Abstract
Purpose of Review
The aim of this review is to summarize current conceptual models of cognitive reserve (CR) and related concepts and to discuss evidence for these concepts within the context of aging and Alzheimer’s disease.
Recent Findings
Evidence to date supports the notion that higher levels of CR, as measured by proxy variables reflective of lifetime experiences, are associated with better cognitive performance, and with a reduced risk of incident mild cognitive impairment/dementia. However, the impact of CR on longitudinal cognitive trajectories is unclear and may be influenced by a number of factors. Although there is promising evidence that some proxy measures of CR may influence structural brain measures, more research is needed.
Summary
The protective effects of CR may provide an important mechanism for preserving cognitive function and cognitive well-being with age, in part because it can be enhanced throughout the lifespan. However, more research on the mechanisms by which CR is protective is needed.
Keywords: Cognitive reserve, Aging, Alzheimer’s disease, Biomarkers, Cognition, Review
Introduction
As the population aged 65 years and older increases, the prevalence of dementia is expected to increase as well [1]. Although Alzheimer’s disease (AD) is the most common cause of dementia and cognitive decline among older individuals [2•], other types of neuropathology are frequently seen [3-6] and make variable contributions to cognitive decline [2•]. According to recent estimates, only about 50% of inter-individual variability in cognitive decline, on average, can be explained by current measures of the most common age-related neuropathologies [2•, 7], suggesting that other factors may also impact cognitive trajectories in non-demented individuals. In light of this, and the lack of effective treatments for dementia, research is increasingly focusing on identifying factors that may delay the onset of cognitive impairment or impact cognitive outcomes. One such factor is the concept of cognitive reserve (CR), a theoretical construct used to describe individual differences in susceptibility to cognitive, functional, or clinical decline due to aging or brain disease [8•].
Defining Cognitive Reserve
The concept of cognitive reserve grew out of the observation that there can be discrepancies between the amount of neuropathology present in the brain and the degree of cognitive or functional impairment among individuals [9, 10]. Although there has been much research on cognitive reserve and related concepts, the term has been defined and used in different ways across studies, research teams, and consensus papers.
Cognitive Reserve, Brain Reserve, and Brain Maintenance
A recent whitepaper published by 31 members of the Reserve, Resilience, and Protective Factors Professional Interest Area, established with the support of the Alzheimer’s Association, defines CR as “adaptability that helps to explain differential susceptibility of cognitive abilities or day-to-day function to brain aging, pathology, or insult.” [8•] This framework postulates that lifetime experiences, in combination or interaction with genetic factors, enable cognitive processes to be resilient by influencing the efficiency, capacity, or flexibility of brain networks, which allow individuals to better cope with brain disease or aging. These experiences include educational and occupational attainment, general cognitive ability or intelligence, and engagement in activities that are cognitively, socially, and physically stimulating. This framework differentiates cognitive reserve (defined above) from the concept of brain reserve, which refers to the structural characteristics of the brain at a given point in time (e.g., premorbid brain volume, white matter integrity) and may protect against age and disease-related brain changes by impacting the threshold at which cognitive or functional decline emerge. The related concept of brain maintenance refers to the process of maintaining or perhaps enhancing the brain through lifetime experiences and their interaction with genetic factors [11]. It encompasses the reduced development of age- or disease-related brain changes (e.g., reduced atrophy over time or preservation of task-related networks) and reduced pathology accumulation over time (e.g., fewer white matter hyperintensities (WMH)). These three processes collectively are thought to operate throughout the lifespan and provide individuals with “resilience” to brain aging, disease, or insult.
Resistance and Resilience
Another conceptual framework specifically proposed for the study of preclinical Alzheimer’s disease suggests two general mechanisms: resistance and resilience [12•]. The concept of brain resistance refers to “the brain processes underlying the ability to better resist pathology” and is measured by absent or lower than expected AD pathology levels. Brain resilience is defined as the ability to cope with AD pathology and is measured by better-than-expected cognitive performance, brain structure, or function given some level of AD pathology. As such, the notion of brain resistance is similar to the concept of brain maintenance in the Stern et al. whitepaper [8•], while brain resilience overlaps with the notion of cognitive reserve.
Scaffolding Theory of Aging and Cognition
According to the Scaffolding Theory of Aging and Cognition (STAC) [13], an individual’s level of cognitive functioning in adulthood is determined by biological aging, genetic factors, and life experiences, via their effects on the brain, as well as by “compensatory scaffolding,” which refers to neural processes that reduce the negative impact of brain aging on brain function and cognition. Similar to the Stern et al. [8•] model, it is postulated that certain life experiences (like education or physical activity) and genetic factors can enhance aspects of brain function and structure (which is similar to promoting brain reserve and brain maintenance), and can enhance the capacity for compensatory scaffolding (which is similar to promoting cognitive reserve), while other factors (like smoking, obesity, and genetics) have negative effects on brain health. The model further postulates that some of the brain mechanisms that support compensatory scaffolding in aging are the same as those used among younger adults under conditions of cognitive and behavioral challenge.
Maintenance, Reserve, and Compensation
Another recent consensus paper published by Cabeza and colleagues [14•] differentiates between reserve, maintenance, and compensation. In this framework, reserve refers to the improvement of brain anatomic or physiological processes involved in cognition (such as the efficiency or capacity of neural processes) above current levels; thereby attenuating the effects of age- or disease-related brain changes, while maintenance refers to the preservation of these processes over time through ongoing cellular, molecular, and systems-level repair and plasticity. It is hypothesized that both reserve and maintenance can be influenced by genetic and environmental factors, like education, exercise, or intelligence. The concept of compensation is defined as the recruitment of neural processes in response to high cognitive demand that enhances cognitive performance. Compensation may be evident in response to age- or disease-related brain changes and, by definition, leads to improved cognitive performance. Although compensation, as defined in this way, appears similar to the concept of cognitive reserve in the Stern et al. [8•] framework, it is viewed as a set of distinct process (i.e., upregulation, selection, and reorganization) that may be differentially related to measures of reserve or age- and disease-related brain changes.
Residual Approach
Another approach to CR, referred to here as the “residual approach,” defines cognitive reserve (or resilience) as the variance in cognition that is not explained by known (i.e., measured) brain variables and demographics [15]. Using this approach, one or more measures of brain structure, function, or pathology, in conjunction with demographic variables, are used as predictors in a model with a cognitive outcome (such as a memory score), and cognitive reserve is measured as the model residual (i.e., unexplained variance). With this approach, the measure of cognitive reserve is, by definition, dependent on the variables in the model and will necessarily differ across studies (for examples, see [15-17, 18•]). Using this residual framework, brain reserve (or brain resilience) has been defined as the residual variance in brain structure not explained by measures of AD pathology [16] or age [18•].
Defining Cognitive Reserve: Common Themes
Despite the different terminology and approaches to measuring reserve, all models seem to agree that certain lifetime experiences, in combination or interaction with genetic factors, can positively or negatively impact (a) brain health (broadly defined; including but not limited to structure, function, vasculature, metabolism, neurochemical transmission, and onset of or rate of pathology accumulation) and (b) the ability of the brain to cope with aging and pathology. The models also appear to agree that as pathology levels or age-related brain changes increase, the ability of the brain to cope with these changes decreases (i.e., level of cognitive reserve [19, 20•], brain resilience [12•], ability for compensatory scaffolding [13], and amount of residual variance [15]). For the sake of consistency, we will use the terms “cognitive reserve,” “brain reserve,” and “brain maintenance,” as defined in the Stern et al. whitepaper, throughout the remainder of this article; however, it is important to note that the evidence discussed has similar implications for the related frameworks reviewed above.
Theoretical Predictions for the Effects of Cognitive Reserve
From a theoretical standpoint, a higher level of CR is thought to impact cognitive and clinical outcomes in multiple ways. Stern’s [19] hypothetical model of CR, for example, hypothesizes that the adaptability provided by higher levels of CR are associated with (1) a higher level of cognitive performance prior to the onset of cognitive decline, as well as (2) a delay in the onset of disease-related cognitive decline. However, because individuals with high levels of CR are thought to be able to compensate for, and therefore sustain, greater amounts of neuropathology, higher levels of CR are also hypothesized to be associated with (3) a faster rate of cognitive decline once neuropathology reaches a level severe enough to impact cognitive functioning. This hypothetical model was originally developed to explain reserve-related differences in cognitive trajectories as a function of the accumulation of AD neuropathology, though it might also account for differences in cognitive trajectories due to the accumulation of other pathologies or other age-related brain changes.
Evidence for Cognitive Reserve
Because cognitive reserve is a theoretical construct, it cannot be directly observed. It is therefore most commonly measured using proxy variables that are descriptive of lifetime experiences, including measures reflective of: educational and occupational attainment, intelligence, level of engagement in lifestyle or leisure activities (e.g., socially, physically, and cognitively stimulating activities); socioeconomic status (SES); and early life experiences (including perinatal and postnatal factors, childhood intelligence, and early life SES). These variables are not mutually exclusive, often overlap, and may continue to be enhanced throughout the lifespan (for a life course model of CR, see [21]). For example, individuals who grow up in wealthier families are more likely to obtain higher levels of education, which may lead to higher occupational attainment, greater income, and greater access to leisure activities. Examining the relative contributions of different CR proxies to risk of cognitive impairment is an active area of research [22-25]. Of note, the literature reviewed below is focused on measures of CR as it relates to aging and AD dementia. However, the concept of CR is applicable to other neurodegenerative diseases [26-29], psychiatric conditions [30-33], traumatic brain injury [34-36], and post-operative delirium [37•, 38, 39], among others.
