Abstract
Hypoxic-ischemic (HI) encephalopathy remains a major cause of perinatal mortality and chronic disability in newborns worldwide (1–6 for 1000 births). The only current clinical treatment is hypothermia, which is efficient for less than 60% of babies. Mainly considered as a waste product in the past, lactate, in addition to glucose, is increasingly admitted as a supplementary fuel for neurons and, more recently, as a signaling molecule in the brain. Our aim was to investigate the neuroprotective effect of lactate in a neonatal (seven day old) rat model of hypoxia-ischemia. Pups received intra-peritoneal injection(s) of lactate (40 μmol). Size and apparent diffusion coefficients of brain lesions were assessed by magnetic resonance diffusion-weighted imaging. Oxiblot analyses and long-term behavioral studies were also conducted. A single lactate injection induced a 30% reduction in brain lesion volume, indicating a rapid and efficient neuroprotective effect. When oxamate, a lactate dehydrogenase inhibitor, was co-injected with lactate, the neuroprotection was completely abolished, highlighting the role of lactate metabolism in this protection. After three lactate injections (one per day), pups presented the smallest brain lesion volume and a complete recovery of neurological reflexes, sensorimotor capacities and long-term memory, demonstrating that lactate administration is a promising therapy for neonatal HI insult.
Keywords: Neonatal hypoxia-ischemia, lactate, neuroprotection, astrocyte to neuron lactate shuttle, MRI
Introduction
Neonatal hypoxic-ischemic (HI) encephalopathy, which occurs in 1–6 out of 1000 births in developed countries, remains a major cause of perinatal mortality and chronic disability in newborns worldwide.1,2 HI insult can be caused by numerous events but independently of the origin, brain injury is ultimately due to impaired cerebral blood flow3 and oxygen delivery to the brain.4 The pathophysiological consequences of an HI event are complex and time-dependent. Several phases can be distinguished.5 The primary energy failure is directly linked to oxygen and glucose deprivation, which leads to a drop in ATP content and, therefore, the failure of the sodium/potassium pump, leading to cell swelling and brain edema. When blood flow is restored, the latent period starts, which duration varies depending on the degree of severity of the insult.6 Then, 6 to 48 h after the HI event, the second energy failure phase occurs, which is more related to oxidative stress, excitotoxicity and inflammation processes.
Currently, the sole clinical treatment in use is moderate hypothermia, which has to be initiated within the first 6 h, therefore during the latent period, which is the optimal time window for therapy, and then continued for 72 h.4 This treatment consists in cooling either the head or the whole body such as the body temperature reaches between 33 and 36.5°C.7 However, this treatment is not beneficial for more than 30% of surviving babies and effective only for moderate HI insults (see Shah,7 which compared 13 clinical trials for hypothermia in infants with HI encephalopathy). Therefore, new therapeutic approaches are eagerly needed.
In the brain, neurons and astrocytes display differences in metabolic profiles. Astrocytes exhibit a high glycolytic metabolism leading to lactate production.8 Neurons are energy-demanding cells but their glycolytic rate is restricted due to the constitutive proteasome-dependent degradation of a key positive regulator of glycolysis, 6-phosphofructo-2-kinase/fructose 2,6-biphosphate 3 (PFKFB3).9,10 Compared to astrocytes, neurons rely more on oxidative metabolism, and, especially in the developing brain, they metabolize a substantial part of glucose through the pentose phosphate pathway.11,12
After a HI insult, despite the high energy demand of the brain, glucose administration has no impact on brain damage,13,14 and even a worsening effect when injected before HI event.15 Nevertheless, neurons could have access to other energetic substrates than glucose to sustain their high energetic demand. After being considered as a metabolic waste product, lactate is now regarded as an important energetic source. Indeed, in the astrocyte to neuron lactate shuttle (ANLS) described by Pellerin and Magistretti,16 it has been proposed that astrocytes fulfill neuronal energetic needs by providing lactate as a fuel through their high glycolytic metabolism capacity. Although this theory is supported by increasing evidence indicating lactate production by astrocytes and the uptake of lactate as preferred neuronal energetic substrate compared to glucose,17–19 it still remains controversial.20
In this context, the use of lactate as a supplementary fuel after a brain insult was already tested, both in animals (stroke model) and in human (traumatic brain injury, TBI). So even if for clinicians, and neuroradiologists in particular, lactate accumulation is considered as a waste product of anaerobic glycolysis and a sign of ischemia associated with severe disease states and poor outcome,21 lactate seems to be promising for neuroprotection. Indeed, if lactate was originally believed to be even the cause of tissue damage in ischemia, recent experiments have shown that lactate administration after middle cerebral artery occlusion (MCAO) in rodents induces a reduction in size of the damaged area.22,23 Moreover, clinical studies in traumatic brain injury (TBI) reported a protective effect of exogenous lactate infusion.24–28 In particular, an increase of the amount of pyruvate and glucose as well as a decrease in glutamate levels was observed after infusion of hypertonic lactate solution in severe TBI, suggesting that lactate can be used as alternative fuel, while glucose would be spared to be utilized for the regulation of other mechanisms such as reactive oxygen species (ROS) production in this context.25 More recently, cerebral microdialysis studies on patients after TBI showed that labelled [3-13C]lactate is metabolized in glutamine.27 Finally, following TBI, reduced lactate/pyruvate ratio within the first 72 h is associated with a better outcome, suggesting that lactate utilization is beneficial in this context.29 All these studies were performed in adults.
Neonatal brain energy metabolism differs from adults. Cerebral glucose utilization is only 10% of the adult value during the first postnatal weeks.30 The neonatal brain has a low capacity for glucose transport due to a low level of glucose transporters (GLUTs). In contrast, compared to adults, it has a higher expression of monocarboxylate transporters (MCTs),31 which allow the use of alternative substrates to glucose, such as lactate and ketone bodies. This substrate transport capacity is therefore adapted to the breastfeeding diet. As in adults, glucose administration in HI newborn pigs has no effect32 or even a deleterious effect on the size of brain damages.33 Moreover, in newborn infants undergoing therapeutic hypothermia after an HI insult, hyperglycemia is associated with a poor outcome.34 In parallel, lactate removal appears to produce more brain damage.35 Although different studies assessed various pharmacological neuroprotective strategies (see Juul and Ferriero36 for review), to our knowledge, no exploration of the neuroprotective role of lactate infusion was performed in neonatal HI insult.
Therefore, considering the recent literature suggesting some possible therapeutic roles of lactate, both in animal and human studies25,37–39 and taking into account the high capacity of the immature brain to use lactate as an energy substrate, we proposed in the current study to evaluate the neuroprotective role of lactate in a HI neonatal rat model.40,41 Brain damages were evaluated as soon as 3 h after the ligation and during the following 48 h by diffusion MRI. In order to investigate the mechanism of this neuroprotection, pyruvate and glucose (isocaloric conditions) injections were compared to the lactate therapy. The enzyme lactate dehydrogenase (LDH) was also blocked with oxamate to assess the implication of lactate metabolism in the neuroprotection.42,43 Motor and behavioral studies were also carried out to evaluate the long-term effect of lactate injection on cognitive functions. Our results demonstrate that both exogenous and endogenous lactate exert a neuroprotective effect on neonatal HI insult and open the way to clinical trials in the future, as already ongoing in human adult TBI.39
Material and methods
Animals
All animal procedures were conducted in accordance with the Animal Experimentation Guidelines of the European Communities Council Directive of 24 November 1986 (86/609/EEC). Protocols met the ethical guidelines of the French Ministry of Agriculture and Forests and the ARRIVE guidelines and were approved by the Bordeaux ethical committee for animal research, n°C2EA-50 (authorization n°9476). Pregnant Wistar RJ-HAN females (Janvier Laboratories, France) were received at day-15 after fertilization and kept on a 12:12-h light:dark cycle with food and water ad libitum.
Model of hypoxic ischemic brain injury
Neonatal Wistar rats post natal day 7 (P7) of both gender (mean body weight 17.8 ± 2.1 g, data were first analyzed with respect to sex but no sex effect was detected) were used for the HI model.40,41 Briefly, rats were anaesthetized with isoflurane (4% for induction and 1.5% for maintenance) and a midline neck incision was made under local anesthesia (lidocaine 0.5%). The left common carotid artery was exposed and permanently ligated with a 7–0 silk thread. After surgery (6–7 min, never exceeded 14 min in total to avoid cardiac or respiratory arrests), animals were allowed to recover (30 min) on a heated mattress to maintain body temperature. Pups were then placed for 2 h in a humidified hypoxic chamber (8% oxygen in 92% nitrogen) submerged in a 36 ± 1°C water bath (Intensive Care Unit Warmer, Harvard Apparatus, France) to maintain a constant thermal environment. Sham-operated pups (n = 10) were kept separated from the dam in a heated atmosphere (33 ± 1°C). These conditions allow to maintain body temperature at 36 ± 1°C (no difference with rectal temperature measured when pups were with the dam). After hypoxic exposure, rat pups were returned to their heated mattress until MRI. Acquisition of DWI was started 180 min after the carotid artery ligation then performed at 24 h and 48 h.
