Abstract
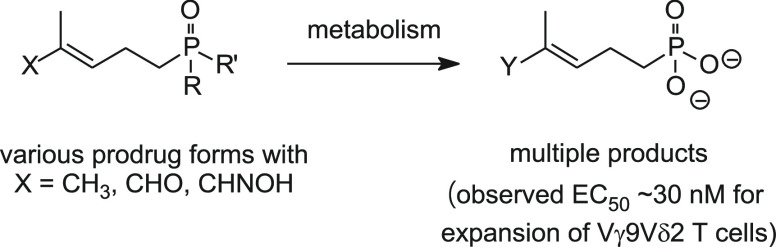
(E)-4-Hydroxy-3-methyl-but-2-enyl diphosphate (HMBPP) and its phosphonate analogs are potent phosphoantigens. HMBPP contains an (E)-allylic alcohol which interacts with the molecular target BTN3A1 giving an antigenic signal to activate Vγ9Vδ2 T cells. As probes of BTN3A1 function, we prepared prodrug derivatives of the HMBPP analog C-HMBP that lack the (E)-allylic alcohol or have modified it to an aldehyde or aldoxime and evaluated their biological activity. Removal of the alcohol completely abrogates phosphoantigenicity in these compounds while the aldoxime modification decreases potency relative to the (E)-allylic alcohol form. However, homoprenyl derivatives oxidized to an aldehyde stimulate Vγ9Vδ2 T cells at nanomolar concentrations. Selection of phosphonate protecting groups (i.e., prodrug forms) impacts the potency of phosphoantigen aldehydes, with mixed aryl acyloxyalkyl forms exhibiting superior activity relative to aryl amidate forms. The activity correlates with the cellular reduction of the aldehyde to the alcohol form. Thus, the functionality on this ligand framework can be altered concurrently with phosphonate protection to promote cellular transformation to highly potent phosphoantigens.
Keywords: Butyrophilin, BTN3A1, ligand, phosphoantigen, prodrugs
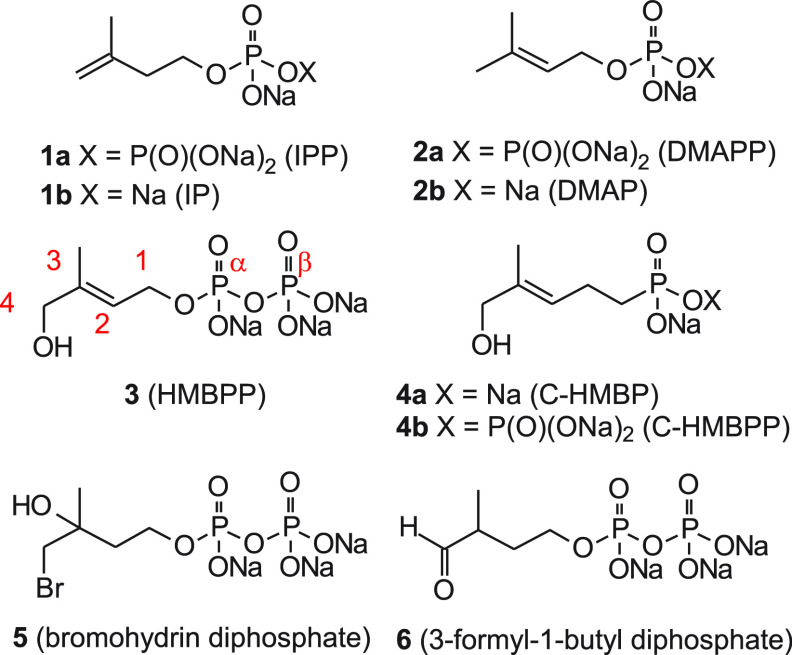
The Vγ9Vδ2 T cells are a subset of human T cells that contribute to immunity against infectious diseases and cancer.1 Unlike most T cells, the Vγ9Vδ2 T cells are activated by small phosphorus-containing molecules known as “phosphoantigens” rather than peptide antigens.2 Isopentenyl diphosphate (IPP, 1a, Figure 1) was the first identified natural phosphoantigen for human Vγ9Vδ2 T cells.3−5 Isomerization of IPP by isopentenyl diphosphate isomerase affords dimethylallyl diphosphate (DMAPP, 2a), which itself may function as a phosphoantigen.6,7 These diphosphates are more active relative to the corresponding monophosphates IP (1b, 233-fold) and DMAP (2b, 3-fold), suggesting that the β phosphate increases specific recognition of the phosphoantigens.8 Additional studies demonstrated that these T cells selectively recognize the methylerythritol 4-phosphate (MEP) pathway intermediate (E)-4-hydroxy-3-methyl-but-2-enyl diphosphate (HMBPP, 3), an immediate precursor to IPP biosynthesis. HMBPP is the most potent natural phosphoantigen, with activity at concentrations 4–6 log units lower than IPP.9−11 Thus, both the (E)-allylic alcohol of HMBPP and the diphosphate group are important determinants of phosphoantigenicity.
Figure 1.
Relevant phosphoantigens. Endogenous phosphoantigens (1a and 2a) and their monophosphate analogs (1b and 2b). The most potent natural ligand for BTN3A1, HMBPP (3). Phosphonate analogs C-HMBP (4a) and C-HMBPP (4b). Bromohydrin diphosphate (5) and 3-formyl-1-butyl diphosphate (6).
