Abstract
Brain-to-brain synchrony has been proposed as an important mechanism underlying social interaction. While first findings indicate that it may be modulated in children with autism spectrum disorder (ASD), no study to date has investigated the influence of different interaction partners and task characteristics. Using functional near-infrared spectroscopy hyperscanning, we assessed brain-to-brain synchrony in 41 male typically developing (TD) children (8–18 years; control sample), as well as 18 children with ASD and age-matched TD children (matched sample), while performing cooperative and competitive tasks with their parents and an adult stranger. Dyads were instructed either to respond jointly in response to a target (cooperation) or to respond faster than the other player (competition). Wavelet coherence was calculated for oxy- and deoxyhemoglobin brain signals. In the control sample, a widespread enhanced coherence was observed for parent–child competition, and a more localized coherence for parent–child cooperation in the frontopolar cortex. While behaviorally, children with ASD showed a lower motor synchrony than children in the TD group, no significant group differences were observed on the neural level. In order to identify biomarkers for typical and atypical social interactions in the long run, more research is needed to investigate the neurobiological underpinnings of reduced synchrony in ASD.
Keywords: fNIRS hyperscanning, brain-to-brain-synchrony, motor synchrony, autism spectrum disorder, parent–child interaction
Introduction
Autism spectrum disorders (ASDs) are a group of neurodevelopmental disorders, characterized by impairments in reciprocal social interaction, communication and repetitive stereotypic behavior (American Psychiatric Association, 2013). Deficits in social interaction are considered as most central to the disorder (Scott-Van Zeeland et al., 2010). Several lines of research have indicated that behavior and brain functions related to diverse aspects of social interaction are altered in ASD, such as theory of mind (e.g. Kimhi, 2014), social reward processing (e.g. Scott-VanZeeland et al., 2010; Kohls et al., 2013; Kruppa et al., 2019), joint attention (e.g. Oberwelland et al., 2017) or spontaneous conversation (e.g. Jasmin et al., 2019). Most studies investigating the underlying mechanisms of social interaction deficits in ASD focused on specific brain regions or networks (‘social brain,’ Pelphrey et al., 2004, 2011) and corresponding behavioral responses. However, in order to capture the dynamic exchange during interpersonal encounters, simultaneous recordings of interacting brains may be necessary (e.g. Hari et al., 2015). This is especially important in the light of evidence that individuals with ASD display less difficulties in social processing during passive observation of static social or non-social stimuli than during real-time social interactions (Bolis and Schilbach, 2018). Hence, examining interacting brains rather than a single person’s brain seems a valuable tool to capture the neural mechanisms of social interaction deficits in ASD.
One important mechanism for the coordination of behavior during social interaction is synchronization. Synchrony describes a specific form of coordinated temporal relationship between events. Across mammalian species, synchrony already occurs during the earliest stages of social development. For example, the bond of parent and child is associated with processes of bio-behavioral synchrony, such as the coupling of parent’s and child’s physiology and behavior during social contact (Feldman, 2012, 2015). Bio-behavioral synchrony has been described as a central element of the socio-emotional development of the child (Feldman, 2012, 2015). Synchrony increases social bonding behaviors and enhances social perception and social cognition (Mogan et al., 2017). In addition to behavioral (Feldman, 2015), physiological (Bornstein and Suess, 2000; Manini et al., 2013) and endocrinological (Papp et al., 2009) synchrony, ‘neural’ synchrony between parent–child dyads has been described recently (Reindl et al., 2018; Miller et al., 2019). Neural synchrony, also termed brain-to-brain synchrony, can be assessed using ‘hyperscanning,’ i.e., the simultaneous measurement of two individuals’ brain activities while interacting with each other (Babiloni and Astolfi, 2014). Neural synchrony is based on joint neural oscillations reflecting bio-behavioral synchrony (Hasson et al., 2012). It can be assumed that being mutually engaged in a given task or situation drives similar cognitive processes in both brains, resulting in similar neural oscillations. An increase in neural synchrony may reflect optimized interactive behavior, driven by internal predictions about the self and the other during (social) interactions (Dai et al., 2018).
In a previous functional near-infrared spectroscopy (fNIRS) hyperscanning study of our research group, we demonstrated enhanced synchronization of parent’s and child’s brain activation (in comparison to stranger–child dyads) in the dorsolateral prefrontal and frontopolar cortex during a cooperative game (Cui et al., 2012) but not during a competitive condition, in which dyads had to respond faster than the partner (Reindl et al., 2018). Such effects in brain-to-brain synchrony may be explained as emerging from mutual interaction during a task, with the interaction being characterized by a common goal and shared attention and behavioral adaptation processes (Reindl et al., 2018). This approach has been applied in several other studies with healthy adults (e.g. Cui et al., 2012; Cheng et al., 2015; Baker et al., 2016; Pan et al., 2017), however, systematic investigation of parent–child dyads remains scarce. Furthermore, the few available studies have focused on infancy (e.g. Leong et al., 2017) or early childhood (e.g. Reindl et al., 2018; Nguyen et al., 2020). Adolescence is a critical phase of life between late childhood and adulthood associated with a number of individual developmental changes which can be particularly challenging in ASD (Anderson et al., 2011). In addition to physical maturation, adolescence is accompanied by profound mental and emotional changes, including the emotional separation from parents together with the development of increased autonomy and stronger identification with peers (Christie and Viner, 2005; Jager et al., 2015). Functional neuroimaging research suggests a fundamental reorganization of the brain during this period (Konrad et al., 2013). In particular, behavioral changes related to social cognition are paralleled by functional changes in the ‘social brain,’ e.g., functional activation of the medial prefrontal cortex during mental state attribution (Blakemore, 2008). Given such marked socio-cognitive and neurodevelopmental changes during adolescence, age-related changes in brain-to-brain synchrony from childhood to adolescence are a particularly important target of investigation, but have not been investigated yet.
Behavioral studies suggest that synchrony is reduced in individuals with ASD (Marsh et al., 2013; Fritzpatrick et al., 2016, 2017; Noel et al., 2017; Curioni et al., 2017). For instance, individuals with ASD showed less coordination of movements with their parents in a rocking chair paradigm (Marsh et al., 2013), synchronized pendulum swings (Fitzpatrick et al., 2016) and exhibited less interpersonal synchrony and complex movements in an interview setting (Noel et al., 2017). The neural mechanism underlying reduced behavioral synchrony in individuals with ASD is poorly understood; hardly any studies have used hyperscanning during social interaction in ASD (but see Tanabe et al., 2012 for fMRI hyperscanning during a joint attention task in adults with ASD). With respect to parent–child dyads in ASD, first evidence was provided by a recent fNIRS hyperscanning study. Wang et al. (2020) found that children with more severe ASD symptoms showed lower levels of behavioral and neural synchronization in the right superior frontal cortex with their parents during an adapted version of a cooperation game (Cui et al., 2012). A competition condition was not investigated, even though this type of control condition would be necessary to ensure that it is not the temporal proximity of the dyads’ responses alone that accounts for the increase in brain-to-brain synchrony during cooperation (see Cui et al., 2012; Reindl et al., 2018). Furthermore, the study did not include typically developing (TD) control subjects. To the best of our knowledge, no study to date has investigated in ASD whether the familiarity of the interaction partner (Pan et al., 2017; Reindl et al., 2018) might modulate brain-to-brain synchrony, taking into account potential age-related changes across childhood and adolescence. Since previous findings have demonstrated altered neural activity in children and adolescence with ASD during interactions with their mother as compared to a female stranger (Oberwelland et al., 2017), influences of familiarity on brain-to-brain synchrony seem likely.
Hence, in the present study, we aimed at investigating brain-to-brain synchrony in ASD and TD participants during social cooperation and competition, while accounting for the influence of the familiarity of the interaction partner as well as possible developmental effects across childhood and adolescence. In order to address these issues, we used a well-established cooperative and competitive computer game (see Reindl et al., 2018) where children and adolescents with ASD played with their parents (as compared to TD children and adolescents and their parents) and with adult strangers, respectively. To assess group effects, a group of participants with ASD was compared to closely age-matched TD participants and to assess developmental effects, we investigated a larger control sample of TD children and adolescents.
Materials and methods
Participants
In total, 22 individuals with ASD (aged between 8 and 18 years) and 55 TD participants (aged between 8 and 18 years) took part in the study with one of their parents, as well as an adult stranger. Children and their parents were recruited at the Department of Child and Adolescent Psychiatry in Aachen (RWTH Aachen University) and from a database of participants from previous studies. Adult strangers were recruited via local advertisement. From the individuals with ASD, four dyads had to be excluded from the final analysis for various reasons (multiple psychotropic medication of parent and/or child, n = 2; multiple co-morbidities with antidepressant medication of the child, n = 1; data quality problems, n = 1). From the TD sample, nine dyads were excluded because of male sex of the parent (except for one ASD-match), in order to reduce potential sex effects (Cheng et al., 2015; Baker et al., 2016). Additional four dyads had to be excluded because of data quality problems and one dyad because of a current neurological diagnosis of the stranger. See Table 1 for demographic information of the final participant sample.
Table 1.
