Abstract
Alcohol is the most ubiquitously consumed and misused mind-altering substance in the world. Various animal models exist to aid in our neurobiological understanding of alcohol addiction. One variable too often taken for granted and not consistently controlled is the “standard” chow diet rodents are maintained on. In this set of experiments, we sought to determine the effect of different commonly used diets on ethanol intake, ethanol preference, and mechanical pain sensitivity in a widely used mouse model of heavy alcohol drinking, the intermittent access to 20% alcohol model. We found that male mice kept on LabDiet 5001 (Diet 2 (LD5001)) and on Teklad Diet 7012 (Diet 3 (H7012)) consistently drank more ethanol than mice kept on Teklad Diet 2918 (Diet 1 (H2918)) as well as compared to mice on LabDiet 5V75 (Diet 4 (LD5V75)). In addition, water intake was consistently lower in mice kept on LabDiet 5001 (Diet 2 (LD5001)), and occasionally in mice kept on Teklad Diet 7012 (Diet 3 (H7012)), compared to the Teklad Diet 2918 (Diet 1 (H2918)) group. We found that male mice showed a strong mechanical allodynia following 8 weeks of intermittent ethanol drinking at 72 hr of withdrawal, compared to water Control mice, regardless of the diet and hence of the different amount of ethanol consumed. Our data provide evidence that the type of rodent diet subject are exposed to is an important variable to report and control, in all ethanol drinking studies.
Keywords: Alcohol, Addiction, Drinking, Dependence, Alcoholism, Animal model, Pain, Hyperalgesia, Food
Introduction
Alcohol is the most ubiquitously consumed and misused mind-altering substance in the world, and it is responsible for 25% of mortality for people aged 20-30 (Hasin and Grant, 2015). Alcohol Use Disorder (AUD), which has a lifetime prevalence in the United States estimated at 29.1% (Hasin and Grant, 2015), is a chronic relapsing condition characterized by compulsive alcohol use, loss of control over alcohol intake, and a negative emotional state when not drinking (Koob and Volkow, 2016). In addition, a large portion of patients seeking treatment for AUD report moderate-to-severe pain, and pain sensitivity has been directly correlated with withdrawal severity (Jochum et al., 2010; Larson et al., 2007). It is, therefore, hypothesized that hyperalgesia may motivate further alcohol consumption (Zale et al., 2015).
Animal models have been crucial for understanding the neurobiology of AUD, as they allow for manipulations of variables not easily changeable in humans. Furthermore, animal models allow researchers to control for a variety of environmental stimuli, including housing conditions (e.g. cage size, number of cage mates, humidity and temperature, etc.). One variable too often taken for granted and not consistently controlled is the laboratory chow diet that the animals are maintained on across laboratories. Unfortunately, no “standard” laboratory chow exists (Barnard et al., 2009). In addition, exact information about the specific chow diet used is rarely reported in manuscripts and, given its influence on a variety of metabolic, behavioral, and molecular outcomes, this represents a significant limitation.
A previous study has described a significant impact of rodent diets on alcohol intake in mice (Marshall et al., 2015). However, whether different chow diets affect alcohol intake in the chronic, intermittent access to 20% alcohol is currently unknown, and whether alcohol intakes correlates (positively or negatively) with the amount of food eaten in a specific diet is also unknown.
The development of heightened pain sensitivity induced by alcohol withdrawal has been shown using multiple alcohol exposure methods (e.g. experimental-controlled, voluntary, etc.) as well as multiple pain tests (e.g. mechanical, thermal, inflammatory) (Egli et al., 2012; Robins et al., 2019). This withdrawal-related phenomenon has been found to correlate with alcohol intake (Kang et al., 2019). However, whether the varying amounts of alcohol consumed as a result of the exposure to the various diets leads to differential degrees of allodynia is currently unknown.
The present study sought to examine whether the use of 4 commonly used standard chow diets resulted in differences in voluntary alcohol drinking and associated heightened pain sensitivity, using a chronic, intermittent access to ethanol model in adult C57Bl/6J mice.
Materials and Methods
Subjects
Male C57BL/6J mice (7 weeks old upon arrival, N=95) were purchased from Jackson laboratory (Bar Harbor, ME, USA). Mice were single-housed with food and water ad libitum in a humidity- and temperature-controlled AAALAC-approved vivarium on a 12 hr reverse light/dark cycle (lights off at 10:00 am). Procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals, the Principles of Laboratory Animal Care, and were approved by the Institutional Animal Care and Use Committee (IACUC) of Boston University.