Because diphosphates of potential clinical importance have low stability, Boëdec and colleagues designed analogs and stereoisomers of the highly potent HMBPP. As a class, the isosteric analogs of HMBPP containing phosphonates, C-HMBP (4a) and C-HMBPP (4b), were more stable than HMBPP, with longer duration of activity.12 The E-olefins were significantly more potent relative to corresponding Z-olefin compounds, with a difference in EC50 values of greater than 500-fold, revealing a strong preference to E-geometry for optimal Vγ9Vδ2 T cell stimulation.13
Phosphoantigens are now known to bind to a shallow, basic binding pocket in the intracellular B30.2 domain of BTN3A1.14−20 BTN3A1 is a single pass transmembrane protein, which associates with other butyrophilins including BTN2A1, BTN3A2, and BTN3A3. Phosphoantigen binding in the intracellular domain causes conformational changes to the extracellular domain which, during interactions between Vγ9Vδ2 T cells and phosphoantigen containing cells, enables the BTN3A1 complex to bind the Vγ9Vδ2 T cell receptor (TCR).21−24 During HMBPP binding to BTN3A1, the β-phosphate interacts directly with arginine residues (R442, 448, and 499) and the α-phosphate interacts indirectly with tryptophan (W421) and methionine (M424) residues. Importantly, the E-allylic alcohol of HMBPP forms a hydrogen bond with the histidine (H381) side chain, an interaction that triggers a conformational transition in H381 and the movement of nearby tryptophan residues that include W421.16 While not fully understood, this key interaction with H381 in the intracellular domain leads to extracellular changes that allow the Vγ9Vδ2 TCR to detect HMBPP-bound BTN3A1 with greater sensitivity than detection of IPP and DMAPP.16,19,25
While IPP and DMAPP lack the (E)-allylic alcohol, these endogenous diphosphates do weakly stimulate Vγ9Vδ2 T cells.8 It is not clear if DMAPP itself functions as a phosphoantigen, or if its activity is due to its cellular conversion to IPP.26 Nevertheless, some studies have focused on increasing intracellular concentrations of these phosphoantigens to provoke TCR-BTN3A1 interactions.27 Because C-HMBP phosphonamidates are more active than HMBPP,28−30 we chose to evaluate whether DMAPP functions as a direct phosphoantigen through preparation of a parallel prodrug analog of C-DMAP. We hypothesized that a C-DMAP phosphonamidate would require lower effective concentrations to stimulate a T cell response comparable to DMAPP. Furthermore, given that the diphosphate 5 is a known phosphoantigen31 and the aldehyde 6 was once proposed to be a natural phosphoantigen,32 the requirement for an allylic alcohol in active compounds may be more apparent than real.
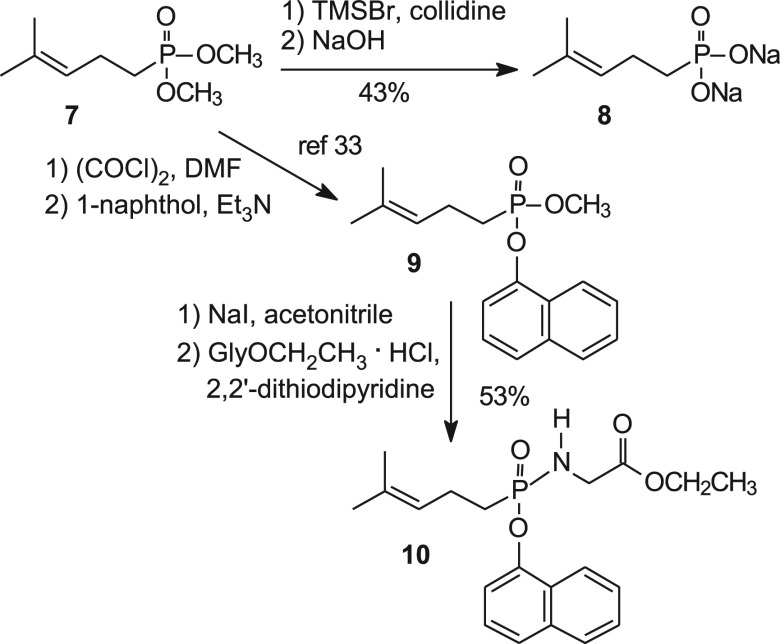
To evaluate the impact of removal of the C-HMBP (E)-allylic alcohol, synthesis of C-DMAP (8) began with the previously reported phosphonate 7 (Scheme 1).19 Treatment of phosphonate 7 with bromotrimethylsilane and collidine followed by sodium hydroxide afforded the sodium salt 8. To access the C-DMAP 1-naphthyl phosphonamidate, treatment of phosphonate 7 with oxalyl chloride and a catalytic amount of dimethylformamide followed by addition of 1-naphthol and triethylamine provided the mixed phosphonate ester 9.33 The remaining methyl ester in phosphonate 9 then was cleaved selectively by reaction with sodium iodide in acetonitrile at reflux, and the resulting salt then was coupled to glycine ethyl ester hydrochloride in the presence of triphenylphosphine and 2,2′-dithiodipyridine to afford phosphonamidate 10. Compound 10 represents a monophosphonate analog of DMAPP protected as the phosphonamidate.
Scheme 1. Synthesis of C-DMAP and Its Phosphonamidate Derivative.
