Abstract
Background
Clinical guidelines recommend anticoagulation for patients with atrial fibrillation (AF) at high risk of stroke; however, studies report 40% of this population is not anticoagulated.
Objective
To evaluate a population health intervention to increase anticoagulation use in high-risk patients with AF.
Methods
We used machine learning algorithms to identify patients with AF from electronic health records at high risk of stroke (CHA2,DS2,-VASc risk score ≥2), and no anticoagulant prescriptions within 12 months.A clinical pharmacist in the anticoagulation service reviewed charts for algorithm-identified patients to assess appropriateness of initiating an anticoagulant. The pharmacist then contacted primary care providers of potentially undertreated patients and offered assistance with anticoagulation management. We used a stepped-wedge design, evaluating the proportion of potentially undertreated patients with AF started on anticoagulant therapy within 28 days for clinics randomised to intervention versus usual care.
Results
Of 1727 algorithm-identified high-risk patients with AF in clinics at the time of randomisation to intervention, 432 (25%) lacked evidence of anticoagulant prescriptions in the prior year. After pharmacist review, only 17% (75 of 432) of algorithm-identified patients were considered potentially undertreated at the time their clinic was randomised to intervention. Over a third (155 of 432) were excluded because they had a single prior AF episode (transient or provoked by serious illness); 36 (8%) had documented refusal of anticoagulation, the remainder had other reasons for exclusion. The intervention did not increase new anticoagulant prescriptions (intervention: 4.1% vs usual care: 4.0%, p=0.86).
Conclusions
Algorithms to identify underuse of anticoagulation among patients with AF in healthcare databases may not capture clinical subtleties or patient preferences and may overestimate the extent of undertreatment. Changing clinician behaviour remains challenging.
BACKGROUND
Atrial fibrillation (AF) represents the most common cardiac arrhythmia, projected to affect over 12 million patients by 2030.1,2 AF is associated with mortality and morbidity from stroke, thromboembolism and related cardiovascular conditions. Extensive evidence demonstrates the effectiveness of oral anticoagulation therapy at preventing stroke and thromboembolism in patients with non-valvular AF, with up to 68% reduction in the risk of ischaemic stroke per year.1,3,4
While oral anticoagulants (OAC) effectively prevent stroke and embolic events in patients with AF, they also increase the risk of major bleeding events.4 To balance benefit and risk, many practice guidelines recommend a tiered approach to management of AF, targeting preventive oral anticoagulation therapy to patients at higher estimated risk of stroke.4–7 Despite these guidelines, studies have reported that 20%–80% of patients with AF at high risk of stroke remain untreated with OACs.1 Identifying these patients can provide an opportunity to increase appropriate anticoagulant use, reduce stroke rates and improve patient outcomes.
Big data and machine learning have been widely touted for improving care.8,9 However, machine learning is rarely used in medicine compared with other industries. Recently, the Food and Drug Administration (FDA) initiated a randomised controlled trial to evaluate an intervention to increase anticoagulant use in patients with AF and high stroke risk (IMPACT-AFib).10 The trial uses four large, national administrative healthcare claims databases. The intervention involves sending letters and educational materials to algorithm-identified patients and their providers with information about stroke risk.
The objective of this study was similar to the ongoing FDA trial. We evaluated whether a population health intervention that used machine learning algorithms to identify potentially undertreated patients with AF from electronic health records (EHR) data and offered support from an anticoagulation management service (AMS) could increase appropriate anticoagulation prescribing compared with usual care.
METHODS
Design
We implemented our intervention using a randomised stepped-wedge design. Fourteen primary care clinics affiliated with Brigham and Women’s Hospital (BWH) participated in the study. Studies were paired by size (patient volume), and one clinic entered the intervention arm every 28 days (online supplementary appendix 1). Clinics were included in the ‘usual care’ arm until their randomised date of entry to the intervention (figure 1). We requested a waiver of patient consent to work with a BWH EHR research database (online supplementary appendix 2). The ClinicalTrials.gov registration number is NCT02734875.
Figure 1.