Diets
Mice were maintained since arrival on 1 of 4 different diets commonly used: Teklad Diet 2918 (Diet 1), LabDiet 5001 (Diet 2), Teklad Diet 7012 (Diet 3), and LabDiet Advanced Protocol Verified 75 IF Diet 5V75 (Diet 4). The diets used have varying ingredient components; Table 1 shows the diet information according to the manufacturers. No specific rationale drove the choice of these specific diets, which are all commonly used as “standard chow” diets in laboratories across the country. We used Teklad Diet 2918 (Diet 1) as the “control” diet for the purpose of post-hoc tests, as this was the standard diet in our animal facility at the time of the study. It is important to note this was chosen a priori. Mice were maintained on each diet for 1 week prior to the introduction of ethanol.
Table 1:
Diet information according to manufacturer.
| Diet 1 | Diet 2 | Diet 3 | Diet 4 | |
|---|---|---|---|---|
| Manufacturer and catalog number | Harlan, 2918 | LabDiet, 5001 | Harlan, 7012 | LabDiet, 5V75 |
| Metabolizable Energy (kcal/g) | 3.1 | 2.9 | 3.1 | 3.3 |
| Calories from protein (%) | 24 | 29 | 25 | 23 |
| Calories from fat (%) | 18 | 13 | 17 | 13 |
| Calories from carbohydrate (%) | 58 | 58 | 58 | 64 |
| Isoflavone content range (mg/kg) | 150-250 | 300-500 | 300-600 | <75 |
| Primary Ingredients | Wheat, corn, wheat middlings, soybean meal, corn gluten meal, soybean oil | Corn, soybean meal, dried plan beet pulp, fish meal, ground oats | Corn, soybean meal, oats, wheat middlings, alfalfa meal, soybean oil | Wheat, wheat middlings, corn gluten meal, ground corn, wheat germ |
Voluntary EtOH Intake: Intermittent Access to 20% Ethanol
Upon arrival, mice were acclimated for 1 week to drink water in their home cage from two drinking bottles made from Corning™ falcon 50 mL conical bottom centrifuge tubes (Fisher Scientific, Pittsburgh, PA) equipped with #6R rubber stoppers with 2.5” straight metal double ball bearing sipper tubes (Ancare, Bellmore, NY). Then, they were given intermittent access to 20% ethanol for 6 weeks, in which one of the water bottles was replaced with a bottle containing 20% v/v ethanol (EtOH) on alternating days (Monday, Wednesday, Friday, “EtOH” group) for 24 hours, as in previous studies (Hwa et al., 2011; Loi et al., 2010). On the remaining days, two water bottles were given. The control, ethanol-naïve group had access to two water bottles at all times. Bottles were presented at the beginning of the third hour of the dark cycle (12pm). Spillage was accounted for by measuring volume lost after placing bottles on two empty cages. Food intake was recorded by weighing food at the beginning of the ethanol access session and then again at the end, during the second, fourth, and sixth week of ethanol access; intake among the 3 sessions was then averaged for each week. For the ethanol study, a total of 34 mice was used (N=8-9/group).
Pain Sensitivity Testing
To assess mechanical sensitivity, a von Frey test was performed using a Dynamic Plantar Anethesiometer (Ugo Basile, Gemonio, Italy) (Bergeson et al., 2016). Mice were placed into a Perspex enclosure covered with a plastic lid, divided into 12 individual compartments by opaque plastic spacers; this animal enclosure laid on a perforated metal platform. The von Frey test uses a metal filament applied vertically to the plantar region of the hind paw of the mouse with increasing force until the paw is withdrawn. Mice were tested at increasing forces (2g, 4g, 6g, and 8g) with a 2 second ramp up of force, similar to other studies (Btesh et al., 2013; Clapper et al., 2010). The force at which the paw was withdrawn was recorded, and the test was repeated 2-3 times per paw. Mean withdrawal forces were averaged to determine the mean paw withdrawal threshold for each animal at each force. For the 4 groups of ethanol naïve mice, pain testing began 8 weeks after arrival, and was performed in a single instance. Pain testing in the 4 groups of ethanol exposed mice plus the group of control mice on Diet 1 was performed in a single instance, though at a separate time compared to ethanol-naïve; it occurred after 8 weeks of ethanol access, at either o hr of withdrawal (i.e. immediately after bottles removal) or 72 hr of withdrawal. Pain sensitivity tests were always performed beginning at 12pm (therefore during the animals’ dark cycle, when bottles normally went on). In all pain sensitivity tests, N=6/group.