Early efforts to determine the organic framework of the more active exogenous phosphoantigen, now thought to be HMBPP, initially assigned the compound as the aldehyde 3-formyl-1-butyl-diphosphate (6).34 Our preparation of C-HMBP phosphonamidates concludes with a selenium dioxide-mediated oxidation, which provides the required E-allylic alcohol as a single olefin isomer.35,36 However, this reaction step can favor an aldehyde product either by increasing reaction temperature or time. In some cases, chromatography of the crude oxidation mixture provided a percentage of recovered starting material in addition to some aldehyde product. In theory, isolation of a mixture of aldehyde and alcohol after minimal purification from the selenium dioxide step would provide material suitable for treatment with manganese dioxide to drive the mixture forward to exclusively aldehyde product. Though aldehydes are generally avoided in drug development due to chemical reactivity, we were interested in evaluation of aldehyde-containing phosphoantigens because that same reactivity can lead to useful biological probes37 as well as occasionally better drug molecules.38
While the identity of the mycobacterial phosphoantigen described by Belmant and colleagues remains unclear, the prenyl phosphate stimulated Vγ9Vδ2 T cell proliferation at nanomolar concentrations.32 Furthermore, their results provided evidence of a terminal aldehyde (C4 in the phosphate which would correspond to C5 in the phosphonate).34 Bromohydrin diphosphate (BrHPP, 5), a modified prenyl derivative, also triggered responses of human Vγ9Vδ2 T cells at nanomolar concentrations.31 Thus, to investigate the impact of other modifications of a known BTN ligand relative to their potential to stimulate an immune response, some C-HMBP aldehyde analogs were prepared for biological evaluation. Considering that recent literature provides strong evidence for the necessity of a ligand to interact with H381 of BTN3A1, we also prepared a phosphonate aldoxime which places a potential hydrogen bond donor off of the C5 position.16,39,40
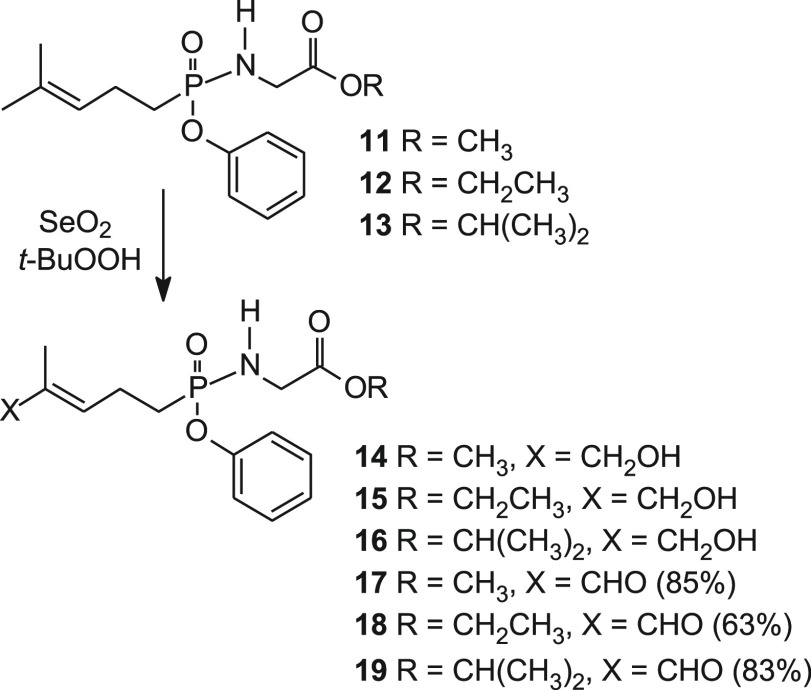
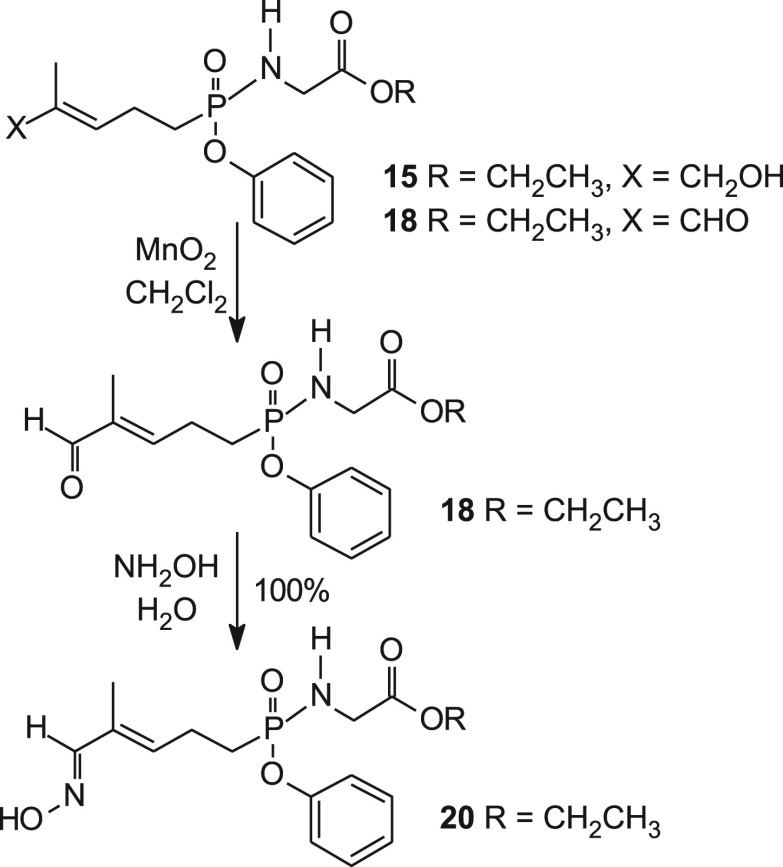
Synthesis of the aldehyde phosphonamidates began with phenyl phosphonamidates 11–13 (Scheme 2).33 Treatment of each of the phosphonamidates 11, 12, and 13 with a catalytic amount of selenium dioxide afforded C-HMBP phosphonamidates 14, 15, and 16, in addition to aldehydes 17, 18, and 19, respectively.
Scheme 2. Synthesis of C-HMBP C5 Aldehydes of Phosphonamidates and Their Previously Reported Alcohols28.
The C-HMBP phosphonamidates containing ethyl esters of glycine provide greater potency, on average, relative to methyl and isopropyl compounds.28 Therefore, the ethyl ester compound 18 was taken to the target aldoxime (20). Treatment of the alcohol/aldehyde mixture (15/18) with manganese dioxide in dichloromethane provided additional aldehyde for the next synthetic step (Scheme 3). Subsequent reaction with hydroxylamine in water allowed for rapid conversion to a quantitative amount of aldoxime 20.
Scheme 3. Synthesis of C-HMBP C5 Modified Aldoxime.
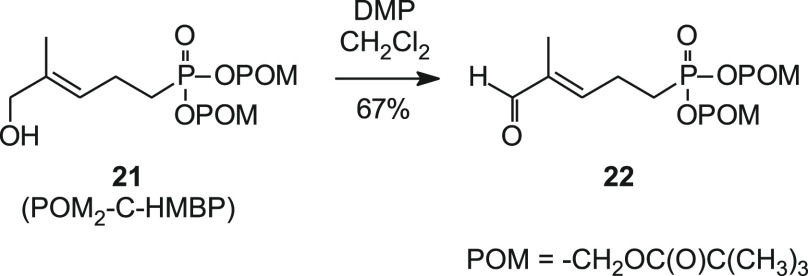
After establishing reaction conditions that modify the (E)-allylic alcohol of C-HMBP to an aldehyde, the bis-pivaloyloxymethyl (POM) derivative 22 and some mixed aryl acyloxyalkyl compounds were prepared to allow for comparison of their activities against other known prodrug forms of C-HMBP. Synthesis of compound 22 began with POM2-C-HMBP (21).19 Treatment of POM2-C-HMBP with Dess–Martin periodinane (DMP) afforded the target bis(acyloxyalkyl) ester 22, in sufficient quantity to allow for bioassay of this aldehyde (Scheme 4).
Scheme 4. Synthesis of POM2-C-HMBP Aldehyde by Oxidation with the Dess–Martin Periodinane.
