Abstract
At least two-thirds of spinal cord injury cases are anatomically incomplete, without complete spinal cord transection, although the initial injuries cause complete loss of sensory and motor functions. The malleability of neural circuits and networks allows varied extend of functional restoration in some individuals after successful rehabilitative training. However, in most cases, the efficiency and extent are both limited and uncertain, largely due to the many obstacles of repair. The restoration of function after anatomically incomplete injury is in part made possible by the growth of new axons or new axon branches through the spared spinal cord tissue and the new synaptic connections they make, either along the areas they grow through or in the areas they terminate. This review will discuss new progress on the understanding of the role of axon guidance molecules, particularly the Wnt family proteins, in spinal cord injury and how the knowledge and tools of axon guidance can be applied to increase the potential of recovery. These strategies, combined with others, such as neuroprotection and rehabilitation, may bring new promises. The recovery strategies for anatomically incomplete spinal cord injuries are relevant and may be applicable to traumatic brain injury and stroke.
Keywords: Spinal cord injury, axon guidance, Wnts, planar cell polarity pathway, Ryk
Introduction
Spinal cord injury is a devastating condition that severely impacts the lives of individuals carrying injury and their families. The total number of people with spinal cord injury in the US is currently 291,000, and there are approximately 54 cases per million people each year in the US (17,730 new cases each year). Depending on the age when spinal cord injury occurs, the lifetime costs are as high as $1.1 to $5.0 million per person, not including indirect costs such as losses in wages (“Spinal Cord Injury (SCI) 2019 Facts and Figures at a Glance” 2019 by National Spinal Cord Injury Statistical Center).
In addition to the loss of sensory and motor functions, cervical and high thoracic spinal injury may cause other complications, such as respiratory failure, hypertension due to loss of injury at T6 or higher (autonomic dysreflexia), and immunodeficiency due to loss of descending tonic control of the sympathetic preganglionic neurons, which leads to greater sympathetic outflow below the lesion.1–3 The quality of life is compromised with inability to empty bladder (neurogenic bladder: incontinence, renal impairment, urinary tract infection, and stones) and control bowel function at T12 or higher (reflex bowel or neurogenic bowel), as well as sexual impairment, cognitive impairment and negative mood states.4–7 Some two-thirds of individuals with SCI sustain chronic neuropathic pain, which are refractory to drug treatment.8,9 Despite many years of efforts, including three to four decades of U.S. Food and Drug Administration (FDA)-approved clinical studies, there has not been any FDA-approved drug or treatment.10 The lack of effective treatment of acute spinal cord injury leads to the continuous increase of cases at chronic stages.
Contrary to common intuition, significant repair and recovery can occur in some people after traumatic injury, especially with anatomically incomplete spinal cord injury (without complete spinal cord transection).11 The underlying mechanisms may be similar to stroke or traumatic brain injury, where functional recovery occurs more frequently and sometimes quite extensively despite acute loss of neurons. These functional recoveries are possible because of the remarkable malleability of the brain networks to form “new circuits,” which may be considered “backup circuits.” This ability appears less extensive in spinal cord injury than in stroke, but still exists in many cases of incomplete spinal cord injury.11 The “new circuits” typically are not identical to the original circuits before injury.
Research in axon growth and guidance has generated tremendous amount of new knowledge and tools. There has been encouraging news that some of the axon guidance molecules are not only present but also play significant roles in regulating the growth of the injured axons or injury-induced axons in adult spinal cord. At least two-thirds of the spinal cord injury cases are anatomically incomplete, leaving spared tissues which can potentially support axon growth and functional recovery. Complete spinal cord injury (compete spinal cord transection), which is not the main focus of this review, is conceivably more challenging to treat and will require more extensive combinatorial approaches. However, once the breakthrough for complete injury treatment is achieved, the approaches to enhance plasticity will likely contribute to the treatment of completely injured individuals.
Spinal cord injury is a complex condition
Spinal cord injury starts with a mechanical injury, which acutely causes the death of neurons and glia as well as other cell types.12 The permeability of blood–brain barrier is increased because of the injury. This initial injury results in secondary damage due to the inflammatory responses, which interacts with the vasculature system. Immune responses promote angiogenesis, which in turn regulates immune responses.13 The secondary injury, which is also caused by ischemia, excitotoxicity and oxidative stress, causes even more cell death, can last from several weeks to a much longer time. A few days after injury, astrogliosis, which is now recognized to be both beneficial and detrimental, starts to unfold, which results, in part, in the formation of glial scar in the next several weeks.14 Axon growth also starts around the same time. The initial growth lasts for a couple of weeks, but some growth likely continues. New axonal branches can grow from the proximal segment of the injured axons or from uninjured axons. But axons or axonal branches grow in the spared tissue and typically cannot grow into and across the lesion sites. Once the glial scar forms and the blood–brain barrier is rebuilt, it is thought that the secondary injury would be slowed down and the lesion would be stable. In complete spinal cord transection, axons will not be able to grow across the lesion without any help. However, in anatomically incomplete injury, growth of axons, including their branches from the injured axons, can continue to grow, bypassing the lesion, and make new synaptic connections along the way. The new connections, like in the developing nervous system, need to be continuously modified to allow for stable functional recovery to occur. If the new axons find stable synaptic targets, they will remain connected rather than being pruned. In light of these many facets of spinal cord injury, a combination of various treatments tailored towards individuals and different injuries will likely be the future of therapy.