Epidemiological and Longitudinal Cohort Studies: Cognitive Reserve Proxies and Risk of Mild Cognitive Impairment and Dementia
Epidemiological and longitudinal cohort studies are uniquely positioned to evaluate the impact of CR on longitudinal cognitive and clinical trajectories, including future risk of dementia; therefore, the below evidence for CR focuses primarily on data from longitudinal studies. The most commonly used proxy variable of CR is years of education and there is considerable evidence that more education is associated with a lower risk of incident mild cognitive impairment (MCI) [40] and dementia [41-44], though not all studies have found these relationships (for reviews and meta-analysis, see [45, 46]); results may also depend on how education is operationalized [47•]. Although easy to measure, years of education do not capture the quality of learning. Additionally, years of education is a static variable that is unlikely to change after early adulthood and thus does not capture lifelong learning and individual differences in the level of engagement in other types of stimulating activities. For these reasons, it has been suggested that literacy, reading ability, or vocabulary may be better proxy measures of reserve [48, 49]. Consistent with this proposal, measures of literacy, reading, or vocabulary tend to show stronger associations with risk of MCI or dementia than years of education [48-52].
Higher occupational attainment or work complexity have also been associated with reduced dementia risk ([53-60], but see [61]), with some data suggesting that certain types of work-related cognitive activity are more protective than others, including information processing and pattern detection [60]. Similarly, older age at retirement was found to be associated with a reduced risk of dementia [62], suggesting that lifelong cognitive engagement is beneficial. Related to occupational complexity, measures of SES, such as greater household income and wealth, have been linked to lower dementia risk [63•, 64-66].
Reduced MCI and dementia risk has furthermore been associated with greater level of engagement in cognitively, socially, and physically stimulating leisure activities, such as reading, playing games, going to museums and concerts, volunteering, or playing music ([67-71], but see [72]). As reviewed by Fratiglioni et al. [73], all three lifestyle components (social, cognitive, and physical) appear to have beneficial effects on dementia risk. Notably, most activities are not one-dimensional and may be beneficial through multiple pathways: social interactions can be cognitively stimulating and physical group activities can have social and/or cognitive components (e.g., aerobics classes, tai-chi). Some studies have therefore suggested that the variety or number of activities is more important than a specific kind of activity [74].
Early life experiences and abilities have also been related to risk of late-life cognitive impairment (for a review, see [75]), and these associations may be independent of adult educational and occupational attainment [54, 76•]. For example, higher childhood school grades [54, 76•], higher scores on cognitive ability tests at age 11 [77, 78], greater childhood SES [79], and greater complexity of writing at age 22 [80] are associated with a reduced risk of dementia, while early life hardship, such as the death of parent, are associated with greater prevalence of AD dementia [81, 82]. Prenatal factors, such as small birth weight and head circumference, may also be related to dementia risk [83•]. The evidence regarding the association between bilingualism and late-life cognitive decline has been mixed, with a recent meta-analysis concluding that bilingualism does not protect from cognitive decline and dementia ([84•], also see [85]).
Taken together, there is strong evidence that higher scores on CR proxy variables are associated with lower risk of MCI and dementia. Assuming that individuals with different levels of CR accumulate neuropathology at the same rate as they age, this provides indirect evidence that those with higher CR can withstand higher levels of neuropathology before becoming symptomatic or showing functional decline, consistent with the theoretical models of reserve reviewed above.
Epidemiological and Longitudinal Cohort Studies: Cognitive Reserve Proxies and Rate of Cognitive Decline
A large body of literature supports the association between higher levels of CR, as measured by proxy variables, and level of cognitive performance among middle-aged and older adults, including years of education [85-92], occupation, and SES [58, 65, 87, 88, 93•, 94, 95•, 96, 97], and leisure activity engagement [23, 70, 71, 98, 99]. This is in line with the predictions of Stern’s model [19], according to which individuals with higher levels of CR continue to perform better than individuals with lower levels of CR as they age and neuropathology develops. The Stern model of CR also predicts that because individuals with higher CR can withstand more pathology before showing cognitive or function decline, they have a delayed onset of disease-related cognitive decline. Consistent with this prediction, several studies have shown that measures of CR are associated with a later onset of MCI [99, 100•] and dementia [101] or cognitive decline [102•, 103].
However, studies examining the effects of CR on longitudinal cognitive trajectories have been mixed. Whereas some have reported reduced rates of cognitive decline among individuals with higher levels of CR [48, 49, 65, 71, 94, 97, 104-106], others have found greater rates of cognitive decline among individuals with higher levels of CR at least on some tests [90, 91, 96, 100•, 102•, 107]. Others still have reported baseline differences in cognition by level of CR, but no difference in cognitive trajectories [58, 85, 86•, 87, 88, 92, 95•, 108, 109].
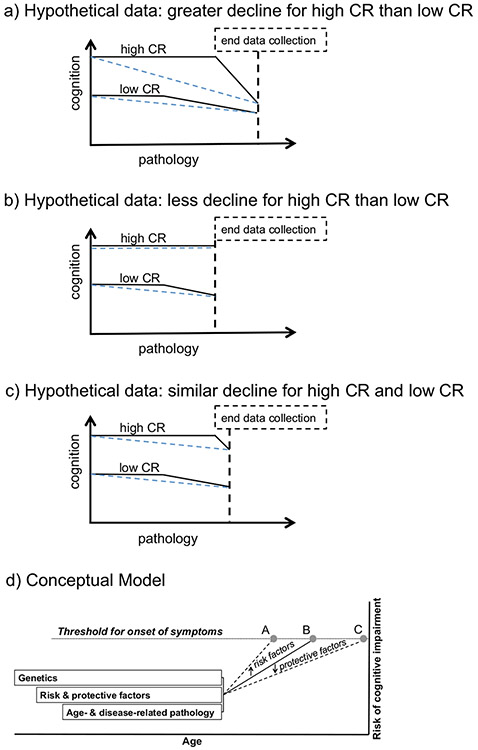
Inconsistencies in prior literature on the relationship between CR and longitudinal clinical and cognitive outcomes may be influenced by a variety of factors, including subject characteristics, methodological or analytical factors, and measurement issues. For example, a large number of prior studies have been conducted among individuals who were non-demented at baseline and likely included individuals with normal cognition as well as individuals with MCI. However, these two clinical groups may have important baseline differences that might confound cognitive and clinical trajectories, including differences in levels of cognitive performance, differences in levels of baseline CR [110, 111], and differences in the amount of underlying neuropathology. Some prior studies that have accounted for clinical impairment (i.e., MCI or dementia) at baseline or follow-up have found that higher levels of CR is associated with greater rates of cognitive decline after clinical symptom onset [100•, 112-114], consistent with Stern’s model [19]. In contrast, level of CR appears to have less of an impact on rates of cognitive change in non-demented aging, and may instead affect cognitive outcomes by resulting in a higher level of cognitive performance (for a review, see [89], see also [99, 100•, 115]), allowing for an improved ability to tolerate the effects of gradually accumulating pathology. Prior results may also be influenced by baseline age or length of follow-up; studies conducted among middle-aged cohorts may require longer follow-up before changes become evident, and studies among older cohorts may be subject to survival effects. See Fig. 1a-c for an illustration of some of these issues.
Fig. 1.
Hypothetical models illustrating the possible associations between longitudinal cognitive trajectories and pathology as a function of CR (as measured by proxy variables), based on Stern’s Fig. 1 from [19]. Although the cognitive trajectories of the high and low CR participants are the same before and after onset of cognitive decline in (a–c) (as illustrated by black solid lines), the study results may differ, depending what part of the trajectory was observed (as illustrated by dashed blue horizontal lines, showing linear slopes of cognitive trajectories (a–c)). Conceptual model illustrating the lifespan impact of genetics, cognitive reserve, and age- and disease-related pathology on an individual’s risk of cognitive impairment as a function of age (d). Evidence to date suggests that CR proxy measures impact the level of cognition (as shown by the intercept effect in (a–c)), which might delay the onset of cognitive decline (as shown by the later cognitive trajectory change point among individuals with high CR (a–c)). Evidence also suggests that CR impacts risk of cognitive impairment (as shown by points A vs. C (d)). While protective factors (such as high levels of CR) may move the threshold of cognitive impairment to a later age (thereby reducing risk of cognitive impairment; point C (d)), risk factors (such as low levels of CR, age- and disease-related brain changes, and other factors not discussed here (e.g., psychiatric conditions; poor health)) may move the threshold for cognitive impairment to a younger age (point A (d)). Of note, genetics and lifestyle factors are hypothesized to act throughout the lifespan, whereas the impact of age- and disease-related pathology may not impact cognition until middle age or later. (Reprinted from: Stern, Y, Cognitive reserve. Neuropsychologia. 2009; 47:2015–2028; with permission from Elsevier) [19]
Methodological limitations may also impact inconsistencies in the literature. As discussed elsewhere ([92, 115, 116], see also [117]), many early studies had statistical limitations that may have biased their results. Few studies [115, 118] have been powered to examine the effects of very low levels of CR, limiting the generalizability of findings to boarder populations. This may be due to methodological factors (e.g., baseline exclusion criteria) or subject characteristics (e.g., volunteer bias, resulting in samples that tend to be highly educated, and of higher SES). Additionally, measures used to index CR may also contribute to inconsistencies in prior research, given different studies collect and operationalize similar CR proxies in different ways (for a discussion, see [119]).
