Abstract
Objective:
Literature describing follow-up vascular ultrasound (VUS) in giant cell arteritis (GCA) is limited. We report our experience with follow-up VUS obtained in clinical care of patients with GCA.
Methods:
We retrospectively identified GCA patients with an abnormal initial VUS, defined as circumferential hypoechoic wall thickening (“halo sign”), or circumferential hyperechoic wall thickening without evidence of arteriosclerosis or arteritis, who subsequently underwent follow-up VUS during 2013-2018. Studies were interpreted as active arteritis, hyperechoic wall thickening without active arteritis, or no arteritis. We compared clinical and laboratory characteristics at time of initial VUS among patients with active arteritis vs. hyperechoic wall thickening without active arteritis. We described whether and how VUS interpretation changed from initial to follow-up VUS. Among individual vessels, we tested whether abnormal findings (e.g. halo sign) persisted at follow-up VUS using McNemar’s test.
Results:
42 patients fulfilled study criteria. Median time between initial and follow-up VUS was 5.1 (IQR 2.6-7.9) months. Characteristics at initial VUS did not differ according to VUS interpretation. Among 36 patients with active arteritis on initial VUS, follow-up VUS showed active arteritis in 25.0%, hyperechoic wall thickening in 33.3% and no arteritis in 41.7%. Among 6 patients with hyperechoic wall thickening on initial VUS, half had no arteritis on follow-up VUS. Sonographic findings tended to persist in axillary arteries and were more likely to change in the superficial temporal arteries.
Conclusion:
Among 42 GCA patients, the majority had a change in VUS interpretation between initial and follow-up VUS. Sonographic findings in the temporal circulation more frequently changed than findings in axillary arteries.
Keywords: giant cell arteritis, ultrasonography, Doppler
INTRODUCTION
Vascular ultrasound (VUS) of temporal and axillary arteries is recommended as a highly specific and sensitive diagnostic test for giant cell arteritis (GCA), but the role of follow-up VUS in GCA remains uncertain(1-3). Studies describing real-world experience with follow-up VUS in GCA are needed. VUS has been utilized for evaluation of GCA at our medical center since 2013. Herein, we report our experience with follow-up VUS obtained in the care of patients with GCA.
METHODS
We performed a retrospective cohort study among newly diagnosed and established GCA patients at a large academic medical center, 2013-2018. We included GCA patients (as diagnosed by the treating rheumatologist) with an abnormal initial VUS who had a follow-up VUS performed as part of clinical care. VUS was defined as abnormal if at least one vessel demonstrated circumferential hypoechoic wall thickening– the well-known halo sign, indicative of active arteritis– or circumferential hyperechoic wall thickening without evidence of arteriosclerosis. The latter finding, which is distinct from both the halo sign and from normal vasculature, has occasionally been referenced in prior literature(4-6). Clinical and laboratory data were extracted through electronic medical record (EMR) review. The Partners HealthCare Institutional Review Board approved all aspects of this study.
Simultaneous color Doppler and duplex ultrasonography were performed using an 8-18 MHz linear transducer (>15 MHz for temporal arteries, <15 MHz for large arteries) (LOGIQ S8 and E9 ultrasound systems; GE Healthcare, Chicago, Illinois). Grey scale was set to the highest available frequency, with dynamic range 40-50 dB and focus set to approximately 5 mm below skin surface. Color Doppler was set to the highest frequency with pulse repetition frequency (PRF) 2 KHz for temporal arteries and lower frequency with PRF 3.5 KHz for large arteries. Frame rate was set high as possible. Color PRF was 2.5 KHz Doppler frequency shift and was readjusted throughout the exam with velocity changes. Color gain was set such that color covered the lumen entirely, and color box angle correction was set to ≤60 degrees. Power Doppler was used if occlusion was suspected. Pulse Doppler settings were 2 KHz for temporal arteries and 3-5 KHz for large arteries and were adjusted according to flow velocities. Doppler sample volume size was the same diameter as the arterial lumen (0.7 mm for temporal arteries; 1 mm for large arteries) and was positioned in the middle of the vessel with angle correction 60 degrees.