Neuroprotection as an approach for promoting functional recovery
Among the first tissue responses of spinal cord injury is the death of neurons, glia, and other cells. The second injury, largely driven by inflammatory responses, ischemia, excitotoxicity, and oxidative stress, leads to continued cell death, expanding the initial injury, which is a major challenge in spinal cord injury. It is not surprising that most of the past and current trials have been based on neuroprotection and efforts to reduce inflammation.12,15 This is relevant to anatomically incomplete spinal cord injury as blocking cell death may not only prevent continued loss of function but also allow more axon growth and greater functional recovery.
Some of the earlier efforts, such as neuroprotective agent, GM1 (monosialotetrahexosylganglioside) Ganglioside, unfortunately showed limited efficacy in its definitive multicenter prospective randomized trial. Methylprednisolone, a synthetic glucocorticoid, is a potent anti-inflammatory agent, which is thought to be protective, as it can reduce secondary damage. However, the benefit has also been limited but the harmful side effects with increased risk of infection including severe pneumonia, sepsis, and gastrointestinal bleeding seem consistent.10,16 Its use has been abandoned by many, although it remains controversial.17 There are currently ongoing clinical trials which are based on neuroprotection, such as Minocycline, Riluzole, glial cell line-derived neurotrophic factor, and hypothermia (moderate intravascular hypothermia, 33°C, for 48 h). In addition, there are more recent promising neuroprotective agents which are starting to be tested in spinal cord injury trials, such as glibenclamide (glyburide), a drug originally for diabetes mellitus type 2. Glibenclamide showed efficacy in rodent models of ischemic and hemorrhagic stroke, traumatic brain injury, and spinal cord injury by mechanisms including reducing edema formation and secondary hemorrhage.18,19 If any of the neuroprotective approaches show benefit, it would be great news for patients and the SCI community; it is largely expected that if more tissue were spared at the injury site, it would be advantageous for downstream regenerative approaches and neuromodulation strategies.
However, it remains possible that merely mitigating secondary damage may have limited effects because of the disruption of axons at the time of injury. More importantly, studies show that neuroinflammation may also be necessary for repair and inhibiting inflammation altogether may be detrimental for repair.20 Hence, meaningful restoration of function may be hard to achieve without interventions to promote axon growth and neural network restoration.
Targeting axon guidance to promote functional recovery
After spinal cord injury, the ascending and descending axon tracts are damaged, whereas the local circuits are largely intact. These longitudinal connections are hard to repair, as the spinal cord is narrow and the strong secondary injury makes it even harder to grow for a long-enough distance, especially across the injured areas. The lack of this long-range communication causes loss of sensory and motor functions as well as other functions mentioned afore. Although it is known that neural plasticity is the underlying mechanism of functional recovery, more and more studies provided direct evidence that newly grown axons do not need to make the original connections to achieve functional recovery.21–24 This type of functional recovery clearly involves the growth of axons, either as branches from injured axons or new sprouts from uninjured axons, and changes of synaptic connections, either by promoting new synapse formation or by changing the functions of existing synaptic connections.
In development, axon guidance molecules play pivotal roles in directing axon growth to find their targets.25,26 Do they play any role on axon growth after spinal cord injury? After the discovery of the key axon guidance molecules, several studies showed that many of them showed increased expression after spinal cord injury, especially Semaphorins, Ephrins, and Wnts.27
The expression of Sema3A after central nervous system was the first axon guidance molecule to be reported to be induced.28 Olfactory nerve regeneration was found improved by the addition of a small molecule, SM-216289 or xanthofulvin, which inhibits Sema3A in vivo.29 5-HT-positive raphespinal tract (RST) axons showed regeneration in the spinal cord when Sema3A signaling is blocked using this small molecule inhibitor in a T8 complete transection model. But the long axons, corticospinal axons, or ascending sensory axons did not show regeneration in the injured spinal cord.30 However, double knockout (KO) of Plexin A3 and A4 did not show any regeneration of either 5-HT-positive RST axons or corticospinal tract (CST) axons in the same T8 complete transection model.31 Therefore, the genetic evidence suggests that blocking Sema3A signaling is not sufficient to induce axon regeneration. Other classes of Semaphorins, Sema4D and Sema7A, are also induced after spinal cord injury.27 We are awaiting genetic evidence to test their functions in vivo. It should be noted that, so far, the functions of Semaphorins have primarily be tested in the context of regeneration across complete transection but not in axon or axonal branch growth in the spared spinal cord tissues in anatomically incomplete injury.