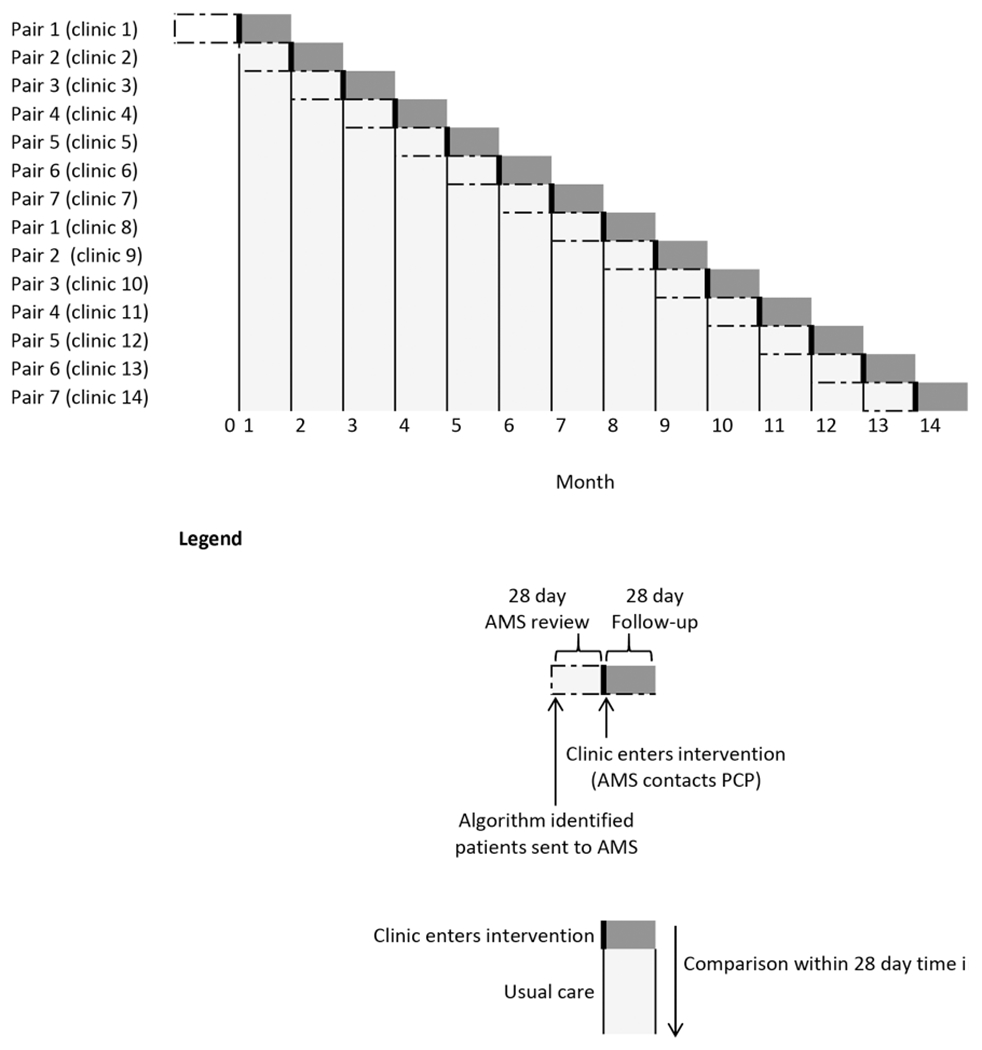
Stepped-wedge randomisation. AMS, anticoagulation management service; PCP, primary care provider.
Intervention
The intervention involved (1) applying algorithms to efficiently identify relevant patients from the EHR, (2) secondary chart review of algorithm-identified patients by clinical staff, (3) an offer to primary care providers (PCP) to assist with anticoagulation management from an established clinical service, and (4) targeted reminders from clinical leaders.
Prior to initiating the intervention, we had multiple discussions with clinic medical directors on the steering committee for the BWH Primary Care PBRN about potential barriers and benefits of deploying the intervention in the network of clinics. During these discussions, we piloted the results of the initial algorithm-based screening with a sample of clinic medical directors to establish the face validity of the population-based screen with respect to their patients.
We deliberately chose not to use EHR-based alerts asking the PCP to take complex actions regarding anticoagulant management. Instead we focused on providing information to an anticoagulation management clinic that could assess clinical subtleties and decide whether it was appropriate to reach out with an offer of support to help PCPs with identified patients. There was consensus from clinic leadership that there was value to the intervention and that bypassing EHR-based alerts with an offer of support from a respected clinical service had the potential to be effective at eliciting action from PCPs. Feedback that we received from one clinical leader was that while he is inundated with alerts and emails, he always pays attention to emails from the AMS.
For each step of the wedge (every 28 days), potentially undertreated patients were identified using previously validated algorithms for identifying AF and known risk factors for stroke and major bleeding from the EHR using validated algorithms with high positive predictive value (PPV) within BWH11 (figure 2, step 1). These algorithms included each component of the CHA2,DS2,-VASc12 risk score for stroke and the HAS-BFED13 risk scores for major bleeding.
Figure 2.