Lactate, pyruvate, glucose and oxamate administration
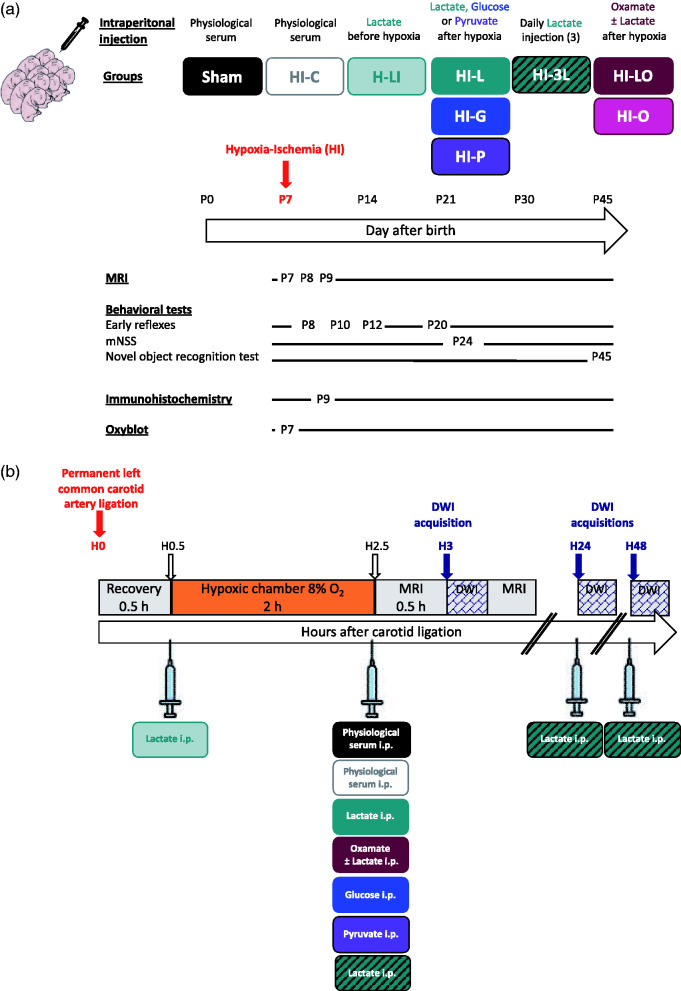
Pups received a single intraperitoneal injection of sodium lactate (534 mmol/L, 4 μl/g pup) before (H-LI group: n = 10) or after hypoxia (HI-L group: n = 14) or three consecutive post-HI event injections (150 min, 24 h and 48 h post-ligation, HI-3L group: n = 10) or the co-injection of lactate and the LDH inhibitor oxamate (HI-LO group: n = 9) (750 mg/kg) or injection of oxamate alone (HI-O group, n = 7, 750 mg/kg). Pups in HI-G and HI-P groups received when leaving the hypoxic chamber a single intraperitoneal injection of glucose (HI-G group: n = 6, 267 mmol/L, 4 μl/g pup) or sodium pyruvate (HI-P group: n = 6, 534 mmol/L, 4 μl/g pup), respectively. Control rats received an injection of NaCl 0.9% 150 min post-ligation (HI-C group: n = 17). A scheme of the experimental design is presented in Figure 1.
Figure 1.
Experimental design and groups. (a) Groups studied and chronology. (b) Time course between carotid artery ligation, hypoxia exposure and DWI acquisition. Nine different groups were studied; the sham group in which pups underwent only the exposition of the left common carotid artery (no HI insult), the HI-C group in which pups were exposed to the HI procedure (left common carotid artery ligation + 2 h hypoxia) without any treatment (intraperitoneal injection of physiological saline solution), the H-LI group in which pups received a single intraperitoneal lactate injection after the carotid artery ligation and before entering the hypoxic chamber, the HI-L group in which pups received a single intraperitoneal lactate injection when leaving the hypoxic chamber (corresponding to 150 min after the carotid artery ligation and 30 min before the DWI acquisition), the HI-G group in which pups received a single intraperitoneal glucose injection when leaving the hypoxic chamber,), the HI-P group in which pups received a single intraperitoneal pyruvate injection when leaving the hypoxic chamber, the HI-3L group in which pups received a daily post-HI injection during three days (when leaving the hypoxic chamber, then 24 h and 48 h after the HI insult) of lactate and the HI-LO group in which both lactate and oxamate (LDH inhibitor) were injected to the pups. HI-O group: HI pups, which received only oxamate.
1H and 13C nuclear magnetic resonance spectroscopy
After hypoxia ended (normoxia condition), four pups were injected intraperitoneally with [3-13C] lactate (200 mg/kg, sodium-L-lactate-3-13C, Cortecnet) and euthanized by cerebral-focused microwaves (2 KW, 1 s, Sacron8000, Sairem) in order to prevent post-mortem metabolism. Contralateral and ipsilateral hemispheres were dissected, weighted and extracted separately using perchloric acid. Lyophilized samples were dissolved in 100 μL D2O containing ethylene glycol (0.04 mol/L, peak at 63 ppm). All samples were analyzed on a Bruker 500/54 ASCEND AEON spectrometer equipped with an HRMAS probe. 1H-NMR spectra were acquired at 4°C with 90° flip angle (measured for each sample), 8 s relaxation delay, 5000 Hz sweep width and 32 K memory size. Residual water signal was suppressed by homonuclear presaturation. Proton-decoupled 13C-NMR spectra were acquired overnight using 60° flip angle, 20 s relaxation delay, 25,063 Hz sweep width and 64 K memory size. Measurements were conducted at 4°C under bi-level broadband gated proton decoupling and D2O lock. Relevant peaks in the spectra were identified and integrated using TopSpin 3.5pl6 software (Bruker Biospin GmbH). Proton-observed carbon-editing (POCE) sequence was used to determine the 13C-specific enrichment at selected metabolite carbon positions using the (13C-1H) heteronuclear multiquanta correlation.44,45 The sequence enabled the successive acquisitions of a first scan corresponding to a standard spin-echo experiment without any 13C excitation and a second scan involving a 13C inversion pulse allowing coherence transfer between coupled 13C and 1H nuclei. Subtraction of two alternate scans resulted in the editing of 1H spins coupled to 13C spins with a scalar coupling constant JCH = 127 Hz. 13C decoupling during the acquisition collapsed the 1H-13C coupling to a single 1H resonance. Flip angles for rectangular pulses were carefully calibrated on both radiofrequency channels before each experiment. The relaxation delay was 8 s for a complete longitudinal relaxation. The fractional 13C-enrichment was calculated as the ratio of the area of a given resonance on the edited 13C-1H spectrum to its area on the spin-echo spectrum.
Acquisition of in vivo MR images
Each pup was studied at 3 h, 24 h and 48 h after the carotid artery ligation on a horizontal 4.7 T Biospec 47/50 system (Bruker, Ettlingen, Germany) equipped with a 12-cm BGA12S gradient system (660 mT/m). For MRI, pups were anesthetized with isoflurane (4% induction, 1.5% maintenance), and respiration was monitored by a ventral pressure sensor. Warmed water circulating in the gradient coil system was used to maintain body temperature. Measurements were performed with Paravision 6.0.1 (Bruker BioSpin, Karlsruhe, Germany). T2-weighted images of the brain were obtained using a RARE sequence, 20 slices, 0.7-mm thick, FOV 2.5 × 2.5 cm, TE 50 ms, TR 3000 ms, rare factor 8, matrix 128 × 128, total duration 4 min 48 s. Brain lesions were assessed by magnetic resonance diffusion-weighted imaging (DWI). DWI was performed using the following parameters: 20 axial slices of 0.7 mm, b-value 1000 s/mm2, TE 24 ms, TR 2 s, 30 directions, Δ = 8.11 ms, δ = 2.5 ms, total duration 17 min 04 s).
For the kinetic study, pups (HI-C, n = 9; HI-L, n = 7 and HI-3L, n = 7) underwent five successive DWI at P7 (30, 47, 64, 81 and 98 min after the end of the HI insult), P8 and P9. Decrease in lesion size was expressed in % and normalized to the size of the lesion measured on the first time point image.
Magnetic resonance imaging analysis
The extent of the lesion was determined on DWI Trace images. ADC maps were computed using the Paravision 6.0.1 (Bruker BioSpin, Karlsruhe, Germany). For each rat, region-of-interest (ROI) was manually drawn, on each slice, to encompass the injured area and determine the global brain area to quantify the lesion volume (in percent of total brain) and ADC values. Analyses were blindly performed by two separate operators (intra-operator variability<1%; inter-operator variability <5%).
Behavioral studies
Righting reflex (P8, P10, P12)46,47
Pups were placed in a supine position and the time required to turn over on all four paws and touch the ground was recorded. Each pup was given three trials and the mean time to perform the reflex was calculated.
Modified neurological severity score (P24)
Neurological functions were assessed using the mNSS,48 which is composed of motor (muscle status), sensory (tactile and proprioceptive), beam balance, reflexes (pinna, corneal and startle) tests. Each rat was graded on the mNSS scale (value <1: no impairment; 1–6: middle impairment, 7–12: moderate impairment and > 13: severe impairment). A non-parametric test (Kruskal–Wallis) was used for statistical analyses of these data.
Novel object recognition (P45)
The pups’ long-term memory was assessed using the novel object recognition test.49 After a habituation period in the open field (50 cm × 50 cm, 10 min) on day 1, pups were further allowed to freely explore two identical objects placed in opposing corners of the arena for 10 min on the next two days. On the last day (day 4), one of the old objects was replaced by a new and different object. Pups were allowed to freely explore the objects for 10 min. Duration of object contacts was measured. A discrimination index ([time with new object minus time with familiar object]/[time with new object plus time with familiar object]) was defined as the parameter for evaluation.