Ephrins family axon guidance molecules were also found induced in multiple cell types after spinal cord injury. However, their function on axon growth after spinal cord injury has not been clear, either. A function-blocking peptide against EphA4 reduced the retraction of CST and RST in a dorsal column lesion, although it did not induce regeneration across the lesion.32 In EphA4−/−, axon regeneration was reported in a T12 left hemisection model. However, because astrogliosis was also found greatly reduced, it is not clear whether it was the reduced astrogliosis that affected axon growth.33 However, subsequent work done by another group and the same group showed that the changes of astrogliosis and astroglial-fibrotic scar formation in EphA4 KO were not replicated.34,35 In addition to EphA4, EphA3 and EphA7 are thought to play roles in astrocytes.27 Astrocyte-specific KO using GFAP-Cre and ephrin-B2 cKO showed reduced astrogliosis and increased axon regeneration and functional recovery in a lateral hemisection model.36 Therefore, conditional KO of Eph receptors in neurons (or Ephrins if it is reverse signaling) will be necessary to determine whether injured adult axons respond to the ephrin family axon guidance molecules or whether the regeneration is caused by reduced astrogliosis.
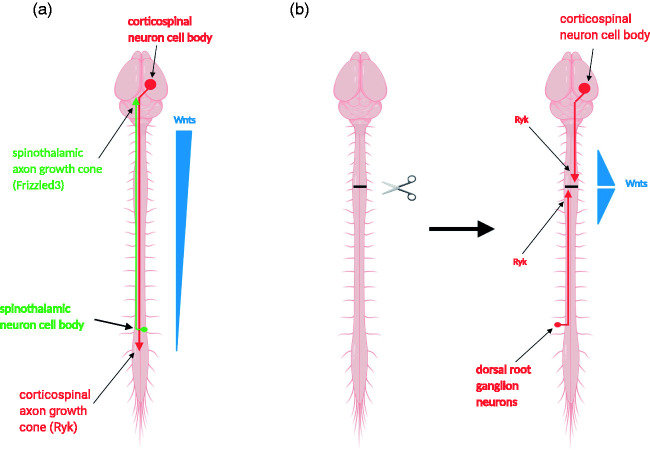
Wnts were first found to be axon guidance molecules directing the growth of axons along the anterior-posterior (rostral-caudal) axis of the rodent spinal cord in development37,38 and direct dorsal-ventral topographic mapping in the chick visual system39 (Figure 1(a)). Subsequent work showed that the function of Wnts in guiding axons along the A-P axis is conserved in a number of animal species, including rodents, chick, zebrafish, and Caenorhabditis elegans,40–45 as well as the function in topographic mapping in Drosophila visual system.46 Wnts attract axons via a non-canonical pathway, planar cell polarity pathway40,47–51 but repel axons which express Ryk as a Wnt coreceptor.38,39 For more detailed discussions about how planar cell polarity pathway mediate Wnt functions in growth cone guidance, please read another recent reviews.50
Figure 1.
The Wnt family axon guidance molecules regulate axon growth after spinal cord injury. (a) An anterior-high-posterior-low gradient of Wnt proteins (blue) provides directional cues for axon guidance along the anterior-posterior axis during development. Spinothalamic axons are attracted by Wnts, mediated by a Wnt receptor Frizzled3 in the axonal growth cones (green), to grow from the spinal cord up to the brain. Corticospinal tract axons are repelled by Wnts, mediated by another Wnt receptor Ryk in the axonal growth cones (red), to grow from the brain down along the spinal cord. (b) Expression of the Wnt signaling system is downregulated in adulthood. After spinal cord injury, Wnts and their receptors are rapidly induced in the injured tissue and in longitudinal axons. The reduced Ryk mediates repulsion by Wnts and inhibits the regeneration and branch growth of ascending and descending axons along the spinal cord.