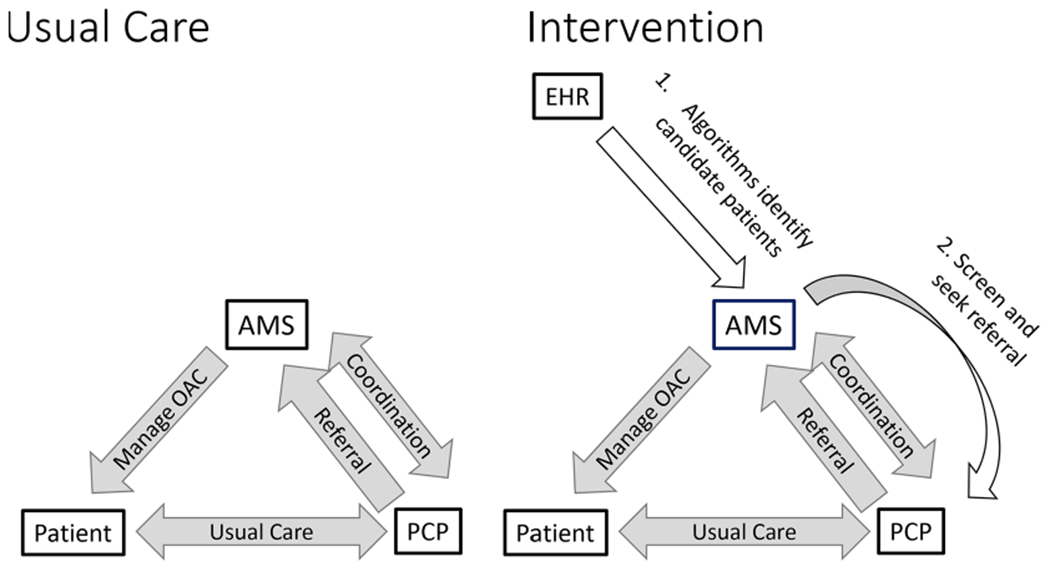
Intervention compared with usual care. AMS, anticoagulation management service; EHR, electronic health record; OAC, oral anticoagulant; PCP, primary care provider.
We ran our algorithms on data from patients with diagnosed AF who had at least two encounters within the BWH system within the prior 2 years, using the most current data extracted from the EHR for research. We further required patients to be alive at the time of secondary chart review and to have: (1) a listed PCP, (2) a calculated CHA2,DS2,-VASc risk of stroke ≥2, and (3) no record for an OAC prescription within 12 months prior to the intervention date.
Twenty-eight days before the date that a clinic was randomised to move from ‘usual care’ to the intervention arm, a list of algorithm-identified patients for the clinic was sent to the AMS, a long-established clinical service at BWH, for review by a staff clinical pharmacist. The clinical pharmacist reviewed the identified patients’ charts in the EHR to assess whether each patient was an appropriate candidate for oral anticoagulation and excluded patients from the study if the three inclusion criteria were not met, AF was transient or provoked, or if the clinical pharmacist identified clearly documented evidence in the chart arguing against anticoagulation. If the clinical pharmacist required help interpreting potentially ambiguous data from the EHR, a clinician coauthor (MAF) provided additional input.
On the date of randomised entry to intervention, research staff double-checked whether there was a new prescription for anticoagulant or if there was a record of patient death that may have occurred after the clinical review. If not, PCPs of potentially undertreated patients were sent an email from the AMS director, a BWH haematologist with >20 years of experience in this role and well known to most PCPs, that listed the patients’ estimated 1-year risk of stroke based on CHA2,DS2,-VASc, risk of bleeding based on HAS-BLED and an offer to discuss and manage anti coagulation with the patient if the PCP chose to make a referral. The brief email ended with a question: what action would the PCP like to take? (figure 2, step 2) PCPs received a follow-up email from AMS at 1 and 2 weeks if they did not respond. At 3 weeks, the Chief of the General Internal Medicine Division sent an email to the Medical Director of the primary care clinic encouraging response. These follow-up emails were specifically designed to highlight the importance that clinical leadership placed on responding to the offer of clinical support from an AMS or documenting why it was not necessary.
Evaluation
We describe the proportion of patients with AF identified as potentially undertreated after (1) validated algorithms, (2) review of EHRs by AMS pharmacist staff, and (3) PCP responses to AMS request for referral. We categorised reasons why patients were excluded from the intervention at successive levels of review and the proportion of patients for whom the PCP responded that they would take action.