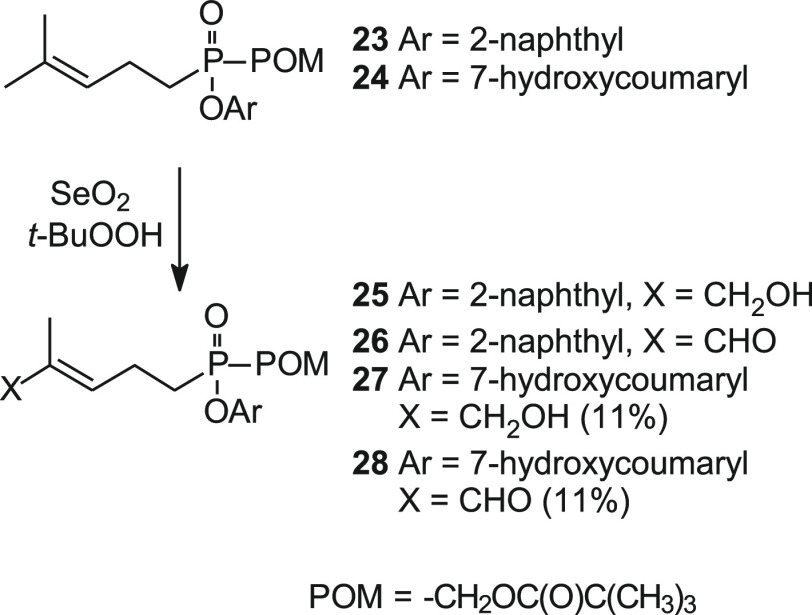
Mixed aryl acyloxyalkyl forms of C-HMBP have been found to increase activity relative to C-HMBP.33,41 To compare the new aldehydes across prodrug classes, we began our synthesis of the C5 aldehyde 26 with the 2-naphthyl phosphonate 23 (Scheme 5).41 The coumaryl phosphonate 24 was prepared by a parallel sequence to that of compound 23 except 2-naphthol was replaced with 7-hydroxycoumarin. Finally, treatment of each mixed phosphonate ester, either 23 or 24, with selenium dioxide resulted in oxidation to afford aldehydes 26 and 28, respectively. Chromatography of the oxidation mixture also provided the corresponding alcohol counterpart (compounds 25(33) and 27) to each of the target aldehydes. Isolation of the aldehydes proceeded in sufficient quantities to allow bioassay without the need for an additional manganese dioxide or DMP oxidation step.
Scheme 5. Synthesis of C-HMBP Aldehydes of Mixed Aryl Acyloxyalkyl Phosphonate Esters.
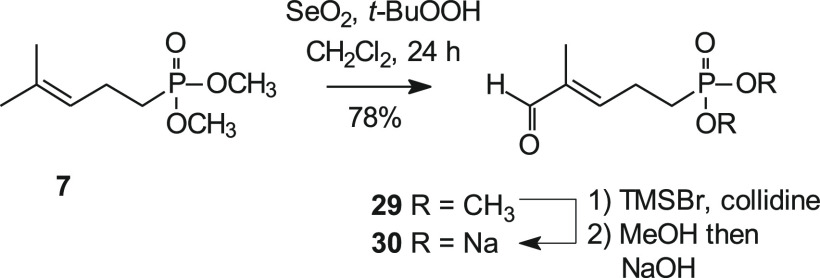
Finally, the last target of interest in this series was the aldehyde salt 30 (Scheme 6), whose activity could be compared to salts studied previously. Unfortunately, this compound proved to be elusive. The dimethyl ester 7 was chosen as the starting phosphonate because methyl esters should be easiest to cleave.42 Oxidation of this phosphonate to the corresponding aldehyde 29 could be accomplished in good yield when the SeO2 oxidation was allowed to proceed for an extended time. However, all attempts to hydrolyze the ester 29 to the corresponding salt 30 under standard conditions went unrewarded. Because this might be due to the instability of the aldehyde itself,38 further efforts were postponed pending the results of the biological studies on the available compounds.
Scheme 6. Attempted Synthesis of the Aldehyde Salt 30.
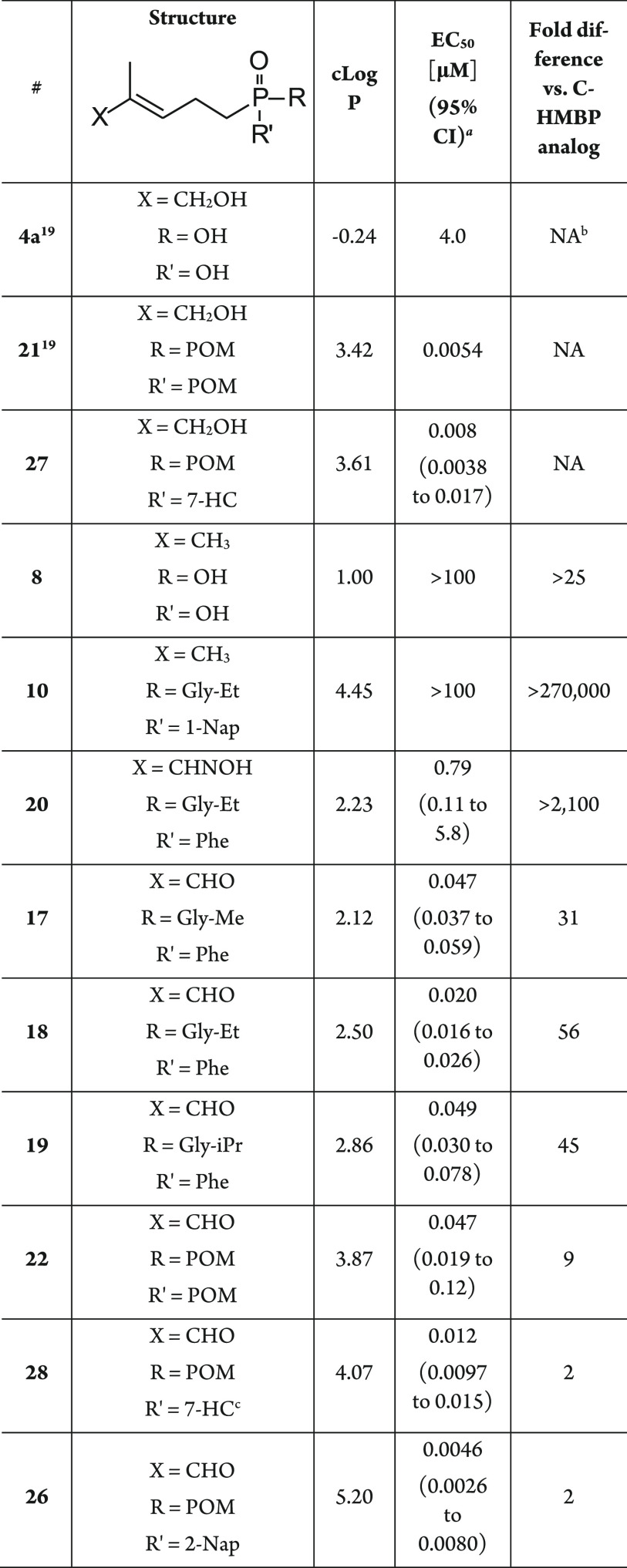
All of the available compounds were tested for cellular toxicity to K562 cells using a cell viability assay (Figure S1). Only compounds 17, 22, and 26 showed cell toxicity, which was weak and only visible at a dose of 100 μM. This indicates that the presence of the aldehyde in these molecules is not inherently toxic to the cells. The newly synthesized compounds were next evaluated for their ability to stimulate proliferation of human Vγ9Vδ2 T cells from peripheral blood using a flow cytometry method to quantify the number of cells positive for the Vγ9Vδ2 TCR after stimulation and growth periods. The C-DMAP sodium salt 8 was completely inactive up to 100 μM. Additionally, C-DMAP phosphonamidate 10 was also completely inactive up to 100 μM (Table 1). This is in contrast to its corresponding C-HMBP 1-naphthyl/GlyOEt phosphonamidate which displayed an EC50 of 0.36 nM, a greater than 270,000-fold difference.19,28 These findings support a model in which it is unlikely that DMAPP functions as a direct phosphoantigen but rather acts through cellular conversion to IPP or another endogenous phosphoantigen, though we cannot exclude an unrecognized defect in conversion of the C-DMAP prodrug to the active form.