In adulthood, the expression level of the Wnt signaling system is drastically downregulated. However, the Wnt signaling system, including the repulsive Wnt receptor, Ryk, is rapidly upregulated after spinal cord injury at the lesion area and in the injured axons.52,53 The induced Wnt-Ryk signaling system causes corticospinal tract axon retraction away from the lesion and inhibits the growth of axon branches from the proximal axon segment (Figure 1(b)).52 The Wnt-Ryk signaling system is also induced in the ascending proprioceptive axons and inhibits the sensory axon regeneration in the conditioning lesion paradigm (Figure 1(b)).54 The increased axon growth leads to improved recovery of sensory and motor functions in a number of experiments, as shown by antibodies against Ryk, Wnt inhibitors (sFRP), or conditional knockout of Ryk in corticospinal tract neurons in a dorsal column lesion model.24,55 The role of Ryk in inhibiting CST axon collateral growth has also been observed independently with a contusion injury model.56
Reorganization of neuronal networks after traumatic injury
It has long been observed that neuronal networks undergo massive reorganization after traumatic injury. The reorganizations are part of the compensatory mechanisms leading to functional recovery. Understanding the network changes will allow us to harness the plasticity to improve repair and may also design therapies to avoid potential detrimental side effects of brain remapping.
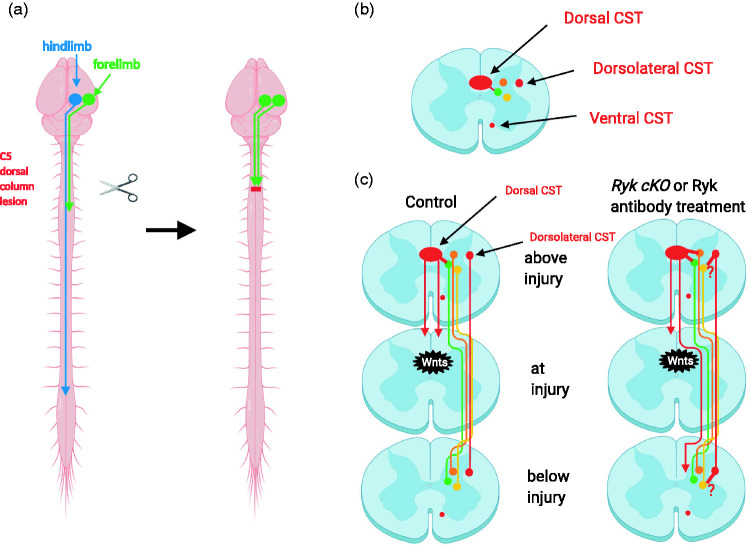
Immediately after C5 dorsal column lesion, the motor output map expanded such that the motor cortical areas that control the forelimb extensor and the hindlimb could also stimulate the forelimb flexor and the forelimb extensor and hindlimb activation were lost due to the serving of the dorsal CST at C5 (Figure 2(a)). With functional recovery, measured by forelimb reach and grasp, over the next two months, the forelimb flexor area reduced back to the original location, whereas the forelimb extensor area appeared in the area that used to be the hindlimb area before injury (Figure 2a). In the Ryk conditional knockout, the areas of both flexor and the new extensor were larger than the control suggesting that increased axon growth in Ryk conditional knockout led to the recruitment of more neurons to regulate both the flexor and extensor, resulting in better recovery of fine motor skills.24 The fact that the hindlimb motor area became recruited to control the forelimb extensor illustrates the remapping as a compensatory mechanism to regain function. It is interesting that the remapping and functional recovery did not occur without continued training after the injury, suggesting that network reorganization also needs to be functionally guided.24
Figure 2.
Reorganization of neuronal networks mediates functional recovery and is regulated by Wnt-Ryk signaling. (a) After recovery from dorsal column lesion, some hindlimb cortical neurons were recruited to regulate the forelimb. (b) Ninety-six percent of the CST in rodents are in the dorsal funiculus; 3% of the CST are in the dorsolateral funiculus; 1% of the CST are in the ventral funiculus. The red dots indicate the relative locations of the different subtypes of CSTs. Only one side was indicated. (c) In control animals, dorsal column lesion causes the sprouting of collateral branches as well as strengthening or recruiting new connections (short red line). Through neuronal networks, some of the information can be relayed beyond the lesion (green). Very few CST axons can grow around the lesion to below injury. When Ryk is conditionally knocked out in the CST axons, the sprouting of CST collateral branches was increased, recruiting more neuronal networks to relay information (green and orange). In addition, more CST axons (still small numbers) were found to grow around the lesion bypassing the glial scar to send information below the injury site in Ryk conditional knockout (long red line). Dorsolateral CST may also project more branches (short red line) to recruit more networks (yellow). The network reorganization occurs bilaterally. Only one side was illustrated. CST: corticospinal tract.