Trained cardiovascular ultrasonographers followed a standardized protocol to visualize the bilateral common superficial temporal arteries and their frontal and parietal branches, and the subclavian and axillary arteries. Trained cardiovascular medicine physicians interpreted each VUS. Ultrasonographers and interpreting cardiovascular medicine physicians were not blinded to clinical data. The overall VUS interpretation was “active arteritis” if at least one vessel had a halo sign, or “hyperechoic wall thickening without active arteritis” if at least one vessel had hyperechoic wall thickening and no vessel had a halo sign. Studies with neither finding were interpreted as no arteritis. Sample images of VUS demonstrating active arteritis, hyperechoic wall thickening and no arteritis are shown in Figures 1 and 2.
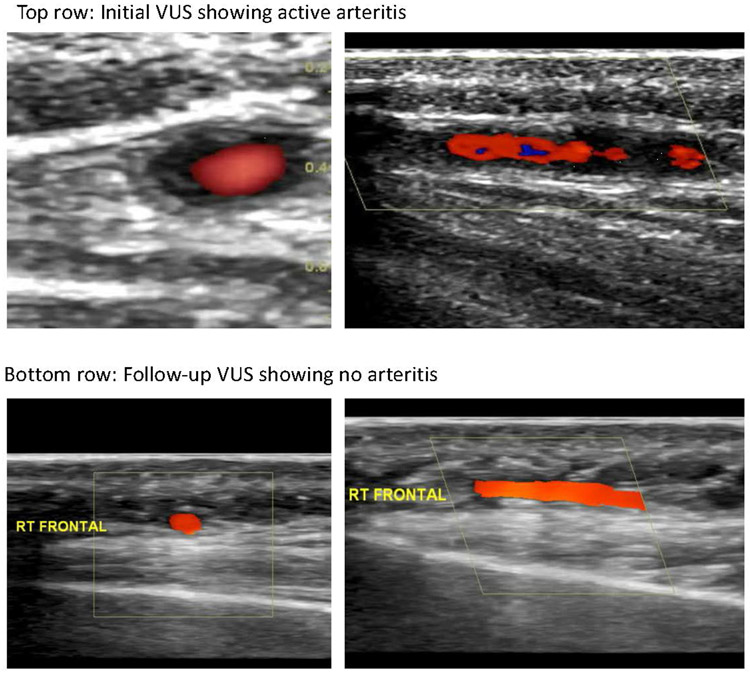
Figure 1.
Vascular ultrasound (VUS) images from a patient in our cohort. Initial VUS demonstrated active arteritis characterized by halo sign (hypoechoic circumferential wall thickening) in the frontal branch of the right temporal artery (top row); follow-up VUS four months later showed no arteritis, with resolution of the halo sign and normal appearance of that same vessel (bottom row).
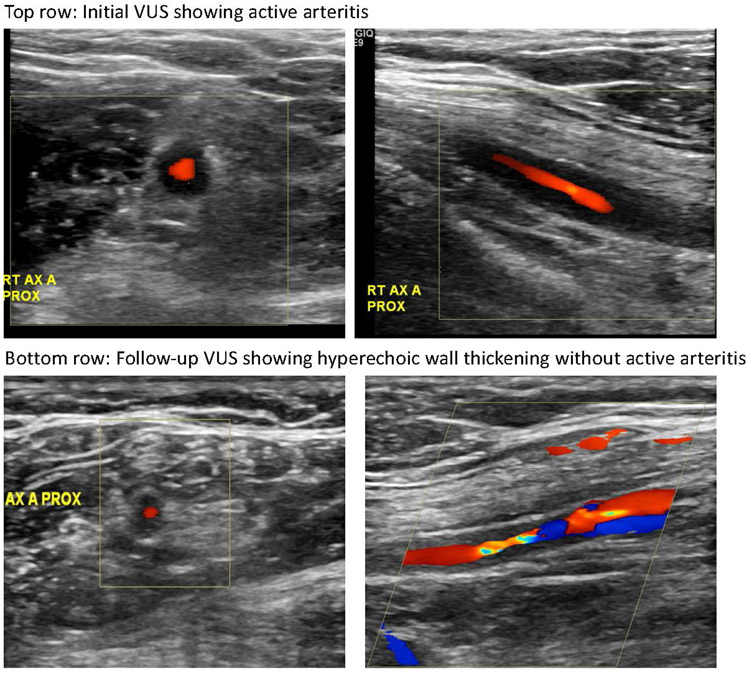
Figure 2.