Statistics
Intake data were analyzed using a mixed design two-way ANOVA, with Time as a within-subject factor and Diet/Group as a between-subjects factor. Paw withdrawal threshold data were analyzed using a mixed design two-way ANOVA, with Applied force as a within-subject factor and Group as a between-subjects factor. Post-hoc comparisons were performed using Dunnett’s test (with EtOH-Diet 1 as control unless otherwise noted).
Results
Effect of Diet on Ethanol Intake
We found a statistically significant effect of Diet on ethanol drinking across the 18 sessions [Diet: F(3,30)= 34.64, p< 0.001; Session: F(17,510)= 2.78, p< 0.001; Diet x Session: F(51,510)= 3.05, p< 0.001]. In particular, as shown in Fig. 1A, mice on LabDiet 5001 (EtOH-Diet 2) consistently drank significantly more than mice maintained on Teklad Diet 2918 (EtOH-Diet 1) (16 out of 18 sessions), mice on Teklad Diet 7012 (EtOH-Diet 3) drank more than those on Teklad Diet 2918 (EtOH-Diet 1) on 12 out of the 18 sessions, and mice on LabDiet 75 (EtOH-Diet 4) drank more than Teklad Diet 2918 (EtOH-Diet 1) on 6 out of the 18 sessions. When cumulative ethanol intake over the course of the 18 sessions was analyzed, it was evident that Diet had a profound influence on the dependent variable (Diet: F(3,30)= 33.57, p< 0.001); specifically, animals on both LabDiet 5001 (EtOH-Diet 2) and Teklad Diet 7012 (EtOH-Diet 3) drank significantly more ethanol than those on Teklad Diet 2918 (EtOH-Diet 1) (69.6% and 30.3%, respectively), but not EtOH-Diet 4 (12.0%).
Figure 1:
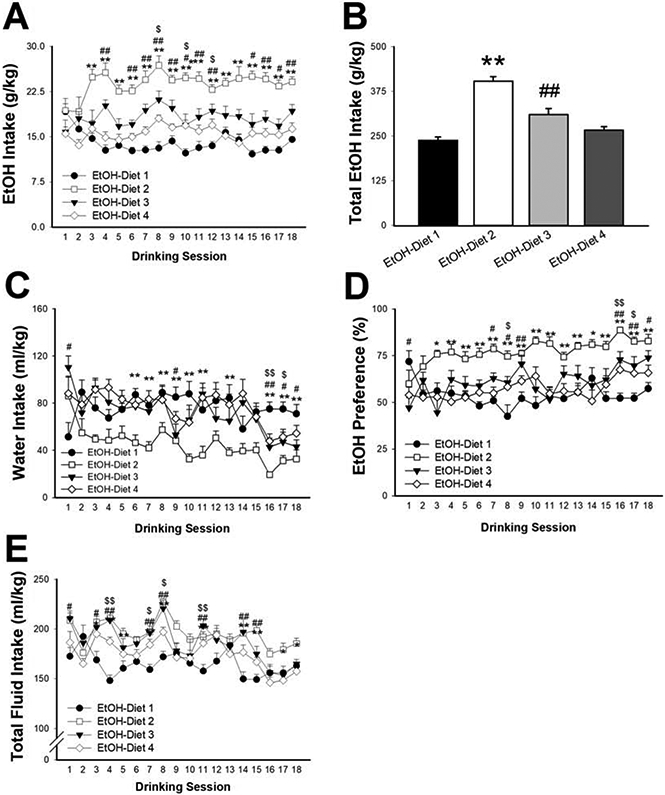
Effect of diet on ethanol (EtOH) intake (A: daily, B: 6-week cumulative), water intake (C), ethanol preference (D) and total fluid intake (E). Data represent Mean ± SEM. * p< 0.05, ** p< 0.01 EtOH-Diet 2 vs. EtOH-Diet 1; # p< 0.05, ## p< 0.01 EtOH-Diet 3 vs EtOH-Diet 1; $ p< 0.05, $$ p< 0.01 EtOH-Diet 4 vs EtOH-Diet 1 (Dunnett’s test).