Lastly, epidemiologic research on CR has generally been limited by a lack of measures of underlying pathology or age-related brain changes. As such, these studies cannot directly examine whether and how measures of CR affect the association between age- and disease-related brain changes and cognitive performance, nor do they provide insight regarding the mechanisms underlying CR. Thus, studies that have incorporated biomarkers, which are considered an indirect reflection of underlying neuropathology and/or brain aging, are of particular importance in clarifying CR-related processes.
Cross-sectional Biomarker Studies
The majority of studies on CR with biomarker measures have been cross-sectional. These studies have repeatedly shown that among non-demented groups, as well as among individuals with MCI or dementia, level of CR (as measured by proxy variables) modulates the relationship between cognition or clinical status and pathology, such as amyloid [120-122] and tau [123, 124], atrophy on magnetic resonance imaging (MRI) [22, 125, 126], WMH [127, 128], metabolism on fluorodeoxyglucose (FDG) positron emission tomography (PET) [120, 129, 130], and cerebral perfusion [131]. These findings suggest that the effects of age- and disease-related brain changes on cognition are reduced in individuals with higher CR, although findings among cognitively normal individuals have been more mixed [122, 124, 130, 132-134].
Cross-sectional studies have also provided a good deal of support for the idea that proxy measures of CR are related to measures of neural and brain reserve, including (but not limited to) neural efficiency and capacity [125, 135•, 136, 137•], structural measures such as brain volume and white matter integrity [125, 138-140], neurotransmission [141, 142], or cerebrovascular health [143]. As an example, fMRI studies have suggested individuals with high CR may compensate for age- or disease-related brain changes by utilizing different neural mechanisms in response to task demands [144, 145]. These types of studies provide insight into the neural mechanisms underlying CR [136, 137•, 145-147], and pathways by which brain reserve may be enhanced. However, evidence for a direct association between proxy measures of CR and level of disease-related pathology is inconclusive [134, 148-158].
Cross-sectional biomarker studies, however, are limited in that they do not allow for inferences about the direction of causality for the relationship between CR, brain integrity, and cognition. Additionally, they do not allow for an examination of the degree to which proxy measures of CR modulate cognitive decline and clinical impairment in the presence of neuropathologic and age-related brain changes, and whether they directly impact rates of change in biomarkers over time.
Longitudinal Biomarker Studies
Only a small number of longitudinal studies have examined the interaction between CR and AD biomarkers on longitudinal clinical and cognitive outcomes. As recently reviewed by Soldan et al. [20•], current evidence suggests that the protective effects of CR on the risk of progression from normal cognition to MCI do not appear to differ across the observed range of amyloid levels (as measured by cerebrospinal fluid (CSF) abeta); instead, CR and abeta have additive effects on the risk of progression to MCI [40, 154, 159]. There is some evidence that as biomarkers of neuronal injury (such as CSF total tau and atrophy on MRI scans) increase, the protective effect of CR on risk of progression to MCI decreases ([151, 154]; but see [40, 153]). This may indicate that the processes that mediate the beneficial effects of CR are less effective as levels of neurodegeneration increase, or that these processes begin to break down with disease progression (for similar findings across the spectrum of AD, see [160•]). Studies among patients with MCI have furthermore shown that given similar levels of cortical thinning, those with more education remain dementia free for a longer period of time than those with less education [101]. There is also some evidence that higher levels of education buffer against the negative impact of WMH on risk of MCI and dementia [161]. In contrast, late-life leisure activities were not found to moderate the relationship between AD biomarkers and risk of progression to dementia [152] in a non-demented cohort, but to our knowledge, this issue has not been examined among individuals with normal cognition at baseline.
Among cognitively normal or non-demented groups, the protective effects of CR on level of cognitive performance appears to be independent of baseline levels of AD biomarkers and cerebrovascular disease [100•, 162, 163]. However, the degree to which CR proxy measures moderate the relationship between baseline biomarker levels and rates of cognitive decline remains unclear [100•, 162, 163] and may depend on the clinical status of individuals and level of pathology. Specifically, one study found that higher CR was associated with faster cognitive decline after symptom onset among those who eventually progressed to MCI or dementia, but did not modify cognitive trajectories among those who remained cognitively normal, independent of baseline biomarker levels [100•]. Similarly, a recent study of individuals across the spectrum of AD found that when atrophy rates were low, those with higher education showed the same or less cognitive decline over time compared to those with low education. By comparison, when atrophy rates were high (i.e., in the range of that seen among individuals with dementia), participants with high education showed greater cognitive decline than those with low education. These results, taken together, are broadly consistent with Stern’s hypothetical model of CR [19] and point to the importance of taking into account both baseline and follow-up diagnosis, as well as biomarker levels, when investigating CR.
Lastly, some studies have examined the relationship between CR proxy variables and rate of change in AD and other biomarkers. A number of studies have shown that among non-demented middle-aged and older adults, greater physical activity is associated with less brain atrophy over time [164•, 165, 166, 167•], though findings have been mixed [155•, 168, 169]. Greater physical fitness and social activities have also been associated with less change in white matter microstructure [167•, 170]. In contrast, studies examining associations between other proxy measures of CR (including cognitive activities, education, occupation, and literacy) among cognitively normal and non-demented participants, and rates of change in AD biomarkers or brain structural measures, have produced mixed results. While a small number of studies reported that higher levels of CR are associated with less change in CSF abeta [171] and hippocampal volume [172, 173•], other studies did not find associations between proxy measures of CR and rates of change in amyloid [154, 155•], medial temporal lobe atrophy [153, 155•, 171], FDG metabolism [155•], and CSF tau and p-tau [154]. Among participants with AD dementia, higher education has been linked to greater cortical thinning over time [174] and greater decreases in cerebral blood flow [175].
Taken together, there is some evidence that greater physical activity levels may attenuate structural changes over time among non-demented groups, including atrophy and white matter microstructure. However, there is only weak evidence that other measures of CR directly affect the rate of change of AD biomarkers or brain structure and function. Notably, current studies are limited by relatively short intervals of longitudinal biomarker collection (2–4 years on average). More research is therefore needed to determine whether CR impacts structural and pathological brain markers over longer follow-up periods.
Summary/Conclusions
Despite differences in terminology, it seems clear that CR, as measured by proxy variables, has beneficial effects on late-life cognitive and clinical outcomes. CR proxy measures seem to be most strongly associated with a higher level of cognition, which might delay the onset of symptoms of cognitive impairment, and with reduced risk of MCI/dementia, even in the presence of pathology (see Fig. 1d). There is relatively little evidence currently, however, that CR impacts the accumulation of disease-related pathology, although there is promising evidence that greater physical activity may be associated with less decline in structural brain measures among non-demented individuals. Additional longitudinal biomarker studies, with large samples and long follow-up intervals, are needed to determine the extent to which lifetime experiences directly impact brain reserve and maintenance. Such studies may help clarify the biological mechanisms underlying the beneficial effects of CR, since these mechanisms remain poorly understood.
To the extent that higher CR protects against the onset of disease-related clinical symptoms, or the onset of age-related cognitive decline, it provides an important mechanism for preserving cognitive function in old age, even if levels of pathology are rising. Broadly speaking, the current data suggests that initiatives that improve economic, social, and educational opportunities may have far reaching consequences for cognitive and brain health with age. For example, providing older adult communities with access to learning opportunities (such as mentoring projects, lifelong learning classes, local libraries), as well as policies that promote social connectedness and physical activity (such as green spaces, swimming pools, sidewalks, and bike lanes) may promote cognitive wellbeing. According to some estimates, delaying the onset of dementia by only 5 years would amount to a 50% decrease in dementia prevalence [176]. As such, interventions that increase level of CR may improve longevity and quality of life with age. Since most CR proxies reflect modifiable experiences that can be enhanced throughout the lifespan, current evidence further highlights the importance of lifelong engagement in cognitive, social, and physical activities.
Acknowledgments
Funding This work was supported by the National Institutes of Health (grant numbers U19-AG033655, P50-AG005146).
Footnotes
Conflict of Interest Anja Soldan and Corinne Pettigrew each declare no potential conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Association, As. 2018 Alzheimer’s disease facts and figures. Alzheimers Dement. 2018;14:367–429. [Google Scholar]
- 2.•.Boyle PA, Yu L, Wilson RS, Leurgans SE, Schneider JA, Bennett DA. Person-specific contribution of neuropathologies to cognitive loss in old age. Ann Neurol. 2018;83:74–83Large study that examines the association between common neuropathologies and rate of cognitive decline prior to death on an individual level; showed that neuropathology is ubiquitous among older adults and more than 230 different combinations of neuropathologies were observed across subjects.