It is encouraging that the functional recovery observed when blocking Wnt-mediated inhibition of axon growth does not require the complete restoration of the original connections. In fact, a small number of neurons can support significant functional recovery by rerouting proprioceptive sensory information via a new connection or strengthening or repurposing existing connections.55 The vast majority of the corticospinal tract axons (96%) project from the brain down the spinal cord in the ventral most part of the dorsal funiculus (dorsal CST), whereas 3% project along the dorsolateral funiculus of the spinal cord (dorsolateral CST) (Figure 2(b)). Ventral CST is minor in rodents (1%), and its function is even less studied. In the dorsal column lesion, only the dorsal CST was injured at cervical level C5. The dorsolateral CST is spared. When Ryk was conditionally knocked out in the corticospinal tract neurons, the dorsal corticospinal tract axon branches showed significantly increased growth and the recovery of the forelimb reach, and grasp was also significantly improved (Figure 2c). After the initial recovery of the cortical control of the forelimb fine motor function, a second dorsal column injury at C3 slightly more rostral to the first dorsal column injury eliminated the enhanced recovery caused by Ryk conditional knockout, suggesting that the increased connections involving the dorsal CST are essential for the maximal recovery.24 Using optogenetic mapping of the motor output, it was shown that the newly recovered cortical control of extensor remained although that of the flexor was eliminated.24 This suggests that recovered cortical control was mediated by a “new circuit” formed by the new axon branches from the proximal segment of the injured dorsal corticospinal tract axons (proposed network reorganization in Figure 2(c)) of the new axon branches and/or synaptic connections from the dorsolateral corticospinal tract (the proposed red axon branches from the dorsolateral CST in Figure 2(c)). The role of the dorsolateral CST in contributing to the recovered cortical control was nicely demonstrating by Designer Receptors Exclusively Activated by Designer Drugs-mediated transient silencing in a skilled locomotion test.57 Although as increase of axon connections from the dorsal CST in Ryk conditional knockout has been observed, whether the connectivity of the dorsolateral CST was increased in Ryk conditional knockout has not been directly tested. However, the synapse density on the CST on the lateral area of the spinal cord below the lesion site was found increased in Ryk conditional knockout (unpublished data from the Zou lab). Therefore, the putative increased sprouting from the dorsolateral CST is labeled with question marks here, awaiting future investigation (Figure 2(c)). Another possibility that an increase of the sprouting from the ventral CST may also contribute to the better functional recovery, especially in the Ryk antibody infusion experiment in rats,24 as sprouting from the ventral CST was found to contribute to spontaneous functional recovery after the complete lesion of the dorsal CST in adult rats.58
Conclusion
Several axon guidance systems have been shown to be induced after spinal cord injury. Among them, the Wnt system has received the most complete and definitive evidence that injured adult axons respond to Wnts and manipulating the responses can lead to improved functional recovery in anatomically incomplete injury.59 Therefore, this further motivates the effort of understanding how Wnts signal to guide axons, including axon attraction, because combining promoting attraction and inhibiting repulsion may lead to greater functional recovery than inhibiting repulsion alone. Studies show that it is the planar cell polarity pathway that directly regulates growth cone polarity and direction of growth in response to Wnts, making the Wnt/planar cell polarity pathway a potential therapeutic target.50,60–62 It will be interesting to ask what are the signals that reactivate the expression of these developmental programs. Changes of neural activity patterns would be one possibility. Local environmental changes, such as inflammatory responses, may lead to the changes of the expression of axon guidance systems.
It remains to be seen whether other axon guidance systems than the Wnt system may also play roles on regulating axon growth, particularly in anatomically incomplete injury. So far, the role of other axon guidance systems, such as Semaphorins and ephrins, remain unconfirmed. However, it should be pointed out that those systems have so far been tested mostly in the context of promoting axon growth across the lesion rather than growing around the lesion through the spared spinal cord tissue in anatomically incomplete injury. In addition to axon guidance molecules, other molecules, such as adhesion molecules, chondroitin sulfate proteoglycan, and myelin-associated inhibitors all affect axon growth and combinatorial approaches of manipulating these pathways with axon guidance molecules may achieve greater benefit.