Behavioral studies were blindly performed by one experimenter, different from the one who acquired MRI, and, to avoid a selection bias, pups from the same dam were assigned to different experimental groups.
Protein carbonyl assay
Protein carbonyls (treated with DNPH) were detected using the Oxidized Protein Western Blot Kit (ab178020). Samples were then separated on a 4–15% SDS polyacrylamide gradient TGX STAIN FREE mini-gel (Biorad 4568086) at 150 V. Gels were activated by UV with chemidoc (Biorad). Proteins were transferred electrophoretically on 0.45 µm-nitrocellulose membranes (7 min, 2.5 A) in Transfer buffer with Trans-Blot Turbo (Biorad). Membranes were blocked 1 h in odyssey buffer (Eurobio 927-40003) and incubated overnight with the primary antibody (1:3000) provided in the kit diluted in odyssey buffer. After three washes with PBS-0.05% Tween 20 membranes were incubated for 1 h with a second antibody (1:2500) (LYCOR) diluted in odyssey buffer. This secondary antibody was detected by fluorescence with odyssey. Total protein was used as loading control.
Statistical analysis
All data are presented as mean ± standard deviation. Statistical analyses were performed with GraphPad Prism v7 using ANOVA non-parametric test (one-way analysis of variance, multiple comparisons followed by Fisher’s LSD test) for lesion volumes, ADC and behavioral studies, except mNSS (Kruskal–Wallis non-parametric test). Unpaired t-test was used to compare oxiblots. A p < 0.05 was considered statistically significant.
Results
Neuroprotection by lactate: Preventive and/or curative?
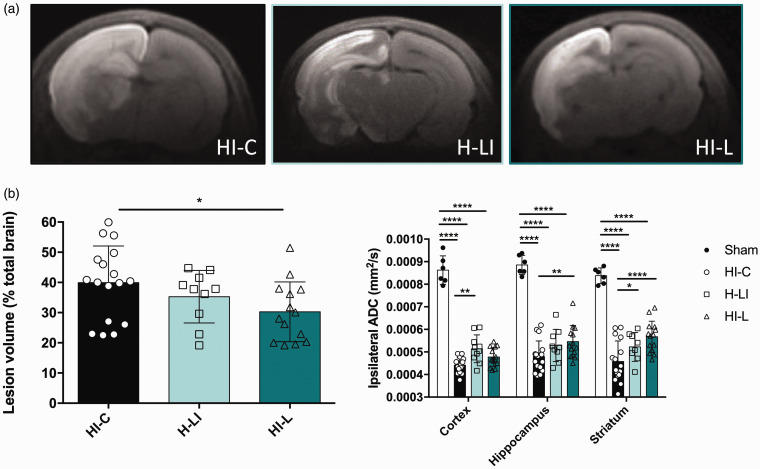
Brain sections of typical trace diffusion-weighted images (DWI) for each animal group were obtained 3 h after the carotid artery ligation with intraperitoneal lactate or physiological serum injection and are presented in Figure 2(a). The more restricted extra-cellular water movements are (cytotoxic edema), the brighter the signal is, allowing to delineate the borders of the brain lesion. Macroscopic evaluation of brain lesions showed a decrease in lesion volumes with lactate therapy (Figure 2(a)). This was confirmed by quantification of the lesion volumes (Figure 2(b)). Pups in the HI-L group showed a significantly reduced damaged area compared to the untreated HI group (% of brain lesion normalized to the total brain volume: 30.3 ± 2.6% in HI-L group compared to 39.9 ± 2.9% in HI-C group, n = 14 and n = 17, respectively, statistically significant, P = 0.016). When lactate was injected before hypoxia (H-LI group), no significant difference was observed in lesion volumes between H-LI and HI-C groups (35.3 ± 2.8% in H-LI group compared to 39.9 ± 2.9% in HI-C group, n = 10 and n = 17, respectively).
Figure 2.
Evaluation of a preventive or curative effect of lactate injection on neonate brain lesions and edema volumes 3 h after carotid artery ligation. Lactate was intraperitoneally injected before (H-LI group) or after (HI-L group) the brain insult. In the control group (HI-C), physiological serum was injected when leaving the hypoxic chamber. (a) Trace DWI of P7 brains (from HI-C, H-LI, and HI-L groups) performed at 4.7 T. Damages appeared as a hypersignal (decrease of water movement, which reflects cytotoxic edema). (b) Quantification of lesion volume (%, relative to total brain volume) for the different groups. (c) Quantification of the ipsilateral ADC in cortex, hippocampus and striatum for sham, HI-C, H-LI and HI-L groups. * P < 0.05, ** P < 0.01, **** P < 0.0001 by one-way ANOVA, followed by Fisher’s LSD test.
From these DWI, apparent diffusion coefficient (ADC) values were calculated for the cortex, the hippocampus and the striatum (Figure 2(c)). A drop in ADC values was observed in all HI groups compared to the sham group, reflecting water diffusion restriction due to cell swelling, a phenomenon typical of this time window (latent period). While the HI-C and H-LI groups presented no statistical difference in ADC values, in the HI-L group, ADC values were significantly higher, indicating that, in addition to reducing the lesion volume, lactate injected after the HI insult reduces the severity of cytotoxic edema. In the present conditions, injection of lactate before ischemia did not provide any neuroprotective effect. Therefore, for the rest of the study, we only explored the impact of lactate injections after the HI insult, which is also more relevant in the perspective of a clinical trial.
Neuroprotection by lactate: A metabolic or signaling effect?
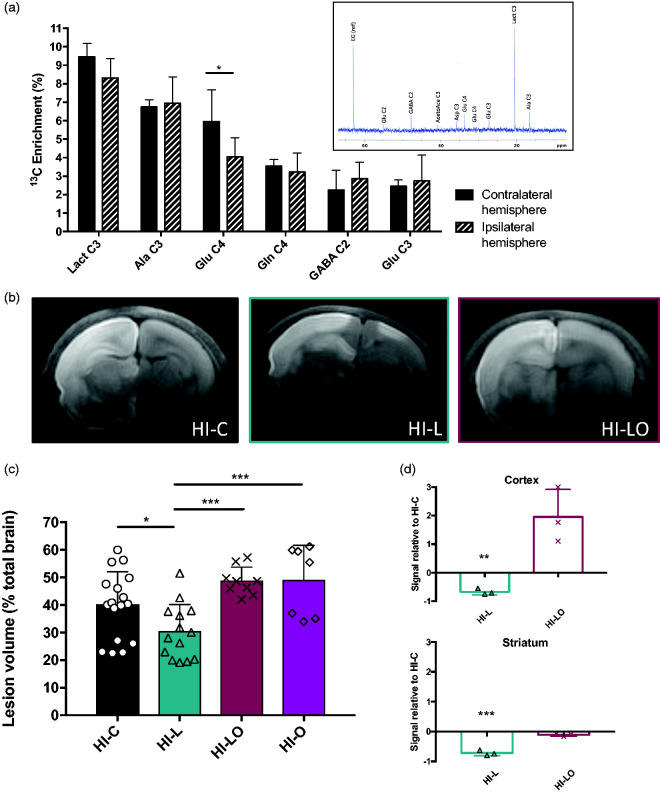
The time window between lactate injection and the first MRI is quite short (30 min). To determine the fate of lactate injected intraperitoneally, we used [3-13C]lactate and followed the incorporation of 13C into cerebral metabolites by ex vivo NMR spectroscopy. A typical 13C-NMR spectrum of brain extract 30 min after i.p. injection of [3-13C]lactate is shown in the insert Figure 3(a). 13C-specific enrichments were measured from the 1H-NMR spectra (POCE spectra, not shown). Results indicated that [3-13C]lactate has reached the brain, crossed the blood–brain barrier and was detected in the healthy brain and in the damaged area ([3-13C]lactate specific enrichment: 9.5 ± 1.0% and 8.3 ± 0.7%; respectively). [3-13C]lactate had also been metabolized in both hemispheres since 13C was incorporated into alanine and glutamate ([3-13C]alanine specific enrichment: 6.8 ± 0.4% and 6.9 ± 1.4%, in the healthy and hypoxic hemispheres, respectively; [4-13C]glutamate specific enrichment: 6.0 ± 1.7% and 4.1 ± 1.0%, respectively). 13C was also incorporated into other amino acids or carbon positions (γ–amino butyric acid (GABA), glutamate C3, glutamine C4 and C3) reflecting its use for cerebral oxidative metabolism through the Krebs cycle.
Figure 3.