- 3.Nascimento C, Di Lorenzo Alho AT, Bazan Conceicao Amaral C, Leite REP, Nitrini R, Jacob-Filho W, et al. Prevalence of transactive response DNA-binding protein 43 (TDP-43) proteinopathy in cognitively normal older adults: systematic review and meta-analysis. Neuropathol Appl Neurobiol. 2018;44: 286–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McAleese KE, Alafuzoff I, Charidimou A, De Reuck J, Grinberg LT, Hainsworth AH, et al. Post-mortem assessment in vascular dementia: advances and aspirations. BMC Med. 2016;14:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schneider JA, Arvanitakis Z, Yu L, Boyle PA, Leurgans SE, Bennett DA. Cognitive impairment, decline and fluctuations in older community-dwelling subjects with Lewy bodies. Brain. 2012;135:3005–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Besser LM, Crary JF, Mock C, Kukull WA. Comparison of symptomatic and asymptomatic persons with primary age-related tauopathy. Neurology. 2017;89:1707–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyle PA, Wilson RS, Yu L, Barr AM, Honer WG, Schneider JA, et al. Much of late life cognitive decline is not due to common neurodegenerative pathologies. Ann Neurol. 2013;74:478–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.•.Stern Y, Arenaza-Urquijo EM, Bartres-Faz D, Belleville S, Cantilon M, Chetelat G, … Conceptual Frameworks, W, Whitepaper: defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement. 2018.Recent consensus paper that defines cognitive reserve, brain reserve, and brain maintenance, and provides framework for utilizing and implementing these concepts in research settings.
- 9.Morris JC, Storandt M, McKeel DW Jr, Rubin EH, Price JL, Grant EA, et al. Cerebral amyloid deposition and diffuse plaques in “normal” aging: evidence for presymptomatic and very mild Alzheimer’s disease. Neurology. 1996;46:707–19. [DOI] [PubMed] [Google Scholar]
- 10.Negash S, Wilson RS, Leurgans SE, Wolk DA, Schneider JA, Buchman AS, et al. Resilient brain aging: characterization of discordance between Alzheimer’s disease pathology and cognition. Curr Alzheimer Res. 2013;10:844–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nyberg L, Lovden M, Riklund K, Lindenberger U, Backman L. Memory aging and brain maintenance. Trends Cogn Sci. 2012;16: 292–305. [DOI] [PubMed] [Google Scholar]
- 12.•.Arenaza-Urquijo EM, Vemuri P. Resistance vs resilience to Alzheimer disease: clarifying terminology for preclinical studies. Neurology. 2018;90:695–703Provides research framework for studying cognitive reserve, as it relates to preclinical AD, and discusses the concepts of resilience and resistance to AD pathology.
- 13.Reuter-Lorenz PA, Park DC. How does it STAC up? Revisiting the scaffolding theory of aging and cognition. Neuropsychol Rev. 2014;24:355–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.•.Cabeza R, Albert M, Belleville S, Craik FIM, Duarte A, Grady CL, et al. Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nat Rev Neurosci. 2018;19:701–10Recent consensus paper that provides consensus definitions for reserve, maintenance, and compensation as they apply to the study of cognitive aging.
- 15.Reed BR, Mungas D, Farias ST, Harvey D, Beckett L, Widaman K, et al. Measuring cognitive reserve based on the decomposition of episodic memory variance. Brain. 2010;133:2196–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hohman TJ, McLaren DG, Mormino EC, Gifford KA, Libon DJ, Jefferson AL, et al. Asymptomatic Alzheimer disease: defining resilience. Neurology. 2016;87:2443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zahodne LB, Manly JJ, Brickman AM, Narkhede A, Griffith EY, Guzman VA, et al. Is residual memory variance a valid method for quantifying cognitive reserve? A longitudinal application. Neuropsychologia. 2015;77:260–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.•.Habeck C, Razlighi Q, Gazes Y, Barulli D, Steffener J, Stern Y. Cognitive reserve and brain maintenance: orthogonal concepts in theory and practice. Cereb Cortex. 2017;27:3962–9Using an approach that is similar to the residual approach, this study found that cognitive reserve and brain reserve are uncorrelated, suggesting they are orthogonal concepts, as hypothesized by some theoretical models.
- 19.Stern Y Cognitive reserve. Neuropsychologia. 2009;47:2015–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.•.Soldan A, Pettigrew C, Albert M. Evaluating cognitive reserve through the prism of preclinical alzheimer disease. Psychiatr Clin North Am. 2018;41:65–77Recent review of longitudinal biomarker studies examining cognitive reserve in preclinical AD.
- 21.Richards M, Deary IJ. A life course approach to cognitive reserve: a model for cognitive aging and development? Ann Neurol. 2005;58:617–22. [DOI] [PubMed] [Google Scholar]
- 22.Chan D, Shafto M, Kievit R, Matthews F, Spink M, Valenzuela M, et al. Lifestyle activities in mid-life contribute to cognitive reserve in late-life, independent of education, occupation, and late-life activities. Neurobiol Aging. 2018;70:180–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gow AJ, Pattie A, Deary IJ. Lifecourse activity participation from early, mid, and later adulthood as determinants of cognitive aging: the Lothian birth cohort 1921. J Gerontol B Psychol Sci Soc Sci. 2017;72:25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kliegel M, Zimprich D, Rott C. Life-long intellectual activities mediate the predictive effect of early education on cognitive impairment in centenarians: a retrospective study. Aging Ment Health. 2004;8:430–7. [DOI] [PubMed] [Google Scholar]
- 25.Parisi JM, Rebok GW, Xue QL, Fried LP, Seeman TE, Tanner EK, et al. The role of education and intellectual activity on cognition. J Aging Res. 2012;2012:416132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hindle JV, Martyr A, Clare L. Cognitive reserve in Parkinson’s disease: a systematic review and meta-analysis. Parkinsonism Relat Disord. 2014;20:1–7. [DOI] [PubMed] [Google Scholar]
- 27.Hindle JV, Hurt CS, Burn DJ, Brown RG, Samuel M, Wilson KC, et al. The effects of cognitive reserve and lifestyle on cognition and dementia in Parkinson’s disease—a longitudinal cohort study. Int J Geriatr Psychiatry. 2016;31:13–23. [DOI] [PubMed] [Google Scholar]
- 28.Rocca MA, Riccitelli GC, Meani A, Pagani E, Del Sette P, Martinelli V, … Filippi M, Cognitive reserve, cognition, and regional brain damage in MS: a 2-year longitudinal study. Mult Scler 2018:1352458517750767. [DOI] [PubMed] [Google Scholar]
- 29.Sumowski JF, Rocca MA, Leavitt VM, Dackovic J, Mesaros S, Drulovic J, et al. Brain reserve and cognitive reserve protect against cognitive decline over 4.5 years in MS. Neurology. 2014;82:1776–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amoretti S, Bernardo M, Bonnin CM, Bioque M, Cabrera B, Mezquida G, et al. The impact of cognitive reserve in the outcome of first-episode psychoses: 2-year follow-up study. Eur Neuropsychopharmacol. 2016;26:1638–48. [DOI] [PubMed] [Google Scholar]
- 31.Hinrichs KH, Easter RE, Angers K, Pester B, Lai Z, Marshall DF, et al. Influence of cognitive reserve on neuropsychological functioning in bipolar disorder: findings from a 5-year longitudinal study. Bipolar Disord. 2017;19:50–9. [DOI] [PubMed] [Google Scholar]
- 32.Koenen KC, Moffitt TE, Roberts AL, Martin LT, Kubzansky L, Harrington H, et al. Childhood IQ and adult mental disorders: a test of the cognitive reserve hypothesis. Am J Psychiatry. 2009;166:50–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang MY, Ho NF, Sum MY, Collinson SL, Sim K. Impact of duration of untreated psychosis and premorbid intelligence on cognitive functioning in patients with first-episode schizophrenia. Schizophr Res. 2016;175:97–102. [DOI] [PubMed] [Google Scholar]
- 34.Leary JB, Kim GY, Bradley CL, Hussain UZ, Sacco M, Bernad M, et al. The association of cognitive reserve in chronic-phase functional and neuropsychological outcomes following traumatic brain injury. J Head Trauma Rehabil. 2018;33:E28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mathias JL, Wheaton P. Contribution of brain or biological reserve and cognitive or neural reserve to outcome after TBI: a meta-analysis (prior to 2015). Neurosci Biobehav Rev. 2015;55:573–93. [DOI] [PubMed] [Google Scholar]
- 36.Steward KA, Kennedy R, Novack TA, Crowe M, Marson DC, Triebel KL. The role of cognitive reserve in recovery from traumatic brain injury. J Head Trauma Rehabil. 2018;33:E18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.•.Cizginer S, Marcantonio E, Vasunilashorn S, Pascual-Leone A, Shafi M, Schmitt EM, et al. The cognitive reserve model in the development of delirium: the successful aging after elective surgery study. J Geriatr Psychiatry Neurol. 2017;30:337–45Large study testing whether different CR proxy variables modify the relationship between post-operative inflammation and the incidence of delirium.