As discussed previously, the complex condition of spinal cord injury may require even greater combination of therapeutic strategies. This review focuses mostly on the progress to promote axon growth in the spared spinal cord tissue, particularly targeting axon guidance pathways. This approach would conceivably work better in combination with others, including neuroprotection, biomaterial, stem cells, neuromodulation and electrical stimulation, and functional rehabilitation. In particular, neuromodulation and electrical stimulation may facilitate the formation of the “new circuits” using the new axons induced by manipulating axon guidance systems, as those approaches may provide instructive signaling to functionally shape the connectivity. In addition, understanding the neural circuits in the spinal cord and brain–spinal cord connections will also provide solid scientific foundation not only to maximize recovery of the brain–spinal cord communication but also to reduce potential side effects. At the moment, the concerns about the potential side effects which may be caused by miswiring through manipulating axon guidance molecules seem remote, because functional rewiring has been only recently achieved with blocking Wnt repulsion.24,59 It may become a consideration if more extensive rewiring can be achieved by manipulating more and more axon guidance molecules. However, given the observation that the newly emerged axonal fibers do not contribute to functional recovery unless the animals undergo continuous training after injury, it is possible that proper functional rehabilitation may not only improve functional recovery but also mitigate potential malconnectivity and undesired side effects.24
Acknowledgments
The author would like to thank Binhai Zheng and Brian Kwon for reading the review and providing insightful comments and Suggestions. The figures were created with BioRender.com.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The studies that identify the role of Wnt family proteins and their receptors in axon guidance and axon growth after spinal cord injury have been supported by NIH grants to Yimin Zou, including R37 NS047484, as well as foundation grants to Yimin Zou, including Wings for Life, International Foundation for Research in Paraplegia and Roman Reed Foundation.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Yimin Zou is the founder of VersaPeutics and has equity, compensation, and interim managerial role. The terms of this arrangement have been reviewed and approved by the University of California, San Diego in accordance with its conflict of interest policies.
References
- 1.Schilero GJ, Bauman WA, Radulovic M.Traumatic spinal cord injury: pulmonary physiologic principles and management. Clin Chest Med 2018; 39: 411–425. [DOI] [PubMed] [Google Scholar]
- 2.Murray TE, Krassioukov AV, Pang EHT, et al. Autonomic dysreflexia in patients with spinal cord injury: what the radiologist needs to know. AJR Am J Roentgenol 2019; 212: 1182–1186. [DOI] [PubMed] [Google Scholar]
- 3.Noble BT, Brennan FH, Popovich PG.The spleen as a neuroimmune interface after spinal cord injury. J Neuroimmunol 2018; 321: 1–11. [DOI] [PubMed] [Google Scholar]
- 4.Taweel WA, Seyam R.Neurogenic bladder in spinal cord injury patients. Res Rep Urol 2015; 7: 85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krassioukov A, Eng JJ, Claxton G, et al. Neurogenic bowel management after spinal cord injury: a systematic review of the evidence. Spinal Cord 2010; 48: 718–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hess MJ, Hough S.Impact of spinal cord injury on sexuality: broad-based clinical practice intervention and practical application. J Spinal Cord Med 2012; 35: 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Craig A, Guest R, Tran Y, et al. Cognitive impairment and mood states after spinal cord injury. J Neurotrauma 2017; 34: 1156–1163. [DOI] [PubMed] [Google Scholar]
- 8.Hagen EM, Rekand T.Management of neuropathic pain associated with spinal cord injury. Pain Ther 2015; 4: 51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siddall PJ.Management of neuropathic pain following spinal cord injury: now and in the future. Spinal Cord 2009; 47: 352–359. [DOI] [PubMed] [Google Scholar]
- 10.Badhiwala JH, Wilson JR, Kwon BK, et al. A review of clinical trials in spinal cord injury including biomarkers. J Neurotrauma 2018; 35: 1906–1917. [DOI] [PubMed] [Google Scholar]
- 11.Khorasanizadeh M, Yousefifard M, Eskian M, et al. Neurological recovery following traumatic spinal cord injury: a systematic review and Meta-analysis. J Neurosurg Spine 2019; 30: 683–699. [DOI] [PubMed] [Google Scholar]
- 12.Liu NK, Xu XM.Neuroprotection and its molecular mechanism following spinal cord injury. Neural Regen Res 2012; 7: 2051–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed A, Patil AA, Agrawal DK.Immunobiology of spinal cord injuries and potential therapeutic approaches. Mol Cell Biochem 2018; 441: 181–189. [DOI] [PubMed] [Google Scholar]