Demographic data of the final participant samples
| TD—complete (N = 41) | ASD (N = 18) | TD—matched (N = 18) | t statistic* | |
|---|---|---|---|---|
| Characteristic | M (s.d.) | M (s.d.) | M (s.d.) | |
| Child age (years) | 12.66 (2.79) | 13.54 (2.96) | 13.53 (2.99) | t(34) = 0.010, P = 0.992, d = −0.00 |
| Parent age (years) | 44.98 (5.14) | 46.49 (5.57) | 47.15 (4.68) | t(34) = −0.386, P = 0.702, d = 0.13 |
| Stranger age (years) | 23.40 (3.72) | 25.00 (2.27) | 23.53 (4.40) | t(30) = 1.190, P = 0.243, d = −0.42 |
| IQa | 111.26 (12.23) | 108.94 (17.47) | 115.76 (14.50) | t(32) = −1.239, P = 0.224, d = 0.43 |
| SRS totalb | 33.81 (20.35) | 84.53 (33.29) | 33.47 (19.03) | t(25.45) = 5.490, P < 0.001, d = −1.88 |
| SCQ totalc | 3.43 (2.52) | 17.72 (6.08) | 3.50 (2.33) | t(21.91) = 9.271, P < 0.001, d = −3.09 |
| FBB-HKS totald | 9.82 (9.23) | 24.94 (13.12) | 7.61 (6.06) | t(22.23) = 4.969, P < 0.001, d = −1.70 |
Intelligence quotient (IQ); TD—complete: 3 missing, ASD: 1 missing, TD—matched: 1 missing.
SRS; TD—complete: 5 missing, ASD: 1 missing, TD—matched: 1 missing.
SCQ; TD—matched: 4 missing.
ADHD rating scale [FBB-HKS]; TD—complete: 3 missing, ASD: 1 missing.
Independent-samples t-tests were conducted (two-sided) to compare ASD and TD-matched participants.
For the final analyses of the TD group (control sample), 41 children and their parents were included. All children, except for one, participated with their mothers. This father–son dyad was included as a match for the ASD sample (as described below). In addition, 32 adult strangers performed identical tasks with the participating children. Seven strangers participated twice and one stranger three times. The sex of the stranger was matched to the sex of the respective parent, but the mean age differed between strangers and parents (see Table 1).
For a group comparison of children with ASD and TD children (matched sample), TD participants were selected from the larger dataset to provide a close match in age with the ASD group (±6 months). In the final matched sample analyses, 18 male individuals with ASD and their parents as well as 18 male TD participants and their parents were included. One child in the ASD and the TD group each participated with their fathers. In this matched-sample, 32 adult strangers participated. Of these, two strangers took part twice and one stranger three times.
All participants with ASD had received a diagnosis by experienced clinicians and reached cut-offs on the autism diagnostic observation schedule-generic (ADOS-G; Lord et al., 2000, one patient with missing values) and/or the autism diagnostic interview-revised (ADI-R; Lord et al., 1994, one patient with missing values). Thirteen of them had a comorbid diagnosis of attention-deficit/hyperactivity disorder (ADHD), in line with the literature reporting comorbidity rates with ASD ranging from approximately one-third (Simonoff et al., 2008) to three-quarters (Lee and Ousley, 2006). One individual diagnosed with ASD had a comorbid hyperkinetic disorder of social behavior. If the patients typically received stimulant treatment, medication was discontinued to ensure stimulant-free testing. One patient did not comply and was thus medicated with stimulants (Methylphenidate; Ritalin LA®) at the time of testing. One mother reported having a depressive episode in remission and was medicated at the time of testing with a selective serotonin reuptake inhibitor (SSRI; Citalopram). All results were replicated after removing the two dyads, which included the medicated child, the child with missing values in ADOS/ADI-R and the medicated mother (see ‘Statistical analyses’). TD participants, their parents and adult strangers had no indication of developmental delay or any neurological or psychiatric disorder, as assessed by a brief clinical interview. All participants filled in questionnaires to control for social communicative abilities (German versions of the social responsiveness scale; SRS; Bölte and Poustka, 2008 and social communication questionnaire; SCQ; Bölte and Poustka, 2006) as well as a German ADHD rating scale (FBB-HKS; Döpfner and Lehmkuhl, 2000) to screen for ADHD symptoms.
The study was approved by the local ethical committee of the University Hospital RWTH Aachen, Aachen, Germany (EK 344/14). All experimental procedures were conducted at the Department of Child and Adolescent Psychiatry, Psychosomatics and Psychotherapy, University Hospital RWTH Aachen in Aachen, Germany, with written informed consent of all participants (for children age <18 years, their legal guardian gave written informed consent and all children gave their verbal assent) after they had received a complete description of the study.
Procedure
The participating children and adolescents were playing a cooperative and competitive computer game, once with their parent and once with an adult stranger in counterbalanced order. Participants were sitting next to each other in front of a computer screen while playing the computer game and being recorded with fNIRS. They were instructed to rest their heads on a chin rest and not to talk during the fNIRS measurement. To reduce the participants’ ability to view each other’s motor activities, a towel was placed over their hands (for a video showing the set-up and procedure, see Reindl et al., 2019).
Experimental task
We employed a computer-based cooperation game, originally introduced by Cui et al. (2012) and modified to be suitable for children by Reindl et al. (2018). At the beginning of each trial, two cartoon dolphins (the game characters of the two players) were shown on the screen. After 2 s, a black circle appeared above the dolphins (‘Ready’ signal). After a random delay of 0.6–1.5 s, the circle turned into a red-white ball (‘Go’ signal).
In the ‘cooperation’ condition, the dyads were asked to respond as simultaneously as possible to the ‘Go’ signal via button press, in order to let their dolphin jump to the ball (feedback screen, presented for 1.5 s) and catch the ball together (result screen, presented for 1.5 s) in order to win shared points. If the difference between the response times (RT) of the two players was above a given threshold (T = 1/8 (RT1 + RT2)), only the faster dolphin jumped to the ball (feedback screen), no dolphin caught the ball (result screen) and both players lost a point.
In the ‘competition’ condition, dyads were instructed to respond faster than the interaction partner to the ‘Go’ signal in order to let their dolphin jump to the ball (feedback screen, presented for 1 s), catch the ball (result screen, presented for 1 s) and win more points than their interaction partner. Only the faster dolphin jumped to the ball and earned a point, whereas the player with the slower dolphin lost a point. However, if both players reacted simultaneously with a margin of error of 50 ms, both dolphins jumped to the ball, caught the ball and each player won a point.
Each task condition consisted of two task blocks with 20 trials each and three 30 s resting blocks: one before the beginning of the first and the second task block, respectively, and one after the second task block. Before the beginning of the experiment, five practice trials of each condition were completed. The order of the two task conditions (cooperation/competition) was counterbalanced across children but kept constant for both dyads (parent-/stranger–child) of each child. Furthermore, the order of playing first with the parent or the adult stranger was counterbalanced accordingly, however, due to the matching procedure, no perfect balance could be achieved in the final sample (see Supplementary Table S1). See Figure 1 for an illustration of the cooperation (A) and competition (B) condition of the task.
Fig. 1.
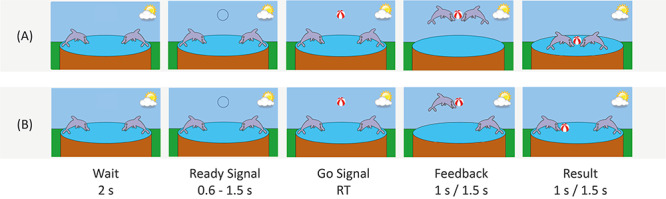
Illustration of the task design. (A) Cooperative game, (B) competitive game. RT is the response time of (A) the slower participant and (B) the faster participant.
Task behavior measurements
Adult’s and child’s response times were recorded during the tasks. As a measure of motor synchrony, the mean of the absolute differences in response times of each dyad was calculated (mean-DRT). Task performance was further quantified by the percentage of joint wins during cooperation and the percentage of child wins and joint wins during competition.
Functional near-infrared spectroscopy
fNIRS data acquisition
fNIRS data were acquired using the ETG-4000 NIRS device (Hitachi Medical Corp., Tokyo, Japan) in order to measure concentration changes in oxygenated hemoglobin (HbO) and deoxygenated hemoglobin (HbR) in two participants simultaneously (sampling frequency = 10 Hz, number of measurement channels (CHs) = 22, source-detector distance = 3 cm). Easycaps (Easycap GmbH, Herrsching, Germany) with an attached measurement patch (3 × 5 optodes) were placed symmetrically over the participants’ foreheads as described elsewhere (Cui et al., 2012; Reindl et al., 2018). For further details of the fNIRS data acquisition protocol, refer to Reindl et al. (2018, 2019).
Estimation of anatomical locations
To estimate the most probable spatial location of the channels, we used the virtual registration method, which is based on simulations (Singh et al., 2005; Tsuzuki et al., 2007). In this method, virtual probe holders are placed on synthetic heads with varying size and shape. Channels are projected on the synthetic brain and positions normalized to MNI space. For Hitachi probe holders and our current probe holder placement, virtual registration results are provided by the Jichi University (http://www.jichi.ac.jp/brainlab/virtual_registration/Result3x5_E.html). For each channel, the Brodmann areas (BA) with the highest probability were determined based on the Talairach Daemon (Lancaster et al., 2000). The brain regions covered by this optode set-up include: BA 8, BA 9, BA 10 and BA 46 (Figure 2).
Fig. 2.