The diet also influenced the amount of water mice drank during the sessions (Diet x Session: F(51,510)= 3.45, p< 0.001; Diet: F(3,30)= 6.32, p< 0.01; Session: F(17,510)= 9.89, p< 0.001), with EtOH-Diet 2 (the group with the highest ethanol intake, as shown in Fig. 1A and 1B) drinking less water (Fig. 1C). Diet also had a significant effect on preference for ethanol [Diet x Session: F(51,510)=3.58, p< 0.001; Diet: F(3,30)=15.36, p< 0.001; Session: F(17,510)=5.37, p< 0.001]. As shown in Fig. 1D, EtOH-Diet 2 mice exhibited increased preference throughout the study, while EtOH-Diet 3 and EtOH-Diet 4 mice showed higher preference for ethanol compared to EtOH-Diet 1 only on 8 and 3 sessions out of the 18, respectively (Fig. 1D). Lastly (Fig. 1E), both EtOH-Diet 2 and EtOH-Diet 3 mice drank more total fluid across most sessions [Diet x Session: F(51,510)=3.14, p< 0.0001; Diet: F(3,30)=7.51, p< 0.0001; Session: F(17,510)=14.52 p< 0.0001].
Effect of Diet on Caloric Intake and Body Weight
When examining food intake in alcohol-exposed animals, we found an effect of diet [Diet x Week: F(6, 60)=4.24, p< 0.001; Diet: F(3,30)=8.52, p< 0.0001; Week: F(2,60)=6.96, p< 0.001]; post-hoc comparisons showed that EtOH-Diet 2 and EtOH-Diet 3 mice ate less food in weeks 4 and 6, compared to EtOH-Diet 1, as shown in Fig. 2A. When accounting for total caloric intake (i.e. calories from food + calories from ethanol), we still see significant effects of the diets on the variable [Diet x Week: F(6, 60)=3.84, p< 0.001; Diet: F(3,30)=4.18, p< 0.01; Week: F(2,60)=4.93, p< 0.01]; interestingly, as shown in Fig. 2B, post-hoc comparisons showed that initially (week 2) EtOH-Diet 2 and EtOH-Diet 4 mice ate more calories compared to EtOH-Diet 1, then came back to the same level during week 4, and finally that EtOH-Diet 3 mice ate less calories during week 6. On the other hand, no effects of diet on body weight were observed at any time point of observation [Diet x Week: F(6, 60)=0.38, n.s; Diet: F(3,30)=0.86, n.s; Week: F(2,60)=93.74, p< 0.0001] (Fig. 2C).
Figure 2:
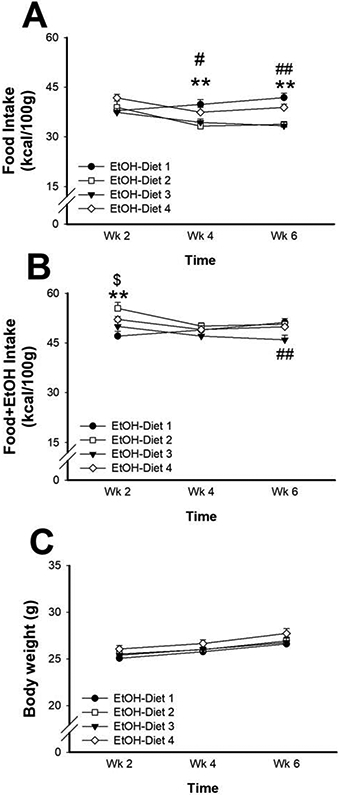
Effect of diet on food intake (A), total caloric intake (B: food + ethanol) and body weight (C) Data represent Mean ± SEM. ** p< 0.01 EtOH-Diet 2 vs. EtOH-Diet 1; # p< 0.05, ## p< 0.01 EtOH-Diet 3 vs. EtOH-Diet 1; $ p< 0.05EtOH-Diet 4 vs. EtOH-Diet 1 (Dunnett’s test).