Metabolic role of lactate in the neuroprotection. (a) 13C-NMR spectrum and 13C-specific enrichments (% of 13C-enriched molecules) of brain metabolites 30 min after [3-13C]lactate intraperitoneal injection. Results are mean values ± SD. * P < 0.05 by one-way ANOVA, followed by Fisher’s LSD test. Insert: Typical 13C-NMR spectrum obtained from brain extract 30 min after i.p. injection of [3-13C]lactate. EG: ethylene glycol, internal reference; AcetoAce C3: acetaoacetate carbon 3; Glu C4, C3 or C2: glutamate carbon 4, 3 or 2; Gln C4, glutamine carbon 4; GABA C2: γ-aminobutyrate carbon 2; Asp C3: aspartate carbon 3; Lact C3: lactate carbon 3; Ala C3: alanine carbon 3. (b, c and d) Effect of oxamate, a lactate dehydrogenase (LDH) inhibitor on neonates’ brain lesions and edema volumes 3 h after the carotid artery ligation. In the HI-L group, lactate was intraperitoneally injected after the brain insult, while in the HI-LO group, both lactate and oxamate (a LDH inhibitor) were co-injected (in the HI-O group, only oxamate was injected). In the control group (HI-C), lactate was replaced by physiological serum. (b) Trace DWI of P7 brains (HI-C, HI-L, and HI-LO groups) obtained at 4.7 T. (c) Quantification of lesion volume for the different groups (%, relative to total brain volume). * P < 0.05, *** P < 0.001, by one-way ANOVA, followed by Fisher’s LSD test. (d) Quantification of oxiblots in cortex and striatum for HI-C, HI-L, and HI-LO groups (n = 3 per group). Results are mean values ± SEM. ** P < 0.01, *** P < 0.001, paired t-test.
In order to determine if the neuroprotective role of lactate was linked to its metabolic use, the LDH inhibitor oxamate was co-injected with lactate (HI-LO group). As control, we also performed experiments with oxamate injection alone (HI-O group, Figure 3(c)). No statistical difference was observed between HI-C and HI-O (39.9 ± 2.9%, n = 17 and 48.8 ± 4.8, n = 7 for HI-C and HI-O groups, respectively) nor between HI-LO and HI-O brain lesion volumes (48.6 ± 1.7, n = 9 and 48.8 ± 4.8, n = 7 for HI-LO and HI-O, respectively). DWI obtained and quantification of lesion volumes are presented (Figure 3(b) and (c), respectively). The co-injection of oxamate completely abolished the neuroprotective effect of lactate (lesion volumes, in percentage of total brain volume: 39.9 ± 2.9%, 30.3 ± 2.6%, and 48.6 ± 1.7% in HI-C, HI-L and HI-LO groups, respectively). The deleterious effect of preventing the metabolic use of lactate was also evaluated via the measurement of ROS production, which is a deleterious event concomitant to the HI insult. Analyses of ROS production by oxiblot showed that post-ischemia intraperitoneal injection of lactate led to a significant decrease in ROS production compared to HI-C group; −68% in the cortex and −72% in the striatum (Figure 3(d)). Conversely, inhibition of lactate metabolism by oxamate prevented the reduction in ROS production compared to the control group in the cortex and in the striatum (moreover, no statistical difference between HI-O and HI-C groups was observed, in both structures, data not shown).
Neuroprotection by lactate: Comparison with pyruvate and glucose administration
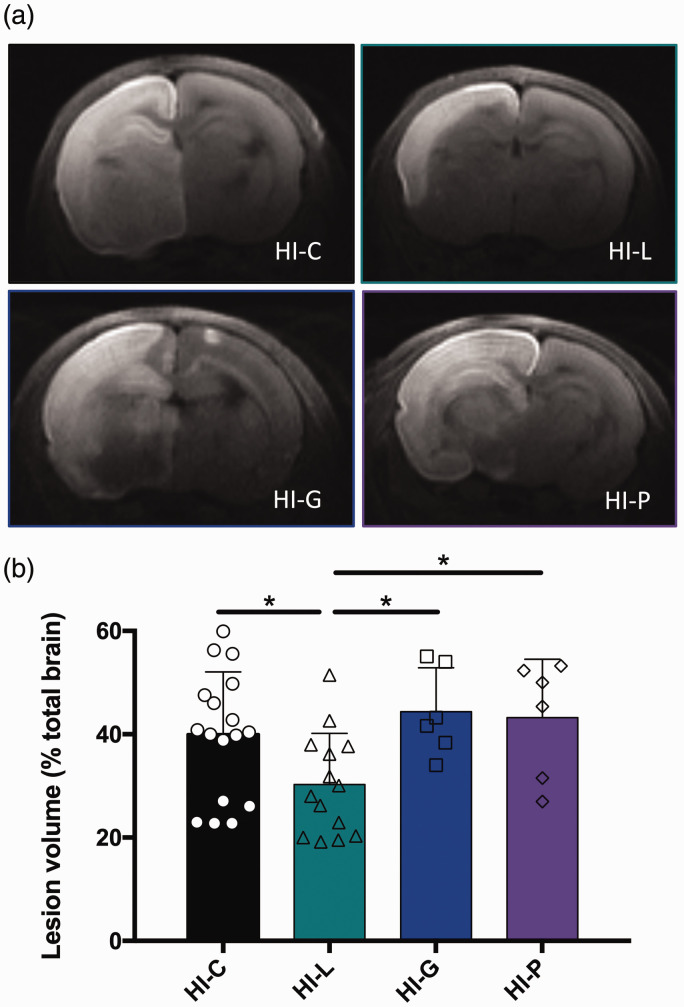
Isocaloric intraperitoneal injections of pyruvate (HI-P group) or glucose (HI-G group) were performed just after the HI insult and brain lesion volumes were measured on DWI and compared with the HI-L group (Figure 4(a) and (b)). No statistical difference was detected between HI-C, HI-P and HI-G groups, whereas pups in the HI-L showed reduced lesion volumes with statistical differences with the other groups.
Figure 4.
Comparison of glucose, pyruvate and lactate injections on neonate brain lesions and edema volumes 3 h after carotid artery ligation. Lactate (HI-L), pyruvate (HI-P), glucose (HI-G) or physiological serum (HI-C) were intraperitoneally injected after the brain insult. (a) Trace DWI of P7 brains. Damages appeared as a hypersignal (decrease of water movement, which reflects edema). (b) Quantification of lesion volume (%, relative to total brain volume) for the different groups. * P < 0.05, by one-way ANOVA, followed by Fisher’s LSD test.
Neuroprotection by lactate: Repeated injections better than a unique dose?
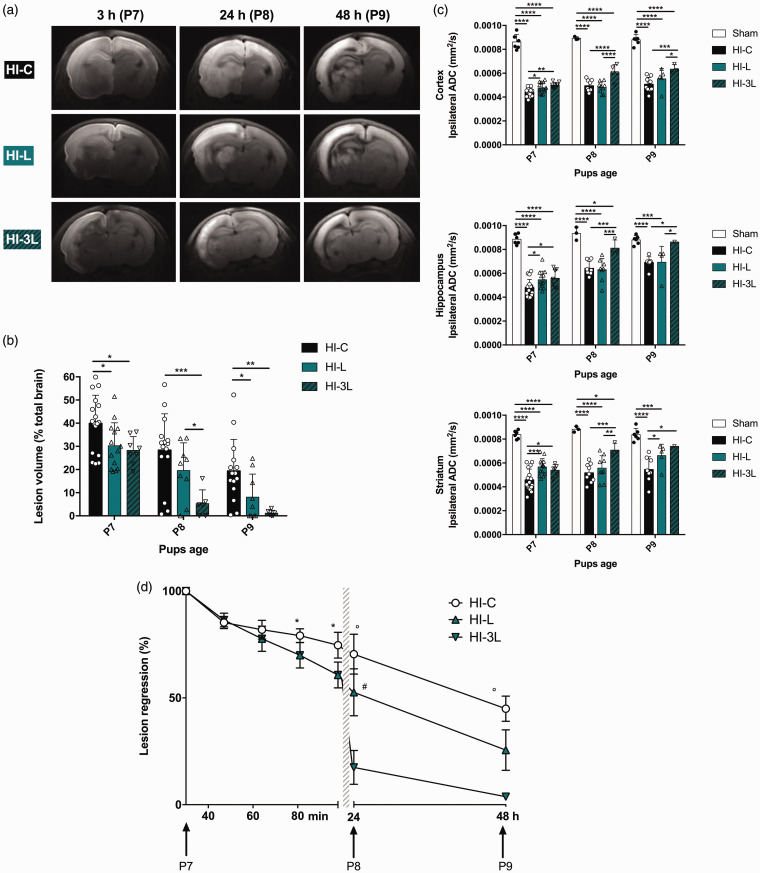
Two injection patterns were compared: a single intraperitoneal injection of lactate (HI-L group) and three daily consecutive lactate injections (HI-3L group). Lesions were followed during three days by DWI (Figure 5(a)) and their volumes were quantified (Figure 5(b)). No difference was observed between HI-L and HI-3L groups at P7 (post-natal seven-day-old pups) (same quantity of lactate injected in both groups). However, as soon as the second day, the HI-3L group had significantly lower lesion volumes compared to the other groups. After three lactate injections, lesion volumes were the smallest in the HI-3L group (lesion volume, in percentage of total brain volume, at P9: 1.2 ± 0.5% and 19.4 ± 3.5% in HI-3L and HI-C group, respectively). ADC values were quantified in the lesion (if present) in cortex, hippocampus and striatum for each group, at each time point (Figure 5(c)). At P9, the HI-3L group presented the best recovery of ADC values in the cortex and the striatum.
Figure 5.