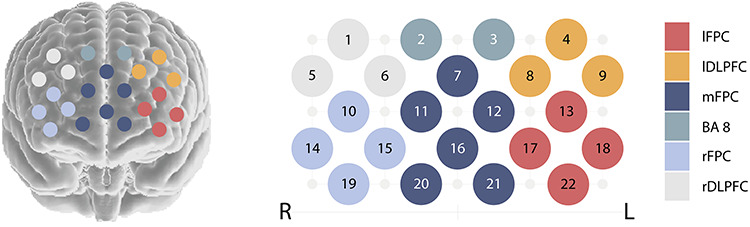
Optode localization and region specification.
Since differences in head size and shape may lead to differences in optode localization, channels were clustered to six regions, in order to enhance the reliability of region specification (see also Nguyen et al., 2020). The assignment of the channels to regions was determined based on their BAs. More specifically, channels located over BA 8 formed one region (BA 8, 2 channels). Channels located over the left and right dorsolateral prefrontal cortex (including BA 9 and BA 46) also formed one region each (lDLPFC and rDLPFC, both 3 channels). The remaining 14 channels, located over BA 10, were clustered to three regions in the left lateral, right lateral and medial frontopolar cortex (lFPC and rFPC both with four channels, mFPC with five channels; Figure 2). For each region, brain-to-brain synchrony values were averaged across channels (see also Baker et al., 2016; Miller et al., 2019). Thus, one synchrony value was obtained for each participant in each region (if at least one channel had valid data).
fNIRS data preprocessing
Preprocessing was conducted using the SPM for fNIRS toolbox (https://www.nitrc.org/projects/spm_fnirs/; SPM12: http://www.fil.ion.ucl.ac.uk/spm/software/spm12/) and the Homer2 toolbox (https://www.nitrc.org/projects/homer2). First, raw attenuation data were converted to optical density (Homer2 function: ‘hmrIntensity2OD’) and motion artifacts were reduced by applying the MARA algorithm (Scholkmann et al., 2010) (Homer2 functions: ‘hmrMotionArtifactByChannel’ and ‘hmrMotionCorrectSpline’; parameters: tMask = 1, std_thresh = 13, amp_thresh = 0.4, tMotion = 1 and P = 0.99, respectively). Next, optical density data were converted to changes in HbO and HbR according to the modified Beer–Lambert law (Homer2 function: ‘hmrOD2Conc’), while the differential pathlength factor was calculated in dependence on wavelength and the participants’ individual age (Scholkmann and Wolf, 2013). Afterwards, data were detrended using a high-pass filter based on a discrete cosine transform set (spm for fnirs function: ‘spm_fnirs_dct,’ cut-off: 128).
Noisy channels were identified based on a combination of objective criteria, including the coefficient of variation, the correlation between HbO and HbR and a ‘flat’ line detection, as well as visual inspection. The procedure is described in more detail in the Supplementary Text S1. Noisy channels were excluded from all further analyses (approximately 5%). If at least 12 channels (≥50%) were classified as ‘noisy,’ the fNIRS measurements of this dyad in the respective condition were excluded. As a result, data were excluded of two dyads in one condition each (1× CoopP, 1× CoopStr) and of one dyad in two conditions (1× CoopP, 1× CompStr).
fNIRS synchrony analysis
As a measure of brain-to-brain synchrony, the wavelet coherence was calculated based on routines from the AStoolbox (Aguiar-Conraria and Soares, 2014) and JLab toolbox (Lilly, 2019) (adapted from Reindl et al., 2018; for more details see Supplementary Text S2). The wavelet coherence was calculated both for the HbO and HbR signals for corresponding channels between adult (parent/stranger) and child. In noisy settings, the mean of the wavelet coherence will be higher than zero. Thus, to increase the robustness of the brain-to-brain synchrony estimator, we only considered ‘salient’ wavelet coherence values that were above a cut-off of 0.65 for HbO and 0.67 for HbR. This cut-off was calculated based on the coherence of surrogate time series, which were constructed by fitting an AR(1) model to the fNIRS signals of the adult and child and building new time series by bootstrapping (for the construction of surrogates, see Aguiar-Conraria and Soares, 2014). As outcome measures, the number of wavelet coherence values higher than the cut-off value in a task-related frequency band between 0.08 and 0.5 Hz (period length: 12.80–2.02 s), relative to the total number of wavelet coherence values in this frequency band, is reported (Reindl et al., 2018). It should be noted that the task-related frequency band includes the trial duration (~7 s for cooperation, ~6 s for competition). Thus, for each dyad and condition (CoopP, CompP, CoopStr and CompStr), one coherence value was obtained in each channel.
Validation procedure
To investigate brain-to-brain synchrony, which is specific to the dyad’s interaction, we implemented a validation strategy correcting for signal similarities of independent subjects performing the same task (see also Reindl et al., 2018). To this end, wavelet coherence was calculated for all random adult–child pairs who did not play together but were recorded under the same experimental condition (for further details, see Supplementary Text S3). For each child, sets of random pairs were constructed by varying the adult partner within the same diagnostic group (TD or ASD), while holding the condition and channel fixed. Using the same blockwise permutation scheme, we additionally calculated sets of random pairs for each adult by holding the adult fixed and varying the corresponding child’s signals. Next, a dyad-, condition- and channel-specific mean random pair coherence value was derived by averaging across the coherence values of both child-fixed and adult-fixed random pair sets in each condition and channel. Finally, all coherence values of the actual dyads were corrected by subtracting the corresponding mean random pair coherence value. This procedure allows us to investigate the dyad’s coherence that goes beyond group, condition, or channel-related brain activity patterns. Importantly, it enables us to control for potential differences in within-brain connectivity (e.g., King et al., 2018) or brain activation patterns between children in the ASD and TD group, which may lead to increased or decreased brain-to-brain synchrony not related to dyadic influences during the interaction. All statistical analyses were performed with this corrected coherence value.
Statistical analyses
Both behavioral and neural data were analyzed in a two-step procedure. First, differences between conditions (CoopP, CompP, CoopStr and CompStr) as well as influences of child’s age were analyzed in the control sample. Second, group differences between the ASD group and TD group were examined in the matched sample. The analysis for the behavioral and neural data is described in more detail below.
Statistical analyses were performed in R (version 3.6.2; R Core Team, 2019). First, outliers, defined as ±3 s.d. from the mean, were winsorized by replacing them with the lower and/or upper boundary values. Winsorizing was conducted across conditions, over all behavioral scores or all coherence values in a specific region for the behavioral and neural data, respectively. To analyze condition, age and group effects, we calculated linear mixed models (LMMs) using the R package ‘lme4’ (Bates et al., 2015). All initial LMMs included the maximal random effects structure justified by the design. Models were then simplified by removing non-significant random slopes backwards using likelihood ratio tests. For statistical inference, the final models were fitted using REML and P-values were derived by the ‘ANOVA’ function of the ‘lmerTest’ package (Kuznetsova et al., 2017) using the Satterthwaite approximation for the degrees of freedom. ‘Pseudo-R2’ for the models was calculated using the R package ‘r2glmm’ (Jaeger, 2017) and the approach by Nakagawa and Schielzeth (2013). Continuous predictors were grand-mean centered. Analyses were checked for potential confounds by (i) including condition order as a predictor and (ii) excluding medicated subjects (including the child with missing values in ADOS/ADI-R), which did not change any of the main findings.
Behavioral analyses
First, to examine differences in motor synchrony in the control sample, LMMs were applied with the mean-DRT (mean absolute differences in response times) as the dependent variable (DV). The full model included the fixed effects of task (0 = competition, 1 = cooperation), partner (0 = stranger, 1 = parent) and the task × partner interaction, as well as a random intercept for subject and by-subject random slopes for task and partner. Differences between parent–child and stranger–child dyads in cooperative and competitive task performance were further investigated by a series of LMMs with task performance as DV (child wins during competition/joint wins during cooperation/joint wins during competition) and partner as fixed effect. Subsequently, the main and interactive effects of child’s age were entered in the LMMs predicting motor synchrony and task behavior. Group differences between children with ASD and TD-controls were analyzed in the matched sample by adding the main and interaction effects of group (0 = TD, 1 = ASD) to the LMMs described above. Relationships between behavioral variables and ASD/ADHD symptom severity were assessed in the ASD group by including the SCQ, SRS and FBB-HKS questionnaire scores as fixed effects in the LMMs.
Neural analyses
For the neural synchrony analysis, we first examined whether the dyad’s brain-to-brain synchrony was higher than brain-to-brain synchrony of dyad-specific random pairs in the same condition and region. To this end, corrected coherence values (as described in ‘Validation procedure’) were subjected to a series of one-sample t-tests, comparing them to zero (one-tailed). P-values were corrected for multiple comparison using FDR correction (48 tests per group) (Benjamini and Hochberg, 1995).
Second, differences between conditions were examined in the control group using LMMs with coherence in the respective region as DV. The full model included the main and interactive effects of task and partner as fixed effects, a random intercept for region and subject, as well as by-subject random slopes for task, partner and task × partner. While these analyses were performed across regions, in a supplementary analysis, we calculated LMMs models for each region and signal type (HbO/HbR) separately to investigate region-specific effects. For these analyses, P-values were adjusted for multiple comparisons (12 tests). Subsequently, to examine potential covariates, the main and interactive effects of child’s age as well as of mean-DRT were entered in separate LMMs. In the matched group analysis, differences between the ASD and TD group were examined by adding the main and interactive effects of group to the LMMs described above.