Effect of Diet on Mechanical Pain Sensitivity
Control ethanol naïve mice on each of the 4 diets did not differ in mechanical sensitivity, as shown in Fig. 3A [Diet: F(3,20)=0.37, n.s; Applied Force x Diet: F(9,60)=0.68, n.s]. Therefore, for the next study, animals on Diet 1 were used as the Control, ethanol-naïve group, to minimize the number of groups and to be able to run the experiment in a single instance thereby reducing variability. Mice exposed to alcohol and tested immediately after the removal of the ethanol bottles (0 hr withdrawal, i.e. while animals were still under the influence of alcohol) showed no differences in mechanical pain sensitivity, as shown in Fig. 3B [Group: F (4,25)= 0.90, n.s.; Applied Force x Group: F(12,75)= 3.02, p< 0.01]; despite the significant interaction observed in the 2-way ANOVA, none of the one-way ANOVAs performed at each force showed any significant effect [2g: F(4,25)= 1.15, n.s.; 4g: F(4,25)= 1.84, n.s.; 6g: F(4,25)= 1.48, n.s.; 8g: F(4,25)= 2.30, n.s.]. On the other hand, at 72 hr withdrawal, differences in mechanical pain sensitivity were observed [Group: F(4,25)= 10.26, p< 0.001; Applied Force x Group: F(12,75)= 6.46, p< 0.001]; post-hoc comparisons showed that animals on all 4 diets exhibited increased mechanical sensitivity compared to control, ethanol-naïve mice on Diet 1, as shown in Fig. 3C, suggesting the development of allodynia independently of the diet of the EtOH mice
Figure 3:
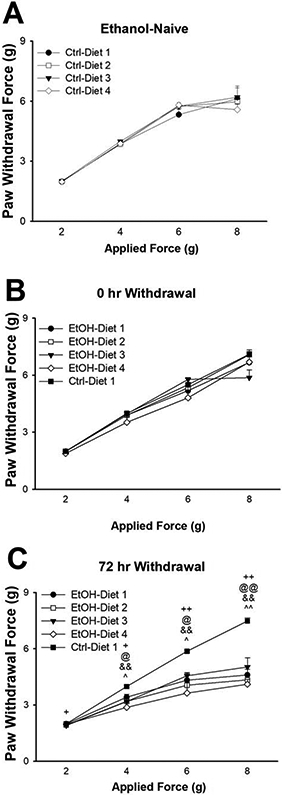
Effect of diet on mechanical sensitivity (paw withdrawal threshold) in alcohol-naïve mice (A). Effect of diet on mechanical sensitivity in ethanol exposed mice at 0 hr withdrawal (B) and 72 hr withdrawal (C). Data represent Mean ± SEM. ^ p< 0.05, ^^ p< 0.01 EtOH-Diet 1 vs. Ctrl-Diet 1; + p< 0.05, ++ p< 0.01 EtOH-Diet 2 vs. Ctrl-Diet 1; @ p< 0.05, @@ p< 0.01 EtOH-Diet 3 vs. Ctrl-Diet 1; && p< 0.01 EtOH-Diet 4 vs. Ctrl-Diet 1 (Dunnett’s test).
Discussion
In this set of experiments, we sought to determine the effect of different diets on ethanol intake, ethanol preference, and allodynic states in a widely used mouse model of heavy alcohol drinking, the intermittent access to 20% alcohol model. The 4 diets chosen for this study all have similar caloric density (Table 1), but slightly differ in the breakdown of their macronutrients. We found that mice kept on Diet 2 (EtOH-Diet 2, LD5001) and on Diet 3 (EtOH-Diet 3, H7012) consistently drank more ethanol than mice kept on Diet 1 (EtOH-Diet 1, H2918, which we choose as our “Control” diet in terms of post-hoc analyses). In addition, water intake was consistently lower in the EtOH-Diet 2 group (LD5001), and it was occasionally lower in the EtOH-Diet 3 (H7012), compared to the EtOH-Diet 1 (H2918) group. EtOH-Diet 2 (LD5001) and 3 (H7012) groups drank more total fluid across the 6 week period, indicating that although the EtOH-Diet 2 group (LD5001) drank less water, it was not sufficient to maintain the same total fluid intake as EtOH-Diet 1(H2918). Lastly, ethanol preference was also higher in the EtOH-Diet 2 (LD5001) group across the entire 6 week period, occasionally higher in the EtOH-Diet 3(H7012), but unaltered in the EtOH-Diet 4 (LD5V75), compared to EtOH-Diet 1(H2918).