Vascular ultrasound (VUS) images in a patient in our cohort. Initial VUS demonstrated active arteritis in the right axillary artery (top row); follow-up VUS approximately three months later showed hyperechoic wall thickening without active arteritis in that same vessel (bottom row).
We used Fisher’s exact and Kruskal-Wallis tests to examine whether clinical and laboratory characteristics at time of initial VUS differed according to initial VUS interpretation (active arteritis or hyperechoic wall thickening without active arteritis) or follow-up VUS interpretation (active arteritis, hyperechoic wall thickening without active arteritis, or no arteritis). We categorized patients according to whether and how VUS changed between the initial and follow-up scan and described the treating rheumatologist’s clinical impression after the follow-up scan. Among individual vessels, we evaluated whether findings on initial VUS (halo sign, hyperechoic wall thickening, or no arteritis) changed on follow-up VUS using McNemar’s test. Analyses were performed using SAS v9.4; threshold for statistical significance p<0.05.
RESULTS
We identified 42 GCA patients (including 28.6% with established GCA at time of VUS) with an abnormal initial VUS and a subsequent follow-up VUS during the study period. The study sample was 71.4% female and 69.1% white, with median age at initial VUS 72.5 years (interquartile range [IQR] 66.6-78.2). Among 26 patients that ever had temporal artery biopsy, 46.2% of biopsies revealed active arteritis on histopathology. The median time between initial and follow-up VUS was 5.1 months (IQR 2.6-7.9). Characteristics at time of initial VUS of the entire sample, and according to initial and follow-up VUS result, are presented in Table 1. Polymyalgia rheumatica (PMR) at time of initial VUS was more common among patients who had hyperechoic wall thickening or no arteritis on follow-up VUS as opposed to active arteritis on follow-up VUS; otherwise, clinical and laboratory characteristics did not significantly differ according to VUS interpretation. Indications for ordering follow-up VUS included assessing ultrasonographic change from initial VUS (45.2%), recurrent/worsening GCA symptoms (38.1%), or rising ESR/CRP (16.7%) in an asymptomatic patient. Twenty-nine patients (69.1%) were using glucocorticoids at time of initial VUS: 11/29 (37.9%) had been commenced on steroids prior to VUS during evaluation of suspected GCA, while 10/29 (34.5%) and 8/29 (27.6%) had been on chronic steroids for prior diagnoses of GCA or PMR, respectively.
Table 1.
Characteristics at the time of initial abnormal VUS, overall and according to initial and follow-up VUS interpretation.
