Abstract
Background
The Lifestyle Interventions and Independence for Elders (LIFE) Study physical activity (PA) intervention was found to be cost-effective compared to health education (HE). However, long-term effects postintervention are unknown.
Method
This was a secondary analysis of LIFE Study data linked to Medicare claims data (2014–2016). Participants were linked via Social Security Numbers to Medicare claims data. Utilization and cost variables were analyzed using generalized linear models with negative binomial and Tweedie distributions. Unadjusted means and 95% confidence intervals were compared by year and overall stratified. Each model compared PA versus HE and adjusted for other baseline characteristics and stratified by study site. Additional models were stratified by baseline physical functioning assessment scores.
Results
Of the 1,635 LIFE Study participants, 804 (53.5%) were linked to Medicare claims with an average of 33 months of follow-up time during the 3-year data linkage period. Mean outpatient (6.6 vs 6.8), inpatient (0.40 vs 0.40), and other utilization metrics were similar between PA and HE groups. Costs were also similar for each group and each type of service, for example, outpatient: $2,070 versus $2,093 and inpatient: $4,704 versus $4,792. Regression results indicated no statistically significant differences between PA and HE groups.
Conclusions
While the LIFE Study demonstrated that PA reduced mobility disability in older adults and was cost-effective, it did not appear to affect long-term health care utilization costs posttrial. These findings suggest that it remains challenging to affect long-term health care costs using PA interventions effects.
Keywords: Physical function, Older adults, Health care costs, Health care utilization
The population over 65 years of age in the United States is expected to nearly double from 43 million to roughly 84 million by 2050 (1). Those 65 years and older currently represent around 13% of the population while accounting for 36% of all health expenditures (2). The combination of the overall aging of the population resulting in higher rates of physical and cognitive disability is expected to lead to high health care expenditures in the near future. This poses a serious concern for the U.S. health care system as these patients live longer in a disabled state.
Promoting healthy behaviors that maintain physical and cognitive function in late life has potential to curb the expected growth in health care spending among seniors. One facet of healthy aging is the promotion and prescription of increased physical activity (PA) among seniors to increase independence and overall health by fostering resilience against medical conditions that contribute to physical disability (3). Increased PA may have beneficial effects in a number of disease-specific (osteoarthritis, cardiovascular, respiratory, cancer, metabolic) and nondisease-specific conditions (muscle and cardiovascular fitness) (1,4–8). Increased PA can delay physical disability and improve cognition (2,9), which play a critical role in maintaining independence and high quality of life in the late years of life. Despite some claims that PA would reduce health care cost (10), there remains a lack of clear evidence for long-term benefit.
A cost-effectiveness analysis was conducted for the Lifestyle Interventions and Independence for Elders (LIFE) Study during the trial time frame. Compared to health education (HE), the PA intervention was found to be cost-effective, costing <$50,000 per major mobility disability (MMD) avoided and per quality-adjusted life year (QALY) gained (11). Despite the knowledge gained from the LIFE Study, there are still uncertainties of the long-term health care cost benefits of PA interventions. The LIFE-Pilot Study showed that there are long-lasting differences in activity levels and physical functioning assessments among the PA intervention arm compared to the HE control arm (12). This contradicted results from the main LIFE Study, which showed no lasting behavioral changes in a 12-month posttrial follow-up visit (13). Therefore, while LIFE-Pilot suggests long-term changes that may accumulate long-term beneficial effects on participant health, the LIFE Study did not support this notion. The objective of this study was to evaluate other metrics related to long-term benefits of the PA intervention versus the HE control related to health care utilization and costs among the main LIFE Study participants to further evaluate this research question (14).
Method
LIFE Study Overview
The LIFE Study was a multicenter, single-blind, parallel randomized trial conducted across eight centers in the United States between February 2010 and December 2013 (14). The study protocol was approved by the institutional review boards of each institution. Written informed consent was obtained from all study participants. The LIFE Study was registered with www.clinicaltrials.gov prior to participant enrollment in the trial (NCT01072500). Details of the study design, rationale, and characteristics of the full study population are described elsewhere (14,15).
Intervention
The PA intervention involved walking, with a goal of 150 minutes per week, strength, flexibility, and balance training. The intervention included attendance at two center-based visits per week and home-based activity three to four times per week for the duration of the study. The HE control intervention included weekly educational workshops during the first 26 weeks, and then monthly sessions thereafter. Workshops included topics relevant to older adults, such as how to effectively negotiate the health care system, how to travel safely, preventive services and screenings recommended at different ages, where to go for reliable health information, nutrition, etc. The workshops did not include any topics related to exercise or PA.