Longitudinal study of the neuroprotective effect of lactate. (a) Brain trace DWI of P7, P8 and P9 pups of HI-C, HI-L and HI-3L groups. Images were obtained at 4.7 T, 3 h, 24 h and 48 h after the insult. (b) Quantification of lesion volumes (%, relative to total brain volume) and comparison between the control group, one injection of lactate and three injections of lactate (daily). (c) Quantification of ADC in cortical, hippocampal and striatal lesions at P7, P8 and P9. (d) Evaluation of lesion regression as a function of time (%, relative to original lesion size). Results are mean values ± SD. (b)(c) * P < 0.05, ** P < 0.01, *** P < 0.001, by one-way ANOVA, followed by Fisher’s LSD test. (c) *statisticaly significant between HI-C and HI-L, °statisticaly significant between HI-C and HI-3L, #statisticaly significant between HI-L and HI-3L.
A temporal profile of brain lesion recovery was established (Figure 5(d)). Longitudinal DWI was performed five times during the first 100 min (P7; 30, 47, 64, 81 and 98 min after the HI insult with lactate or physiological serum injection, in HI-L and HI-C groups, respectively) and at P8 and P9. A nearly complete recovery was observed in the HI-3L group.
Neuroprotection by lactate: An acute as well as a long-term effect?
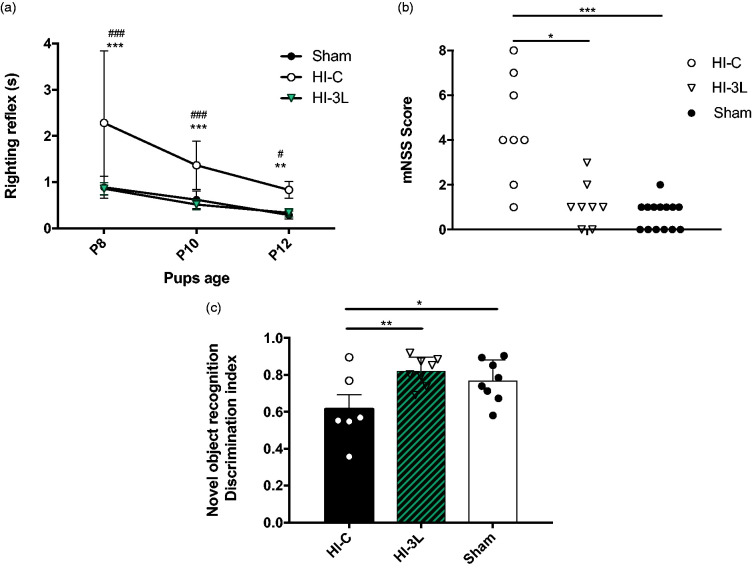
Pups in the HI-3L group had the smallest lesion volumes measured by MRI during the first 48 h after the HI insult but the impact on functional recovery and long-term outcome was unknown. Therefore, behavioral tests at different time points were performed. Early reflexes were analyzed to evaluate the impact of three daily consecutive lactate injections after a HI event. The HI-C group presented significantly poorer performances in the righting reflex, while no difference was measured between sham and HI-3L pups (Figure 6(a)).
Figure 6.
Effect of lactate therapy on neurological reflexes as well as juvenile somatosensory and memory capacities. (a) Performances on righting reflexes at P8, P10 and P12. Significantly different from sham group: ** P < 0.01, *** P < 0.001, significantly different from HI-3L: # P < 0.05, ### P < 0.001. (b) Modified neurological severity scores (mNSS) performed at P24. * P < 0.05, *** P < 0.001. (c) Novel object recognition test performed at P45. Results are mean values +/_ SEM. * P < 0.05, ** P < 0.01.
Sensorimotor deficits were also evaluated using the modified Neurological Severity Scores (mNSS) (Figure 6(b)). The mNSS scores of pups in HI-C and HI-3L groups were 4.5 ± 0.8 and 1.1 ± 0.4, respectively, while the one for sham pups was 0.6 ± 0.3. No statistical difference was found between HI-3L and sham groups.
Long-term memory was tested using the novel object recognition test (Figure 6(c)). HI insult induced a significant decrease in discrimination compared to the sham group. Such a deleterious effect was prevented by three consecutive daily injections of lactate (0.77 ± 0.04; 0.62 ± 0.08; 0.82 ± 0.03, for sham, HI-C and HI-3L groups, respectively). No statistical difference was found between HI-3L and sham groups.
Discussion
The metabolic cooperation between neurons and astrocytes, and more precisely the astrocyte-neuron lactate shuttle (ANLS) proposed by Pellerin and Magistretti16 more than 20 years ago, has led to a new vision of neuroenergetics. In this shuttle, glutamate, a neurotransmitter released by neurons during brain activity, is taken up by astrocytes and its entry stimulates the production of glycolytic lactate, which will be transferred to neurons in which it will be used as an energetic substrate.16,50 While still debated,20,51 a growing number of studies have led to results indicating that neurons efficiently consume lactate18,19 and that lactate metabolism is linked to functional activity and memory.52,53 In the brain, monocarboxylate transporters (MCTs) mediate the transport of lactate, pyruvate and ketone bodies. These transporters are key elements in this hypothesis since different isoforms exist, with different kinetic parameters. MCT2 is found in neurons,54 while MCT1 is predominantly located on endothelial cells as well as on glial cells including astrocytes, these latter expressing in addition MCT4.55 In vitro, it has already been reported that the transcriptional factor hypoxia-inducible factor 1-α regulates the expression of MCT4, which could play a crucial role for neuronal recovery after an ischemic episode.56 In the context of ischemic insults, in vitro and in vivo studies have shown that lactate is the major energy substrate for surviving neurons, and it has been reported that lactate can protect neurons from glutamate-induced neurotoxicity,57,58 while administration of glucose before an ischemic stroke led to an increase in lesion size in a rat model of global cerebral ischemia.15 Moreover, in hippocampal slices, after a period of oxygen deprivation, accumulated brain lactate allows the recovery of neuronal functions upon reoxygenation.59,60 In vivo, in rats undergoing a transient global cerebral ischemia, the inhibition of monocarboxylate transporter, reducing lactate transport, increased neuronal damages leading the authors to conclude that lactate is a critical oxidative energy substrate in the post-ischemic rat brain.15 Considering the aforementioned data together with the fact that the immature brain readily metabolizes ketones as well as lactate,30 and that MCTs are overexpressed during the entire breastfeeding period,61,62 led us to investigate whether lactate administration after a neonatal HI insult could be neuroprotective.
In this study, we demonstrate the therapeutic efficacy of lactate in a neonatal HI rat model. We used the well-established hypoxia-ischemia model of Rice-Vannucci on P7 pups that is commonly accepted to be equivalent to the human term newborn.41 Ligature of the left common carotid artery followed by a hypoxic episode systematically induced an ipsilateral brain lesion. Lactate was intraperitoneally injected before or after the hypoxic insult. Our results clearly indicate that lactate exerts a therapeutic rather than a preventive effect, which, from a clinical point of view, is far more interesting, since smaller brain lesions were observed when lactate was injected after the insult when compared to lactate injection before hypoxia.
This therapeutic effect of lactate was observed as soon as 30 min after its administration. Therefore, we first wanted to confirm that lactate injected intraperitoneally was able to reach the brain within this short time period. To follow the fate of lactate, we used 13C-labeled lactate.63 Half an hour after its intraperitoneal administration, 13C-labeled lactate was found in the brain (on 13C-NMR spectra of brain perchloric extracts) and it was also metabolized, both in the healthy and the ischemic hemisphere, since 13C was incorporated into different brain metabolites. Metabolism of [3-13C]lactate was previously investigated in another study,64,65 which showed, in adult rats, that [3-13C]lactate perfused in the blood circulation crossed the blood–brain barrier and was efficiently metabolized, almost exclusively in the neuronal compartment (metabolism of [3-13C]lactate observed in the brain after both 1 h- or 20 min-[3-13C]lactate infusions). Therefore, lactate injected intraperitoneally after the insult is entering the brain, essentially through the MCTs, and is metabolized by the brain of HI pups.
When lactate metabolism is blocked using oxamate, a LDH inhibitor, the neuroprotection was completely lost, indicating that lactate consumption by brain cells is mandatory to get a therapeutic effect. This is confirmed by the fact that the efficacy of lactate is more important when injected after the insult, therefore when normoxia and oxidative metabolism are restored. In this reperfusion period, the use of lactate rather than glucose as an oxidative energetic substrate is neuroprotective. Indeed, if lactate is replaced by glucose, no neuroprotection could be observed. We can hypothesize that the metabolic use of lactate as an energetic substrate (precursor) for the Krebs cycle allows to spare glucose for other important cellular repair mechanisms, such as the pentose phosphate pathway, which activity is crucial for the production of glutathione, a ROS scavenger.11,66,67 This hypothesis is strongly supported by the results obtained by oxiblots, which show that the huge increase in protein oxidation after a HI insult is prevented by lactate administration. Once again, the co-injection of oxamate with lactate prevents the beneficial effect of lactate on protein oxidation, confirming the need for lactate metabolism to obtain the effect on ROS production. In this context, it may seem surprising to observe that pyruvate does not provide neuroprotection. Pyruvate is efficiently transported by the same neuronal monocarboxylate transporter than lactate. It is also metabolized similarly to lactate as the former must be converted into pyruvate intracellularly before being used in oxidative metabolism. The main difference between the two is the contribution of lactate to the redox potential since the conversion of lactate to pyruvate requires the conversion of NAD+ into NADH. The concomitant production of NADH with lactate metabolism might be a critical factor for its neuroprotective effect.