Countless “standard” chow diets exist and are commonly used in preclinical laboratories. However, this variable has often been overlooked, and the exact diet used in each study is often not mentioned in scientific papers, which may definitely affect studies’ reproducibility. The model of alcohol drinking we used here was initially proposed in 1973 by Wise in rats (Wise, 1973); it has subsequently been readopted in several laboratories with some modifications, in both rats and mice (Darcq et al., 2015; Rosenwasser et al., 2013). A significant variability in the baseline of drinking has been reported across studies, using this alcohol drinking procedure, ranging from 5 to 25 g/kg (Anderson et al., 2016; Darcq et al., 2015; Fu et al., 2016; Rosenwasser et al., 2013), which may in part depend on the type of chow diet animals were maintained on. In this study, we found differences in ethanol intake as large as +80% (EtOH-Diet 2 (LD5001) vs. EtOH-Diet 1 (H2918)) during the last week of observation.
A previous study has described a significant impact of rodent diets on alcohol intake in mice (Marshall et al., 2015). However, a few differences can be identified between the two studies; a) different standard chow diets were tested; b) different models of alcohol drinking were used (drinking in the dark and continuous access vs. intermittent access to 20% alcohol); c) food intake measurements were taken in all groups in this study but not in the previous; d) this study also examined differences in alcohol withdrawal induced-mechanical allodynia in mice kept on the different chow diets.
The underlying causes of the observed differences in ethanol intake across diets, although not directly tested here, could be various. A possible influence of the different diets on the palatability of gustatory stimuli, including that of ethanol, can be hypothesized. Previous studies have indeed indicated that different chow diets can affect the intake of sweet and bitter solutions (Tordoff, 2007); in addition, certain diets that are associated with higher ethanol intake also lead to higher sucrose and quinine intake (Marshall et al., 2015). Therefore, it is conceivable that Diet 2 (LD5001; and, to a minor extent, Diet 3 (H7012)) may have affected gustatory preference by increasing preference for either the sweet and/or for the bitter component of ethanol taste.
It may be argued that the increased alcohol intake associated with some of the chow diets may simply be a compensatory change to reduced food intake. Indeed, the group that showed the highest ethanol intake (EtOH-Diet 2 (LD5001)) also ate less food. However, it is evident from our data that, temporally, the increased ethanol intake preceded the decreased food intake, leading initially (week 2) to a higher cumulative −ethanol+food− caloric intake, and then to a normalization of food intake, likely to maintain energy homeostasis. Therefore, we can conclude that the higher ethanol intake associated with Diet 2 (LD5001) was not a response to an alteration in food intake, but rather that the increased ethanol intake drove the decreased food intake. Interestingly, mice in the EtOH-Diet 3 (H7012) group showed a similar pattern of food intake behavior as the EtOH-Diet 2 (LD5001) group, but were perhaps less efficient at maintaining homeostasis, as their total caloric intake stayed lower throughout the experiment. Importantly, ethanol naïve mice maintained on these diets do not differ in food intake (data not shown), suggesting that differences in food intake must be due to an interaction between the diets and the presence of the ethanol solution.
The 4 chow diets used in this study differ, although not majorly so, in macronutrient composition (Table 1). For example, Diets 2 (LD5001) and Diet 3 (H7012), which were associated with highest ethanol intake, also have the highest protein content (29% and 25% calories from protein, respectively, vs. Diet 1 (H2918) and Diet 4 (LD5V75): 24% and 23%, respectively) and can be classified as “high protein” diets, according to some authors (Tome et al., 2019). Since high protein diets have been associated with increased alcohol drinking in rats, mice, pigs, and humans (Forsander, 1998; Forsander and Sinclair, 1988; Hauser and Iber, 1989), this is a factor that may explain our observations. On the other hand, carbohydrate content and fat content, which also have been correlated (negatively) with alcohol intake, do not seem to play a role in our study based on the relative diet composition (Gelineau et al., 2017). One limitation of this study is that we did not measure blood alcohol levels in the mice kept on the different chow diets; therefore, potential dietary influences on ethanol pharmacokinetics and metabolism cannot be excluded based on the present data.