- 38.Martins S, Paiva JA, Simoes MR, Fernandes L. Delirium in elderly patients: association with educational attainment. Acta Neuropsychiatr. 2017;29:95–101. [DOI] [PubMed] [Google Scholar]
- 39.Tow A, Holtzer R, Wang C, Sharan A, Kim SJ, Gladstein A, et al. Cognitive reserve and postoperative delirium in older adults. J Am Geriatr Soc. 2016;64:1341–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roe CM, Fagan AM, Grant EA, Marcus DS, Benzinger TL, Mintun MA, et al. Cerebrospinal fluid biomarkers, education, brain volume, and future cognition. Arch Neurol. 2011;68:1145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kukull WA, Higdon R, Bowen JD, McCormick WC, Teri L, Schellenberg GD, et al. Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol. 2002;59:1737–46. [DOI] [PubMed] [Google Scholar]
- 42.Lindsay J, Laurin D, Verreault R, Hebert R, Helliwell B, Hill GB, et al. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian Study of health and aging. Am J Epidemiol. 2002;156:445–53. [DOI] [PubMed] [Google Scholar]
- 43.Tyas SL, Manfreda J, Strain LA, Montgomery PR. Risk factors for Alzheimer’s disease: a population-based, longitudinal study in Manitoba, Canada. Int J Epidemiol. 2001;30:590–7. [DOI] [PubMed] [Google Scholar]
- 44.Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer’s disease. JAMA. 1994;271:1004–10. [PubMed] [Google Scholar]
- 45.Valenzuela MJ, Sachdev P. Brain reserve and dementia: a systematic review. Psychol Med. 2006;36:441–54. [DOI] [PubMed] [Google Scholar]
- 46.Sharp ES, Gatz M. Relationship between education and dementia: an updated systematic review. Alzheimer Dis Assoc Disord. 2011;25:289–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.•.Then FS, Luck T, Angermeyer MC, Riedel-Heller SG. Education as protector against dementia, but what exactly do we mean by education? Age Ageing. 2016;45:523–8Demonstrated that different methods of operationalizing education can impact the degree to which education protects against dementia risk.
- 48.Manly JJ, Touradji P, Tang MX, Stern Y. Literacy and memory decline among ethnically diverse elders. J Clin Exp Neuropsychol. 2003;25:680–90. [DOI] [PubMed] [Google Scholar]
- 49.Manly JJ, Schupf N, Tang MX, Stern Y. Cognitive decline and literacy among ethnically diverse elders. J Geriatr Psychiatry Neurol. 2005;18:213–7. [DOI] [PubMed] [Google Scholar]
- 50.Brewster PW, Melrose RJ, Marquine MJ, Johnson JK, Napoles A, MacKay-Brandt A, et al. Life experience and demographic influences on cognitive function in older adults. Neuropsychology. 2014;28:846–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaup AR, Nettiksimmons J, Harris TB, Sink KM, Satterfield S, Metti AL, et al. Cognitive resilience to apolipoprotein E epsilon4: contributing factors in black and white older adults. JAMA Neurol. 2015;72:340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pettigrew C, Soldan A, Li S, Lu Y, Wang MC, Seines OA, et al. Relationship of cognitive reserve and APOE status to the emergence of clinical symptoms in preclinical Alzheimer's disease. Cogn Neurosci. 2013;4:136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Andel R, Crowe M, Pedersen NL, Mortimer J, Crimmins E, Johansson B, et al. Complexity of work and risk of Alzheimer’s disease: a population-based study of Swedish twins. J Gerontol B Psychol Sci Soc Sci. 2005;60:P251–8. [DOI] [PubMed] [Google Scholar]
- 54.Dekhtyar S, Wang HX, Scott K, Goodman A, Koupil I, Herlitz A. A life-course study of cognitive reserve in dementia—from childhood to old age. Am J Geriatr Psychiatry. 2015;23:885–96. [DOI] [PubMed] [Google Scholar]
- 55.Karp A, Andel R, Parker MG, Wang HX, Winblad B, Fratiglioni L. Mentally stimulating activities at work during midlife and dementia risk after age 75: follow-up study from the Kungsholmen project. Am J Geriatr Psychiatry. 2009;17:227–36. [DOI] [PubMed] [Google Scholar]
- 56.Kroger E, Andel R, Lindsay J, Benounissa Z, Verreault R, Laurin D. Is complexity of work associated with risk of dementia? The Canadian Study of health and aging. Am J Epidemiol. 2008;167: 820–30. [DOI] [PubMed] [Google Scholar]
- 57.Qiu C, Karp A, von Strauss E, Winblad B, Fratiglioni L, Bellander T. Lifetime principal occupation and risk of Alzheimer’s disease in the Kungsholmen project. Am J Ind Med. 2003;43:204–11. [DOI] [PubMed] [Google Scholar]
- 58.Rusmaully J, Dugravot A, Moatti JP, Marmot MG, Elbaz A, Kivimaki M, et al. Contribution of cognitive performance and cognitive decline to associations between socioeconomic factors and dementia: a cohort study. PLoS Med. 2017;14:e1002334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang HX, MacDonald SW, Dekhtyar S, Fratiglioni L. Association of lifelong exposure to cognitive reserve-enhancing factors with dementia risk: a community-based cohort study. PLoS Med. 2017;14:e1002251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Then FS, Luck T, Heser K, Ernst A, Posselt T, Wiese B, et al. Which types of mental work demands may be associated with reduced risk of dementia? Alzheimers Dement. 2017;13:431–40. [DOI] [PubMed] [Google Scholar]
- 61.Helmer C, Letenneur L, Rouch I, Richard-Harston S, Barberger-Gateau P, Fabrigoule C, et al. Occupation during life and risk of dementia in French elderly community residents. J Neurol Neurosurg Psychiatry. 2001;71:303–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dufouil C, Pereira E, Chene G, Glymour MM, Alperovitch A, Saubusse E, et al. Older age at retirement is associated with decreased risk of dementia. Eur J Epidemiol. 2014;29:353–61. [DOI] [PubMed] [Google Scholar]
- 63.•.Cadar D, Lassale C, Davies H, Llewellyn DJ, Batty GD, Steptoe A. Individual and area-based socioeconomic factors associated with dementia incidence in England: evidence from a 12-year follow-up in the English longitudinal study of ageing. JAMA Psychiatry. 2018;75:723–32Large study of two independent, nationally representative age cohorts showing that wealth in late life is associated risk of dementia independent of education.
- 64.Koster A, Penninx BW, Bosma H, Kempen GI, Newman AB, Rubin SM, et al. Socioeconomic differences in cognitive decline and the role of biomedical factors. Ann Epidemiol. 2005;15:564–71. [DOI] [PubMed] [Google Scholar]
- 65.Ouvrard C, Meillon C, Dartigues JF, Avila-Funes JA, Amieva H. Psychosocioeconomic precariousness, cognitive decline and risk of developing dementia: a 25-year study. Dement Geriatr Cogn Disord. 2016;41:137–45. [DOI] [PubMed] [Google Scholar]
- 66.Sattler C, Toro P, Schonknecht P, Schroder J. Cognitive activity, education and socioeconomic status as preventive factors for mild cognitive impairment and Alzheimer’s disease. Psychiatry Res. 2012;196:90–5. [DOI] [PubMed] [Google Scholar]
- 67.Fancourt D, Steptoe A, Cadar D. Cultural engagement and cognitive reserve: museum attendance and dementia incidence over a 10-year period. Br J Psychiatry. 2018;213:661–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fabrigoule C, Letenneur L, Dartigues JF, Zarrouk M, Commenges D, Barberger-Gateau P. Social and leisure activities and risk of dementia: a prospective longitudinal study. J Am Geriatr Soc. 1995;43:485–90. [DOI] [PubMed] [Google Scholar]
- 69.Roberts RO, Cha RH, Mielke MM, Geda YE, Boeve BF, Machulda MM, et al. Risk and protective factors for cognitive impairment in persons aged 85 years and older. Neurology. 2015;84:1854–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scarmeas N, Levy G, Tang MX, Manly J, Stern Y. Influence of leisure activity on the incidence of Alzheimer’s disease. Neurology. 2001;57:2236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Verghese J, Lipton RB, Katz MJ, Hall CB, Derby CA, Kuslansky G, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348:2508–16. [DOI] [PubMed] [Google Scholar]
- 72.Bickel H, Cooper B. Incidence and relative risk of dementia in an urban elderly population: findings of a prospective field study. Psychol Med. 1994;24:179–92. [DOI] [PubMed] [Google Scholar]
- 73.Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3:343–53. [DOI] [PubMed] [Google Scholar]
- 74.Carlson MC, Parisi JM, Xia J, Xue QL, Rebok GW, Bandeen-Roche K, et al. Lifestyle activities and memory: variety may be the spice of life. The women’s health and aging study II. J Int Neuropsychol Soc. 2012;18:286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Borenstein AR, Copenhaver CI, Mortimer JA. Early-life risk factors for Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20: 63–72. [DOI] [PubMed] [Google Scholar]
- 76.•.Dekhtyar S, Wang HX, Fratiglioni L, Herlitz A. Childhood school performance, education and occupational complexity: a life-course study of dementia in the Kungsholmen Project. Int J Epidemiol. 2016;45:1207–15Study showed that lower childhood school grades were associated with greater risk of dementia after accounting for subsequent educational and occupational attainment, as well as vascular co-morbidities and depressive symptoms.