Linkage to Medicare Claims Data
The original LIFE Study informed consent included voluntary consent for collection of Social Security Numbers (SSNs) and long-term use for research purposes. Data were held by the data management coordinating site (Wake Forest University) who served as an honest broker for this study. SSNs were sent to the Center for Medicare and Medicaid Services’ (CMS) third-party data vendor for linkage. A cross-walk file between SSNs and Medicare beneficiary identification numbers was returned and a final cross-walk between de-identified LIFE Study identifiers was created. Linked files included all inpatient, outpatient, skill nursing, and hospice administrative claims data for the 2014, 2015, and 2016 calendar years which represented the first full year after LIFE Study termination (December 2013) and the most recent data available.
Linkage success was evaluated based on calendar year enrollment for 12 months or by enrollment until date of death. Participants were required to have Medicare Parts A and B to ensure adequate capture of inpatient and outpatient utilization. Those enrolled in Medicare Part C (Medicare Advantage plans) and those with only Part A were considered not linked.
Health Care Utilization and Cost Analyses
Health care utilization and associated costs were divided by the type of service and included: home health care, hospice, skilled nursing facility, inpatient, and outpatient services. Utilization and costs were evaluated by calendar years, which started in January 1 and followed participants through the whole year or until they were lost to follow-up due to death or disenrollment in Medicare health insurance. All analyses were corrected for differential follow-up using a time offset. The summed total utilization and costs were cumulative for each category. Health care utilization variables were analyzed using a generalized linear model with a log link function and a negative binomial distribution. Health care costs were analyzed using a generalized linear model with a Tweedie distribution.
The primary exposure of interest was the intervention arm assignment (PA or HE) coded as a binary variable. Baseline characteristics included age, sex, race, medical history of heart attack, heart failure, cardiovascular disease, diabetes, arthritis, and chronic lung disease as well as baseline Short Physical Performance Battery (SPPB) score were adjusted and were compared via chi-squared or t tests where appropriate. The base regression models included all study participants and additional subgroup analyses included stratification by baseline SPPB score (≤7 or >7) and whether participants experienced the primary outcome (MMD) during the trial. All analyses were conducted using PROC GENMOD or PROC GLM in SAS version 9.4 (SAS Institute, Cary, NC) with an alpha level of 0.05 for statistical testing.
Results
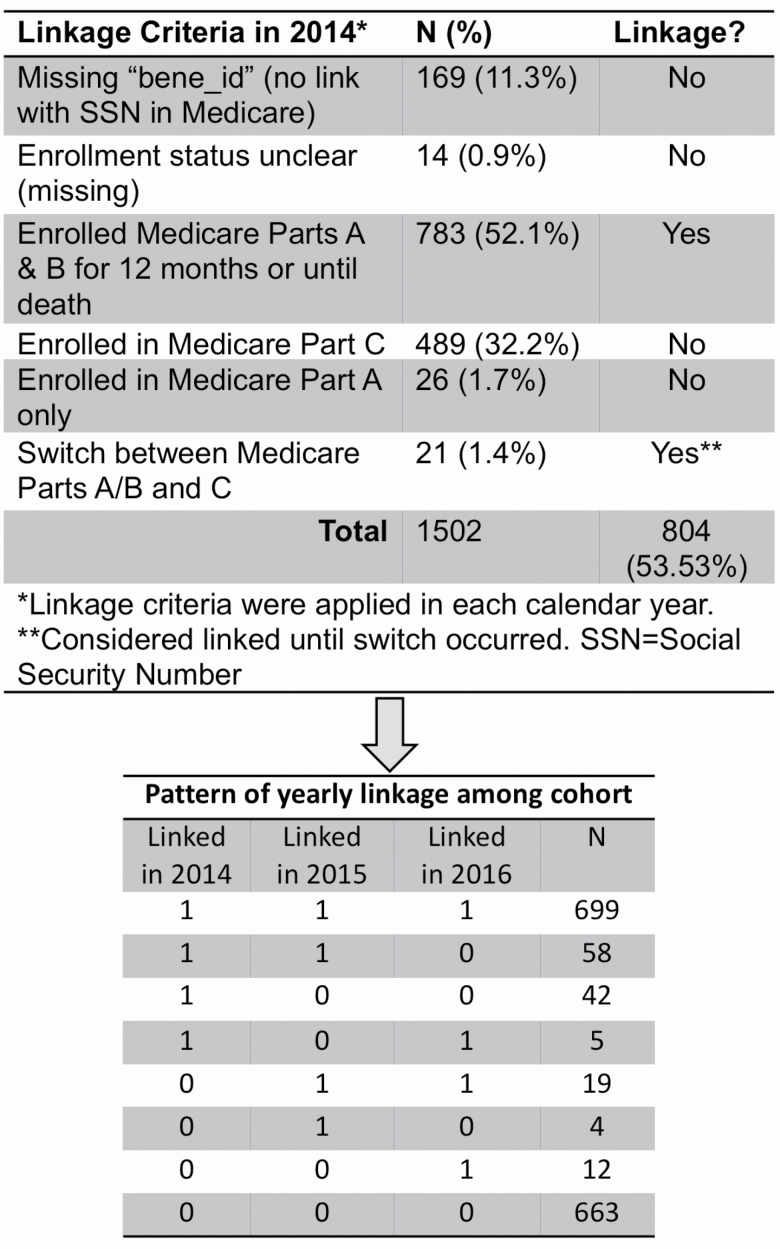