Our primary outcome was a comparison of the proportion of algorithm-identified eligible patients prescribed an OAC in the 28 days after entering the intervention or ‘usual care’ arm at each step in the stepped wedge. AMS clinical screening of the algorithm-identified patients for clinics in the intervention arm was considered part of the intervention. Additional details are provided in online supplementary appendix 3. In preliminary work, roughly 5% of algorithm-identified eligible patients were prescribed an anticoagulant within 28 days. The estimated power to detect a change from 5% to 10% with a p value <0.05 was over 80%. We used Cran R V.3.0.3, package lme4, and report a two-sided p value.
Results of assessment
The validated algorithms identified 1727 patients with AF who were at high risk of stroke and had a listed PCP in the 14 affiliated primary care clinics at the time they were randomised to intervention. Of these, 25% (n=432) did not have evidence of OAC prescription within 12 months. Only 17% of the 432 algorithm-identified patients were still considered potentially undertreated after AMS pharmacist staff review. There were often numerous reasons identified for excluding algorithm-identified patients. We created a hierarchy of reasons for exclusion and report the top reason for each patient (figure 3). Out of 432 reviewed patients with AMS, 2% were dead, 23% had evidence of OAC prescription from unstructured text, notes, or EHR fields otherwise inaccessible for automated identification, 3% were no longer with the listed PCP and 37% were excluded because patients had transient AF—usually only a single distant episode. For 6%, notes indicated that OAC had been discontinued due to adverse events or comorbidities and 7% stated that OAC was contraindicated. Overall, ignoring the hierarchy, 8.2% had EHR documentation that patients had declined OAC. The AMS contacted 43 PCPs by email about 75 patients who remained eligible after clinical review; 25 PCPs (58%) responded about 44 patients (59%). Of these 44 patients, PCPs indicated they would consider action regarding OAC for 4 (9.0%) (figure 4). PCPs reported that 7% of the patients were dead, 30% were no longer under their care, 28% had transient AF, 21% had contraindications and 7% of patients had undocumented refusal of OAC.
Figure 3.
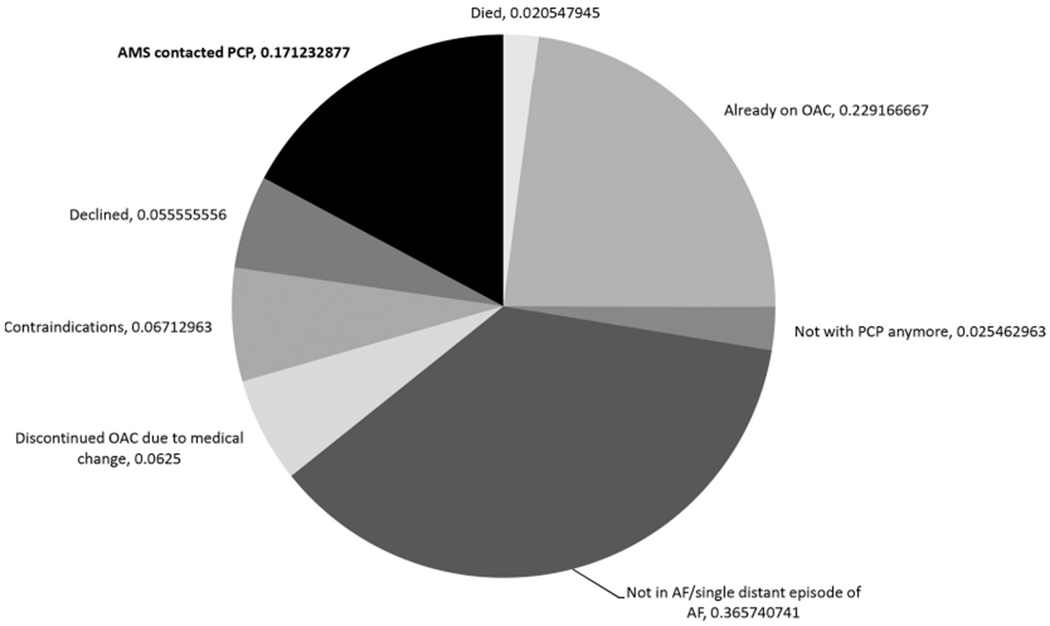
Anticoagulation management service (AMS) review of algorithm-identified patients (n=432). AF, atrial fibrillation; OAC, oral anticoagulant; PCP, primary care provider.
Figure 4.