- 14.Bradbury EJ, Burnside ER.Moving beyond the glial scar for spinal cord repair. Nat Commun 2019; 10: 3879–3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joaquim AF, Daniel JW, Schroeder GD, et al. Neuroprotective agents as an adjuvant treatment in patients with acute spinal cord injuries: a qualitative systematic review of randomized trials. Clin Spine Surg 2020; 33: 65–75. [DOI] [PubMed] [Google Scholar]
- 16.Donovan J, Kirshblum S.Clinical trials in traumatic spinal cord injury. Neurotherapeutics 2018; 15: 654–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fehlings MG, Wilson JR, Tetreault LA, et al. A clinical practice guideline for the management of patients with acute spinal cord injury: recommendations on the use of methylprednisolone sodium succinate. Global Spine J 2017; 7: 203S–211S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurland DB, Tosun C, Pampori A, et al. Glibenclamide for the treatment of acute CNS injury. Pharmaceuticals (Basel) 2013; 6: 1287–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minnema AJ, Mehta A, Boling WW, et al. SCING-Spinal cord injury neuroprotection with glyburide: a pilot, open-label, multicentre, prospective evaluation of oral glyburide in patients with acute traumatic spinal cord injury in the USA. BMJ Open 2019; 9: e031329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bollaerts I, Van Houcke J, Andries L, et al. Neuroinflammation as fuel for axonal regeneration in the injured vertebrate central nervous system. Mediat Inflamm 2017; 2017: 9478542–9478502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bareyre FM, Kerschensteiner M, Raineteau O, et al. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci 2004; 7: 269–277.nn1195 [pii]. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Haiss F, Sydekum E, et al. Rewiring of hindlimb corticospinal neurons after spinal cord injury. Nat Neurosci 2010; 13: 97–104. [DOI] [PubMed] [Google Scholar]
- 23.van den Brand R, Heutschi J, Barraud Q, et al. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science 2012; 336: 1182–1185. [DOI] [PubMed] [Google Scholar]
- 24.Hollis ER, II, Ishiko N, Yu T, et al. Ryk controls remapping of motor cortex during functional recovery after spinal cord injury. Nat Neurosci 2016; 19: 697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stoeckli ET.Understanding axon guidance: are we nearly there yet? Development 2018; 145: dev151415. [DOI] [PubMed] [Google Scholar]
- 26.Chedotal A.Roles of axon guidance molecules in neuronal wiring in the developing spinal cord. Nat Rev Neurosci 2019; 20: 380–396. [DOI] [PubMed] [Google Scholar]
- 27.Giger RJ, Hollis ER, II, Tuszynski MH.Guidance molecules in axon regeneration. Cold Spring Harb Perspect Biol 2010; 2: a001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pasterkamp RJ, Giger RJ, Ruitenberg MJ, et al. Expression of the gene encoding the chemorepellent semaphorin III is induced in the fibroblast component of neural scar tissue formed following injuries of adult but not neonatal CNS. Mol Cell Neurosci 1999; 13: 143–166. [DOI] [PubMed] [Google Scholar]
- 29.Kikuchi K, Kishino A, Konishi O, et al. In vitro and in vivo characterization of a novel semaphorin 3A inhibitor, SM-216289 or xanthofulvin. J Biol Chem 2003; 278: 42985–42991. [DOI] [PubMed] [Google Scholar]
- 30.Kaneko S, Iwanami A, Nakamura M, et al. A selective Sema3A inhibitor enhances regenerative responses and functional recovery of the injured spinal cord. Nat Med 2006; 12: 1380–1389. [DOI] [PubMed] [Google Scholar]
- 31.Lee JK, Chow R, Xie F, et al. Combined genetic attenuation of myelin and semaphorin-mediated growth inhibition is insufficient to promote serotonergic axon regeneration. J Neurosci 2010; 30: 10899–10904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fabes J, Anderson P, Brennan C, et al. Regeneration-enhancing effects of EphA4 blocking peptide following corticospinal tract injury in adult rat spinal cord. Eur J Neurosci 2007; 26: 2496–2505. [DOI] [PubMed] [Google Scholar]
- 33.Goldshmit Y, Galea MP, Wise G, et al. Axonal regeneration and lack of astrocytic gliosis in EphA4-deficient mice. J Neurosci 2004; 24: 10064–10073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herrmann JE, Shah RR, Chan AF, et al. EphA4 deficient mice maintain astroglial-fibrotic scar formation after spinal cord injury. Exp Neurol 2010; 223: 582–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dixon KJ, Munro KM, Boyd AW, et al. Partial change in EphA4 knockout mouse phenotype: loss of diminished GFAP upregulation following spinal cord injury. Neurosci Lett 2012; 525: 66–71. [DOI] [PubMed] [Google Scholar]
- 36.Ren Z, Chen X, Yang J, et al. Improved axonal regeneration after spinal cord injury in mice with conditional deletion of ephrin B2 under the GFAP promoter. Neuroscience 2013; 241: 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lyuksyutova AI, Lu CC, Milanesio N, et al. Anterior-posterior guidance of commissural axons by wnt-frizzled signaling. Science 2003; 302: 1984–1988. [DOI] [PubMed] [Google Scholar]
- 38.Liu Y, Shi J, Lu CC, et al. Ryk-mediated wnt repulsion regulates posterior-directed growth of corticospinal tract. Nat Neurosci 2005; 8: 1151–1159. [DOI] [PubMed] [Google Scholar]