Of the original 1,635 LIFE Study participants, N = 1,502 (91.9%) consented for SSN use for research purposes and were alive at the end of their follow-up period in the LIFE Study (Figure 1). Of these, 183 (12.2%) did not have a Medicare ID match or were not enrolled in the Medicare program. A further 489 were enrolled in Part C plans and 26 (1.7%) were enrolled only in Part A—all of which were excluded due to inability to link to Medicare claims and missing utilization information, respectively. Successful linkages, that is, those with at least one calendar year enrollment in Parts A and B between 2014 and 2016, included 782 participants enrolled for the entire calendar year and 22 participants that were enrolled until they switched plans or died during the year for a total of 804 (53.5%) successful linkage to Medicare claims. Patterns of linkages during this 3-year period included 699 participants enrolled all 3 years (Figure 1).
Figure 1.
Cohort selection, linkage criteria, and linkage pattern for integration of Lifestyle Interventions and Independence for Elders (LIFE) Study trial data and Medicare administrative claims data.
The original LIFE Study intervention arms were compared on baseline information to determine if the randomization of the original trial was still apparent (Table 1). There were few observed differences including slightly higher proportions of diabetes (28.2% vs 21.9%, p = .042) and heart failure (7.4% vs 4.1%, p = .045) in the HE group compared to the PA group. There were no other differences between the two intervention groups and the cohort was interpreted as generally maintaining randomization after data linkage. Follow-up time in the linked data was also similar during the extended follow-up time in linked data at roughly 33 months in each group of the whole 36-month follow-up period. We also compared baseline characteristics among linked and unlinked groups. These groups differed in age, sex, race, and education status (Supplementary Table 1).
Table 1.
Comparison of Select Baseline Characteristic Between Physical Activity (PA) and Health Education (HE) Trial Groups Who Were Successfully Linked to Medicare Claims Data
| Patient characteristics | PA (N = 393) | HE (N = 411) | p Value |
|---|---|---|---|
| Age, mean (SD) | 79.1 (5.3) | 79.4 (5.2) | .3722 |
| Subgroup age | .9084 | ||
| 70–79 | 211 (53.7%) | 219 (53.3%) | |
| ≥80 | 182 (46.3%) | 192 (46.7%) | |
| Female | 264 (67.2%) | 267 (65.0%) | .5079 |
| Race | .5229 | ||
| Black | 59 (15.0%) | 53 (13.0%) | |
| White | 317 (80.7%) | 333 (81.4%) | |
| Other | 17 (4.3%) | 23 (5.6%) | |
| Education, >high school | 282 (71.8%) | 287 (70.0%) | .5842 |
| Smoking | .0826 | ||
| Never | 184 (47.4%) | 222 (55.0%) | |
| Former | 191 (49.2%) | 167 (41.3%) | |
| Current | 13 (3.4%) | 15 (3.7%) | |
| Body mass index | 29.7 (5.6) | 29.9 (6.1) | .6376 |
| Cerebrovascular disease | 106 (27.0%) | 128 (31.1%) | .1930 |
| Diabetes | 86 (21.9%) | 115 (28.2%) | .0417 |
| Heart attack | 33 (8.4%) | 48 (11.7%) | .1195 |
| Heart failure | 16 (4.1%) | 30 (7.4%) | .0450 |
| Arthritis | 74 (19.0%) | 88 (21.5%) | .3812 |
| Chronic lung disease | 69 (17.6%) | 65 (16.0%) | .5480 |
| SPPB ≤7 | 169 (43.0%) | 185 (45.0%) | .5661 |
| Gait speed (m/s) | 0.76 (0.2) | 0.76 (0.2) | .7224 |
| Cognitive assessment (3MSE), mean (SD) | 91.8 (5.1) | 91.8 (5.4) | .9063 |
Notes: PA = physical activity intervention; HE = health education control arm; SD = standard deviation; SPPB = Short Physical Performance Battery; m/s = meters per second; 3MSE = Modified Mini Mental State Examination.