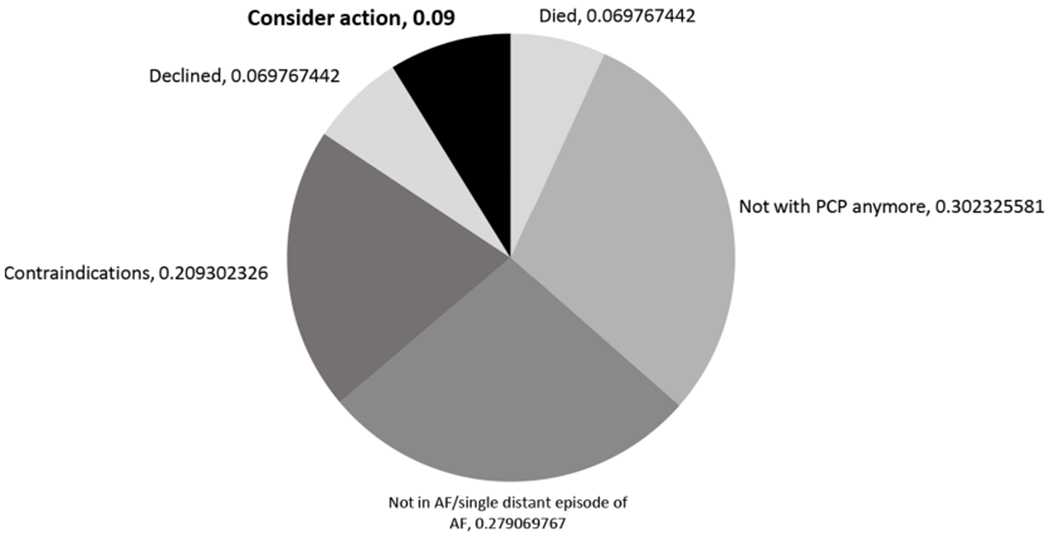
Primary care provider (PCP) review of anticoagulation management service screened patients (n=44 patients). AF, atrial fibrillation.
Of the 39 patients excluded due to reported contraindication in AMS review of EHRs or PCP response, 64% had a prior major bleed, 23% had a history of falls or frailty, 10% had alcohol/substance abuse problems, 5% were judged to be unable to adhere to therapy and 5% had other contraindications such as amyloid angiopathy, arteriovenous malformation or severe dementia. Some patients had multiple contraindications.
The free-text PCP responses had several themes. These included that the PCP had discussed anticoagulation with the patient, but the patient declined; that the patient was not able to follow through on treatment or had other contraindications; and that the patient’s cardiologist should make the decision to prescribe anticoagulant (box 1).
Box 1 Example of primary care provider reasons for not referring patient to anticoagulation management service (AMS) or prescribing anticoagulant.
‘Patient X refused anticoagulation numerous times. Well documented that the patient understands risks.’
‘Patient X abuses alcohol, has inconsistent adherence to meds, and a history of falls. Inability to cooperate with medical management was considered a contraindication.’
‘I have been trying to get Patient X to go to cardiology to discuss this. His old cardiologist did not want him on OAC [oral anticoagulant]. I do, but he wants to see cardiologist to discuss and then does not go, cancels, does not schedule etc.’
‘Patient X is followed by cardiology, they should decide.’
Selected quotes chosen to emphasise themes. These have been edited for grammar and spelling and may combine quotes from multiple primary care providers.
There were 412 algorithm-identified patients who met eligibility criteria in intervention clinics 28 days before as well as on the day their clinic entered the intervention arm. There were 3364 algorithm-identified patients in ‘usual care’ clinics who met eligibility criteria at the same time points. Within 28 days of entering the intervention arm, 4.1% (n=17) of algorithm-identified patients had a prescription for an anticoagulant. Over the same time period, 4.0% (n=136) of algorithm-identified patients in usual care clinics had an anticoagulant prescription. There was little evidence to support a difference between intervention and ‘usual care’ clinics (p=0.97).
Of the 17 patients in the intervention arm with a prescription for an anticoagulant observed within 28 days in Enterprise Data Warehouse (EDW) data, 14 had been excluded by AMS staff in clinical review because the live medical chart indicated that the patient had an anticoagulant prescription within the prior year (unstructured text or fields that were not updated in EDW). Two patients who initiated anticoagulant were excluded during AMS clinical review because the patients had single episodes of AF in the past but were not currently in AF. The final patient was included in the intervention. Although the PCP responded to the AMS by stating that the patient had a single distant episode of AF in the past and did not make a referral or indicate that action would be taken, a new prescription for an anticoagulant for this patient was observed in EDW data within 28 days of contact from AMS.
DISCUSSION
We evaluated an intervention to increase appropriate prescribing of anticoagulation for patients with AF at high risk of stroke. The intervention was designed to address identified barriers to appropriate anticoagulation therapy among patients with AF such as lack of physician awareness, exaggerated perception of bleeding risk and lack of time to address AF due to competing comorbid conditions.3,14
We found that the proportion of patients with AF who should have been receiving anticoagulation but were not was considerably lower than most published estimates. For patients identified as candidates for anticoagulation, we found that despite intensive resources and support from clinical leaders, non-response from PCPs was high and the intervention had little impact on OAC prescribing. Our findings have important implications for how EHR and other healthcare data are used to measure the quality of care, for how interventions to address gaps in care are designed and implemented, and for ongoing research.