Interestingly, the neuroprotection seems not to be equivalent in all brain regions. Indeed, striatal regions were first protected, as observed either on diffusion MRI and on oxiblots. Such a regional difference, already observed during lactate neuroprotection in a mouse model of stroke,68 is not surprising since brain metabolism is known to differ depending on the cerebral structure69 as well as brain development and pathologies.70
The use of oxamate also demonstrates that the neuroprotection offered by lactate is linked to its metabolic use rather than to its signaling effect. Indeed, a lactate receptor, called GPR81 or HCAR1, was recently found in the brain.71 In adult, it was proposed that neuroprotection by lactate was due to both its metabolic and its signaling roles.23 Results obtained in this study indicate that the metabolic consumption of lactate is essential to observe neuroprotection, since when the conversion of lactate into pyruvate is blocked by oxamate, then the neuroprotective effect of lactate is lost. Even if it did not reach statistical significance, there was a tendency to observe bigger brain lesion volumes when oxamate was injected compared to control animals (Figure 3(c); HI-C compared to HI-LO or HI-O, P = 0.054 and 0.068, respectively). This suggests that endogenous lactate produced during hypoxia, and its conversion into pyruvate during the reperfusion period, is already neuroprotective. However, we cannot exclude a role of the receptor-mediated signaling pathway at a different time point. GPR81 is a G-protein coupled receptor, which activation leads to a decrease in cAMP content. cAMP is known to modulate glucose metabolism, and, in particular, to activate glycogenolysis.72 Therefore, activation of GPR81 after lactate administration could also decrease cAMP content, decrease the release of glucose from glycogen and, finally, limit deleterious effect of glucose excess after a stroke.73
MRI is a non-invasive technique that allows longitudinal studies. In addition to anatomical images, parametric images can also be acquired. With DWI, “trace” images reflect extracellular water diffusion restriction due to cell swelling (with also a T2 contribution). Even if quantification of brain lesion volumes and severity of edema by MRI is considered a gold standard in the field to determine the efficacy of a treatment, an evaluation of functional recovery over several days and even weeks after initiating the treatment is mandatory. In our study, the long-term neuroprotection of lactate was monitored with different behavioral tests. Pups subjected to HI insult presented deficits in early reflexes. A complete recovery was observed after three consecutive daily injections of lactate. Later, the same was observed in juvenile rats in the mNSS test. After three consecutive daily injections of lactate, rats performed the test as well as sham pups, while non-treated rats presented somatosensory deficits and asymmetric dysfunctions. Such deficits have been reported in a MCAO ischemic rat model.74 These results demonstrate that multiple lactate injections counteract the deleterious effects of a hypoxic-ischemic event on neurological reflexes as well as on juvenile somatosensory capacities and asymmetric functions.
Previous studies have shown that white matter damages after hypoxic-ischemic injury in preterm children correlated with late cognitive impairments.75 Hypoxic-ischemic rodents also display short- and long-term memory deficits.49,76–78 Neuroprotection by lactate of long-term memory was assessed with the novel object recognition test. Juveniles that have received at P7, P8 and P9 a daily injection of lactate after the HI event completely recovered. Interestingly, a role of lactate and MCTs was recently demonstrated in memory processes.53 The authors suggested that astrocytic glycogenolysis leads to lactate production and release from astrocytes via MCT1 or MCT4 and uptake into neurons via MCT2 which appears to be required for the consolidation step of long-term memory. As expected, in our study, rats subjected to HI insult displayed long-term memory deficits. They were, however, completely protected by multiple injections of lactate. These data highlight a therapeutic role of lactate on hippocampal-dependent long-term memory in a context of hypoxic-ischemic stroke.
There are limitations to this study. First, the route of lactate administration is not similar to the one that should be used in the clinic. Indeed, lactate should be injected through a continuous blood circulation infusion, such as the one already performed in TBI patients.25,39 However, this was not compatible with the rat model, in which the pups return to the dam just after the end of the MRI. Another way to continuously administered lactate would be the use of osmotic pumps. Unfortunately, the smallest one offered by the manufacturer (for mice) is still too big to be implanted in 16–20 g rat pups. Second, after rodent studies and before being translated to the clinic, a pre-clinical study on larger animals, such as pigs, should be performed. However, if this step-by-step approach is a prerequisite to test potential side effect(s) of a new drug, in the case of lactate, which is a “natural” molecule, this is not really relevant. Lactate infusions are protocols already tested in some clinical trials. For example, after TBI, hypertonic sodium lactate is infused as soon as possible after the insult, at a rate of 30 to 40 μmol/kg body weight/min, during 3 h,39 which corresponds to the administration of 5.4 to 7.2 mmol/kg. In our protocol, the three consecutive daily administrations of lactate correspond to a slightly lower dose, 2.14 mmol/kg. Thus, its direct translation to the clinic should therefore be possible more easily.
Additional preclinical evaluation of lactate administration under a hypothermia condition will be needed before lactate therapy can be clinically investigated, since hypothermia is the only efficient treatment currently used.4 However, since this standard procedure does not imply injection of a chemical product, no deleterious association with lactate is expected, which was not the case with tissue plasminogen activator, for example, in an adult rat model of stroke.79
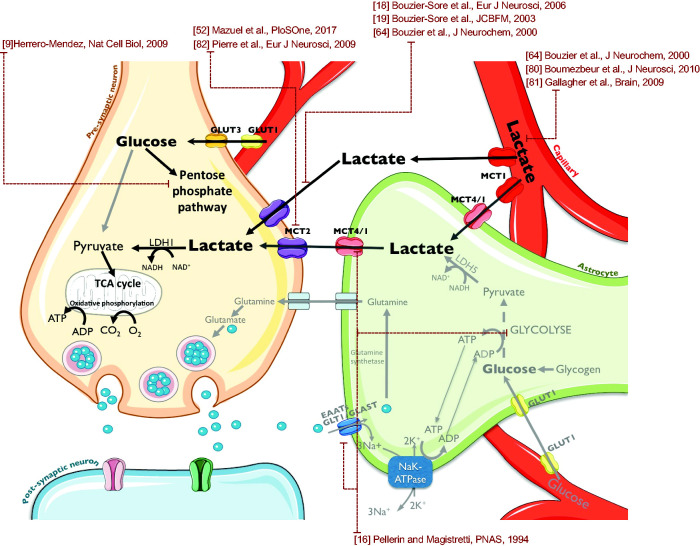
Our study demonstrates for the first time the neuroprotective and curative roles of multiple lactate injections by reducing brain damages and improving sensorimotor deficits as well as cognitive impairments. This neuroprotection could be due to the use of lactate as an energetic source and redox potential contributor. Indeed, blood lactate can be taken up and metabolized by the brain, as shown by our data (using 13C-lactate injection in pups), as well as by previous studies performed in adult animals.64,80,81 This lactate can then be used as an energetic substrate, preferentially by neurons, as demonstrated both in vitro18,19 and in vivo.64 In addition to blood-borne lactate, lactate can be also provided by astrocytes, through the astrocyte-neuron lactate shuttle,16 which becomes prominent during brain activation as it is driven by astrocytic glutamate uptake. Lactate enters neurons through MCT2,82 which expression was recently correlated with the BOLD response, a surrogate marker of neuronal activity.52 The use of lactate as an energetic source for neurons could then spare glucose for its use in the pentose phosphate pathway (PPP), a biochemical series of reactions that has been shown to be essential for neuronal survival through glutathione reduction for protection against ROS.9 In this latter study, when glycolysis was upregulated in neurons, this led to their death, emphasizing the importance of the PPP. The neuroprotective and curative roles of lactate injections in neonatal hypoxia-ischemia can be therefore explained by the model summarized in Figure 7. Our data obtained using oxamate and the oxiblot technique are consistent with such a mechanistic neuroprotective pathway and prompt for further investigations.
Figure 7.
Mechanistic hypothesis of lactate neuroprotection on HI damages. Injected lactate could be taken from the bloodstream by astrocytes. Then, through the astrocyte-neuron lactate shuttle, lactate could be metabolized by neurons as an energy source to overcome the lack of glucose induced by HI insult. The small amount of available neuronal glucose could thus be saved to integrate the pentose phosphate pathway to maintain the Redox balance together with the NADH provided by lactate metabolism.
Footnotes
Author's note: Luc Pellerin is now affiliated with Inserm U1082/Université de Poitiers, Poitiers, France.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the TRAIL (Translational Research and Advanced Imaging Laboratory) cluster of excellence of Bordeaux University. AKBS has received financial support from the program IdEx and the LabEx TRAIL, reference ANR-10-IDEX and ANR-10-LABX-57. AKBS and LP have received financial support from an international French (ANR)/Swiss (FNS) grant, references ANR-15-CE37-0012 and FNS n° 310030E-164271. LP received financial support from the program IdEx Bordeaux ANR-10-IDEX-03-02.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: AKBS has conceived, designed, acquired and analyzed data, and wrote the article. HR, UD, SS, LM, JB, GR has acquired and analyzed data. JFC and LP have contributed to the final drafting of the article.