| Initial VUS interpretation | Follow-up VUS interpretation | |||||
|---|---|---|---|---|---|---|
| Characteristic at the time of initial VUS |
All patients (n=42) |
Active arteritis (n=36) |
Hyperechoic wall thickening without active arteritis (n=6) |
Active arteritis (n=10) |
Hyperechoic wall thickening without active arteritis (n=14) |
No arteritis (n=18) |
| Age, years | 72.5 (66.6-78.2) | 72.5 (64.9-78.0) | 73.4 (68.8-78.4) | 72.5 (69.4, 77.2) | 74.2 (68.8, 79.8) | 70.8 (61.6, 77.8) |
| Female | 71.4 | 66.7 | 100.0 | 60.0 | 78.6 | 72.2 |
| White | 69.1 | 63.9 | 100.0 | 70.0 | 64.3 | 72.2 |
| Symptom duration | ||||||
| Less than 1 week | 2.4 | 2.8 | 0.0 | 0.0 | 0.0 | 5.6 |
| 1-3 weeks | 11.9 | 8.3 | 33.3 | 0.0 | 0.0 | 27.8 |
| ≥3 weeks | 73.8 | 75.0 | 66.7 | 80.0 | 92.9 | 55.6 |
| Unclear | 11.9 | 13.9 | 0.0 | 20.0 | 7.1 | 11.1 |
| Clinical features at time of symptom onset | ||||||
| Headache | 35.7 | 33.3 | 50.0 | 30.0 | 42.9 | 33.3 |
| Fever | 14.3 | 11.1 | 33.3 | 20.0 | 14.3 | 11.1 |
| Jaw claudication | 31.0 | 33.3 | 16.7 | 10.0 | 50.0 | 27.8 |
| Temporal artery tenderness | 21.4 | 22.2 | 16.7 | 20.0 | 14.3 | 27.8 |
| Scalp tenderness | 21.4 | 25.0 | 0.0 | 10.0 | 28.6 | 22.2 |
| Fatigue | 33.3 | 30.6 | 50.0 | 10.0 | 28.6 | 50.0 |
| Weight loss | 16.7 | 16.7 | 16.7 | 40.0 | 7.1 | 11.1 |
| Transient vision loss | 9.5 | 11.1 | 0.0 | 0.0 | 21.4 | 5.6 |
| Polymyalgia rheumatica | 33.3 | 30.6 | 50.0 | 0.0* | 35.7* | 50.0* |
| GCA diagnosis prior to initial VUS | 28.6 | 30.6 | 16.7 | 20.0 | 14.3 | 44.4 |
| CRP, mg/L (median, IQR) | 28.8 (8.4-87.6) | 29.0 (8.4-89.3) | 22.9 (3.3-38.0) | 69.3 (5.7, 215.7) | 27.8 (10.4, 51.1) | 30.6 (3.3, 81.9) |
| ESR, mm/hr (median, IQR) | 59 (34-90) | 59 (34-91) | 50 (31-78) | 77 (71, 85) | 49 (34, 95) | 55 (26, 68) |
| Current glucocorticoid use | 69.1 | 69.4 | 66.7 | 50.0 | 71.4 | 77.8 |
| Prednisone equivalent daily dose** | ||||||
| Low (>0 to 15mg) | 37.9 | 36.0 | 50.0 | 0.0 | 40.0 | 50.0 |
| Moderate (≥15 to 40mg) | 17.2 | 16.0 | 25.0 | 20.0 | 10.0 | 21.4 |
| High (≥40mg) | 44.8 | 48.0 | 25.0 | 80.0 | 50.0 | 28.6 |
| Prednisone duration** | ||||||
| >0 days to <1 week | 34.5 | 36.0 | 25.0 | 60.0 | 40.0 | 21.4 |
| ≥1 week to <3 weeks | 3.5 | 4.0 | 0.0 | 20.0 | 0.0 | 0.0 |
| ≥3 weeks | 62.1 | 60.0 | 75.0 | 20.0 | 60.0 | 78.6 |
| Methotrexate use | 9.5 | 11.1 | 0.0 | 0.0 | 7.1 | 16.7 |
Change in VUS interpretation from initial to follow-up VUS is illustrated in Figure 3. Among 36 patients with active arteritis on initial ultrasound, follow-up ultrasound showed no arteritis in 15 (41.7%), active arteritis in 9 (25.0%), and hyperechoic wall thickening without active arteritis in 12 (33.3%). Median time between the initial and follow-up VUS was shorter among patients with persistent active arteritis on the follow-up scan (2.7 months, IQR 0.5-7.9) compared to those with no arteritis on follow-up scan (6.1 months, IQR 4.2-13.4). Of the 6 patients with hyperechoic wall thickening without active arteritis on initial VUS, follow-up VUS revealed no arteritis in 3, active arteritis in 1, and persistent hyperechoic wall thickening without active arteritis in 2. After a follow-up VUS with no arteritis, the treating rheumatologist (who was not blinded to VUS result) felt that GCA was inactive/not flaring in 11/18 (61.1%). After a follow-up VUS with hyperechoic wall thickening without active arteritis, the treating rheumatologist felt that GCA was felt to be inactive/not flaring in 9/14 (64.3%).
