathway, inhalation of microplastics should also be considered as a significant contributing factor. Contamination from the operation theater environment, during transportation or within the laboratory cannot be totally excluded. However, the negative procedural blanks does provide assurance on the quality of this study.
Filament or fiber was the most common shape found in our study. This observation is similar to studies on the environment and other studies on cetaceans, fish, and mammals 15 which further support the validity of our findings. In contrast, fragments and films were more common than fibers in the study on human stools. 14 We cannot explain this disparity; we did not collect stool samples from our subjects for comparison. Instead of colored filaments commonly found in marine organisms, 33 our human samples had more transparent filaments. We hypothesized that ingested colored microplastics may be later “bleached” by digestive enzymes during prolonged colonic transit within humans. While we are unsure of the actual bleaching agent(s) or processes in‐vivo, we postulate that bile salts could be involved. Future in‐vitro tests with microplastics exposed to bile aspirated during endoscopy could help determine if bleaching of colors occurs over time.
In humans, toxicity from microplastics exposure is still unclear due to limited data. Based on ecotoxicology studies in marine species, microplastics could elicit gut inflammation through changes in intestinal permeability and dysbiosis. 16 From studies in human cell lines, including a recent report on polystyrene, some degree of cytotoxicity by microplastics have been documented especially at high concentration and with smaller particles. 20 , 34 , 35 Furthermore, evidence from occupational risk studies indicate that exposure to plastics might be a potential cause of colorectal cancer. 36 , 37 Although our study reported the presence of microplastics in human colon, the relationship between microplastics exposure and colon cancer in humans remains speculative. However, individuals with increased intestinal permeability for example, in those with inflammatory bowel diseases might have a higher risk of microplastic particle translocation, especially into deeper tissue and blood vessels which could potentially result in systemic adverse effects. 38
We would like to highlight several physical characteristics of the polymers that were identified in this study. PC is a durable and robust thermoplastic polymer commonly found in electronic components and construction materials, but also individual drinking bottles and food containers. Bisphenol‐A, a by‐product of PC, is a known endocrine disruptor that may cause colon cancer. 39 PA is a component of nylon, commonly found in fishnets, and may cause direct inflammatory responses in the colon and indirectly from chemical additives. 40 PP is a thermoplastic polymer and because of its inert character, PP is commonly used in medical applications which include sutures and meshes. In this present study, we are not able to explain the differences in the relative abundance of these various polymers. It is postulated that differential degradation of polymers by gastric and intestinal secretions may be a factor; however, further studies are needed to prove this.
We acknowledge that there are limitations to our study. Colectomy specimens were only obtained as part of the subject's clinical management, which explains the small sample size in our study. While stool analysis is not absolutely necessary, if available this may assist in explaining the possible discrepancy in polymer characteristics found in stool versus those from colonic mucosal samples. For example, PC was most abundant in our colonic specimens; however, stool studies from Schwabl et al. found that PP was the most frequent polymer detected. 14 We also did not attempt to determine the dietary sources of microplastics; this could have involved seafood, food packaging, or even airborne particles. In view of this, having a food diary which lists down what the subjects consumed may be helpful for future studies. Only a subset of filaments and limited number of polymer types were investigated due to the time‐consuming nature of processing the samples; there were also financial constraints. Due to the exploratory nature of our study and the limitations listed above, further confirmatory analysis is needed using specimens from a larger human cohort. Future research should include concentrations of colonic microplastics in normal and colorectal carcinoma subjects, or even in those with other bowel ailments to elucidate the cut‐off points of these plastic particles and their relation to colonic diseases.
In conclusion, our preliminary study provides evidence that microplastics exist in human colonic tissue samples.
Acknowledgments
The work was supported by the Ministry of Higher Education (MOHE) of Malaysia through the following research grants; FRGS 59457 and FRGS 203.PPSP.6171192. We wish to acknowledge UMT (Centre Lab & INOS) and USM for providing research facilities, and UNESCO/IOC WESTPAC for training as well as related technical support.
Declaration of conflict of interest: None.
Author contribution: Yusof Shuaib Ibrahim and Yeong Yeh Lee were responsible for the conception and design of the work. Andee Dzulkarnaen, Zaidi Zakaria, Nazri Mustaffa, and Sharifah Emilia Tuan Sharif were involved in sample acquisition and processing. All authors performed the data analysis and interpretation. Yusof Shuaib Ibrahim, Nazri Mustaffa, and Yeong Yeh Lee wrote the manuscript and all authors approved the final version.