Results
Behavioral results
Control sample
In the control sample, LMMs revealed a significant effect of task (F(1, 40.275) = 19.998, P < 0.001) and partner (F(1, 39.986) = 8.770, P = 0.005) on mean-DRT, however, no significant interaction (see Supplementary Table S2 for descriptive results and Supplementary Table S3 for LMM results). The full model explained R2 = 12% of the variance. Dyads were more synchronous in their response times during competition than during cooperation. Moreover, stranger–child dyads were more synchronous than parent–child dyads. The child’s age did not significantly influence mean-DRT (no main effect or interactive effects with child’s age, Ps > 0.10).
Further examining task performance, results indicate no significant differences between parent–child and stranger–child dyads in the number of joint wins during cooperation and the number of joint wins during competition (Supplementary Table S3). However, during competition, children won more often against the parent than against the stranger (partner effect: F(1, 40.034) = 9.581, P = 0.004, R2 = 0.08), and older children won more often (age effect: F(1, 39.256) = 4.456, P = 0.041, R2 = 0.14).
Matched sample
In the matched sample, LMMs yielded a main effect of task (F(1, 34) = 23.411, P < 0.001), partner (F(1, 68) = 5.793, P = 0.019) and group (F(1, 34) = 5.433, P = 0.026) on mean-DRT (R2 = 0.20; see Supplementary Table S2 for descriptive results and Supplementary Table S4 for LMM results). In line with the results in the control sample, dyads were more synchronous during competition compared to cooperation and more synchronous with the stranger compared to the parent. Moreover, children in the ASD group were less synchronous, i.e., had a higher difference in response times than children in the TD group across conditions and interaction partners.
While no significant group effect was observed for the number of child wins during competition (Supplementary Table S4), the number of joint wins during competition and cooperation differed between children in the TD and ASD group (competition: F(1, 68) = 7.830, P = 0.007, R2 = 0.15; cooperation: F(1, 34) = 8.756, P = 0.006, R2 = 0.14). Consistent with the findings on mean-DRT, children in the TD group had a higher number of joint wins than children in the ASD group, irrespective of interaction partner (Supplementary Table S2). Within the ASD group, both mean-DRT and dyad’s number of joint wins (cooperation/competition) were neither significantly predicted by ASD symptom severity (SCQ or SRS total score) nor by ADHD symptom severity (FBB-HKS total score).
To summarize, dyads were more synchronous during competition as compared to cooperation. Importantly, children in the ASD group showed a lower motor synchrony than children in the TD group both during competition and during cooperation.
Neural results
Control sample
To examine whether brain-to-brain synchrony was higher in the actual dyads as compared to the random pairs, we subjected the corrected coherence values in each region and condition to a series of one-sample t-tests. Results showed an increased, widespread coherence for the actual dyads in the CompP condition in five regions for HbO (rDLPFC, lDLPFC, rFPC, lFPC and mFPC) and in five regions for HbR (BA8, rDLPFC, rFPC, lFPC and mFPC) (Table 2). Moreover, increased coherence was observed for CoopP in the rFPC for HbO as well as in the rFPC and lFPC for HbR, respectively. No significantly increased coherence was observed for CoopStr and CompStr after FDR-correction of the P-values.
Table 2.
Results of one-sample t-tests examining coherence increase in actual dyads compared to random pairs
| TD—complete | TD—matched | |||
|---|---|---|---|---|
| M (s.d.) | t statistic | M (s.d.) | t statistic | |
| CompP—HbO | ||||
| rDLPFC | 0.032 (0.055) | t(37) = 3.624, Padj = 0.005, d = 0.59 | ||
| lDLPFC | 0.025 (0.059) | t(39) = 2.729, Padj = 0.025, d = 0.43 | ||
| rFPC | 0.034 (0.051) | t(40) = 4.272, Padj = 0.001, d = 0.67 | 0.044 (0.062) | t(17) = 3.043, Padj = 0.044, d = 0.72 |
| lFPC | 0.015 (0.037) | t(40) = 2.561, Padj = 0.031, d = 0.40 | 0.031 (0.042) | t(17) = 3.121, Padj = 0.044, d = 0.74 |
| mFPC | 0.024 (0.037) | t(40) = 4.178, Padj = 0.001, d = 0.65 | ||
| CompP—HbR | ||||
| BA8 | 0.014 (0.028) | t(33) = 2.979, Padj = 0.018, d = 0.51 | ||
| rDLPFC | 0.014 (0.030) | t(37) = 2.841, Padj = 0.022, d = 0.46 | ||
| rFPC | 0.020 (0.037) | t(40) = 3.490, Padj = 0.006, d = 0.55 | 0.021 (0.026) | t(17) = 3.400, Padj = 0.041, d = 0.80 |
| lFPC | 0.017 (0.033) | t(40) = 3.232, Padj = 0.010, d = 0.50 | ||
| mFPC | 0.020 (0.029) | t(40) = 4.402, Padj = 0.001, d = 0.69 | 0.023 (0.028) | t(17) = 3.476, Padj = 0.041, d = 0.82 |
| CoopP—HbO | ||||
| rFPC | 0.017 (0.046) | t(40) = 2.355, Padj = 0.043, d = 0.37 | ||
| CoopP—HbR | ||||
| rFPC | 0.013 (0.032) | t(40) = 2.571, Padj = 0.031, d = 0.40 | ||
| lFPC | 0.011 (0.029) | t(40) = 2.465, Padj = 0.036, d = 0.38 | ||
Notes: One-sample t-tests were conducted (one-sided) for each region, examining whether the sample mean is greater than zero. Padj = P-values were adjusted for multiple comparisons (48 tests) using FDR correction; CompP, parent–child competition; CoopP, parent–child cooperation
In the next step, we directly compared the different conditions using LMMs with the corrected coherence value as the DV, and task, partner and the task × partner interaction as fixed effects (Table 3). For HbR, results showed a highly significant effect of task, with higher coherence for competition as compared to cooperation across regions, as well as a marginally significant effect of partner, with higher coherence for parent–child compared to stranger–child dyads. For HbO, a marginally significant effect of partner and marginally significant interaction between task × partner were observed across regions. Coherence was highest in the CompP condition for HbO and HbR, as indicated by Figure 3. Separate LMMs analyses for each region and signal type did not yield any significant effects after P-value adjustment (except for one marginally significant task effect for HbR in the rFPC, see Supplementary Text S4).
Table 3.
Fixed effects estimates (top) and variance-covariance estimates (bottom) for the LMM predicting coherence in the control sample
| HbO | HbR | |||||||
|---|---|---|---|---|---|---|---|---|
| Est (SE) | F(P) | Est (SE) | F(P) | Est (SE) | F(P) | Est (SE) | F(P) | |
| Fixed effect | ||||||||
| Intercept | 0.003 (0.005) | 0.003 (0.005) | 0.007 (0.004) | 0.007 (0.004) | ||||
| Task | 0.004 (0.007) | 1.302 (0.261) | 0.004 (0.007) | 1.276 (0.266) | −0.005 (0.002) | 13.699 (<0.001) | −0.005 (0.002) | 13.680 (<0.001) |
| Partner | 0.023 (0.009) | 3.667 (0.063) | 0.023 (0.009) | 3.586 (0.066) | 0.008 (0.004) | 3.576 (0.066) | 0.008 (0.004) | 3.474 (0.070) |
| Age | <0.001 (0.002) | 1.526 (0.224) | <−0.001 (0.001) | 0.001 (0.978) | ||||
| Task × partner | −0.019 (0.010) | 3.222 (0.080) | −0.019 (0.010) | 3.275 (0.078) | −0.003 (0.003) | 0.660 (0.417) | −0.003 (0.003) | 0.630 (0.427) |
| Task × age | 0.003 (0.002) | 0.095 (0.760) | <0.001 (0.001) | 4.989 (0.026) | ||||
| Partner × age | 0.002 (0.003) | 0.100 (0.753) | 0.001 (0.001) | 0.008 (0.928) | ||||
| Task × partner × age | −0.005 (0.004) | 1.795 (0.188) | −0.003 (0.001) | 5.685 (0.017) | ||||
| Random part | ||||||||
| Intercept ID | 0.001 (0.026) | <0.001 (0.027) | <0.001 (0.018) | <0.001 (0.018) | ||||
| Task by ID | 0.001 (0.039) | 0.001 (0.038) | ||||||
| Partner by ID | 0.003 (0.053) | 0.003 (0.053) | <0.001 (0.018) | <0.001 (0.018) | ||||
| Task × partner by ID | 0.004 (0.060) | 0.004 (0.060) | ||||||
| Intercept region | <0.000 (0.005) | <0.001 (0.005) | <0.001 (0.003) | <0.001 (0.003) | ||||
| Residual | 0.001 (0.034) | 0.001 (0.034) | <0.001 (0.026) | <0.001 (0.026) | ||||
| R 2 | 0.03 | 0.04 | 0.02 | 0.03 | ||||
Notes: For the fixed effects, estimates are presented with the SE in parenthesis. For the random part, variances are presented with the s.d. in parenthesis. Partner is coded 0 = Stranger, 1 = Parent. Task is coded 0 = Competition, 1 = Cooperation. Age is the child’s age in years (centered): ID is the child’s subject number. Significance was calculated using the ANOVA function and Satterthwaite approximation for degrees of freedom.
Fig. 3.