Figure 3. Change in VUS interpretation over time.
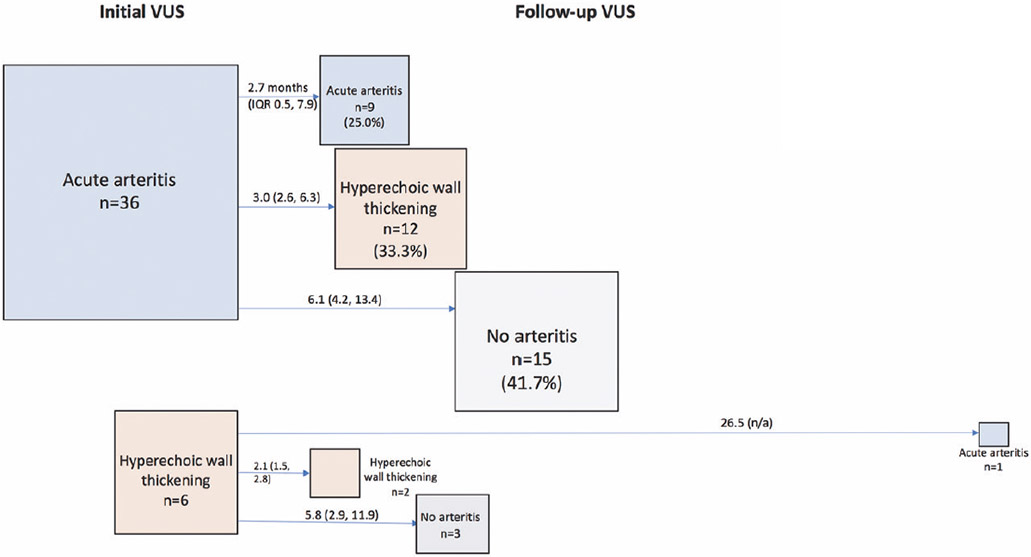
Arrows are labeled with the median (interquartile range) number of months between initial and follow-up VUS.
At the individual vessel level, abnormalities tended to remain concordant between initial and follow-up VUS in the axillary and subclavian arteries according to McNemar’s test (p>0.05). For example, among 9 right subclavian arteries with halo sign on initial VUS, 1 had no arteritis, 4 had halo sign and 4 had hyperechoic wall thickening on follow-up VUS. Among 9 right axillary arteries with halo sign on initial VUS, 1 had no arteritis, 3 had halo sign and 5 had hyperechoic wall thickening on follow-up VUS. Abnormal findings in the superficial temporal arteries on initial VUS often had no arteritis on follow-up VUS (McNemar’s p<0.05). For example, of 12 right superficial temporal arteries with halo sign on initial VUS, 8 had no arteritis, 2 had halo sign and 2 had hyperechoic wall thickening on follow-up VUS.
DISCUSSION
Among 42 GCA patients with an abnormal initial VUS and a follow-up VUS obtained as part of clinical care, the majority (73.8%) had a different VUS interpretation between the initial and follow-up scan (median of 5 months later). Clinical/laboratory parameters including steroid exposure did not statistically differ among patients according to VUS findings, with the exception of PMR being more common among those patients without active arteritis on follow-up VUS, though small sample size limited the power to detect such differences. In this observational study, the median time between the initial and follow-up scan was shorter among patients with persistent active arteritis on VUS compared with those whose active arteritis resolved. Findings in the superficial temporal arteries often changed between initial and follow-up VUS, while axillary and subclavian artery findings often remained stable.