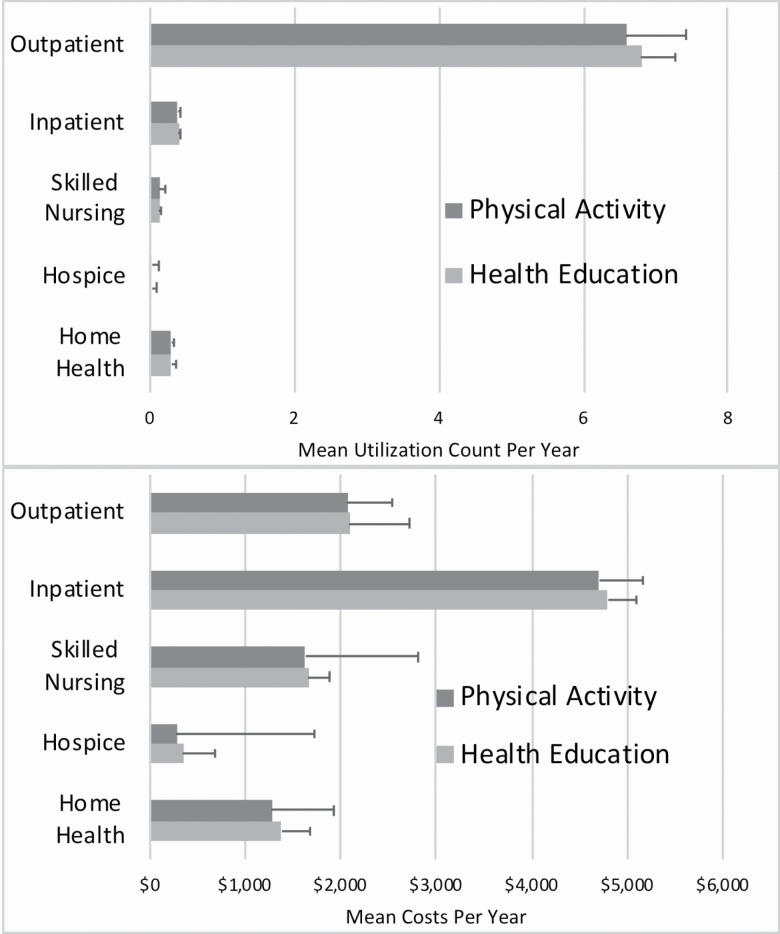
Mean utilization and costs for each type of utilization are reported for the overall group and among those with that type of utilization (Figure 2). Complete summary data overall and by each year with regression estimates are provided in Supplementary Materials. Negative binomial regression models included the main exposure variable of trial intervention group, age, sex, race, medical conditions at baseline, and SPPB score at baseline. Consistently across all measures of utilization, intervention arm (ie, PA vs HE) showed no statistically significant differences. A model with a Tweedie distribution for health care costs also showed no statistically significant differences for the primary comparison between PA and HE groups. Individual year comparisons for utilization and costs further showed no differences. Similar findings were observed when the sample was restricted to participants with SPPB ≤7 or >7 at LIFE Study baseline.
Figure 2.
Mean health care utilization and costs stratified by Lifestyle Interventions and Independence for Elders (LIFE) Study intervention by type of health care service. All comparisons between the physical activity and successful aging trial arms were not statistically significant in unadjusted analyses as well as adjusted regression models controlling for baseline characteristics. Full details are available by year and overall in Supplementary Materials.
Discussion
This long-term posttrial follow-up, enabled via linkage of LIFE Study data to Medicare claims data, showed no long-term effect of the PA intervention versus HE on health care resource utilization or costs. These findings persisted for stratifications by year and by SPPB stratification.