We found that mice showed mechanical allodynia following 8 weeks of intermittent ethanol drinking 72 hr after (but not immediately after) the removal of the ethanol bottles, as assessed with von Frey filaments, compared to ethanol naïve, water Control mice. Our finding is in line with what has been shown before with several rodent models of alcohol drinking, where chronic exposure to ethanol results in allodynia (non-noxious stimuli becoming noxious) and hyperalgesia (sensitization of the pain response) (Alongkronrusmee et al., 2016; Dina et al., 2006). Interestingly, ethanol mice kept on all 4 diets showed a significantly lower force threshold compared to controls, despite the different amount of ethanol they consumed across the weeks of observation. Consistent with studies that correlate alcohol intake to hyperalgesia severity (Kang et al., 2019), finding suggests that there may be a threshold of ethanol intake that elicits allodynia (which all 4 groups of mice likely reached) above which the degree of the resulting allodynia does not change. Importantly, ethanol-naïve control mice on the 4 different diets did not differ in mechanical sensitivity, and therefore control animals on a single diet (Diet 1 (H2918)) were used as the ethanol-naïve control group in this allodynia study, to avoid an unnecessary number of groups.
Our data provide evidence that the type of rodent diet is an important variable not only to report but also perhaps to control in ethanol drinking studies. To the best of our knowledge, this is the first study showing the effect of specific laboratory chow diets on ethanol in the widely used chronic, intermittent access to 20% alcohol model. Future studies will need to determine whether the “ranking” of the 4 diets observed with ethanol intake in this model is generalizable to other models of alcohol drinking, as differences have been reported in the drinking in the dark vs. continuous access model (Marshall et al., 2015). The current study was performed exclusively on male mice; future studies will need to determine whether different chow diets would also affect ethanol drinking and allodynia in an analogous way in female mice. Furthermore, the effect of these diets on the consumption of other reinforcing substances, such as sucrose and saccharin, remains to be determined.
Highlights.
The effect of four chow diets on ethanol intake and pain sensitivity was assessed.
Specific chow diets lead to significant increases in alcohol intake and preference.
Ethanol drinking mice showed mechanical allodynia regardless of type of diet.
Type of rodent diet is an important variable in all ethanol drinking studies.
Acknowledgments
We thank Kristin Doucette, Yasmine Sami, and Sean Tanino for their technical help. This publication was made possible thanks to grant numbers AA024439 (VS), AA025038 (VS), and AA026051 (PC) from the National Institute on Alcohol and Alcoholism (NIAAA), the Peter Paul Career Development Professorship (PC), and the Boston University's Undergraduate Research Opportunities Program (UROP). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. The authors declare no conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alongkronrusmee D, Chiang T, van Rijn RM, 2016. Involvement of delta opioid receptors in alcohol withdrawal-induced mechanical allodynia in male C57BL/6 mice. Drug and alcohol dependence 167, 190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RI, Lopez MF, Becker HC, 2016. Forced swim stress increases ethanol consumption in C57BL/6J mice with a history of chronic intermittent ethanol exposure. Psychopharmacology 233(11), 2035–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard DE, Lewis SM, Teter BB, Thigpen JE, 2009. Open- and closed-formula laboratory animal diets and their importance to research. Journal of the American Association for Laboratory Animal Science : JAALAS 48(6), 709–713. [PMC free article] [PubMed] [Google Scholar]
- Bergeson SE, et al. , 2016. Binge Ethanol Consumption Increases Inflammatory Pain Responses and Mechanical and Cold Sensitivity: Tigecycline Treatment Efficacy Shows Sex Differences. Alcoholism, clinical and experimental research 40(12), 2506–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Btesh J, Fischer MJM, Stott K, McNaughton PA, 2013. Mapping the binding site of TRPV1 on AKAP79: implications for inflammatory hyperalgesia. The Journal of neuroscience : the official journal of the Society for Neuroscience 33(21), 9184–9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapper JR, et al. , 2010. Anandamide suppresses pain initiation through a peripheral endocannabinoid mechanism. Nature neuroscience 13(10), 1265–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darcq E, Warnault V, Phamluong K, Besserer GM, Liu F, Ron D, 2015. MicroRNA-30a-5p in the prefrontal cortex controls the transition from moderate to excessive alcohol consumption. Molecular psychiatry 20(10), 1261. [DOI] [PubMed] [Google Scholar]