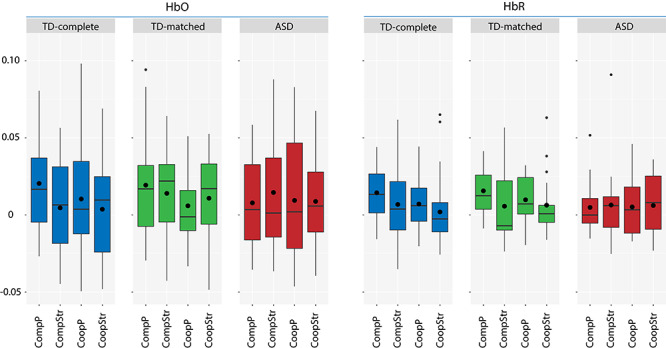
Differences between parent–child cooperation (CoopP), parent–child competition (CompP), stranger–child cooperation (CoopStr) and stranger–child competition (CompStr) in coherence, measured in HbO and HbR, for the TD-complete, TD-matched and ASD group. Boxplots are depicted. The lower and upper hinges correspond to the 25 and 75% percentiles. The lower and upper whiskers extend from the hinge to the lowest/largest values with a maximum of 1.5 times the inter-quartile range. The median value is represented by the horizontal bar and the mean by the black circle.
While no significant influences were found for child’s age on HbO coherence, results showed a significant three-way interaction between task × partner × age for HbR (Table 3). Breaking down this interaction, we found a significant two-way interaction between task × age only for parent-child (F(1, 417.25) = 10.357, P = 0.001, R2 = 0.04) but not for stranger–child dyads (F(1, 427.98) = 0.010, P = 0.92, R2 = 0.01). Age had a positive slope for CompP and a negative slope for CoopP, indicating that coherence in the CompP condition increased with higher age, while coherence in the CoopP condition decreased. However, it should be noted that age effects were not significant in separate models for CompP (P = 0.16) and CoopP (P = 0.09).
In an exploratory analysis, we further examined whether task differences in coherence can be explained by differences in motor synchrony. To this end, the main and interactive effects of mean-DRT were added to the LMMs. Neither in the model for HbO nor in the model for HbR the mean-DRT emerged as a significant predictor (no main or interactive effects with mean-DRT, Ps > 0.05; see Supplementary Table S5). Thus, increased coherence for competition compared to cooperation in HbR is unlikely to be explained by increased motor synchrony.
To summarize, we observed a strong and widespread brain-to-brain synchrony for parent–child competition in comparison to random pairs consistently in the control sample. Moreover, significant increases were observed for parent–child cooperation in more localized regions, located over the rFPC (and lFPC only for HbR). These results were consistent for both HbO and HbR. Furthermore, findings for HbR indicate that parent–child brain-to-brain synchrony during cooperation and competition is differentially influenced by the child’s age from childhood to adolescence. To account for these age effects, ASD participants were compared to age-matched TD participants in the subsequent analyses.
Matched sample
In the ASD sample, no significant increases in coherence were observed for actual dyads in comparison to random pairs (one-sample t-tests, FDR correction). In the control sample, higher coherence of the actual dyads compared to random pairs was observed for CompP in the rFPC and lFPC for HbO and in the rFPC and mFPC for HbR (Table 2). Furthermore, no significant group differences between the ASD and TD group were found in the LMMs, both across regions (Supplementary Table S6) and for each region separately. In an exploratory analysis based on the findings of Wang et al. (2020), we examined group differences in LMMs separately for the parent conditions (CoopP, CompP). In line with the findings of the one-sample t-tests, we found a marginally significant effect of group for CompP across regions for HbR (F(1, 34.66) = 3.860, P = 0.058, R2 = 0.03), indicating that there was a trend for higher scoherence in the TD compared to the ASD group (Figure 3).
To conclude, while we partly replicated our findings of the complete control/TD group in the smaller matched control/TD group, which showed an increased brain-to-brain synchrony during parent–child competition, no significantly increased brain-to-brain synchrony was found in the ASD group. Group comparisons between the ASD and matched control group did not yield significant differences.
Discussion
Using fNIRS hyperscanning, we investigated brain-to-brain synchrony in parent–child dyads with and without ASD and the influence of the familiarity of the interaction partner, taking into account potential age-related changes across childhood and adolescence. We administered a social cooperation (joint button press with interaction partner) and competition (faster button press than interaction partner) game in a larger sample of control participants and compared groups in a smaller matched sample of children and adolescents with and without ASD. On the behavioral level, we observed overall smaller differences in response times (i.e., increased synchrony) during competition than during cooperation across all dyads and increased synchrony for stranger–child dyads in comparison to parent–child dyads. For dyads in the ASD group, a lower motor synchrony was evident in comparison to the TD group. On the neural level, we found enhanced widespread brain-to-brain synchrony during competition with parents in the control sample, paralleled by increased brain-to-brain synchrony during parent–child cooperation in specific regions (i.e., frontopolar cortex; FPC). Parent–child synchrony in HbR signals was modulated by the child’s age, indicating that across childhood and adolescence synchrony may increase for competition and decrease for cooperation. In contrast to the control group, no significantly increased brain-to-brain synchrony was found for children and adolescents with ASD. Direct group comparisons in the matched sample yielded merely marginally significant effects.
Motor synchrony
In line with Reindl et al. (2018), dyads in the control sample showed a higher synchronization of their response times during competition than during cooperation for both parent–child and stranger–child dyads. Moreover, we found higher synchronization in stranger–child dyads than in parent–child dyads across task conditions (i.e. during both cooperation and competition). Particularly for competition, response times between strangers and children were more similar than between parents and children, which most likely induced the overall higher synchronization in stranger–child dyads. It seems plausible that strangers were more competitive than parents, or that parents may have been less motivated to win themselves and have let their children win occasionally to avoid frustration (Reindl et al., 2018).
In the matched sample, children in the ASD group showed a lower overall motor synchrony than children in the TD group during both competition and cooperation, independent of the familiarity of the interaction partner. The lower motor synchrony in the ASD group is in line with previous findings, demonstrating that lower levels of action synchronization in children with ASD are associated with higher ASD severity symptoms during cooperation with their parents (Wang et al., 2020). In the present study, however, we did not find any associations with symptom severity scores, as assessed by parent-rated questionnaires (SRS, SCQ). Previous studies have consistently reported lower motor synchrony in ASD for a range of motor-related synchrony tasks (e.g. Marsh et al., 2013; Fitzpatrick et al., 2016, 2017; Curioni et al., 2017; Noel et al., 2017). Motor impairments, which are frequently observed in children with ASD (Bhat et al., 2011; Kaur et al., 2018), may contribute to difficulties in the ability to synchronize movements with others. Furthermore, since most of these studies involve synchronizing one’s movements while directly observing the other person, lower synchrony in ASD could be explained by lower sensitivity and decreased attention to the movements of the other person (e.g. Fitzpatrick et al., 2016). In the present study, however, participants had no direct sight of the partner’s moving hand and no instruction to explicitly synchronize movements. Thus, differences in synchrony might not only result from motor impairments and deficits in attention to motor cues. Alternatively, differences in higher order cognitive processes, such as the ability to predict the behavior of the other person, could also play an important role.
To sum up, the present finding of a lower motor synchrony in ASD as compared with their TD peers parallels other studies administering social motor synchrony tasks. Since previous studies further showed that social motor synchrony was related to social competences and ASD symptom severity in children with ASD (Fritzpatrick et al., 2017), it may potentially be a useful marker for early identification of children at risk for ASD.
Neural synchrony
In the control sample, a widespread enhanced brain-to-brain synchrony during competition with the parent was observed, paralleled by localized increases in brain-to-brain synchrony for cooperation with parents in the FPC. Furthermore, these parent-specific effects were differentially modulated by age, with a tendency for increased overall synchrony with age during competition and decreased synchrony during cooperation.
Previous studies typically found higher brain-to-brain synchrony during cooperation with the more familiar interaction partner (Cui et al., 2012; Pan et al., 2017; Reindl et al., 2018; Miller et al., 2019; Wang et al., 2020), but not all of these studies have investigated cooperative and competitive contexts within the same experimental task. Furthermore, these studies investigated either younger ages with a smaller age range (Reindl et al., 2018; Miller et al., 2019; Wang et al., 2020), or adults (Cui et al., 2012; Pan et al., 2017). Competitive conditions within a social context (such as in the present study), however, also may induce social comparison processes between the interaction partners (Balconi and Vanutelli, 2018) and thus involve enhanced socio-cognitive processing. Accordingly, other studies have also reported enhanced brain-to-brain synchrony during competitive tasks that involve taking the actions of the interaction partner into account, such as in a simplified poker game in a fNIRS hyperscanning study (Piva et al., 2017). However, in the present study, the best strategy to compete would be to focus on one’s own action (i.e., faster button press) in order to perform better than the interaction partner, which would not necessarily involve enhanced socio-cognitive processing.