Based on findings from the LIFE-Pilot Study and from an analysis in a nationally representative cohort (12,16), the current study hypothesized that these long-term effects may lead to long-term cumulative health benefits which could be captured via surrogate outcomes of health care utilization and cost. While utilization and cost outcomes are nonspecific and all-cause, long-term benefits associated with PA in cardiovascular disease, reduced falls, and other health and well-being effects should translate to reductions in these metrics. Further, outcomes related to utilization and costs are more relevant to health care payers (ie, health insurance providers, Medicare) as stakeholders and evidence from this perspective could lead to broader implementation of PA interventions (10).
Cost-effectiveness analyses of both LIFE-Pilot and LIFE Study data found that PA was cost-effective compared to HE using common cutoff metrics (17). However, health care costs did not significantly differ between the two intervention groups despite differences in mobility disability. Although costs were based on self-reported utilization is those analyses, the results of those prior health economic analyses suggest that health care costs may be hard to affect with PA interventions in older adults.
In a separate analysis of LIFE Study data, participants in the PA arm did not have significantly improved activity levels (behavioral change) and showed similar physical functioning (functional change) at a 1-year extended follow-up compared to those in the HE control arm (13). The authors pointed out differences between the intervention and study population in LIFE-Pilot and LIFE including key differences in the intervention such as transition to home-based intervention after initial group sessions, and the much longer (24 vs 12 months) intervention which could have led to waning interest. Using accelerometry data, which has been suggested as a means to measure the intensity of PA in older adults for utilization outcomes (18), larger differences in moderate PA were noted between the comparison arms in LIFE-Pilot compared to LIFE (12,13). This led to a pattern of waning PA in the LIFE trial that was not observed in the shorter LIFE-Pilot Study that could have contributed to the lack of long-term benefits (13).
While this long-term extension of the LIFE study via linkage to administrative claims data showed similar effects of the interventions on health care utilization and costs, this does not imply lack of impact of PA. With a 24-month intervention, mean follow-up of 2.9 years, a postintervention follow-up roughly 12 months after that, the length of time between intervention and data linkage here was up to 2–5 years postintervention (2). Thus, any impact of the PA, which was demonstrated for primary and secondary outcomes, may have diminished over this extended period of time. It also suggests that consistency and maintenance of the PA treatment is needed to promote and ensure its long-term benefits (13,19). Further health care utilization and costs are surrogate outcomes for overall health and may not be sensitive enough measures to capture effects of PA interventions.
Limitations
This analysis linked LIFE Study participants who had Medicare FFS benefits. Those that were not linked differed with the linked group in age (1-year mean difference), sex (5% fewer females in linked group), race (more white in linked group), and education (more college educated in linked group). This is likely indicative of the demographic differences in those who enroll in Medicare FFS versus Medicare Advantage plans. We would not expect any other differences between these groups nor for results to be different among those who were not linked after controlling for these characteristics. Lastly, while we measured health care utilization and costs, these measures were all-cause and not cause-specific. A PA intervention may not be suitable for prevention of all conditions in older adults. However, as many events were rare and we had a smaller sample size after linkage, focusing on cause-specific events (eg, falls, fractures, cardiovascular disease) was not possible.
Conclusion
Long-term follow-up of LIFE trial participants via linkage to Medicare claims data did not reveal any differences in health care utilization and costs between a HE and PA intervention. These results imply that effects of such interventions may wane over time, that HE provides similar benefits to PA, or that potential differences are not detectable using utilization and cost metrics.
Funding
This project was supported by a Claude D. Pepper Older American Independence Centers Junior Scholar Award from the University of Florida Institute on Aging through support from the National Institute on Aging at the National Institutes of Health (P30AG028740). The sponsor had no input into the conduct of the study and the result and conclusions of the study do not represent the official views of the sponsor.
Conflict of Interest
None reported.
Supplementary Material
Acknowledgments
Author contributions: J.D.B., T.M., M.P., E.J.G. conceived of and designed the study. J.D.B. and C.-Y.W. conducted data analysis. J.D.B. drafted the initial manuscript. All authors provided feedback to the study and critically revised the study materials.