Table 1. Activity of C5 Modified C-HMBP Compounds for Expansion of Vγ9Vδ2 T Cells from Peripheral Blood Mononuclear Cellsd.
Because the allylic alcohol of HMBPP plays an important role in the immune response of Vγ9Vδ2 T cells, we became interested in evaluation of a modified C-HMBP analog with a different hydrogen bond donor at this position. Despite the presence of a hydrogen bond donor at the C5 position, the aldoxime 20 was about 40-fold less potent relative to the corresponding aldehyde 18, with an EC50 of 790 nM vs 20 nM, respectively. It is possible that differences in molecular geometry including bond length and position contribute to the observed difference in potency.
Evaluation of the new aldehydes revealed strong stimulants of Vγ9Vδ2 T cell proliferation at nanomolar concentrations. In this assay, the most potent of the aldehyde phosphonamidate set proved to be phenyl phosphonamidate 18 bearing an ethyl ester of glycine, with an EC50 of 20 nM. However, as a class, the aldehyde phosphonamidates (17–19) required an average of 44-fold higher concentrations to stimulate Vγ9Vδ2 T cells proliferation relative to their corresponding C-HMBP phenyl phosphonamidates. Nevertheless, the aldehydes required comparable concentrations to those reported for 3-formyl-1-butyl-diphosphate by Belmant and colleagues.34 It is possible that the activity reported then was due to HMBPP itself, rather than an aldehyde. However, our findings clearly indicate that the presence of an aldehyde at a comparable position within the butyrophilin ligand affords potent derivatives of the analog of HMBPP (i.e., C-HMBP) which require nanomolar concentrations to elicit an immune response. Furthermore, these bioactivity results bolster our earlier SAR finding with C-HMBP phosphonamidates of glycine, showing increased potency for ethyl ester compounds relative to methyl and isopropyl esters.
The bis(acyloxyalkyl) ester 22 stimulated proliferation of Vγ9Vδ2 T cells at comparable effective concentrations to phosphonamidates 17 and 19, with EC50 values of 47 nM and 49 nM, respectively. However, the activity of compound 22 was more similar to that of POM2-C-HMBP (21), with the aldehyde only 9-fold less potent than its corresponding alcohol.
Aldehydes 26 and 28 of the mixed acyloxy aryloxy class41 stimulate proliferation of Vγ9Vδ2 T cells at low nanomolar levels, with EC50 values of 4.6 nM and 12 nM, respectively. These two mixed aryl acyloxyalkyl esters with an aldehyde stimulated T cell proliferation at concentrations comparable to their corresponding alcohol, only requiring 2-fold higher concentrations, which was not statistically significant by 2-way ANOVA. A similar though less pronounced pattern of activity was observed in ELISA assays for detection of interferon γ (Table S2) produced by Vγ9Vδ2 T cells after exposure to phosphoantigen loaded K562 cells. Therefore, the allylic functionality on this prenyl phosphate framework can be altered concurrently with phosphonate protection to retain high potency.
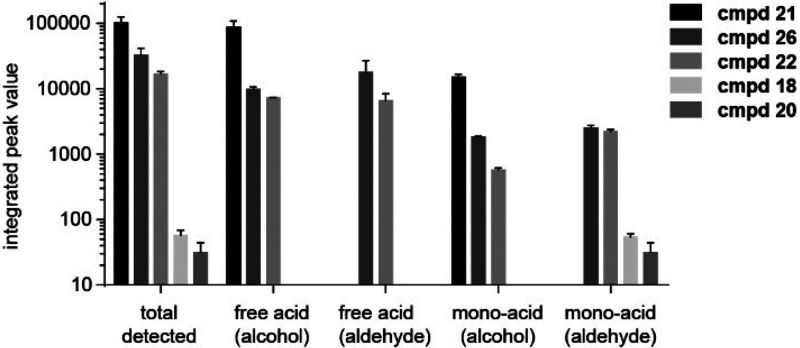
Because the aldehydes may be reduced in the cytosol to the corresponding C-HMBP or form glutathione conjugates, we investigated the cellular metabolism of selected compounds. We used basic extraction conditions43 and ion pairing LCMS44 to quantify the phosphonate metabolites formed following treatment with selected prodrugs (Supporting Information). As expected, treatment of K562 cells for 1 h with the control prodrug 21 produced the free phosphonic acid payload in its allylic alcohol form, as well as the monoacid intermediate (Figure 2). Compound 21 was not converted to aldehyde forms. All of the compounds examined in this study (18, 20, 22, and 26) were at least partially converted to the monoacid in its aldehyde (or aldoxime) form. Compounds 22 and 26 additionally drove formation of the free phosphonic acid aldehyde. Interestingly, both compounds also formed the free acid alcohol. Measurable reduction of the aldehydes to their alcohol forms was observed in the intact prodrugs, the monoacids, and the free acids. Masses consistent with compound 22 metabolites in [M+307–H]− form were also found, indicative of glutathione conjugation. Dehydration to the DMAP monoacid was also observed. Taken together, these aldehydes are reduced to the alcohol forms in cells at significant levels, and the amount of alcohol form generated correlates with the potency of the prodrug forms in cellular assays.