ORCID iD: Jean-François Chateil https://orcid.org/0000-0002-6143-7607
References
- 1.Kurinczuk JJ, White-Koning M, Badawi N.Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Develop 2010; 86: 329–338. [DOI] [PubMed] [Google Scholar]
- 2.Davidson JO, Wassink G, van den Heuij LG, et al. Therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy – where to from here? Front Neurol 2015; 6: 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shalak L, Perlman JM.Hypoxic-ischemic brain injury in the term infant-current concepts. Early Hum Develop 2004; 80: 125–141. [DOI] [PubMed] [Google Scholar]
- 4.Cotten CM, Shankaran S.Hypothermia for hypoxic-ischemic encephalopathy. Exp Rev Obstetr Gynecol 2010; 5: 227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen KA, Brandon DH.Hypoxic ischemic encephalopathy: pathophysiology and experimental treatments. Newborn Infant Nursing Rev 2011; 11: 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwata O, Iwata S, Thornton JS, et al. “Therapeutic time window” duration decreases with increasing severity of cerebral hypoxia-ischaemia under normothermia and delayed hypothermia in newborn piglets. Brain Res 2007; 1154: 173–180. [DOI] [PubMed] [Google Scholar]
- 7.Shah PS.Hypothermia: a systematic review and meta-analysis of clinical trials. Semin Fetal Neonatal Med 2010; 15: 238–246. [DOI] [PubMed] [Google Scholar]
- 8.Bouzier-Sore AK, Pellerin L.Unraveling the complex metabolic nature of astrocytes. Front Cell Neurosci 2013; 7: 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herrero-Mendez A, Almeida A, Fernandez E, et al. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nat Cell Biol 2009; 11: 747–752. [DOI] [PubMed] [Google Scholar]
- 10.Bolanos JP.Bioenergetics and redox adaptations of astrocytes to neuronal activity. J Neurochem 2016; 139(Suppl 2): 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morken TS, Brekke E, Haberg A, et al. Neuron-astrocyte interactions, pyruvate carboxylation and the pentose phosphate pathway in the neonatal rat brain. Neurochem Res 2014; 39: 556–569. [DOI] [PubMed] [Google Scholar]
- 12.Brekke E, Morken TS, Sonnewald U.Glucose metabolism and astrocyte-neuron interactions in the neonatal brain. Neurochem Int 2015; 82: 33–41. [DOI] [PubMed] [Google Scholar]
- 13.Chew W, Kucharczyk J, Moseley M, et al. Hyperglycemia augments ischemic brain injury: in vivo MR imaging/spectroscopic study with nicardipine in cats with occluded middle cerebral arteries. AJNR Am J Neuroradiol 1991; 12: 603–609. [PMC free article] [PubMed] [Google Scholar]
- 14.Myers RE, Yamaguchi S.Nervous system effects of cardiac arrest in monkeys. Preservation of vision. Arch Neurol 1977; 34: 65–74. [DOI] [PubMed] [Google Scholar]
- 15.Schurr A, Payne RS, Miller JJ, et al. Blockade of lactate transport exacerbates delayed neuronal damage in a rat model of cerebral ischemia. Brain Res 2001; 895: 268–272. [DOI] [PubMed] [Google Scholar]
- 16.Pellerin L, Magistretti PJ.Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A 1994; 91: 10625–10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bouzier-Sore AK, Merle M, Magistretti PJ, et al. Feeding active neurons: (re)emergence of a nursing role for astrocytes. J Physiol Paris 2002; 96: 273–282. [DOI] [PubMed] [Google Scholar]
- 18.Bouzier-Sore AK, Voisin P, Bouchaud V, et al. Competition between glucose and lactate as oxidative energy substrates in both neurons and astrocytes: a comparative NMR study. Eur J Neurosci 2006; 24: 1687–1694. [DOI] [PubMed] [Google Scholar]
- 19.Bouzier-Sore AK, Voisin P, Canioni P, et al. Lactate is a preferential oxidative energy substrate over glucose for neurons in culture. J Cereb Blood Flow Metab 2003; 23: 1298–1306. [DOI] [PubMed] [Google Scholar]
- 20.Barros LF, Weber B.CrossTalk proposal: an important astrocyte-to-neuron lactate shuttle couples neuronal activity to glucose utilisation in the brain. J Physiol 2018; 596: 347–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kraut JA, Madias NE.Lactic acidosis. N Engl J Med 2014; 371: 2309–2319. [DOI] [PubMed] [Google Scholar]
- 22.Berthet C, Castillo X, Magistretti PJ, et al. New evidence of neuroprotection by lactate after transient focal cerebral ischaemia: extended benefit after intracerebroventricular injection and efficacy of intravenous administration. Cerebrovasc Dis 2012; 34: 329–335. [DOI] [PubMed] [Google Scholar]
- 23.Castillo X, Rosafio K, Wyss MT, et al. A probable dual mode of action for both L- and D-lactate neuroprotection in cerebral ischemia. J Cereb Blood Flow Metab 2015; 35: 1561–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jalloh I, Helmy A, Shannon RJ, et al. Lactate uptake by the injured human brain: evidence from an arteriovenous gradient and cerebral microdialysis study. J Neurotrauma 2013; 30: 2031–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouzat P, Sala N, Suys T, et al. Cerebral metabolic effects of exogenous lactate supplementation on the injured human brain. Intens Care Med 2014; 40: 412–421. [DOI] [PubMed] [Google Scholar]
- 26.Sala N, Suys T, Zerlauth JB, et al. Cerebral extracellular lactate increase is predominantly nonischemic in patients with severe traumatic brain injury. J Cereb Blood Flow Metab 2013; 33: 1815–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glenn TC, Martin NA, Horning MA, et al. Lactate: brain fuel in human traumatic brain injury: a comparison with normal healthy control subjects. J Neurotrauma 2015; 32: 820–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alessandri B, Schwandt E, Kamada Y, et al. The neuroprotective effect of lactate is not due to improved glutamate uptake after controlled cortical impact in rats. J Neurotrauma 2012; 29: 2181–2191. [DOI] [PubMed] [Google Scholar]
- 29.Timofeev I, Carpenter KL, Nortje J, et al. Cerebral extracellular chemistry and outcome following traumatic brain injury: a microdialysis study of 223 patients. Brain 2011; 134: 484–494. [DOI] [PubMed] [Google Scholar]
- 30.Nehlig A, Pereira de Vasconcelos A.Glucose and ketone body utilization by the brain of neonatal rats. Prog Neurobiol 1993; 40: 163–221. [DOI] [PubMed] [Google Scholar]
- 31.Vannucci SJ, Simpson IA.Developmental switch in brain nutrient transporter expression in the rat. Am J Physiol Endocrinol Metab 2003; 285: E1127–1134. [DOI] [PubMed] [Google Scholar]
- 32.LeBlanc MH, Huang M, Patel D, et al. Glucose given after hypoxic ischemia does not affect brain injury in piglets. Stroke 1994; 25: 1443–1447; discussion 1448. [DOI] [PubMed] [Google Scholar]
- 33.LeBlanc MH, Huang M, Vig V, et al. Glucose affects the severity of hypoxic-ischemic brain injury in newborn pigs. Stroke 1993; 24: 1055–1062. [DOI] [PubMed] [Google Scholar]
- 34.Chouthai NS, Sobczak H, Khan R, et al. Hyperglycemia is associated with poor outcome in newborn infants undergoing therapeutic hypothermia for hypoxic ischemic encephalopathy. J Neonatal Perinatal Med 2015; 8: 125–131. [DOI] [PubMed] [Google Scholar]
- 35.Ohki S, Togari H, Sobajima H, et al. Lactate attenuates neuron specific enolase elevation in newborn rats. Pediatr Neurol 1999; 21: 543–547. [DOI] [PubMed] [Google Scholar]
- 36.Juul SE, Ferriero DM.Pharmacologic neuroprotective strategies in neonatal brain injury. Clin Perinatol 2014; 41: 119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taher M, Leen WG, Wevers RA, et al. Lactate and its many faces. Eur J Paediatr Neurol 2016; 20: 3–10. [DOI] [PubMed] [Google Scholar]
- 38.Patet C, Quintard H, Suys T, et al. Neuroenergetic response to prolonged cerebral glucose depletion after severe brain injury and the role of lactate. J Neurotrauma 2015; 32: 1560–1566. [DOI] [PubMed] [Google Scholar]
- 39.Quintard H, Patet C, Zerlauth JB, et al. Improvement of neuroenergetics by hypertonic lactate therapy in patients with traumatic brain injury is dependent on baseline cerebral lactate/pyruvate ratio. J Neurotrauma 2016; 33: 681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brissaud O, Villega F, Pieter Konsman J, et al. Short-term effect of erythropoietin on brain lesions and aquaporin-4 expression in a hypoxic-ischemic neonatal rat model assessed by magnetic resonance diffusion weighted imaging and immunohistochemistry. Pediatr Res 2010; 68: 123–127. [DOI] [PubMed] [Google Scholar]
- 41.Rice JE, 3rd, Vannucci RC, Brierley JB.The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol 1981; 9: 131–141. [DOI] [PubMed] [Google Scholar]
- 42.Cremillieux Y, Salvati R, Dumont U, et al. Online (1) H-MRS measurements of time-varying lactate production in an animal model of glioma during administration of an anti-tumoral drug. NMR Biomed 2018; 31: e3861. [DOI] [PubMed] [Google Scholar]