- Dina OA, Messing RO, Levine JD, 2006. Ethanol withdrawal induces hyperalgesia mediated by PKCepsilon. The European journal of neuroscience 24(1), 197–204. [DOI] [PubMed] [Google Scholar]
- Egli M, Koob GF, Edwards S, 2012. Alcohol dependence as a chronic pain disorder. Neuroscience and biobehavioral reviews 36(10), 2179–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsander OA, 1998. Dietary influences on alcohol intake: a review. Journal of studies on alcohol 59(1), 26–31. [DOI] [PubMed] [Google Scholar]
- Forsander OA, Sinclair JD, 1988. Protein, carbohydrate, and ethanol consumption: interactions in AA and ANA rats. Alcohol 5(3), 233–238. [DOI] [PubMed] [Google Scholar]
- Fu R, et al. , 2016. Ablation of mu opioid receptor-expressing GABA neurons in rostromedial tegmental nucleus increases ethanol consumption and regulates ethanol-related behaviors. Neuropharmacology 107, 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelineau RR, Arruda NL, Hicks JA, Monteiro De Pina I, Hatzidis A, Seggio JA, 2017. The behavioral and physiological effects of high-fat diet and alcohol consumption: Sex differences in C57BL6/J mice. Brain and behavior 7(6), e00708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Grant BF, 2015. The National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Waves 1 and 2: review and summary of findings. Social psychiatry and psychiatric epidemiology 50(11), 1609–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser MB, Iber FL, 1989. Nutritional advice and diet instruction in alcoholism treatment. Alcohol Health & Research World 13(3), 261–271. [Google Scholar]
- Hilderbrand ER, Lasek AW, 2018. Studying Sex Differences in Animal Models of Addiction: An Emphasis on Alcohol-Related Behaviors. ACS chemical neuroscience 9(8), 1907–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, Miczek KA, 2011. Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcoholism, clinical and experimental research 35(11), 1938–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochum T, Boettger MK, Burkhardt C, Juckel G, Bar KJ, 2010. Increased pain sensitivity in alcohol withdrawal syndrome. Eur J Pain 14(7), 713–718. [DOI] [PubMed] [Google Scholar]
- Kang S, et al. , 2019. Downregulation of M-channels in lateral habenula mediates hyperalgesia during alcohol withdrawal in rats. Scientific reports 9(1), 2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND, 2016. Neurobiology of addiction: a neurocircuitry analysis. The lancet. Psychiatry 3(8), 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson MJ, Paasche-Orlow M, Cheng DM, Lloyd-Travaglini C, Saitz R, Samet JH, 2007. Persistent pain is associated with substance use after detoxification: a prospective cohort analysis. Addiction 102(5), 752–760. [DOI] [PubMed] [Google Scholar]
- Loi B, et al. , 2010. Increase in alcohol intake, reduced flexibility of alcohol drinking, and evidence of signs of alcohol intoxication in Sardinian alcohol-preferring rats exposed to intermittent access to 20% alcohol. Alcoholism, clinical and experimental research 34(12), 2147–2154. [DOI] [PubMed] [Google Scholar]
- Marshall SA, Rinker JA, Harrison LK, Fletcher CA, Herfel TM, Thiele TE, 2015. Assessment of the Effects of 6 Standard Rodent Diets on Binge-Like and Voluntary Ethanol Consumption in Male C57BL/6J Mice. Alcoholism, clinical and experimental research 39(8), 1406–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins MT, Heinricher MM, Ryabinin AE, 2019. From Pleasure to Pain, and Back Again: The Intricate Relationship Between Alcohol and Nociception. Alcohol Alcohol 54(6), 625–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwasser AM, Fixaris MC, Crabbe JC, Brooks PC, Ascheid S, 2013. Escalation of intake under intermittent ethanol access in diverse mouse genotypes. Addiction biology 18(3), 496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tome D, Chaumontet C, Even PC, Darcel N, Azzout-Marniche D, 2019. Protein status modulates the rewarding value of foods and meals to maintain an adequate protein intake. Physiology & behavior 206, 7–12. [DOI] [PubMed] [Google Scholar]
- Tordoff MG, 2007. Taste solution preferences of C57BL/6J and 129X1/SvJ mice: influence of age, sex, and diet. Chemical senses 32(7), 655–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, 1973. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia 29(3), 203–210. [DOI] [PubMed] [Google Scholar]
- Zale EL, Maisto SA, Ditre JW, 2015. Interrelations between pain and alcohol: An integrative review. Clinical psychology review 37, 57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]