Figure 2.
Conversion of selected aldehyde (or aldoxime) prodrugs to their free acid alcohol forms in K562 cells. Treatment for 1 h with the indicated compound results in a two-step biological release of the phosphoantigen payload from the prodrug groups to generate the phosphono monoacid and free-acid forms. Simultaneously, the allylic aldehyde is reduced to the alcohol form (n = 2).
In conclusion, these studies report novel modifications to the allylic framework of C-HMBP and their impact on human Vγ9Vδ2 T cell proliferation. Removal of the (E)-allylic alcohol completely abrogates activity, while replacement with an aldoxime (i.e., 20) reduces but does not eliminate activity. In contrast, aldehyde replacements are well tolerated. Six examples including phosphonamidate, bis-acyloxy ester, and mixed acyloxy aryloxy ester forms of C-HMBP are provided, all modified to include an aldehyde and all function as stimulants of Vγ9Vδ2 T cell proliferation at nanomolar concentrations, with two aldehydes (26 and 28) having similar activity to alcohol 21. The latter two compounds were metabolized in cells to produce the corresponding alcohol form. We suspect that the combination of the aldehyde with a rapidly metabolized prodrug form45 enables cell entry and conversion to the alcohol form, whereas the slower uptake of phosphonamidate forms may allow for extracellular aldehyde metabolism that slows cell entry. In theory, this aldehyde modification could serve as a synthetic handle for further derivation of C-HMBP analogs. One example of such a modification, the quantitative conversion of an aldehyde (18) to its corresponding C5 aldoxime (20) is reported here.
Acknowledgments
We thank the UI Graduate College for Ballard-Seashore Fellowships (to N.A.L. and B.J.F.), the GAANN Program at the University of Iowa (P200A150065) for a fellowship (to N.M.H.), and the American Cancer Society – Kirby Foundation for a Postdoctoral Fellowship (PF-18-119-01-LIB to M.M.P.). Financial support from the NIH (CA186935 and AI150869 to A.J.W.), the Herman Frasch Foundation for Chemical Research, Bank of America, N.A., Trustee (HF17 to A.J.W.), and the Roy J. Carver Charitable Trust through its Research Program of Excellence (01-224 to D.F.W.) is gratefully acknowledged.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.0c00586.
Experimental procedures for synthetic chemistry, bioassay protocols, LC traces, and 1H, 13C, and 31P NMR spectra (PDF)
Author Contributions
∇ N.A.L. and C.M.S. contributed equally to this manuscript. The manuscript was written through contributions of all authors, and all authors have given approval to the final version of the manuscript.
The authors declare the following competing financial interest(s): A.J.W. and D.F.W. own shares in Terpenoid Therapeutics, Inc. The current work did not involve the company. The other authors have no financial conflicts of interest.
Supplementary Material
References
- Vantourout P.; Hayday A. Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nat. Rev. Immunol. 2013, 13 (2), 88–100. 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita C. T.; Jin C.; Sarikonda G.; Wang H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vgamma2Vdelta2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol. Rev. 2007, 215, 59–76. 10.1111/j.1600-065X.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- Sicard H.; Fournie J. J. Metabolic routes as targets for immunological discrimination of host and parasite. Infect. Immun. 2000, 68 (8), 4375–4377. 10.1128/IAI.68.8.4375-4377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemer A. J.; Hohl R. J.; Wiemer D. F. The Intermediate Enzymes of Isoprenoid Metabolism as Anticancer Targets. Anti-Cancer Agents Med. Chem. 2009, 9 (5), 526–542. 10.2174/187152009788451860. [DOI] [PubMed] [Google Scholar]
- Wiemer A. J.; Hsiao C. H. C.; Wiemer D. F. Isoprenoid Metabolism as a Therapeutic Target in Gram-Negative Pathogens. Curr. Top. Med. Chem. 2010, 10 (18), 1858–1871. 10.2174/156802610793176602. [DOI] [PubMed] [Google Scholar]
- Lombard J.; Moreira D. Origins and Early Evolution of the Mevalonate Pathway of Isoprenoid Biosynthesis in the Three Domains of Life. Mol. Biol. Evol. 2011, 28 (1), 87–99. 10.1093/molbev/msq177. [DOI] [PubMed] [Google Scholar]
- Holstein S. A.; Hohl R. J. Isoprenoids: remarkable diversity of form and function. Lipids 2004, 39 (4), 293–309. 10.1007/s11745-004-1233-3. [DOI] [PubMed] [Google Scholar]
- Tanaka Y.; Morita C. T.; Tanaka Y.; Nieves E.; Brenner M. B.; Bloom B. R. Natural and Synthetic Nonpeptide Antigens Recognized by Human Gamma-Delta T-Cells. Nature 1995, 375 (6527), 155–158. 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- Hintz M.; Reichenberg A.; Altincicek B.; Bahr U.; Gschwind R. M.; Kollas A. K.; Beck E.; Wiesner J.; Eberl M.; Jomaa H. Identification of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human gamma delta T cells in Escherichia coli. FEBS Lett. 2001, 509 (2), 317–322. 10.1016/S0014-5793(01)03191-X. [DOI] [PubMed] [Google Scholar]
- Song Y. C.; Zhang Y. H.; Wang H.; Raker A. M.; Sanders J. M.; Broderick E.; Clark A.; Morita C. T.; Oldfield E. Synthesis of chiral phosphoantigens and their activity in gamma delta T cell stimulation. Bioorg. Med. Chem. Lett. 2004, 14 (17), 4471–4477. 10.1016/j.bmcl.2004.06.052. [DOI] [PubMed] [Google Scholar]