An alternative explanation might be synchronized emotional responses, induced by the competitive character of the task, where participants are affectively involved during similar periods of the game. Emotional valence and arousal have been shown to promote a synchronization of brain activities, for instance in participants listening to the same affective story (Nummenmaa et al., 2014) or watching the same movie clip (Nummenmaa et al., 2012). Time-locked increases and decreases in emotional arousal (e.g., in response to the ‘ready’ signal, ‘go’ signal or feedback) may not only lead to an enhanced neural synchrony but also to an enhanced synchrony in other biological systems, such as the autonomic nervous system (ANS). For example, interpersonal synchronization of the heart rate has been observed during cooperative and competitive games in electrocardiography studies (Järvelä et al., 2014), in particular in a competitive context (Chanel et al., 2012). Although the heart rate (~1 Hz) is outside the task-related frequency band (0.08–0.5 Hz), cardiovascular influences generally show a strong effect on the fNIRS signal (for instance, respiration at ~0.3 Hz or arterial blood pressure waves, so called ‘Mayer waves,’ at ~0.1 Hz; Yücel et al., 2016). Furthermore, the enhanced coherence during competition being widespread rather than localized may indicate that it is not solely caused by neural synchrony. Thus, widespread coherence might reflect a mixture of ANS influences as well as socio-cognitive, and emotional processing. Accordingly, bio-behavioral synchrony is considered a multimodal phenomenon (Semin, 2007; Hari et al., 2015), which may be established in different behavioral but also biological systems, including ANS activities or hormonal levels, and which may also be influenced by ASD symptoms (Baker et al., 2015).
In line with Reindl et al. (2018) and other studies, we observed localized increases in brain-to-brain synchrony during cooperation with parents in the FPC. This localized synchronization during cooperation may reflect more socio-cognitive processes, such as continuous attending to each other’s actions and adapting responses in relation to the anticipated response time of the interaction partner. It may thus reflect mutual interaction, which is characterized by a common goal and shared attention and adaptation processes (Reindl et al., 2018).
Across regions, brain-to-brain synchrony during competition and cooperation with parents was modulated by increasing age (i.e., higher synchrony for competition and lower synchrony for cooperation with parents). Such age effects may also explain differences to our previous study in 5–9-year-old children, in which localized increases in brain-to-brain synchrony were found for parent–child cooperation but no effects for parent–child competition (Reindl et al., 2018). Together, this pattern of results might be associated with developmental processes related to adolescence. Adolescence is characterized by changes in the relationship with parents and peers, including a normative development of increasing separation and experience of autonomy from parents towards more relying on age mates (Jager et al., 2015). Considering the described developmental changes from childhood to adolescence, competitive, as compared to cooperative aspects during the interaction with parents seem likely to play a role in the investigated age-range of the present study (for a review about the development of parent–adolescent relationships and conflict interactions, see Branje, 2018). It might be speculated that these developmental processes in adolescence are associated with more emotional arousal and related bio-behavioral, and specifically neural, synchrony during competition and/or with less socio-cognitive processing and associated neural synchrony during cooperation. The notion of a link between emotional arousal and increased synchrony in adolescents is supported by previous findings relating adolescence to enhanced risk-taking (Smith et al., 2013), increased intensity and frequency of emotions, particularly in social contexts (Guyer et al., 2016), as well as increased emotional responses and arousal (Lanteigne, 2011) along with adjustments in the capacity of emotion regulation (Ahmed et al., 2015). Furthermore, other studies suggest a direct link between adolescents’ emotional responses and their parent’s emotional arousal and capacities of emotion regulation (Turpyn et al., 2018). It might be speculated that synchronized emotional arousal (in our study particularly during competition conditions) in turn induces brain-to-brain synchrony and may contribute to this link. Nevertheless, the present findings should be interpreted with caution since they were only observed for HbR and not for HbO. Future studies should investigate the changes in emotional arousal, regulation and social cognitive processing during adolescence and potential interrelations with changes in brain-to-brain synchrony using a wider range of paradigms targeting specific aspects of these functions. This would be important to disentangle the contribution of mutual arousal and the ANS to processes of bio-behavioral synchrony and their influence on brain-to-brain synchrony.
In the matched sample, no group differences between TD and ASD dyads could be observed after rigorous control of random effects. Note, we used a very strict analysis strategy to rule out any possible influence of group-wise overall activation, or dyad differences on the measure for brain-to-brain synchrony (e.g. group- and channel-wise random pairs). Although we could observe reduced behavioral synchrony (also in comparison to TD), we could not replicate reductions of brain-to-brain synchrony in relation to ASD symptomatology as demonstrated by Wang et al. (2020) during parent–child cooperation using the same experimental task. However, this study differed in several respects: participants were much younger (5–11 years) and this study did not include a control group. Furthermore, synchrony was assessed for the same cooperation task as in our study, but in comparison to a control condition where the child responded as fast as possible and the parent merely observed. Therefore, it cannot be ruled out that the observed effects, including correlations with symptom severity, could be more related to motor synchrony rather than coordinated socio-cognitive processes during cooperation driving synchrony.
In an exploratory analysis, a marginally significant group effect for competition with parents was observed, suggesting enhanced brain-to-brain synchrony during competition with the parent in the TD but not ASD group. Furthermore, we did not find significantly increased synchrony in our sample of children and adolescents with ASD in comparison to random pairs, but effects were evident in a matched TD sample. This pattern of results is in line with the notion that synchronization during competition may be elicited via emotional arousal, and that this effect could be reduced in ASD, as has been shown in a study investigating electrodermal activity as a measure of synchrony in the ANS (Baker et al., 2015). However, further studies directly targeting joint emotional arousal and neural synchrony in parent-ASD dyads are needed to investigate this potential link.
To conclude, brain-to-brain synchrony in tasks with ‘minimal’ motor-synchrony related interaction may either not or only slightly be different in patients with ASD at older ages. Future studies should use more naturalistic designs (for example eye contact and joint attention tasks; Oberwelland et al., 2017; see also Tanabe et al., 2012 for a fMRI hyperscanning study in adults with ASD) or joint problem solving tasks (Nguyen et al., 2020). More naturalistic tasks could potentially reveal higher effects sizes for synchrony which is driven by social-cognitive processing and could be better suited to reveal differences in children and adolescents with ASD also on the neural level.
Limitations
The present study has some limitations with respect to the sample and fNIRS methodology that should be considered. All of our participants with ASD were male and high-functioning, so the inference drawn to the autism population in general is limited. Future studies may include participants with lower functioning ASD as well as female individuals with ASD, considering that gender differences have been reported in previous hyperscanning studies (Cheng et al., 2015; Baker et al., 2016). Given the high comorbidity of ASD with ADHD, future studies may also include an ADHD patient group. Methodologically, the precise localization of the fNIRS channels has to be considered with caution. Future studies may use a 3D digitizer to localize fNIRS channels onto the subject’s own structural MRI scan or an age-appropriate template (Tsuzuki and Dan, 2014). Furthermore, the present findings indicate the need for multimodal hyperscanning studies, such as fNIRS-EEG or fNIRS-ANS studies, or short-distance measurements in order to differentiate between neural and non-neural sources in hemodynamic-based brain measurements. Moreover, while our optode set-up covered mainly prefrontal brain areas, no conclusion can be drawn about other brain regions. In particular, ‘social brain’ regions in the temporal cortex and at the temporoparietal junction are implicated in socio-cognitive processes (Frith and Frith, 2003) and play a role in brain-to-brain synchrony during social interaction (Piva et al. 2017).
Conclusions and future directions
Taken together, the present findings indicate that brain-to-brain synchrony may be influenced by both the child’s age and the familiarity of the interaction partner in late childhood and adolescence. This highlights the importance of an age-matched control sample and contrasting conditions, in order to elucidate the brain mechanisms underlying social interactions in children and adolescents with ASD. Adolescence is a critical developmental period with marked changes in the relationship with parents and peers, as well as changes in emotional arousal, probably influencing brain-to-brain synchrony across childhood and adolescence. Although cross-sectional developmental differences between children and adolescents in brain-to-brain synchrony, as indicated by our results, are an important finding, further systematic longitudinal studies are warranted. Our results further reveal, in line with other studies, that children and adolescents with ASD show reduced motor synchrony with interaction partners, although this was not reflected on the neural level. While in the current study a highly standardized task was employed, group differences may be observed in more naturalistic designs with higher levels of social interaction. To conclude, more research is needed to investigate the neurobiological underpinnings of a reduced social (motor) synchrony in ASD, with the long-term goal to identify biomarkers for typical and atypical social interactions in order to identify at-risk subjects and evaluate treatments.
Supplementary Material
Acknowledgements
The authors would like to thank Dr Wolfgang Scharke for his contributions to the development of the dolphin games, Julia Pfeil for assisting with participant recruitment, data collection and data entry as well as Laura Bell and Simon Kohl for their helpful advice. The authors would further like to thank all participants who took part in this study and made an indispensable contribution.
Contributor Information
Jana A Kruppa, Child Neuropsychology Section, Department of Child and Adolescent Psychiatry, Psychosomatics and Psychotherapy, Medical Faculty, RWTH Aachen University, 52074 Aachen, Germany; JARA-Brain Institute II, Molecular Neuroscience and Neuroimaging (INM-11), RWTH Aachen & Research Centre Jülich, 52428 Jülich, Germany.
Vanessa Reindl, Child Neuropsychology Section, Department of Child and Adolescent Psychiatry, Psychosomatics and Psychotherapy, Medical Faculty, RWTH Aachen University, 52074 Aachen, Germany; JARA-Brain Institute II, Molecular Neuroscience and Neuroimaging (INM-11), RWTH Aachen & Research Centre Jülich, 52428 Jülich, Germany.
Christian Gerloff, Child Neuropsychology Section, Department of Child and Adolescent Psychiatry, Psychosomatics and Psychotherapy, Medical Faculty, RWTH Aachen University, 52074 Aachen, Germany; JARA-Brain Institute II, Molecular Neuroscience and Neuroimaging (INM-11), RWTH Aachen & Research Centre Jülich, 52428 Jülich, Germany; Department of Psychiatry, Psychosomatics, and Psychotherapy, University Hospital RWTH Aachen, 52074 Aachen, Germany.