- 77.Whalley LJ, Starr JM, Athawes R, Hunter D, Pattie A, Deary IJ. Childhood mental ability and dementia. Neurology. 2000;55: 1455–9. [DOI] [PubMed] [Google Scholar]
- 78.McGurn B, Deary IJ, Starr JM. Childhood cognitive ability and risk of late-onset Alzheimer and vascular dementia. Neurology. 2008;71:1051–6. [DOI] [PubMed] [Google Scholar]
- 79.Zhang Z, Gu D, Hayward MD. Early life influences on cognitive impairment among oldest old Chinese. J Gerontol B Psychol Sci Soc Sci. 2008;63:S25–33. [DOI] [PubMed] [Google Scholar]
- 80.Snowdon DA, Kemper SJ, Mortimer JA, Greiner LH, Wekstein DR, Markesbery WR. Linguistic ability in early life and cognitive function and Alzheimer’s disease in late life. Findings from the Nun Study. JAMA. 1996;275:528–32. [PubMed] [Google Scholar]
- 81.Norton MC, Smith KR, Ostbye T, Tschanz JT, Schwartz S, Corcoran C, et al. Early parental death and remarriage of widowed parents as risk factors for Alzheimer disease: the Cache County study. Am J Geriatr Psychiatry. 2011;19:814–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ravona-Springer R, Beeri MS, Goldbourt U. Younger age at crisis following parental death in male children and adolescents is associated with higher risk for dementia at old age. Alzheimer Dis Assoc Disord. 2012;26:68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.•.Mosing MA, Lundholm C, Cnattingius S, Gatz M, Pedersen NL. Associations between birth characteristics and age-related cognitive impairment and dementia: a registry-based cohort study. PLoS Med. 2018;15:e1002609.Study of twins reporting that small birth weight for gestational age and small head size are risk factors for cognitive dysfunction in late life, after controlling for childhood SES and education in adulthood, highlighting the importance of fetal and prenatal grown for later development.
- 84.•.Mukadam N, Sommerlad A, Livingston G. The relationship of bilingualism compared to monolingualism to the risk of cognitive decline or dementia: a systematic review and meta-analysis. J Alzheimers Dis. 2017;58:45–54Recent systematic review and meta-analysis of retrospective and prospective studies on bilingualism and risk of cognitive decline and dementia.
- 85.Mungas D, Early DR, Glymour MM, Zeki Al Hazzouri A, Haan MN. Education, bilingualism, and cognitive trajectories: Sacramento Area Latino Aging Study (SALSA). Neuropsychology. 2018;32:77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.•.Berggren R, Nilsson J, Lovden M. Education does not affect cognitive decline in aging: a Bayesian assessment of the association between education and change in cognitive performance. Front Psychol. 2018;9:1138.Using a Bayesian hypothesis testing approach for quantifying the evidence in favor of the null hypothesis, this study reports that education affects level of cognitive performance across different cognitive domains, but not rate of decline in cognition.
- 87.Gonzalez HM, Tarraf W, Bowen ME, Johnson-Jennings MD, Fisher GG. What do parents have to do with my cognitive reserve? Life course perspectives on twelve-year cognitive decline. Neuroepidemiology. 2013;41:101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Karlamangla AS, Miller-Martinez D, Aneshensel CS, Seeman TE, Wight RG, Chodosh J. Trajectories of cognitive function in late life in the United States: demographic and socioeconomic predictors. Am J Epidemiol. 2009;170:331–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lenehan ME, Summers MJ, Saunders NL, Summers JJ, Vickers JC. Relationship between education and age-related cognitive decline: a review of recent research. Psychogeriatrics. 2015;15:154–62. [DOI] [PubMed] [Google Scholar]
- 90.Glymour MM, Tzourio C, Dufouil C. Is cognitive aging predicted by one’s own or one’s parents’ educational level? Results from the three-city study. Am J Epidemiol. 2012;175:750–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Proust-Lima C, Amieva H, Letenneur L, Orgogozo JM, Jacqmin-Gadda H, Dartigues JF. Gender and education impact on brain aging: a general cognitive factor approach. Psychol Aging. 2008;23:608–20. [DOI] [PubMed] [Google Scholar]
- 92.Zahodne LB, Glymour MM, Sparks C, Bontempo D, Dixon RA, MacDonald SW, et al. Education does not slow cognitive decline with aging: 12-year evidence from the Victoria longitudinal study. J Int Neuropsychol Soc. 2011;17:1039–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.•.Cadar D, Robitaille A, Clouston S, Hofer SM, Piccinin AM, Muniz-Terrera G. An international evaluation of cognitive reserve and memory changes in early old age in 10 European Countries. Neuroepidemiology. 2017;48:9–20Large-scale study of over 10,000 individuals from 10 European countries showing strong evidence that education and income affect baseline-levels of cognitive performance but have little effect on rates of decline.
- 94.Jokinen H, Melkas S, Madureira S, Verdelho A, Ferro JM, Fazekas F, et al. Cognitive reserve moderates long-term cognitive and functional outcome in cerebral small vessel disease. J Neurol Neurosurg Psychiatry. 2016;87:1296–302. [DOI] [PubMed] [Google Scholar]
- 95.•.Lane AP, Windsor TD, Andel R, Luszcz MA. Is occupational complexity associated with cognitive performance or decline? Results from the Australian longitudinal study of ageing. Gerontology. 2017;63:550–9Examined whether occupational complexity with data, people, and things modifies baseline levels of and rates of change across different cognitive domains, controlling for physical demands of work, postretirement leisure activities, and retirement age.
- 96.Singh-Manoux A, Marmot MG, Glymour M, Sabia S, Kivimaki M, Dugravot A. Does cognitive reserve shape cognitive decline? Ann Neurol. 2011;70:296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Then FS, Luck T, Luppa M, Konig HH, Angermeyer MC, Riedel-Heller SG. Differential effects of enriched environment at work on cognitive decline in old age. Neurology. 2015;84: 2169–76. [DOI] [PubMed] [Google Scholar]
- 98.Hultsch DF, Hertzog C, Small BJ, Dixon RA. Use it or lose it: engaged lifestyle as a buffer of cognitive decline in aging? Psychol Aging. 1999;14:245–63. [DOI] [PubMed] [Google Scholar]
- 99.Pettigrew C, Shao Y, Zhu Y, Grega M, Brichko R, Wang MC, … Soldan A, Self-reported lifestyle activities in relation to longitudinal cognitive trajectories. Alzheimer Dis Assoc Disord. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.•.Soldan A, Pettigrew C, Cai Q, Wang J, Wang MC, Moghekar A, et al. Cognitive reserve and long-term change in cognition in aging and preclinical Alzheimer’s disease. Neurobiol Aging. 2017;60: 164–72Showed that higher CR, as measured by proxies, did not modify the rate of change in cognition among individuals who remained cognitively normal, nor among those who progressed to MCI prior to symptom onset. However, higher CR was associated with faster decline after the onset of symptoms and with greater age of symptom onset.
- 101.Querbes O, Aubry F, Pariente J, Lotterie JA, Demonet JF, Duret V, et al. Early diagnosis of Alzheimer’s disease using cortical thickness: impact of cognitive reserve. Brain. 2009;132:2036–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.•.Aguirre-Acevedo DC, Lopera F, Henao E, Tirado V, Munoz C, Giraldo M, et al. Cognitive decline in a Colombian kindred with autosomal dominant Alzheimer disease: a retrospective cohort study. JAMA Neurol. 2016;73:431–8Large study of individuals with autosomal dominant AD demonstrating a delayed onset of cognitive decline among mutation carriers with high education compared to those with low education and faster decline after onset among those with high education and SES.