- Eberl M.; Hintz M.; Reichenberg A.; Kollas A. K.; Wiesner J.; Jomaa H. Microbial isoprenoid biosynthesis and human gamma delta T cell activation. FEBS Lett. 2003, 544 (1–3), 4–10. 10.1016/S0014-5793(03)00483-6. [DOI] [PubMed] [Google Scholar]
- Kilcollins A. M.; Li J.; Hsiao C. H. C.; Wiemer A. J. HMBPP Analog Prodrugs Bypass Energy-Dependent Uptake To Promote Efficient BTN3A1-Mediated Malignant Cell Lysis by V gamma 9V delta 2 T Lymphocyte Effectors. J. Immunol. 2016, 197 (2), 419–428. 10.4049/jimmunol.1501833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boedec A.; Sicard H.; Dessolin J.; Herbette G.; Ingoure S.; Raymond C.; Belmant C.; Kraus J. L. Synthesis and biological activity of phosphonate analogues and geometric isomers of the highly potent phosphoantigen (E)-1-hydroxy-2-methylbut-2-enyl 4-diphosphate. J. Med. Chem. 2008, 51 (6), 1747–1754. 10.1021/jm701101g. [DOI] [PubMed] [Google Scholar]
- Salim M.; Knowles T. J.; Baker A. T.; Davey M. S.; Jeeves M.; Sridhar P.; Wilkie J.; Willcox C. R.; Kadri H.; Taher T. E.; Vantourout P.; Hayday A.; Mehellou Y.; Mohammed F.; Willcox B. E. BTN3A1 Discriminates gamma delta T Cell Phosphoantigens from Nonantigenic Small Molecules via a Conformational Sensor in Its B30.2 Domain. ACS Chem. Biol. 2017, 12 (10), 2631–2643. 10.1021/acschembio.7b00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poe M. M.; Agabiti S. S.; Liu C.; Li V.; Teske K. A.; Hsiao C. H. C.; Wiemer A. J. Probing the Ligand-Binding Pocket of BTN3A1. J. Med. Chem. 2019, 62 (14), 6814–6823. 10.1021/acs.jmedchem.9b00825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. Y.; Li L. P.; Yuan L. J.; Zhou X. Y.; Duan J. X.; Xiao H. Y.; Cai N. N.; Han S.; Ma X. Q.; Liu W. D.; Chen C. C.; Wang L. L.; Li X.; Chen J. H.; Kang N.; Chen J.; Shen Z. X.; Malwal S. R.; Liu W. L.; Shi Y.; Oldfield E.; Guo R. T.; Zhang Y. H. A Structural Change in Butyrophilin upon Phosphoantigen Binding Underlies Phosphoantigen-Mediated V gamma 9V delta 2 T Cell Activation. Immunity 2019, 50 (4), 1043–1053. 10.1016/j.immuni.2019.02.016. [DOI] [PubMed] [Google Scholar]
- Shippy R. R.; Lin X. C.; Agabiti S. S.; Li J.; Zangari B. M.; Foust B. J.; Poe M. M.; Hsiao C. H. C.; Vinogradova O.; Wiemer D. F.; Wiemer A. J. Phosphinophosphonates and Their Tris-pivaloyloxymethyl Prodrugs Reveal a Negatively Cooperative Butyrophilin Activation Mechanism. J. Med. Chem. 2017, 60 (6), 2373–2382. 10.1021/acs.jmedchem.6b00965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom A.; Peigne C. M.; Leger A.; Crooks J. E.; Konczak F.; Gesnel M. C.; Breathnach R.; Bonneville M.; Scotet E.; Adams E. J. The Intracellular B30.2 Domain of Butyrophilin 3A1 Binds Phosphoantigens to Mediate Activation of Human V gamma 9V delta 2 T Cells. Immunity 2014, 40 (4), 490–500. 10.1016/j.immuni.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao C. H. C.; Lin X. C.; Barney R. J.; Shippy R. R.; Li J.; Vinogradova O.; Wiemer D. F.; Wiemer A. J. Synthesis of a Phosphoantigen Prodrug that Potently Activates V gamma 9V delta 2 T-Lymphocytes. Chem. Biol. 2014, 21 (8), 945–954. 10.1016/j.chembiol.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Harly C.; Guillaume Y.; Nedellec S.; Peigne C. M.; Monkkonen H.; Monkkonen J.; Li J. Q.; Kuball J.; Adams E. J.; Netzer S.; Dechanet-Merville J.; Leger A.; Herrmann T.; Breathnach R.; Olive D.; Bonneville M.; Scotet E. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human gamma delta T-cell subset. Blood 2012, 120 (11), 2269–2279. 10.1182/blood-2012-05-430470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigau M.; Ostrouska S.; Fulford T. S.; Johnson D. N.; Woods K.; Ruan Z.; McWilliam H. E. G.; Hudson C.; Tutuka C.; Wheatley A. K.; Kent S. J.; Villadangos J. A.; Pal B.; Kurts C.; Simmonds J.; Pelzing M.; Nash A. D.; Hammet A.; Verhagen A. M.; Vairo G.; Maraskovsky E.; Panousis C.; Gherardin N. A.; Cebon J.; Godfrey D. I.; Behren A.; Uldrich A. P. Butyrophilin 2A1 is essential for phosphoantigen reactivity by gamma delta T cells. Science 2020, 367 (6478), 642. [DOI] [PubMed] [Google Scholar]
- Karunakaran M. M.; Willcox C. R.; Salim M.; Paletta D.; Fichtner A. S.; Noll A.; Starick L.; Nohren A.; Begley C. R.; Berwick K. A.; Chaleil R. A. G.; Pitard V.; Dechanet-Merville J.; Bates P. A.; Kimmel B.; Knowles T. J.; Kunzmann V.; Walter L.; Jeeves M.; Mohammed F.; Willcox B. E.; Herrmann T. Butyrophilin-2A1 Directly Binds Germline-Encoded Regions of the V gamma 9V delta 2 TCR and Is Essential for Phosphoantigen Sensing. Immunity 2020, 52 (3), 487. 10.1016/j.immuni.2020.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann T.; Fichtner A. S.; Karunakaran M. M.. An Update on the Molecular Basis of Phosphoantigen Recognition by V gamma 9V delta 2 T Cells. Cells 2020, 9 ( (6), ). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemer A. J. Structure-Activity Relationships of Butyrophilin 3 Ligands. ChemMedChem 2020, 15 (12), 1030–1039. 10.1002/cmdc.202000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen K.; Li J.; Puthenveetil R.; Lin X.; Poe M. M.; Hsiao C. C.; Vinogradova O.; Wiemer A. J.. The butyrophilin 3A1 intracellular domain undergoes a conformational change involving the juxtamembrane region. FASEB J. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthelot K.; Estevez Y.; Deffieux A.; Peruch F. Isopentenyl diphosphate isomerase: A checkpoint to isoprenoid biosynthesis. Biochimie 2012, 94 (8), 1621–1634. 10.1016/j.biochi.2012.03.021. [DOI] [PubMed] [Google Scholar]