References
- 1. Ostle C, Thompson RC, Broughton D, Gregory L, Wootton M, Johns DG. The rise in ocean plastics evidenced from a 60‐year time series. Nat. Commun. 2019; 10: 1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cai M, He H, Liu M et al Lost but can't be neglected: huge quantities of small microplastics hide in the South China Sea. Sci. Total Environ. 2018; 633: 1206–16. [DOI] [PubMed] [Google Scholar]
- 3. El‐Zoghby SM, Soltan EM, Salama HM. Impact of the COVID‐19 pandemic on mental health and social support among adult Egyptians. J. Community Health. 2020; 45: 689–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Khalik WMAWM, Ibrahim YS, Tuan Anuar S, Govindasamy S, Baharuddin NF. Microplastics analysis in Malaysian marine waters: a field study of Kuala Nerus and Kuantan. Mar. Pollut. Bull. 2018; 135: 451–7. [DOI] [PubMed] [Google Scholar]
- 5. Yusof SI, Azmi A, Abdul Shukor S, Anuar S, Abdullah S. Microplastics ingestion by Scapharca cornea at Setiu Wetland, Terengganu, Malaysia. Middle East J. Sci. Res. 2016; 24: 2129–36. [Google Scholar]
- 6. Ibrahim YS, Rathnam R, Anuar ST, Khalik WMAWM. Isolation and characterisation of microplastic abundance in Lates calcarifer from Setiu wetlands, Malaysia Malaysian. J. Anal. Sci. 2017; 21: 1054–64. [Google Scholar]
- 7. Smith M, Love DC, Rochman CM, Neff RA. Microplastics in seafood and the implications for human health. Curr. Environ. Health Rep. 2018; 5: 375–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Allen S, Allen D, Phoenix VR et al Atmospheric transport and deposition of microplastics in a remote mountain catchment. Nat. Geosci. 2019; 12: 339–44. [Google Scholar]
- 9. Thompson RC, Moore CJ, vom Saal FS, Swan SH. Plastics, the environment and human health: current consensus and future trends. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009; 364: 2153–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. He P, Chen L, Shao L, Zhang H, Lü F. Municipal solid waste (MSW) landfill: a source of microplastics? ‐ evidence of microplastics in landfill leachate. Water Res. 2019; 159: 38–45. [DOI] [PubMed] [Google Scholar]
- 11. Cox KD, Covernton GA, Davies HL, Dower JF, Juanes F, Dudas SE. Human consumption of microplastics. Environ. Sci. Technol. 2019; 53: 7068–74. [DOI] [PubMed] [Google Scholar]
- 12. Abbasi S, Turner A. Human exposure to microplastics: a study in Iran. J. Hazard. Mater. 2021; 403: 123799. [DOI] [PubMed] [Google Scholar]
- 13. Zhang K, Shi H, Peng J et al Microplastic pollution in China's inland water systems: a review of findings, methods, characteristics, effects, and management. Sci. Total Environ. 2018; 630: 1641–53. [DOI] [PubMed] [Google Scholar]
- 14. Schwabl P, Köppel S, Königshofer P et al Detection of various microplastics in human stool: a prospective case series. Ann. Intern. Med. 2019; 171: 453–7. [DOI] [PubMed] [Google Scholar]
- 15. Cole M, Lindeque P, Halsband C, Galloway TS. Microplastics as contaminants in the marine environment: a review. Mar. Pollut. Bull. 2011; 62: 2588–97. [DOI] [PubMed] [Google Scholar]
- 16. Qiao R, Sheng C, Lu Y, Zhang Y, Ren H, Lemos B. Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Sci. Total Environ. 2019; 662: 246–53. [DOI] [PubMed] [Google Scholar]
- 17. Prata JC, da Costa JP, Lopes I, Duarte AC, Rocha‐Santos T. Environmental exposure to microplastics: an overview on possible human health effects. Sci. Total Environ. 2020; 702: 134455. [DOI] [PubMed] [Google Scholar]
- 18. Masura J, Baker JE, Foster GD, Arthur C, Herring C. Laboratory methods for the analysis of microplastics in the marine environment: recommendations for quantifying synthetic particles in waters and sediments. NOAA Tech. Memo NOS‐OR&R‐48, 2015.