Eileen Oberwelland Weiss, Child Neuropsychology Section, Department of Child and Adolescent Psychiatry, Psychosomatics and Psychotherapy, Medical Faculty, RWTH Aachen University, 52074 Aachen, Germany; JARA-Brain Institute II, Molecular Neuroscience and Neuroimaging (INM-11), RWTH Aachen & Research Centre Jülich, 52428 Jülich, Germany.
Julia Prinz, Child Neuropsychology Section, Department of Child and Adolescent Psychiatry, Psychosomatics and Psychotherapy, Medical Faculty, RWTH Aachen University, 52074 Aachen, Germany.
Beate Herpertz-Dahlmann, Department of Child and Adolescent Psychiatry, Psychosomatics and Psychotherapy, Medical Faculty, RWTH Aachen University, 52074 Aachen, Germany.
Kerstin Konrad, Child Neuropsychology Section, Department of Child and Adolescent Psychiatry, Psychosomatics and Psychotherapy, Medical Faculty, RWTH Aachen University, 52074 Aachen, Germany; JARA-Brain Institute II, Molecular Neuroscience and Neuroimaging (INM-11), RWTH Aachen & Research Centre Jülich, 52428 Jülich, Germany.
Martin Schulte-Rüther, Child Neuropsychology Section, Department of Child and Adolescent Psychiatry, Psychosomatics and Psychotherapy, Medical Faculty, RWTH Aachen University, 52074 Aachen, Germany; JARA-Brain Institute II, Molecular Neuroscience and Neuroimaging (INM-11), RWTH Aachen & Research Centre Jülich, 52428 Jülich, Germany; Department of Child and Adolescent Psychiatry and Psychotherapy, University Medical Center Göttingen, 37075 Göttingen, Germany.
Funding
This work was supported by the START-Programme of the medical faculty of the RWTH Aachen University (grant to MSR). The work of CG was performed as part of the Helmholtz School for Data Science in Life, Earth and Energy (HDS-LEE). The Hitachi NIRS system was supported by funding from the German Research Foundation DFG (INST 948/18–1 FUGG). MSR was supported by the excellence initiative of the federal German states.
Conflict of interest
The authors declare no conflict of interest.
References
- Aguiar-Conraria L., Soares M.J. (2014). The continuous wavelet transform: moving beyond uni-and bivariate analysis. Journal of Economic Surveys, 28(2), 344–75. [Google Scholar]
- Ahmed S.P., Bittencourt-Hewitt A., Sebastian C.L. (2015). Neurocognitive bases of emotion regulation development in adolescence. Developmental Cognitive Neuroscience, 15, 11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders, 5th edn, Washington, DC: American Psychiatric Association. [Google Scholar]
- Anderson D.K., Maye M.P., Lord C. (2011). Changes in maladaptive behaviors from mid childhood to young adulthood in autism spectrum disorder. American Journal on Intellectual and Developmental Disabilities, 116(5), 381–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiloni F., Astolfi L. (2014). Social neuroscience and hyperscanning techniques: past present and future. Neuroscience and Biobehavioral Reviews, 44, 76–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J.K., Fenning R.M., Howland M.A., Baucom B.R., Moffitt J., Erath S.A. (2015). Brief report: a pilot study of parent-child biobehavioral synchrony in autism spectrum disorder. Journal of Autism and Developmental Disorders, 45(12), 4140–6. [DOI] [PubMed] [Google Scholar]
- Baker J.M., Liu N., Cui X., et al. (2016). Sex differences in neural and behavioral signatures of cooperation revealed by fNIRS hyperscanning. Scientific Reports, 6(26), 492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balconi M., Vanutelli M.E. (2018). Functional EEG connectivity during competition. BMC Neuroscience, 19(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D., Mächler M., Bolker B., Walker S. (2015). Fitting linear mixed-effects models. Using lme4. Journal of Statistical Software, 67, 1–48. [Google Scholar]
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, 57(1), 289–300. [Google Scholar]
- Bhat A.N., Landa R.J., Galloway J.C. (2011). Current perspectives on motor functioning in infants, children, and adults with autism spectrum disorders. Physical Therapy, 91(7), 1116–29. [DOI] [PubMed] [Google Scholar]
- Blakemore S.J. (2008). The social brain in adolescence. Nature Reviews. Neuroscience, 9(4), 267–77. [DOI] [PubMed] [Google Scholar]
- Bolis D., Schilbach L. (2018). Observing and participating in social interactions: action perception and action control across the autistic spectrum. Developmental Cognitive Neuroscience, 29, 168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölte S., Poustka F. (2006). Fragebogen zur sozialen Kommunikation (FSK) [Social Communication Questionnaire], Bern: Huber. [Google Scholar]
- Bölte S., Poustka F. (2008). Skala zur Erfassung sozialer Reaktivität: dimensionale Autismus-Diagnostik; SRS; deutsche Fassung der Social Responsiveness Scale (SRS) von John N. Constantino und Christan P. Gruber; Manual, Bern: Huber. [Google Scholar]
- Bornstein M.H., Suess P.E. (2000). Child and mother cardiac vagal tone: continuity, stability, and concordance across the first 5 years. Developmental Psychology, 36(1), 54–65. [PubMed] [Google Scholar]
- Branje S. (2018). Development of parent–adolescent relationships: conflict interactions as a mechanism of change. Child Development Perspectives, 12(3), 171–6. [Google Scholar]
- Chanel G., Kivikangas J.M., Ravaja N. (2012). Physiological compliance for social gaming analysis: cooperative versus competitive play. Interacting with Computers, 24(4), 306–16. [Google Scholar]
- Cheng X., Li X., Hu Y. (2015). Synchronous brain activity during cooperative exchange depends on gender of partner: a fNIRS-based hyperscanning study. Human Brain Mapping, 36(6), 2039–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie D., Viner R. (2005). Adolescent development. British Medical Journal, 330(7486), 301–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X., Bryant D.M., Reiss A.L. (2012). NIRS-based hyperscanning reveals increased interpersonal coherence in superior frontal cortex during cooperation. NeuroImage, 59(3), 2430–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curioni A., Minio-Paluello I., Sacheli L.M., Candidi M., Aglioti S.M. (2017). Autistic traits affect interpersonal motor coordination by modulating strategic use of role-based behavior. Molecular Autism, 8(1), 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai B., Chen C., Long Y., et al. (2018). Neural mechanisms for selectively tuning in to the target speaker in a naturalistic noisy situation. Nature Communications, 9(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döpfner M., Lehmkuhl G. (2000). DISYPS-KJ: Diagnostik-System für psychische Störungen im Kindes-und Jugendalter nach ICD-10 und DSM-IV; klinische Diagnostik-Elternurteil-Erzieher-und Lehrerurteil-Selbsturteil; Manual, Bern: Huber. [Google Scholar]
- Feldman R. (2012). Parent-infant synchrony: a biobehavioral model of mutual influences in the formation of affiliative bonds. Monographs of the Society for Research in Child Development, 77(2), 42–51. [Google Scholar]
- Feldman R. (2015). Mutual influences between child emotion regulation and parent–child reciprocity support development across the first 10 years of life: implications for developmental psychopathology. Development and Psychopathology, 27(4pt1), 1007–23. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick P., Frazier J.A., Cochran D.M., Mitchell T., Coleman C., Schmidt R.C. (2016). Impairments of social motor synchrony evident in autism spectrum disorder. Frontiers in Psychology, 7, 1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick P., Romero V., Amaral J.L., et al. (2017). Social motor synchronization: insights for understanding social behavior in autism. Journal of Autism and Developmental Disorders, 47(7), 2092–107. [DOI] [PubMed] [Google Scholar]
- Frith U., Frith C.D. (2003). Development and neurophysiology of mentalizing. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 358(1431), 459–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer A.E., Silk J.S., Nelson E.E. (2016). The neurobiology of the emotional adolescent: from the inside out. Neuroscience and Biobehavioral Reviews, 70, 74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari R., Henriksson L., Malinen S., Parkkonen L. (2015). Centrality of social interaction in human brain function. Neuron, 88(1), 181–93. [DOI] [PubMed] [Google Scholar]
- Hasson U., Ghazanfar A.A., Galantucci B., Garrod S., Keysers C. (2012). Brain-to-brain coupling: a mechanism for creating and sharing a social world. Trends in Cognitive Sciences, 16(2), 114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager J., Yuen C.X., Putnick D.L., Hendricks C., Bornstein M.H. (2015). Adolescent-peer relationships, separation and detachment from parents, and internalizing and externalizing behaviors: linkages and interactions. Journal of Early Adolescence, 35(4), 511–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger B. (2017). R2glmm: computes R squared for mixed (multilevel) models. R package version 0.1.2 Available:https://CRAN.R-project.org/package=r2glmm[Accessed March 21, 2020].