- Zhou X.; Gu Y.; Xiao H.; Kang N.; Xie Y.; Zhang G.; Shi Y.; Hu X.; Oldfield E.; Zhang X.; Zhang Y. Combining Vgamma9Vdelta2 T Cells with a Lipophilic Bisphosphonate Efficiently Kills Activated Hepatic Stellate Cells. Front. Immunol. 2017, 8, 1381. 10.3389/fimmu.2017.01381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentini N. A.; Foust B. J.; Hsiao C. H. C.; Wiemer A. J.; Wiemer D. F. Phosphonamidate Prodrugs of a Butyrophilin Ligand Display Plasma Stability and Potent V gamma 9 V delta 2 T Cell Stimulation. J. Med. Chem. 2018, 61 (19), 8658–8669. 10.1021/acs.jmedchem.8b00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentini N. A.; Hsiao C. H. C.; Crull G. B.; Wiemer A. J.; Wiemer D. F. Synthesis and Bioactivity of the Alanyl Phosphonamidate Stereoisomers Derived from a Butyrophilin Ligand. ACS Med. Chem. Lett. 2019, 10 (9), 1284–1289. 10.1021/acsmedchemlett.9b00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.; Lentini N. A.; Wiemer D. F.; Wiemer A. J. A luciferase lysis assay reveals in vivo malignant cell sensitization by phosphoantigen prodrugs. Biochem. Pharmacol. 2019, 170, 113668. 10.1016/j.bcp.2019.113668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa E.; Belmant C.; Pont F.; Luciani B.; Poupot R.; Romagne F.; Brailly H.; Bonneville M.; Fournie J. J. Chemical synthesis and biological activity of bromohydrin pyrophosphate, a potent stimulator of human gamma delta T cells. J. Biol. Chem. 2001, 276 (21), 18337–18344. 10.1074/jbc.M100495200. [DOI] [PubMed] [Google Scholar]
- Poquet Y.; Constant P.; Halary F.; Peyrat M. A.; Gilleron M.; Davodeau F.; Bonneville M.; Fournie J. J. A novel nucleotide-containing antigen for human blood gamma delta T lymphocytes. Eur. J. Immunol. 1996, 26 (10), 2344–2349. 10.1002/eji.1830261011. [DOI] [PubMed] [Google Scholar]
- Foust B. J.; Poe M. M.; Lentini N. A.; Hsiao C. C.; Wiemer A. J.; Wiemer D. F. Mixed Aryl Phosphonate Prodrugs of a Butyrophilin Ligand. ACS Med. Chem. Lett. 2017, 8 (9), 914–918. 10.1021/acsmedchemlett.7b00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmant C.; Espinosa E.; Poupot R.; Peyrat M. A.; Guiraud M.; Poquet Y.; Bonneville M.; Fournie J. J. 3-formyl-1-butyl pyrophosphate A novel mycobacterial metabolite-activating human gamma delta T cells. J. Biol. Chem. 1999, 274 (45), 32079–32084. 10.1074/jbc.274.45.32079. [DOI] [PubMed] [Google Scholar]
- Bhalerao U. T.; Rapoport H. Stereochemistry of Allylic Oxidation with Selenium Dioxide - Stereospecific Oxidation of Gem-Dimethyl Olefins. J. Am. Chem. Soc. 1971, 93 (19), 4835–4840. 10.1021/ja00748a028. [DOI] [Google Scholar]
- Sharpless K. B.; Lauer R. F. Selenium dioxide oxidation of olefins - evidence for intermediacy of allylseleninic acids. J. Am. Chem. Soc. 1972, 94 (20), 7154–7155. 10.1021/ja00775a050. [DOI] [Google Scholar]
- London N.; Miller R. M.; Krishnan S.; Uchida K.; Irwin J. J.; Eidam O.; Gibold L.; Cimermancic P.; Bonnet R.; Shoichet B. K.; Taunton J. Covalent docking of large libraries for the discovery of chemical probes. Nat. Chem. Biol. 2014, 10 (12), 1066–1072. 10.1038/nchembio.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gampe C.; Verma V. A.. Curse or Cure? A Perspective on the Developability of Aldehydes as Active Pharmaceutical Ingredients. J. Med. Chem. 2020. [DOI] [PubMed] [Google Scholar]
- Damljanovic I.; Vukicevic M.; Vukicevic R. D. A simple synthesis of oximes. Monatsh. Chem. 2006, 137 (3), 301–305. 10.1007/s00706-005-0427-3. [DOI] [Google Scholar]
- Yamamoto T.; Fujita K.; Asari S.; Chiba A.; Kataba Y.; Ohsumi K.; Ohmuta N.; Iida Y.; Ijichi C.; Iwayama S.; Fukuchi N.; Shoji M. Synthesis and evaluation of isoxazole derivatives as lysophosphatidic acid (LPA) antagonists. Bioorg. Med. Chem. Lett. 2007, 17 (13), 3736–3740. 10.1016/j.bmcl.2007.04.024. [DOI] [PubMed] [Google Scholar]
- Foust B. J.; Li J.; Hsiao C. C.; Wiemer D. F.; Wiemer A. J. Stability and Efficiency of Mixed Aryl Phosphonate Prodrugs. ChemMedChem 2019, 14 (17), 1597–1603. 10.1002/cmdc.201900344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsagi N.; Radai Z.; Kiss N. Z.; Szigetvari A.; Keglevich G. Two step acidic hydrolysis of dialkyl arylphosphonates. Mendeleev Commun. 2020, 30 (1), 38–39. 10.1016/j.mencom.2020.01.012. [DOI] [Google Scholar]
- Tong H. X.; Kuder C. H.; Wasko B. M.; Hohl R. J. Quantitative determination of isopentenyl diphosphate in cultured mammalian cells. Anal. Biochem. 2013, 433 (1), 36–42. 10.1016/j.ab.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Joachimiak L.; Janczewski L.; Ciekot J.; Boratynski J.; Blazewska K. Applying the prodrug strategy to alpha-phosphonocarboxylate inhibitors of Rab GGTase - synthesis and stability studies. Org. Biomol. Chem. 2015, 13 (24), 6844–6856. 10.1039/C5OB00281H. [DOI] [PubMed] [Google Scholar]
- Wiemer A. J. Metabolic Efficacy of Phosphate Prodrugs and the Remdesivir Paradigm. ACS Pharmacology & Translational Science 2020, 3 (4), 613–626. 10.1021/acsptsci.0c00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.