Multiple smaller prospective studies and one large retrospective study investigating VUS in GCA diagnosis also reported data on follow-up VUS after initiation of treatment(3,6-13). These studies reported a wide range of mean time to halo sign disappearance, e.g. 16 days to 11 weeks, with one study finding that 10 of 26 patients had persistent halo signs 6 months into treatment despite being in clinical remission(7,11,13). In most of these studies, VUS was performed at protocolized intervals, in contrast to the present study which included VUS obtained in the course of longitudinal patient care for a variety of indications. Furthermore, only several of the above studies included the axillary or subclavian arteries in the ultrasonographic assessment(3,5,6). In our cohort, less than half of patients with active arteritis (i.e. halo sign) on initial VUS had resolution of findings on follow-up VUS after median 5 months. A possible explanation for the relatively low frequency of halo sign resolution despite treatment in our cohort could be confounding by indication (e.g. more symptomatic patients may have had follow-up VUS performed sooner than asymptomatic patients). We also observed that findings in the superficial temporal arteries were more likely to change from active arteritis to no arteritis between the initial and follow-up VUS, whereas findings in the axillary arteries often remained stable. That abnormalities of proximal arm arteries tend to change appearance more slowly with time compared to temporal arteries has been previously observed by Schmidt and colleagues(5).
Circumferential hyperechoic wall thickening without sonographic evidence for active arteritis or arteriosclerosis was observed in 14% of initial VUS in our cohort. Hyperechoic wall thickening has been infrequently described in prior literature and is of unclear clinical significance. Schmidt and colleagues described a patient with extracranial GCA in which hypoechoic wall thickening of the axillary, brachial, carotid and subclavian arteries became hyperechoic 1 year after commencing treatment, hypothesizing that hyperechogenicity may represent fibrosis due to chronic disease(4). A subsequent study by Schmidt et al. of 40 follow-up VUS in GCA patients with large vessel involvement noted “vasculitic wall swelling became brighter at follow-up examinations”(5). Aschwanden et al. performed follow-up VUS 6 months after initial VUS in 9 patients with halo signs involving the extracranial large arteries. In the majority of examined segments, findings did not normalize but rather “a marginally enhanced echogenicity of the vessel wall persisted”(6). In our cohort, the 6 patients with hyperechoic wall thickening and no active arteritis on initial VUS did not differ from patients with active arteritis in terms of clinical or laboratory parameters, prior diagnosis of GCA, or prednisone exposure, though our small sample size prevents meaningful clinical conclusions. We observed that hyperechoic wall thickening on the initial ultrasound was not necessarily permanent, as 3 of these 6 patients had resolution of findings on the follow-up ultrasound and 1 patient developed new halo sign. The majority of patients with hyperechoic wall thickening on follow-up VUS were ultimately felt to have inactive disease by their treating rheumatologists.
Strengths of our study include application of a standardized VUS protocol including the extracranial arteries in a clinic-based cohort, which examined the real-world use of follow-up VUS in GCA. Limitations include small sample size, restricting our ability to detect differences between subgroups, as well as short follow-up period. Approximately one-third of our cohort (35.7%) presented with headache at time of initial abnormal VUS, which is perhaps unexpectedly low compared to other GCA cohorts. The relatively low frequency of headache in our cohort may be explained by the fact that our cohort included patients with established disease who were undergoing treatment, rather than exclusively patients with a new presentation of GCA. Some patients in our cohort had predominantly large vessel involvement, which may also explain the low prevalence of headache.
In summary, in this retrospective cohort of 42 GCA patients who underwent follow-up VUS after initial abnormal VUS as part of clinical care, the majority had a different VUS interpretation between initial and follow-up scan. Abnormalities in the superficial temporal arteries tended to change, whereas abnormalities in the subclavian and axillary arteries tended to persist. Though more studies are needed, follow-up VUS to monitor GCA disease activity may be informative, particularly in the temporal circulation.
ACKNOWLEDGEMENTS
We would like to thank the Brigham and Women’s Hospital vascular ultrasound technicians.