- Järvelä S., Kivikangas J.M., Kätsyri J., Ravaja N. (2014). Physiological linkage of dyadic gaming experience. Simulation and Gaming, 45(1), 24–40. [Google Scholar]
- Jasmin K., Gotts S.J., Xu Y., et al. (2019). Overt social interaction and resting state in young adult males with autism: core and contextual neural features. Brain, 142(3), 808–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur M., Srinivasan S.M., Bhat A.N. (2018). Comparing motor performance, praxis, coordination, and interpersonal synchrony between children with and without autism Spectrum disorder (ASD). Research in Developmental Disabilities, 72, 79–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimhi Y. (2014). Theory of mind abilities and deficits in autism spectrum disorders. Topics in Language Disorders, 34(4), 329–43. [Google Scholar]
- King J.B., Prigge M.B., King C.K., et al. (2018). Evaluation of differences in temporal synchrony between brain regions in individuals with autism and typical development. JAMA Network Open, 1(7), e184777–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohls G., Schulte-Rüther M., Nehrkorn B., et al. (2013). Reward system dysfunction in autism spectrum disorders. Social Cognitive and Affective Neuroscience, 8(5), 565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad K., Firk C., Uhlhaas P.J. (2013). Brain development during adolescence: neuroscientific insights into this developmental period. Deutsches Ärzteblatt International, 110(25), 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruppa J.A., Gossen A., Oberwelland Weiß E., et al. (2019). Neural modulation of social reinforcement learning by intranasal oxytocin in male adults with high-functioning autism spectrum disorder: a randomized trial. Neuropsychopharmacology, 44(4), 749–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova A., Brockhoff P.B., Christensen R.H.B. (2017). lmerTest package: tests in linear mixed effects models. Journal of Statistical Software, 82(13), 1–26. [Google Scholar]
- Lancaster J.L., Woldorff M.G., Parsons L.M., et al. (2000). Automated Talairach atlas labels for functional brain mapping. Human Brain Mapping, 10(3), 120–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanteigne D.M. (2011). Patterns among emotional experience, arousal, and expression in adolescence. Doctoral dissertation.
- Lee D.O., Ousley O.Y. (2006). Attention-deficit hyperactivity disorder symptoms in a clinic sample of children and adolescents with pervasive developmental disorders. Journal of Child and Adolescent Psychopharmacology, 16(6), 737–46. [DOI] [PubMed] [Google Scholar]
- Leong V., Byrne E., Clackson K., Georgieva S., Lam S., Wass S. (2017). Speaker gaze increases information coupling between infant and adult brains. Proceedings of the National Academy of Sciences of the United States of America, 114(50), 13290–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly J. M. (2019), jLab: a data analysis package for Matlab, v. 1.6.6. Available:http://www.jmlilly.net/jmlsoft.html[Accessed January 30, 2020].
- Lord C., Rutter M., Le Couteur A. (1994). Autism diagnostic interview—revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24(5), 659–85. [DOI] [PubMed] [Google Scholar]
- Lord C., Risi S., Lambrecht L., et al. (2000). The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders, 30(3), 205–23. [PubMed] [Google Scholar]
- Manini B., Cardone D., Ebisch S., Bafunno D., Aureli T., Merla A. (2013). Mom feels what her child feels: thermal signatures of vicarious autonomic response while watching children in a stressful situation. Frontiers in Human Neuroscience, 7, 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh K.L., Isenhower R.W., Richardson M.J., et al. (2013). Autism and social disconnection in interpersonal rocking. Frontiers in Integrative Neuroscience, 7, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J.G., Vrtička P., Cui X., et al. (2019). Inter-brain synchrony in mother-child dyads during cooperation: an fNIRS hyperscanning study. Neuropsychologia, 124, 117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogan R., Fischer R., Bulbulia J.A. (2017). To be in synchrony or not? A meta-analysis of synchrony’s effects on behavior, perception, cognition and affect. Journal of Experimental Social Psychology, 72, 13–20. [Google Scholar]
- Nakagawa S., Schielzeth H. (2013). A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods in Ecology and Evolution, 4(2), 133–42. [Google Scholar]
- Nguyen T., Schleihauf H., Kayhan E., Matthes D., Vrtička P., Hoehl S. (2020). The effects of interaction quality on neural synchrony during mother-child problem solving. Cortex, 124, 235–49. [DOI] [PubMed] [Google Scholar]
- Noel J.P., De Niear M.A., Lazzara N.S., Wallace M.T. (2017). Uncoupling between multisensory temporal function and nonverbal turn-taking in autism spectrum disorder. IEEE Transactions on Cognitive and Developmental Systems, 10(4), 973–82. [Google Scholar]
- Nummenmaa L., Glerean E., Viinikainen M., Jääskeläinen I.P., Hari R., Sams M. (2012). Emotions promote social interaction by synchronizing brain activity across individuals. Proceedings of the National Academy of Sciences of the United States of America, 109(24), 9599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummenmaa L., Saarimäki H., Glerean E., et al. (2014). Emotional speech synchronizes brains across listeners and engages large-scale dynamic brain networks. NeuroImage, 102, 498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberwelland E., Schilbach L., Barisic I., et al. (2017). Young adolescents with autism show abnormal joint attention network: a gaze contingent fMRI study. Neuroimage Clinical, 14, 112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Cheng X., Zhang Z., Li X., Hu Y. (2017). Cooperation in lovers: an fNIRS-based. Hyperscanning study. Human Brain Mapping, 38(2), 831–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp L.M., Pendry P., Adam E.K. (2009). Mother-adolescent physiological synchrony in naturalistic settings: within-family cortisol associations and moderators. Journal of Family Psychology, 23(6), 882–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey K., Adolphs R., Morris J.P. (2004). Neuroanatomical substrates of social cognition dysfunction in autism. Developmental Disabilities Research Reviews, 10(4), 259–71. [DOI] [PubMed] [Google Scholar]
- Pelphrey K.A., Shultz S., Hudac C.M., Vander Wyk B.C. (2011). Research review: constraining heterogeneity: the social brain and its development in autism spectrum disorder. Journal of Child Psychology and Psychiatry, 52(6), 631–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piva M., Zhang X., Noah J.A., Chang S.W., Hirsch J. (2017). Distributed neural activity patterns during human-to-human competition. Frontiers in Human Neuroscience, 11, 571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2019). R: a language and environment for statistical computing. Available:https://www.R-project.org[Accessed January 30, 2020].
- Reindl V., Gerloff C., Scharke W., Konrad K. (2018). Brain-to-brain synchrony in parent-child dyads and the relationship with emotion regulation revealed by fNIRS-based hyperscanning. NeuroImage, 178, 493–502. [DOI] [PubMed] [Google Scholar]
- Reindl V., Konrad K., Gerloff C., Kruppa J.A., Bell L., Scharke W. (2019). Conducting hyperscanning experiments with functional near-infrared spectroscopy. Journal of Visualized Experiments, (143), e58807. [DOI] [PubMed] [Google Scholar]
- Scholkmann F., Spichtig S., Muehlemann T., Wolf M. (2010). How to detect and reduce movement artifacts in near-infrared imaging using moving standard deviation and spline interpolation. Physiological Measurement, 31(5), 649–62. [DOI] [PubMed] [Google Scholar]
- Scholkmann F., Wolf M. (2013). General equation for the differential pathlength factor of the frontal human head depending on wavelength and age. Journal of Biomedical Optics, 18(10), 105004. [DOI] [PubMed] [Google Scholar]
- Scott-van Zeeland A.A., Dapretto M., Ghahremani D.G., Poldrack R.A., Bookheimer S.Y. (2010). Reward processing in autism. Autism Research, 3(2), 53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semin G.R. (2007). Grounding communication: synchrony In: Kruglanski A.W., Higgins E.T., editors. Social Psychology: Handbook of Basic Principles, New York: The Guilford Press, pp. 630–49. [Google Scholar]
- Simonoff E., Pickles A., Charman T., Chandler S., Loucas T., Baird G. (2008). Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. Journal of the American Academy of Child and Adolescent Psychiatry, 47(8), 921–9. [DOI] [PubMed] [Google Scholar]
- Singh A.K., Okamoto M., Dan H., Jurcak V., Dan I. (2005). Spatial registration of multichannel multi-subject fNIRS data to MNI space without MRI. NeuroImage, 27(4), 842–51. [DOI] [PubMed] [Google Scholar]
- Smith A.R., Chein J., Steinberg L. (2013). Impact of socio-emotional context, brain development, and pubertal maturation on adolescent risk-taking. Hormones and Behavior, 64(2), 323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe H.C., Kosaka H., Saito D.N., et al. (2012). Hard to ‘tune in’: neural mechanisms of live face-to-face interaction with high-functioning autistic spectrum disorder. Frontiers in Human Neuroscience, 6, 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuzuki D., Jurcak V., Singh A.K., Okamoto M., Watanabe E., Dan I. (2007). Virtual spatial registration of stand-alone fNIRS data to MNI space. NeuroImage, 34(4), 1506–18. [DOI] [PubMed] [Google Scholar]
- Tsuzuki D., Dan I. (2014). Spatial registration for functional near-infrared spectroscopy: from channel position on the scalp to cortical location in individual and group analyses. NeuroImage, 85, 92–103. [DOI] [PubMed] [Google Scholar]
- Turpyn C.C., Poon J.A., Ross C.E., Thompson J.C., Chaplin T.M. (2018). Associations between parent emotional arousal and regulation and adolescents’ affective brain response. Social Development, 27(1), 3–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Han Z., Hu X., et al. (2020). Autism symptoms modulate interpersonal neural synchronization in children with autism spectrum disorder in cooperative interactions. Brain Topography, 33(1), 112–22. [DOI] [PubMed] [Google Scholar]
- Yücel M.A., Selb J., Aasted C.M., et al. (2016). Mayer waves reduce the accuracy of estimated hemodynamic response functions in functional near-infrared spectroscopy. Biomedical Optics Express, 7(8), 3078–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