Measuring the quality of care for AF
Underuse of anticoagulation for patients with AF has been identified in the medical literature for many years, but precise measurement has been a challenge, with estimates of undertreatment in different populations ranging from 20% to 80%.1 Our algorithms applied in EHR warehouse data identified 25% of patients with AF who apparently did not have a prescription for an OAC within the last year; however, 23% of these patients had anticoagulant use documented in free text or other areas of the live EHR inaccessible to our algorithms. Several possible factors may explain why our results differed from those in the prior literature.
Our use of EHR-based data as opposed to claims data may have resulted in overestimation of the proportion of patients receiving anticoagulation. The EHR records anticoagulant prescriptions but does not verify whether they were filled. Given the well-documented rates of medication non-adherence,15–17 the actual proportion of patients in our study not receiving anticoagulation may be higher than what we identified. We did not have claims or pharmacy data to assess adherence, however such information is becoming more readily available as healthcare data systems become more integrated.18 Those developing quality of care measures will need to consider how to classify situations where a clinician has recommended and prescribed anticoagulation but the patient is not adherent.
Relatedly, patient refusal to use OAC accounted for 9% of algorithm-identified patients. Documentation of patient refusal was not uniform, in some cases the pharmacist reviewing charts identified patient refusal and in others it was reported by PCPs. An important area for future study would be determining how to incorporate patient refusal into measurement of undertreatment. Many patients had prior complications with OAC or had contraindications documented in the EHR. There is no current consensus on how these patients should be counted when measuring the quality of AF care.
Other possible explanations for differences between our findings and prior literature are that rates of OAC use in patients with AF have increased over time or are higher in the healthcare system we studied than in other healthcare systems. Neither of these possibilities seem likely since other recent studies continue to estimate non-treatment rates higher than we identified, including at least one study from our own institution.19,20 A sizeable fraction of patients had a single time-limited episode of AF in the past, often with a provoking clinical event; the proportion of patients in this group was similar in our study to that in another recent primary care-based study of AF and OAC.21 Clinicians did not consider OAC initiation to be indicated for these patients, and current clinical guidelines do not address this subset specifically.22 Guideline-developing bodies should address how this subgroup should be treated.
Challenges for quality of care interventions
The factors described above contributed to identification of a smaller than expected number of patients with ‘actionable’ AF who were considered undertreated. Recognising that alert fatigue could reduce the impact of an EHR alert-based intervention, we implemented a clinically nuanced intervention with stronger encouragement to increase appropriate anticoagulant prescribing.21,23–25
Despite this the response rate from clinicians was very low, with the intervention having essentially no impact. New starts of OAC were identified for 4% of patients in both the intervention and control groups, identical to a similar recent study.21
The struggle to measure clinical subtleties and patient preferences from EHR data as well as low PCP response rates are not unique to our study. Primary care clinicians are already struggling with daunting demands when attempting to manage ageing and increasingly complex patients in time-limited office visits. The challenges we experienced, in spite of pharmacist screening of algorithm-identified patients to minimise PCP contacts for patients in whom a discussion about anticoagulation was clinically inappropriate and offers clinical support, illustrate the many hurdles for clinicians, researchers and health system leaders attempting to design pragmatic trials to improve quality of care. These challenges are especially relevant in light of the ongoing FDA Catalyst IMPACT-Afib10 trial and similar electronic alert and informational material-based interventions21,26,27 that have used algorithm-based identification of target patients from large healthcare data. Future studies should evaluate whether other means of engaging with PCPs or patient-directed interventions might be more successful.
Limitations
This intervention had several limitations. First, the validated algorithms for AF were not designed to capture clinical subtleties such as transient AF provoked by serious illness. Furthermore, problem list entries and diagnosed conditions were carried forward indefinitely in the EHR. Second, there was high non-response from PCPs when contacted by AMS about potentially undertreated patients with AF. Third, while our intervention provided a calculated HAS-BLED score based on algorithms run against the EHR, we did not address individual perceived contraindications. Fourth, while the data on eligible patients for the intervention arm were captured every month during the study, data on eligible patients in the ‘usual care’ arm were extracted at the end of the study; after all 14 clinics had entered the intervention. Fifth, there was limited pilot testing, and we did not have formal evidence that the intervention would be effective at changing physician behaviour prior to beginning the trial.