- 103.Hall CB, Derby C, LeValley A, Katz MJ, Verghese J, Lipton RB. Education delays accelerated decline on a memory test in persons who develop dementia. Neurology. 2007;69:1657–64. [DOI] [PubMed] [Google Scholar]
- 104.Bourne VJ, Fox HC, Deary IJ, Whalley LJ. Does childhood intelligence predict variation in cognitive change in later life? Personal Individ Differ. 2007;42:1551–9. [Google Scholar]
- 105.Olaya B, Bobak M, Haro JM, Demakakos P. Trajectories of verbal episodic memory in middle-aged and older adults: evidence from the English longitudinal Study of ageing. J Am Geriatr Soc. 2017;65:1274–81. [DOI] [PubMed] [Google Scholar]
- 106.Wilson RS, Scherr PA, Schneider JA, Tang Y, Bennett DA. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology. 2007;69:1911–20. [DOI] [PubMed] [Google Scholar]
- 107.Gottesman RF, Rawlings AM, Sharrett AR, Albert M, Alonso A, Bandeen-Roche K, et al. Impact of differential attrition on the association of education with cognitive change over 20 years of follow-up: the ARIC neurocognitive study. Am J Epidemiol. 2014;179:956–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Everson-Rose SA, Mendes de Leon CF, Bienias JL, Wilson RS, Evans DA. Early life conditions and cognitive functioning in later life. Am J Epidemiol. 2003;158:1083–9. [DOI] [PubMed] [Google Scholar]
- 109.Wilson RS, Scherr PA, Hoganson G, Bienias JL, Evans DA, Bennett DA. Early life socioeconomic status and late life risk of Alzheimer’s disease. Neuroepidemiology. 2005;25:8–14. [DOI] [PubMed] [Google Scholar]
- 110.Bosma H, van Boxtel MP, Ponds RW, Jelicic M, Houx P, Metsemakers J, et al. Engaged lifestyle and cognitive function in middle and old-aged, non-demented persons: a reciprocal association? Z Gerontol Geriatr. 2002;35:575–81. [DOI] [PubMed] [Google Scholar]
- 111.Small BJ, Dixon RA, McArdle JJ, Grimm KJ. Do changes in lifestyle engagement moderate cognitive decline in normal aging? Evidence from the Victoria longitudinal study. Neuropsychology. 2012;26:144–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Andel R, Vigen C, Mack WJ, Clark LJ, Gatz M. The effect of education and occupational complexity on rate of cognitive decline in Alzheimer's patients. J Int Neuropsychol Soc. 2006;12: 147–52. [DOI] [PubMed] [Google Scholar]
- 113.Scarmeas N, Albert SM, Manly JJ, Stern Y. Education and rates of cognitive decline in incident Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2006;77:308–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stern Y, Albert S, Tang MX, Tsai WY. Rate of memory decline in AD is related to education and occupation: cognitive reserve? Neurology. 1999;53:1942–7. [DOI] [PubMed] [Google Scholar]
- 115.Zahodne LB, Stern Y, Manly JJ. Differing effects of education on cognitive decline in diverse elders with low versus high educational attainment. Neuropsychology. 2015;29:649–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Glymour MM, Weuve J, Berkman LF, Kawachi I, Robins JM. When is baseline adjustment useful in analyses of change? An example with education and cognitive change. Am J Epidemiol. 2005;162:267–78. [DOI] [PubMed] [Google Scholar]
- 117.Ghisletta P, Bickel JF, Lovden M. Does activity engagement protect against cognitive decline in old age? Methodological and analytical considerations. J Gerontol B Psychol Sci Soc Sci. 2006;61:P253–61. [DOI] [PubMed] [Google Scholar]
- 118.Ngandu T, von Strauss E, Helkala EL, Winblad B, Nissinen A, Tuomilehto J, et al. Education and dementia: what lies behind the association? Neurology. 2007;69:1442–50. [DOI] [PubMed] [Google Scholar]
- 119.Jones RN, Manly J, Glymour MM, Rentz DM, Jefferson AL, Stern Y. Conceptual and measurement challenges in research on cognitive reserve. J Int Neuropsychol Soc. 2011;17:593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kemppainen NM, Aalto S, Karrasch M, Nagren K, Savisto N, Oikonen V, et al. Cognitive reserve hypothesis: Pittsburgh compound B and fluorodeoxyglucose positron emission tomography in relation to education in mild Alzheimer’s disease. Ann Neurol. 2008;63:112–8. [DOI] [PubMed] [Google Scholar]
- 121.Roe CM, Mintun MA, D'Angelo G, Xiong C, Grant EA, Morris JC. Alzheimer disease and cognitive reserve: variation of education effect with carbon 11-labeled Pittsburgh compound B uptake. Arch Neurol. 2008;65:1467–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rentz DM, Locascio JJ, Becker JA, Moran EK, Eng E, Buckner RL, et al. Cognition, reserve, and amyloid deposition in normal aging. Ann Neurol. 2010;67:353–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hoenig MC, Bischof GN, Hammes J, Faber J, Fliessbach K, van Eimeren T, et al. Tau pathology and cognitive reserve in Alzheimer's disease. Neurobiol Aging. 2017;57:1–7. [DOI] [PubMed] [Google Scholar]
- 124.Rentz DM, Mormino EC, Papp KV, Betensky RA, Sperling RA, Johnson KA. Cognitive resilience in clinical and preclinical Alzheimer’s disease: the Association of Amyloid and tau Burden on cognitive performance. Brain Imaging Behav. 2017;11:383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sole-Padulles C, Bartres-Faz D, Junque C, Vendrell P, Rami L, Clemente IC, et al. Brain structure and function related to cognitive reserve variables in normal aging, mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2009;30:1114–24. [DOI] [PubMed] [Google Scholar]
- 126.Liu Y, Julkunen V, Paajanen T, Westman E, Wahlund LO, Aitken A, et al. Education increases reserve against Alzheimer’s disease—evidence from structural MRI analysis. Neuroradiology. 2012;54:929–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dufouil C, Alperovitch A, Tzourio C. Influence of education on the relationship between white matter lesions and cognition. Neurology. 2003;60:831–6. [DOI] [PubMed] [Google Scholar]
- 128.Brickman AM, Siedlecki KL, Muraskin J, Manly JJ, Luchsinger JA, Yeung LK, et al. White matter hyperintensities and cognition: testing the reserve hypothesis. Neurobiol Aging. 2011;32:1588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Alexander GE, Furey ML, Grady CL, Pietrini P, Brady DR, Mentis MJ, et al. Association of premorbid intellectual function with cerebral metabolism in Alzheimer’s disease: implications for the cognitive reserve hypothesis. Am J Psychiatry. 1997;154:165–72. [DOI] [PubMed] [Google Scholar]
- 130.Garibotto V, Borroni B, Kalbe E, Herholz K, Salmon E, Holtoff V, et al. Education and occupation as proxies for reserve in aMCI converters and AD: FDG-PET evidence. Neurology. 2008;71: 1342–9. [DOI] [PubMed] [Google Scholar]
- 131.Stern Y, Alexander GE, Prohovnik I, Mayeux R. Inverse relationship between education and parietotemporal perfusion deficit in Alzheimer’s disease. Ann Neurol. 1992;32:371–5. [DOI] [PubMed] [Google Scholar]
- 132.Ewers M, Insel PS, Stern Y, Weiner MW, Alzheimer's Disease Neuroimaging, I. Cognitive reserve associated with FDG-PET in preclinical Alzheimer disease. Neurology. 2013;80:1194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nebes RD, Meltzer CC, Whyte EM, Scanlon JM, Halligan EM, Saxton JA, et al. The relation of white matter hyperintensities to cognitive performance in the normal old: education matters. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2006;13:326–40. [DOI] [PubMed] [Google Scholar]
- 134.Vemuri P, Weigand SD, Przybelski SA, Knopman DS, Smith GE, Trojanowski JQ, et al. Cognitive reserve and Alzheimer’s disease biomarkers are independent determinants of cognition. Brain. 2011;134:1479–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.•.Anthony M and Lin F, A systematic review for functional neuroimaging studies of cognitive reserve across the cognitive aging spectrum. Arch Clin Neuropsychol. 2017.Systematic review of studies evaluating the neural basis of CR using resting-state and task-based fMRI across the aging and AD spectrum. Identified distinct regions associated with neural reserve and neural compensation, with neural compensation being more common among those with MCI or dementia.
- 136.Stern Y, Zarahn E, Habeck C, Holtzer R, Rakitin BC, Kumar A, et al. A common neural network for cognitive reserve in verbal and object working memory in young but not old. Cereb Cortex. 2008;18:959–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.•.Stern Y, Gazes Y, Razlighi Q, Steffener J, Habeck C. A task-invariant cognitive reserve network. Neuroimage. 2018;178:36–45Study of 255 individuals, aged 20–80 years that identified a task-invariant covariance pattern of regions that was active across 12 different cognitive task and correlated with IQ, a proxy of CR. The network moderated between cortical thickness and reasoning performance, suggesting it represents a task-invariant CR network.