- 43.Sada N, Lee S, Katsu T, et al. Epilepsy treatment. Targeting LDH enzymes with a stiripentol analog to treat epilepsy. Science 2015; 347: 1362–1367. [DOI] [PubMed] [Google Scholar]
- 44.Freeman R, Mareci TH, Morris GA.Weak satellite signals in high-resolution NMR spectra: separating the wheat from the chaff. J Magn Reson 1981; 42: 341–345. [Google Scholar]
- 45.Rothman DL, Behar KL, Hetherington HP, et al. 1H-Observe/13C-decouple spectroscopic measurements of lactate and glutamate in the rat brain in vivo. Proc Natl Acad Sci U S A 1985; 82: 1633–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lubics A, Reglodi D, Tamas A, et al. Neurological reflexes and early motor behavior in rats subjected to neonatal hypoxic-ischemic injury. Behav Brain Res 2005; 157: 157–165. [DOI] [PubMed] [Google Scholar]
- 47.Karalis F, Soubasi V, Georgiou T, et al. Resveratrol ameliorates hypoxia/ischemia-induced behavioral deficits and brain injury in the neonatal rat brain. Brain Res 2011; 1425: 98–110. [DOI] [PubMed] [Google Scholar]
- 48.Chen J, Li Y, Wang L, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke 2001; 32: 1005–1011. [DOI] [PubMed] [Google Scholar]
- 49.Arteaga O, Revuelta M, Uriguen L, et al. Pretreatment with resveratrol prevents neuronal injury and cognitive deficits induced by perinatal hypoxia-ischemia in rats. PLoS One 2015; 10: e0142424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Magistretti PJ, Pellerin L.Cellular bases of brain energy metabolism and their relevance to functional brain imaging: evidence for a prominent role of astrocytes. Cereb Cortex 1996; 6: 50–61. [DOI] [PubMed] [Google Scholar]
- 51.Bak LK, Walls AB.CrossTalk opposing view: lack of evidence supporting an astrocyte-to-neuron lactate shuttle coupling neuronal activity to glucose utilisation in the brain. J Physiol 2018; 596: 351–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mazuel L, Blanc J, Repond C, et al. A neuronal MCT2 knockdown in the rat somatosensory cortex reduces both the NMR lactate signal and the BOLD response during whisker stimulation. PLoS One 2017; 12: e0174990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suzuki A, Stern SA, Bozdagi O, et al. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 2011; 144: 810–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pierre K, Magistretti PJ, Pellerin L.MCT2 is a major neuronal monocarboxylate transporter in the adult mouse brain. J Cereb Blood Flow Metab 2002; 22: 586–595. [DOI] [PubMed] [Google Scholar]
- 55.Pierre K, Pellerin L.Monocarboxylate transporters in the central nervous system: distribution, regulation and function. J Neurochem 2005; 94: 1–14. [DOI] [PubMed] [Google Scholar]
- 56.Rosafio K, Pellerin L.Oxygen tension controls the expression of the monocarboxylate transporter MCT4 in cultured mouse cortical astrocytes via a hypoxia-inducible factor-1alpha-mediated transcriptional regulation. Glia 2014; 62: 477–490. [DOI] [PubMed] [Google Scholar]
- 57.Bergersen LH.Is lactate food for neurons? Comparison of monocarboxylate transporter subtypes in brain and muscle. Neuroscience 2007; 145: 11–19. [DOI] [PubMed] [Google Scholar]
- 58.Ros J, Pecinska N, Alessandri B, et al. Lactate reduces glutamate-induced neurotoxicity in rat cortex. J Neurosci Res 2001; 66: 790–794. [DOI] [PubMed] [Google Scholar]
- 59.Schurr A, Payne RS, Miller JJ, et al. Brain lactate, not glucose, fuels the recovery of synaptic function from hypoxia upon reoxygenation: an in vitro study. Brain Res 1997; 744: 105–111. [DOI] [PubMed] [Google Scholar]
- 60.Cater HL, Chandratheva A, Benham CD, et al. Lactate and glucose as energy substrates during, and after, oxygen deprivation in rat hippocampal acute and cultured slices. J Neurochem 2003; 87: 1381–1390. [DOI] [PubMed] [Google Scholar]
- 61.Rafiki A, Boulland JL, Halestrap AP, et al. Highly differential expression of the monocarboxylate transporters MCT2 and MCT4 in the developing rat brain. Neuroscience 2003; 122: 677–688. [DOI] [PubMed] [Google Scholar]
- 62.Pellerin L, Pellegri G, Martin JL, et al. Expression of monocarboxylate transporter mRNAs in mouse brain: support for a distinct role of lactate as an energy substrate for the neonatal vs. adult brain. Proc Natl Acad Sci U S A 1998; 95: 3990–3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bouzier-Sore AK, Serres S, Canioni P, et al. Lactate involvement in neuron-glia metabolic interaction: (13)C-NMR spectroscopy contribution. Biochimie 2003; 85: 841–848. [DOI] [PubMed] [Google Scholar]
- 64.Bouzier AK, Thiaudiere E, Biran M, et al. The metabolism of [3-(13)C]lactate in the rat brain is specific of a pyruvate carboxylase-deprived compartment. J Neurochem 2000; 75: 480–486. [DOI] [PubMed] [Google Scholar]
- 65.Sampol D, Ostrofet E, Jobin ML, et al. Glucose and lactate metabolism in the awake and stimulated rat: a (13)C-NMR study. Front Neuroenerget 2013; 5: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ben-Yoseph O, Boxer PA, Ross BD.Oxidative stress in the central nervous system: monitoring the metabolic response using the pentose phosphate pathway. Dev Neurosci 1994; 16: 328–336. [DOI] [PubMed] [Google Scholar]
- 67.Brekke EM, Morken TS, Wideroe M, et al. The pentose phosphate pathway and pyruvate carboxylation after neonatal hypoxic-ischemic brain injury. J Cereb Blood Flow Metab 2014; 34: 724–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berthet C, Lei H, Thevenet J, et al. Neuroprotective role of lactate after cerebral ischemia. J Cereb Blood Flow Metab 2009; 29: 1780–1789. [DOI] [PubMed] [Google Scholar]
- 69.Fridman EA, Beattie BJ, Broft A, et al. Regional cerebral metabolic patterns demonstrate the role of anterior forebrain mesocircuit dysfunction in the severely injured brain. Proc Natl Acad Sci U S A 2014; 111: 6473–6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Camandola S, Mattson MP.Brain metabolism in health, aging, and neurodegeneration. EMBO J 2017; 36: 1474–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bergersen LH, Gjedde A.Is lactate a volume transmitter of metabolic states of the brain? Front Neuroenerget 2012; 4: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sutherland EW, Robison GA.The role of cyclic AMP in the control of carbohydrate metabolism. Diabetes 1969; 18: 797–819. [DOI] [PubMed] [Google Scholar]
- 73.Pan Y, Cai X, Jing J, et al. Stress hyperglycemia and prognosis of minor ischemic stroke and transient ischemic attack: the CHANCE study (clopidogrel in high-risk patients with acute nondisabling cerebrovascular events). Stroke 2017; 48: 3006–3011. [DOI] [PubMed] [Google Scholar]
- 74.Bouet V, Freret T, Ankri S, et al. Predicting sensorimotor and memory deficits after neonatal ischemic stroke with reperfusion in the rat. Behav Brain Res 2010; 212: 56–63. [DOI] [PubMed] [Google Scholar]
- 75.Sanches EF, Arteni NS, Nicola F, et al. Early hypoxia-ischemia causes hemisphere and sex-dependent cognitive impairment and histological damage. Neuroscience 2013; 237: 208–215. [DOI] [PubMed] [Google Scholar]
- 76.Balduini W, De Angelis V, Mazzoni E, et al. Long-lasting behavioral alterations following a hypoxic/ischemic brain injury in neonatal rats. Brain Res 2000; 859: 318–325. [DOI] [PubMed] [Google Scholar]
- 77.Ikeda T, Mishima K, Yoshikawa T, et al. Selective and long-term learning impairment following neonatal hypoxic-ischemic brain insult in rats. Behav Brain Res 2001; 118: 17–25. [DOI] [PubMed] [Google Scholar]
- 78.Wagner BP, Nedelcu J, Martin E.Delayed postischemic hypothermia improves long-term behavioral outcome after cerebral hypoxia-ischemia in neonatal rats. Pediatr Res 2002; 51: 354–360. [DOI] [PubMed] [Google Scholar]
- 79.Guggisberg N.Combined treatment of lactate and rTPA for the management of acute ischemic strokes. Mémoire Maîtrise Méd 2016; 3394: 1–31. [Google Scholar]
- 80.Boumezbeur F, Petersen KF, Cline GW, et al. The contribution of blood lactate to brain energy metabolism in humans measured by dynamic 13C nuclear magnetic resonance spectroscopy. J Neurosci 2010; 30: 13983–13991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gallagher CN, Carpenter KL, Grice P, et al. The human brain utilizes lactate via the tricarboxylic acid cycle: a 13C-labelled microdialysis and high-resolution nuclear magnetic resonance study. Brain 2009; 132: 2839–2849. [DOI] [PubMed] [Google Scholar]
- 82.Pierre K, Chatton JY, Parent A, et al. Linking supply to demand: the neuronal monocarboxylate transporter MCT2 and the alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionic acid receptor GluR2/3 subunit are associated in a common trafficking process. Eur J Neurosci 2009; 29: 1951–1963. [DOI] [PubMed] [Google Scholar]