References
- 1. Kemmler W, von Stengel S, Engelke K, Haberle L, Kalender WA. Exercise effects on bone mineral density, falls, coronary risk factors, and health care costs in older women: the randomized controlled senior fitness and prevention (SEFIP) study. Arch Intern Med. 2010;170:179–185. doi: 10.1001/archinternmed.2009.499 [DOI] [PubMed] [Google Scholar]
- 2. Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311:2387–2396. doi: 10.1001/jama.2014.5616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Williamson J, Pahor M. Evidence regarding the benefits of physical exercise. Arch Intern Med. 2010;170:124–125. doi: 10.1001/archinternmed.2009.491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van der Bij AK, Laurant MG, Wensing M. Effectiveness of physical activity interventions for older adults: a review. Am J Prev Med. 2002;22: 120–133. doi: 10.1016/s0749-3797(01)00413-5 [DOI] [PubMed] [Google Scholar]
- 5. Taylor AH, Cable NT, Faulkner G, Hillsdon M, Narici M, Van Der Bij AK. Physical activity and older adults: a review of health benefits and the effectiveness of interventions. J Sports Sci. 2004;22:703–725. doi: 10.1080/02640410410001712421 [DOI] [PubMed] [Google Scholar]
- 6. Liu-Ambrose T, Nagamatsu LS, Graf P, Beattie BL, Ashe MC, Handy TC. Resistance training and executive functions: a 12-month randomized controlled trial. Arch Intern Med. 2010;170:170–178. doi: 10.1001/archinternmed.2009.494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Varma VR, Tan EJ, Wang T, et al. Low-intensity walking activity is associated with better health. J Appl Gerontol. 2014;33:870–887. doi: 10.1177/0733464813512896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gill TM, Pahor M, Guralnik JM, et al. ; LIFE Study Investigators Effect of structured physical activity on prevention of serious fall injuries in adults aged 70-89: randomized clinical trial (LIFE Study). BMJ. 2016;352:i245. doi: 10.1136/bmj.i245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Etgen T, Sander D, Huntgeburth U, Poppert H, Forstl H, Bickel H. Physical activity and incident cognitive impairment in elderly persons: the INVADE study. Arch Intern Med. 2010;170:186–193. doi: 10.1001/archinternmed.2009.498 [DOI] [PubMed] [Google Scholar]
- 10. Pahor M. Consideration of insurance reimbursement for physical activity and exercise programs for patients with diabetes. JAMA. 2011;305: 1808–1809. doi: 10.1001/jama.2011.572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Groessl EJ, Kaplan RM, Castro Sweet CM, et al. Cost-effectiveness of the LIFE physical activity intervention for older adults at increased risk for mobility disability. J Gerontol A Biol Sci Med Sci. 2016;71:656–662. doi: 10.1093/gerona/glw001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rejeski WJ, Marsh AP, Chmelo E, et al. The Lifestyle Interventions and Independence for Elders Pilot (LIFE-P): 2-year follow-up. J Gerontol A Biol Sci Med Sci. 2009;64:462–467. doi: 10.1093/gerona/gln041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Henderson RM, Miller ME, Fielding RA, et al. Maintenance of physical function 1 year after exercise intervention in at-risk older adults: follow-up from the life study. J Gerontol A Biol Sci Med Sci. 2018;73:688–694. doi: 10.1093/gerona/glx231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fielding RA, Rejeski WJ, Blair S, et al. The Lifestyle Interventions and Independence for Elders Study: design and methods. J Gerontol A Biol Sci Med Sci. 2011;66:1226–1237. doi: 10.1093/gerona/glr123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marsh AP, Lovato LC, Glynn NW, et al. Lifestyle interventions and independence for elders study: recruitment and baseline characteristics. J Gerontol A Biol Sci Med Sci. 2013;68:1549–1558. doi: 10.1093/gerona/glt064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cheng Y, Goodin AJ, Pahor M, Manini T, Brown JD. Healthcare utilization and physical functioning in older adults in the United States. J Am Geriatr Soc. 2020;68:266–271. doi: 10.1111/jgs.16260 [DOI] [PubMed] [Google Scholar]
- 17. Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371:796–797. doi: 10.1056/NEJMp1405158 [DOI] [PubMed] [Google Scholar]
- 18. Resnick B, Boltz M. The impact of psychological status, social well-being, and physical function on healthcare utilization. J Am Geriatr Soc. 2020;68:241–243. doi: 10.1111/jgs.16331 [DOI] [PubMed] [Google Scholar]
- 19. Pahor M, Guralnik JM, Anton SD, et al. Impact and Lessons From the Lifestyle Interventions and Independence for Elders (LIFE) clinical trials of physical activity to prevent mobility disability. J Am Geriatr Soc. 2020; 68(4):872–881. doi: 10.1111/jgs.16365 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.