Funding: This work was supported by the Brigham and Women’s Hospital Faculty Career Development Award, William P. Docken Inflammatory Autoimmune Disease Research Program, and the National Institutes of Health (K23 AR075070).
REFERENCES
- 1.Duftner C, Dejaco C, Sepriano A, Falzon L, Schmidt WA, Ramiro S. Imaging in diagnosis, outcome prediction and monitoring of large vessel vasculitis: a systematic literature review and meta-analysis informing the EULAR recommendations. RMD open. 2018. February 2;4(1):e000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dejaco C, Ramiro S, Duftner C, Besson FL, Bley TA, Blockmans D, et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis. 2018. May;77(5):636–43. [DOI] [PubMed] [Google Scholar]
- 3.Monti S, Floris A, Ponte CB, Schmidt WA, Diamantopoulos AP, Pereira C, et al. The proposed role of ultrasound in the management of giant cell arteritis in routine clinical practice. Rheumatology. 2018. January 1;57(1):112–9. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt WA, Kraft HE, Borkowski A, Gromnica-Ihle EJ. Color duplex ultrasonography in large-vessel giant cell arteritis. Scand J Rheumatol. 1999;28(6):374–6. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt WA, Moll A, Seifert A, Schicke B, Gromnica-Ihle E, Krause A. Prognosis of large-vessel giant cell arteritis. Rheumatology (Oxford). 2008. September 4;47(9):1406–8. [DOI] [PubMed] [Google Scholar]
- 6.Aschwanden M, Kesten F, Stern M, Thalhammer C, Walker UA, Tyndall A, et al. Vascular involvement in patients with giant cell arteritis determined by duplex sonography of 2x11 arterial regions. Ann Rheum Dis. 2010. July 1;69(7):1356–9. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt WA, Kraft HE, Vorpahl K, Völker L, Gromnica-Ihle EJ. Color duplex ultrasonography in the diagnosis of temporal arteritis. N Engl J Med. 1997. November 6;337(19):1336–42. [DOI] [PubMed] [Google Scholar]
- 8.Salvarani C, Silingardi M, Ghirarduzzi A, Lo Scocco G, Macchioni P, Bajocchi G, et al. Is Duplex Ultrasonography Useful for the Diagnosis of Giant-Cell Arteritis? Ann Intern Med. 2002. August 20;137(4):232. [DOI] [PubMed] [Google Scholar]
- 9.Pfadenhauer K, Weber H. Duplex sonography of the temporal and occipital artery in the diagnosis of temporal arteritis. A prospective study. J Rheumatol. 2003;30:2177–81. [PubMed] [Google Scholar]
- 10.Karahaliou M, Vaiopoulos G, Papaspyrou S, Kanakis MA, Revenas K, Sfikakis PP. Colour duplex sonography of temporal arteries before decision for biopsy: a prospective study in 55 patients with suspected giant cell arteritis. Arthritis Res Ther. 2006;8(4):R116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pérez López J, Solans Laqué R, Bosch Gil JA, Molina Cateriano C, Huguet Redecilla P, Vilardell Tarrés M. Colour-duplex ultrasonography of the temporal and ophthalmic arteries in the diagnosis and follow-up of giant cell arteritis. Clin Exp Rheumatol. 27(1 Suppl 52):S77–82. [PubMed] [Google Scholar]
- 12.Habib HM, Essa AA, Hassan AA. Color duplex ultrasonography of temporal arteries: role in diagnosis and follow-up of suspected cases of temporal arteritis. Clin Rheumatol. 2012. February 9;31(2):231–7. [DOI] [PubMed] [Google Scholar]
- 13.De Miguel E, Roxo A, Castillo C, Peiteado D, Villalba A, Martín-Mola E. The utility and sensitivity of colour Doppler ultrasound in monitoring changes in giant cell arteritis. Clin Exp Rheumatol. 30(1 Suppl 70):S34–8. [PubMed] [Google Scholar]