- 39.Schmitt AM, Shi J, Wolf AM, et al. Wnt-Ryk signalling mediates medial-lateral retinotectal topographic mapping. Nature 2006; 439: 31–37. [DOI] [PubMed] [Google Scholar]
- 40.Fenstermaker AG, Prasad AA, Bechara A, et al. Wnt/planar cell polarity signaling controls the anterior-posterior organization of monoaminergic axons in the brainstem. J Neurosci 2010; 30: 16053–16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pan CL, Howell JE, Clark SG, et al. Multiple wnts and frizzled receptors regulate anteriorly directed cell and growth cone migrations in Caenorhabditis elegans. Dev Cell 2006; 10: 367–377. [DOI] [PubMed] [Google Scholar]
- 42.Hilliard MA, Bargmann CI.Wnt signals and frizzled activity orient anterior-posterior axon outgrowth in C. elegans. Dev Cell 2006; 10: 379–390. [DOI] [PubMed] [Google Scholar]
- 43.Prasad BC, Clark SG.Wnt signaling establishes anteroposterior neuronal polarity and requires retromer in C. elegans. Development 2006; 133: 1757–1766. [DOI] [PubMed] [Google Scholar]
- 44.Domanitskaya E, Wacker A, Mauti O, et al. Sonic hedgehog guides post-crossing commissural axons both directly and indirectly by regulating wnt activity. J Neurosci 2010; 30: 11167–11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun SD, Purdy AM, Walsh GS.Planar cell polarity genes Frizzled3a, Vangl2, and scribble are required for spinal commissural axon guidance. BMC Neurosci 2016; 17: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sato M, Umetsu D, Murakami S, et al. DWnt4 regulates the dorsoventral specificity of retinal projections in the Drosophila melanogaster visual system. Nat Neurosci 2006; 9: 67–75. [DOI] [PubMed] [Google Scholar]
- 47.Shafer B, Onishi K, Lo C, et al. Vangl2 promotes wnt/planar cell polarity-like signaling by antagonizing Dvl1-mediated feedback inhibition in growth cone guidance. Dev Cell 2011; 20: 177–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Onishi K, Shafer B, Lo C, et al. Antagonistic functions of dishevelleds regulate Frizzled3 endocytosis via filopodia tips in Wnt-Mediated growth cone guidance. J Neurosci 2013; 33: 19071–19085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Onishi K, Zou Y.Sonic Hedgehog switches on wnt/planar cell polarity signaling in commissural axon growth cones by reducing levels of Shisa2. Elife 2017; 6: e25269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zou Y.Breaking symmetry – cell polarity signaling pathways in growth cone guidance and synapse formation. Curr Opin Neurobiol 2020; 63: 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Onishi K, Tian R, Feng B, et al. LRRK2 mediates axon development by regulating Frizzled3 phosphorylation and growth cone-growth cone communication. Proc Natl Acad Sci USA 2020; 117: 18037–18048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Y, Wang X, Lu CC, et al. Repulsive wnt signaling inhibits axon regeneration after CNS injury. J Neurosci 2008; 28: 8376–8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hollis ER, II, Zou Y.Expression of the wnt signaling system in central nervous system axon guidance and regeneration. Front Mol Neurosci 2012; 5: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hollis ER, II, Zou Y.Reinduced wnt signaling limits regenerative potential of sensory axons in the spinal cord following conditioning lesion. Proc Natl Acad Sci USA 2012; 109: 14663–14668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hollis ER, II, Ishiko N, Pessian M, et al. Remodelling of spared proprioceptive circuit involving a small number of neurons supports functional recovery. Nat Commun 2015; 6: 6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miyashita T, Koda M, Kitajo K, et al. Wnt-Ryk signaling mediates axon growth inhibition and limits functional recovery after spinal cord injury. J Neurotrauma 2009; 26: 955–964. [DOI] [PubMed] [Google Scholar]
- 57.Hilton BJ, Anenberg E, Harrison TC, et al. Re-establishment of cortical motor output maps and spontaneous functional recovery via spared dorsolaterally projecting corticospinal neurons after dorsal column spinal cord injury in adult mice. J Neurosci 2016; 36: 4080–4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weidner N, Ner A, Salimi N, et al. Spontaneous corticospinal axonal plasticity and functional recovery after adult central nervous system injury. Proc Natl Acad Sci USA 2001; 98: 3513–3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hilton BJ, Bradke F.Can injured adult CNS axons regenerate by recapitulating development? Development 2017; 144: 3417–3429. [DOI] [PubMed] [Google Scholar]
- 60.Zou Y.Wnt signaling in axon guidance. Trends Neurosci 2004; 27: 528–532. [DOI] [PubMed] [Google Scholar]
- 61.Zou Y.Does planar cell polarity signaling steer growth cones? Curr Top Dev Biol 2012; 101: 141–160. [DOI] [PubMed] [Google Scholar]
- 62.Onishi K, Hollis E, Zou Y.Axon guidance and injury-lessons from wnts and wnt signaling. Curr Opin Neurobiol 2014; 27: 232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]