There were numerous challenges with using EHR data to triage and identify relevant patients. A few months before the intervention launched, BWH transitioned from a home-grown longitudinal medical record system to an EPIC EHR system. The transition affected both how healthcare encounters were coded and how the data were exported to Research Patient Data Registry (RPDR) for research purposes. The added workload of adopting a new EHR may have made PCPs less willing to respond to our intervention. Additionally, RPDR extracts of EHR data were not available to the research team in real time. However, we had an AMS pharmacist screen the algorithm-identified patients from RPDR and used EDW data to check if exclusion criteria were still met the day prior to a clinic entering the intervention arm. The EDW is fed by numerous information systems using different identifiers. Crosswalks to connect across relational data tables for these systems were imperfect. Furthermore, clinical notes were not available for use in the algorithms. These issues contributed to our inability to identify some of the anticoagulant prescriptions that were observable in the live medical record during the course of the intervention.
CONCLUSIONS
Commonly used algorithms to count patients with AF in large healthcare databases do not capture subtleties in AF status, potentially including patients with only distant histories of AF. Nor do such algorithms reflect patient profiles beyond known risk factors for stroke and major bleeding or the complexity of decision-making processes in accordance with patient preferences. The clinical context surrounding the decision to prescribe anticoagulants for AF is complex and goes beyond risk of stroke or major bleeding. Factors such as perceived ability of the patient to stay on the regimen and transient, provoked AF are difficult to measure in EHRs. This can complicate measurement of clinical performance and quality with respect to anticoagulation for AF.28 Although there are challenges with using large healthcare databases for population health interventions and previously published reports may overestimate the magnitude of the problem, nevertheless, it is clear that there are potentially undertreated patients with AF who should be anticoagulated to prevent stroke and that more work is needed to identify interventions that can close this gap in care.
Supplementary Material
Acknowledgements
This study used data from the Partners Healthcare System Research Patient Data Registry (RPDR).
The authors thank Shawn Murphy and Henry Chueh and the Partners Healthcare System RPDR group for facilitating use of their database.
Funding This project was funded by the Agency for Healthcare Research and Quality (R00HS022193).
Competing interests SVW was supported by a grant from the Laura and John Arnold Foundation. She is a paid consultant to Aetion, a software company, and principal investigator on investigator-initiated grants from Novartis, Boehringer Ingelheim and J&J to BWH for unrelated work. During the conduct of this study, JRR was a paid consultant to Aetion, for unrelated work. MAF is Director of the National Resource Center for Academic Detailing, which is supported by AHRQ (Grant No R18HS023236).
Footnotes
► Additional material is published online only. To view please visit the journal online (http://dx.doi.Org/10.1136/bmjqs-2019-009367).
Early results were presented at the International Conference for Pharmacoepidemiology in Montreal, August 2017.
Publisher's Disclaimer: Disclaimer The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
Patient consent for publication Not required.
Ethics approval This study was approved by the BWH Institutional Review Board and BWH Primary Care Practice Based Research Network (PBRN) steering committee.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available upon reasonable request.
REFERENCES
- 1.Ogilvie IM, Newton N, Welner SA, et al. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med 2010;123:638–45. [DOI] [PubMed] [Google Scholar]
- 2.Colilla S, Crow A, Petkun W et al. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol 2013;112:1142–7. [DOI] [PubMed] [Google Scholar]
- 3.Bungard TJ, Ghali WA, Teo KK, et al. Why do patients with atrial fibrillation not receive warfarin? Arch Intern Med 2000;160:41–6. [DOI] [PubMed] [Google Scholar]
- 4.Camm AJ, Lip GYH, De Caterina R, et al. 2012 focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation. developed with the special contribution of the European heart rhythm association. Eur Heart J 2012;33:2719–47. [DOI] [PubMed] [Google Scholar]
- 5.Atrial fibrillation: management. NICE Guidelines [CG180]. NICE Guidance 2014, 2016. Available: https://www.nice.org.uk/guidance/cg180 [Google Scholar]