- 138.Carreiras M, Seghier ML, Baquero S, Estevez A, Lozano A, Devlin JT, et al. An anatomical signature for literacy. Nature. 2009;461:983–6. [DOI] [PubMed] [Google Scholar]
- 139.Lovden M, Wenger E, Martensson J, Lindenberger U, Backman L. Structural brain plasticity in adult learning and development. Neurosci Biobehav Rev. 2013;37:2296–310. [DOI] [PubMed] [Google Scholar]
- 140.Piras F, Cherubini A, Caltagirone C, Spalletta G. Education mediates microstructural changes in bilateral hippocampus. Hum Brain Mapp. 2011;32:282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Garibotto V, Tettamanti M, Marcone A, Florea I, Panzacchi A, Moresco R, et al. Cholinergic activity correlates with reserve proxies in Alzheimer’s disease. Neurobiol Aging. 2013;34:2694 e13–8. [DOI] [PubMed] [Google Scholar]
- 142.Robertson IH. A noradrenergic theory of cognitive reserve: implications for Alzheimer’s disease. Neurobiol Aging. 2013;34:298–308. [DOI] [PubMed] [Google Scholar]
- 143.Davenport MH, Hogan DB, Eskes GA, Longman RS, Poulin MJ. Cerebrovascular reserve: the link between fitness and cognitive function? Exerc Sport Sci Rev. 2012;40:153–8. [DOI] [PubMed] [Google Scholar]
- 144.Kennedy KM, Rodrigue KM, Bischof GN, Hebrank AC, Reuter-Lorenz PA, Park DC. Age trajectories of functional activation under conditions of low and high processing demands: an adult lifespan fMRI study of the aging brain. NeuroImage. 2015;104: 21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Steffener J, Reuben A, Rakitin BC, Stern Y. Supporting performance in the face of age-related neural changes: testing mechanistic roles of cognitive reserve. Brain Imaging Behav. 2011;5:212–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Franzmeier N, Buerger K, Teipel S, Stern Y, Dichgans M, Ewers M, et al. Cognitive reserve moderates the association between functional network anti-correlations and memory in MCI. Neurobiol Aging. 2017;50:152–62. [DOI] [PubMed] [Google Scholar]
- 147.Speer ME, Soldan A. Cognitive reserve modulates ERPs associated with verbal working memory in healthy younger and older adults. Neurobiol Aging. 2015;36:1424–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Almeida RP, Schultz SA, Austin BP, Boots EA, Dowling NM, Gleason CE, et al. Effect of cognitive reserve on age-related changes in cerebrospinal fluid biomarkers of Alzheimer disease. JAMA Neurol. 2015;72:699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dumurgier J, Paquet C, Benisty S, Kiffel C, Lidy C, Mouton-Liger F, et al. Inverse association between CSF Abeta 42 levels and years of education in mild form of Alzheimer’s disease: the cognitive reserve theory. Neurobiol Dis. 2010;40:456–9. [DOI] [PubMed] [Google Scholar]
- 150.Landau SM, Marks SM, Mormino EC, Rabinovici GD, Oh H, O'Neil JP, et al. Association of lifetime cognitive engagement and low beta-amyloid deposition. Arch Neurol. 2012;69:623–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pettigrew C, Soldan A, Zhu Y, Wang MC, Brown T, Miller M, et al. Cognitive reserve and cortical thickness in preclinical Alzheimer’s disease. Brain Imaging Behav. 2017;11:357–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Reijs BLR, Vos SJB, Soininen H, Lotjonen J, Koikkalainen J, Pikkarainen M, et al. Association between later life lifestyle factors and Alzheimer’s disease biomarkers in non-demented individuals: a longitudinal descriptive cohort study. J Alzheimers Dis. 2017;60:1387–95. [DOI] [PubMed] [Google Scholar]
- 153.Soldan A, Pettigrew C, Lu Y, Wang MC, Selnes O, Albert M, et al. Relationship of medial temporal lobe atrophy, APOE genotype, and cognitive reserve in preclinical Alzheimer's disease. Hum Brain Mapp. 2015;36:2826–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Soldan A, Pettigrew C, Li S, Wang MC, Moghekar A, Selnes OA, et al. Relationship of cognitive reserve and cerebrospinal fluid biomarkers to the emergence of clinical symptoms in preclinical Alzheimer’s disease. Neurobiol Aging. 2013;34:2827–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.•.Vemuri P, Lesnick TG, Przybelski SA, Knopman DS, Machulda M, Lowe VJ, et al. Effect of intellectual enrichment on AD biomarker trajectories: longitudinal imaging study. Neurology. 2016;86:1128–35Reported that CR proxy variables, including education and cognitive and physical activities, were not associated with short-term rate of change in AD biomarkers in a non-demented sample, including PET amyloid, FDG PET metabolism, and hippocampal volume.
- 156.Vemuri P, Lesnick TG, Przybelski SA, Knopman DS, Roberts RO, Lowe VJ, et al. Effect of lifestyle activities on Alzheimer disease biomarkers and cognition. Ann Neurol. 2012;72:730–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wirth M, Villeneuve S, La Joie R, Marks SM, Jagust WJ. Geneenvironment interactions: lifetime cognitive activity, APOE genotype, and beta-amyloid burden. J Neurosci. 2014;34:8612–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wirth M, Haase CM, Villeneuve S, Vogel J, Jagust WJ. Neuroprotective pathways: lifestyle activity, brain pathology, and cognition in cognitively normal older adults. Neurobiol Aging. 2014;35:1873–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Roe CM, Fagan AM, Williams MM, Ghoshal N, Aeschleman M, Grant EA, et al. Improving CSF biomarker accuracy in predicting prevalent and incident Alzheimer disease. Neurology. 2011;76: 501–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.•.Mungas D, Gavett B, Fletcher E, Farias ST, DeCarli C, Reed B. Education amplifies brain atrophy effect on cognitive decline: implications for cognitive reserve. Neurobiol Aging. 2018;68: 142–50Reported that high education was associated with slower decline in individuals with lesser brain atrophy but with faster decline in those with greater atrophy, suggesting that the protective effects of education are reduced with increasing levels of neurodegeneration.
- 161.Mortamais M, Portet F, Brickman AM, Provenzano FA, Muraskin J, Akbaraly TN, et al. Education modulates the impact of white matter lesions on the risk of mild cognitive impairment and dementia. Am J Geriatr Psychiatry. 2014;22:1336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Vemuri P, Lesnick TG, Przybelski SA, Knopman DS, Preboske GM, Kantarci K, et al. Vascular and amyloid pathologies are independent predictors of cognitive decline in normal elderly. Brain. 2015;138:761–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yaffe K, Weston A, Graff-Radford NR, Satterfield S, Simonsick EM, Younkin SG, et al. Association of plasma beta-amyloid level and cognitive reserve with subsequent cognitive decline. JAMA. 2011;305:261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.•.Kim RE, Yun CH, Thomas RJ, Oh JH, Johnson HJ, Kim S, et al. Lifestyle-dependent brain change: a longitudinal cohort MRI study. Neurobiol Aging. 2018;69:48–57Large-scale study (N = 984) of middle-aged and older adults showing that intense physical activity is associated with reduced brain atrophy in men.
- 165.Smith JC, Nielson KA, Woodard JL, Seidenberg M, Durgerian S, Hazlett KE, et al. Physical activity reduces hippocampal atrophy in elders at genetic risk for Alzheimer’s disease. Front Aging Neurosci. 2014;6:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yuki A, Lee S, Kim H, Kozakai R, Ando F, Shimokata H. Relationship between physical activity and brain atrophy progression. Med Sci Sports Exerc. 2012;44:2362–8. [DOI] [PubMed] [Google Scholar]
- 167.•.Ritchie SJ, Tucker-Drob EM, Cox SR, Dickie DA, Del CVHM, Corley J, et al. Risk and protective factors for structural brain ageing in the eighth decade of life. Brain Struct Funct. 2017;222:3477–90Large-scale study examining the relationship between CR proxies and brain gray matter, white matter, white matter hyperintensities, and white matter microstructure. Only physical fitness predicted baseline and rate of change in brain structure.
- 168.Podewils LJ, Guallar E, Beauchamp N, Lyketsos CG, Kuller LH, Scheltens P Physical activity and white matter lesion progression: assessment using MRI. Neurology. 2007;68:1223–6. [DOI] [PubMed] [Google Scholar]
- 169.Kooistra M, Boss HM, van der Graaf Y, Kappelle LJ, Biessels GJ, Geerlings MI, et al. Physical activity, structural brain changes and cognitive decline. The SMART-MR study. Atherosclerosis. 2014;234:47–53. [DOI] [PubMed] [Google Scholar]
- 170.Kohncke Y, Laukka EJ, Brehmer Y, Kalpouzos G, Li TQ, Fratiglioni L, et al. Three-year changes in leisure activities are associated with concurrent changes in white matter microstructure and perceptual speed in individuals aged 80 years and older. Neurobiol Aging. 2016;41:173–86. [DOI] [PubMed] [Google Scholar]
- 171.Lo RY, Jagust WJ, Alzheimer’s Disease Neuroimaging, I. Effect of cognitive reserve markers on Alzheimer pathologic progression. Alzheimer Dis Assoc Disord. 2013;27:343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Suo C, Leon I, Brodaty H, Trollor J, Wen W, Sachdev P, et al. Supervisory experience at work is linked to low rate of hippocampal atrophy in late life. NeuroImage. 2012;63:1542–51. [DOI] [PubMed] [Google Scholar]
- 173.•.Elbejjani M, Fuhrer R, Abrahamowicz M, Mazoyer B, Crivello F, Tzourio C, et al. Life-course socioeconomic position and hippocampal atrophy in a prospective cohort of older adults. Psychosom Med. 2017;79:14–23Study evaluating the relationship between childhood, early adulthood, and mid-life socioeconomic measures in relationship to rates of hippocampal atrophy among a large sample of older adults.
- 174.Cho H, Jeon S, Kim C, Ye BS, Kim GH, Noh Y, et al. Higher education affects accelerated cortical thinning in Alzheimer’s disease: a 5-year preliminary longitudinal study. Int Psychogeriatr. 2015;27:111–20. [DOI] [PubMed] [Google Scholar]
- 175.Hanyu H, Sato T, Shimuzu S, Kanetaka H, Iwamoto T, Koizumi K. The effect of education on rCBF changes in Alzheimer’s disease: a longitudinal SPECT study. Eur J Nucl Med Mol Imaging. 2008;35:2182–90. [DOI] [PubMed] [Google Scholar]
- 176.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88:1337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]