- 19. Erni‐Cassola G, Zadjelovic V, Gibson MI, Christie‐Oleza JA. Distribution of plastic polymer types in the marine environment: a meta‐analysis. J. Hazard. Mater. 2019; 369: 691–8. [DOI] [PubMed] [Google Scholar]
- 20. Campanale C, Massarelli C, Savino I, Locaputo V, Uricchio VF. A detailed review study on potential effects of microplastics and additives of concern on human health. Int. J. Environ. Res. Public Health. 2020; 17: 1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Daniel DB, Ashraf PM, Thomas SN. Abundance, characteristics and seasonal variation of microplastics in Indian white shrimps (Fenneropenaeus indicus) from coastal waters off Cochin, Kerala, India. Sci. Total Environ. 2020; 737: 139839. [DOI] [PubMed] [Google Scholar]
- 22. Galgani F, Hanke G, Werner S et al Guidance on monitoring of marine litter in European seas publications office of the European Union. Luxembourg: Publications Office of the European Union, 2013; 128 10.2788/99475. [DOI] [Google Scholar]
- 23. Hossain MS, Sobhan F, Uddin MN et al Microplastics in fishes from the Northern Bay of Bengal. Sci. Total Environ. 2019; 690: 821–30. [DOI] [PubMed] [Google Scholar]
- 24. Hossain MS, Rahman MS, Uddin MN et al Microplastic contamination in Penaeid shrimp from the Northern Bay of Bengal. Chemosphere. 2020; 238: 124688. [DOI] [PubMed] [Google Scholar]
- 25. Veerasingam S, Ranjani M, Venkatachalapathy R et al Contributions of Fourier transform infrared spectroscopy in microplastic pollution research: a review. Crit. Rev. Environ. Sci. Technol. 2020: 1–63. 10.1080/10643389.2020.1807450 [DOI] [Google Scholar]
- 26. Zhang Q, Xu EG, Li J et al A review of microplastics in table salt, drinking water, and air: direct human exposure. Environ. Sci. Technol. 2020; 54: 3740–51. [DOI] [PubMed] [Google Scholar]
- 27. Nelms SE, Barnett J, Brownlow A et al Microplastics in marine mammals stranded around the British coast: ubiquitous but transitory? Sci. Rep. 2019; 9: 1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lusher AL, McHugh M, Thompson RC. Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Mar. Pollut. Bull. 2013; 67: 94–9. [DOI] [PubMed] [Google Scholar]
- 29. Ahmad NI, Wan Mahiyuddin WR, Tengku Mohamad TR et al Fish consumption pattern among adults of different ethnics in Peninsular Malaysia. Food Nutr. Res. 2016; 60: 32697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee YY, Ismail AW, Mustaffa N et al Sociocultural and dietary practices among Malay subjects in the north‐eastern region of Peninsular Malaysia: a region of low prevalence of Helicobacter pylori infection. Helicobacter. 2012; 17: 54–61. [DOI] [PubMed] [Google Scholar]
- 31. Lavers JL, Bond AL. Exceptional and rapid accumulation of anthropogenic debris on one of the world's most remote and pristine islands. Proc. Natl. Acad. Sci. 2017; 114: 6052–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Md Amin R, Sohaimi ES, Anuar ST, Bachok Z. Microplastic ingestion by zooplankton in Terengganu coastal waters, southern South China Sea. Mar. Pollut. Bull. 2020; 150: 110616. [DOI] [PubMed] [Google Scholar]
- 33. Barboza LGA, Dick Vethaak A, Lavorante B, Lundebye AK, Guilhermino L. Marine microplastic debris: an emerging issue for food security, food safety and human health. Mar. Pollut. Bull. 2018; 133: 336–48. [DOI] [PubMed] [Google Scholar]
- 34. Hwang J, Choi D, Han S, Jung SY, Choi J, Hong J. Potential toxicity of polystyrene microplastic particles. Sci. Rep. 2020; 10: 7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yong CQY, Valiyaveetill S, Tang BL. Toxicity of microplastics and nanoplastics in mammalian systems. Int. J. Environ. Res. Public Health. 2020; 17: 1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Acquavella JF, Douglass TS, Vernon S, Hughes JI, Thar WE. Assessment of colorectal cancer screening outcomes among workers involved in polypropylene manufacture. J. Occup. Med. 1989; 31: 785–91. [DOI] [PubMed] [Google Scholar]
- 37. Oddone E, Modonesi C, Gatta G. Occupational exposures and colorectal cancers: a quantitative overview of epidemiological evidence. World J. Gastroenterol. 2014; 20: 12431–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schmidt C, Lautenschlaeger C, Collnot EM et al Nano‐ and microscaled particles for drug targeting to inflamed intestinal mucosa: a first in vivo study in human patients. J. Control. Release. 2013; 165: 139–45. [DOI] [PubMed] [Google Scholar]
- 39. Gao H, Yang BJ, Li N et al Bisphenol A and hormone‐associated cancers: current progress and perspectives. Medicine. 2015; 94: e211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McGregor JR, Galloway DJ, Jarrett F, Brown IL, George WD. Anastomotic suture materials and experimental colorectal carcinogenesis. Dis. Colon Rectum. 1991; 34: 987–92. [DOI] [PubMed] [Google Scholar]