- 6.Cairns JA, Connolly S, McMurtry S, et al. Canadian cardiovascular Society atrial fibrillation guidelines 2010: prevention of stroke and systemic thromboembolism in atrial fibrillation and flutter. Can J Cardiol 2011;27:74–90. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein LB, Adams R, Alberts MJ, et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association stroke Council: cosponsored by the atherosclerotic peripheral vascular disease interdisciplinary Working group; cardiovascular nursing Council; clinical cardiology Council; nutrition, physical activity, and metabolism Council; and the quality of care and outcomes research interdisciplinary Working group: the American Academy of Neurology affirms the value of this guideline. Stroke 2006;37:1583–633. [DOI] [PubMed] [Google Scholar]
- 8.Longhurst CA, Harrington RA, Shah NH. A ‘Green Button’ For Using Aggregate Patient Data At The Point Of Care. Health Affairs 2014;33:1229–35. [DOI] [PubMed] [Google Scholar]
- 9.Gallego B, Walter SR, Day RO, et al. Bringing cohort studies to the bedside: framework for a ’green button’ to support clinical decision-making. J Comp Eff Res 2015;4:191–7. [DOI] [PubMed] [Google Scholar]
- 10.Pokorney SC, Al-Khalidi N;, Haynes H;, et al. Implementation of a randomized controlled trial to improve treatment with oral anticoagulants in patients with atrial fibrillation (IMPACT-AFib). Available: https://www.sentinelinitiative.org/FDA-catalyst/projects/implementation-randomized-controlled-trial-improve-treatment-oral-anticoagulants-patients [Accessed 20 Nov 2018]. [DOI] [PMC free article] [PubMed]
- 11.Wang SV, Rogers JR, Jin Y, et al. Use of electronic healthcare records to identify complex patients with atrial fibrillation for targeted intervention. Journal of the American Medical Informatics Association 2016;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.GYH L, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart survey on atrial fibrillation. Chest 2010;137:263–72. [DOI] [PubMed] [Google Scholar]
- 13.Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation. Chest 2010;138:1093–100. [DOI] [PubMed] [Google Scholar]
- 14.Closing the Quality Gap. A critical analysis of quality improvement strategies: volume 7—Care coordination. Rockland, MD: Quality AfHRa, 2007. [PubMed] [Google Scholar]
- 15.Benner JS, Glynn RJ, Mogun H. Long-term persistence in use of statin therapy in elderly patients. JAMA 2002;288:455–61. [DOI] [PubMed] [Google Scholar]
- 16.Fischer MA, Stedman MR, Lii J, et al. Primary medication Non-Adherence: analysis of 195,930 electronic prescriptions. Journal of General Internal Medicine 2010;25:284–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer MA, Choudhry NK, Brill G, et al. Trouble getting started: predictors of primary medication nonadherence. The American Journal of Medicine 2011;124:1081.e9–1081.e22. [DOI] [PubMed] [Google Scholar]
- 18.Lin KJ, Glynn RJ, Singer DE, et al. Out-of-system care and recording of patient characteristics critical for comparative effectiveness research. Epidemiology 2018;29:356–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashburner JM, Singer DE, Lubitz SA, et al. Changes in use of anticoagulation in patients with atrial fibrillation within a primary Care network associated with the introduction of direct oral anticoagulants. The American Journal of Cardiology 2017;120:786–91. [DOI] [PubMed] [Google Scholar]
- 20.Piazza G, Karipineni N, Goldberg HS, et al. Underutilization of Anticoagulation for Stroke Prevention in Atrial Fibrillation. Journal of the American College of Cardiology 2016;67:2444–6. [DOI] [PubMed] [Google Scholar]
- 21.Ashburner JM, Atlas SJ, Khurshid S, et al. Electronic physician notifications to improve guideline-based anticoagulation in atrial fibrillation: a randomized controlled trial. Journal of General Internal Medicine 2018;33:2070–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manning WS, D E; Lip GYH. Specific patient groups: short duration atrial fibrillaation. Atrial fibrillation. Anticoagulant therapy to prevent embolization, 2017. [Google Scholar]
- 23.Nwulu U, Brooks H, Richardson S, et al. Electronic risk assessment for venous thromboembolism: investigating physicians’ rationale for bypassing clinical decision support recommendations. BMJ Open 2014;4:e005647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCoy AB, Thomas EJ, Krousel-Wood M, et al. Clinical decision support alert appropriateness: a review and proposal for improvement. The Ochsner journal 2014;14:195–202. [PMC free article] [PubMed] [Google Scholar]
- 25.van der Sijs H, Aarts J, Vulto A, et al. Overriding of drug safety alerts in computerized physician order entry. Journal of the American Medical Informatics Association 2006;13:138–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cook DA, Enders F, Caraballo PJ, et al. An automated clinical alert system for Newly-Diagnosed atrial fibrillation. Plos One 2015;10:e0122153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eckman MH, Lip GYH, Wise RE, et al. Using an atrial fibrillation decision support tool for thromboprophylaxis in atrial fibrillation: effect of sex and age. Journal of the American Geriatrics Society 2016;64:1054–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heidenreich PA, Solis P, Mark Estes NA, et al. 2016 ACC/AHA clinical performance and quality measures for adults with atrial fibrillation or atrial flutter. Circ Cardiovasc Qual Outcomes 2016;9:443–88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


