ABSTRACT
Background:
Strongly associated with obesity, non-alcoholic fatty liver disease is considered the hepatic manifestation of the metabolic syndrome. It presents as simple steatosis and steatohepatitis, which can progress to cirrhosis and its complications. Among the therapeutic alternatives is bariatric surgery.
Aim:
To compare the effect of the two most frequent bariatric procedures (sleeve and bypass) on liver disease regarding to epidemiological, demographic, clinical and laboratory parameters.
Methods:
The results of intraoperative and 12 months after surgery liver biopsies were used. The NAFLD activity score (NAS) was used to assess and compare the stages of liver disease.
Results:
Sixteen (66.7%) patients underwent Bypass procedure and eight (33.3%) Sleeve. It was observed that the variation in the NAFLD activity score was significantly greater in the Bypass group than in Sleeve (p=0.028) and there was a trend regarding the variation in fibrosis (p=0.054).
Conclusion:
Both surgical techniques were effective in improving the hepatic histology of most operated patients. When comparing sleeve and bypass groups, bypass showed better results, according to the NAS score.
HEADINGS: Bypass, Sleeve, Non-alcoholic fatty liver disease, Morbid obesity, Bariatric surgery
RESUMO
Racional:
Fortemente associada à obesidade, a doença hepática gordura não alcoólica é considerada a manifestação hepática da síndrome metabólica. Ela apresenta-se como esteatose simples e esteato-hepatite, podendo evoluir para cirrose e suas complicações. Entre as alternativas terapêuticas está a cirurgia bariátrica.
Objetivo:
Comparar o efeito sobre a doença hepática dos dois procedimentos bariátricos mais frequentes - sleeve e bypass - e comparar dados epidemiológicos, demográficos, parâmetros clínicos e laboratoriais.
Métodos:
Utilizou-se o resultado das biópsias hepáticas realizadas no intra-operatório e 12 meses após a operação. O NAFLD activity score foi utilizado para avaliar e comparar os estágios da doença hepática.
Resultados:
Dezesseis (66,7%) pacientes foram submetidos ao bypass e oito (33,3%) ao sleeve. Observou-se melhora significativa no IMC e glicemia nas duas técnicas cirúrgicas enquanto que os níveis de fosfatase alcalina, ferritina, Gama-GT e TGP reduziram com significância apenas no grupo bypass. A redução no NAFLD activity score foi significativamente maior no grupo bypass que no sleeve (p=0,040).
Conclusão:
Ambas as técnicas foram eficazes em promover a melhora da histologia hepática da maior parte dos pacientes operados. Quando comparadas o bypass apresentou melhores resultados.
DESCRITORES: Bypass, Sleeve, Doença hepática gordurosa não alcoólica, Obesidade mórbida, Cirurgia bariátrica
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) has a global distribution and is considered nowadays the most common liver disease in the west industrialized countries, probably because of the association with obesity, type 2 diabetes (DM2), dyslipidemia and metabolic syndrome. The prevalence of NAFLD is reported as 6-35%, with an average of 20%, in the general population 33 .
There is a strong evidence that NAFLD represents the hepatic component of the metabolic syndrome, characterized by obesity, hyperinsulinemia, insulin resistance (IR), DM2, hypertriglyceridemia and systemic arterial hypertension (SAH). Obesity is a common and well-documented risk factor for NAFLD. In patients with severe obesity undergoing bariatric surgery, the prevalence of NAFLD can exceed 90%, with up to 50% of patients presenting steatohepatitis (NASH) and 5% cirrhosis 6 , 25 .
Abdominal ultrasound is widely used as the first line in the investigation of NAFLD and evidence the accumulation of fat when more than 33% of hepatocytes have steatosis. Liver biopsy is the gold standard method for the diagnosis of NAFLD, being able to assess the degree of fatty infiltration, hepatocellular damage, inflammation and fibrosis. The presence of hepatocellular ballooning in association with steatosis is the key to the differential diagnosis between simple steatosis and NASH 29 .
All patients with NAFLD should undergo interventions to promote a healthier lifestyle and strict control of the metabolic risk factors associated with NAFLD 17 . Weight loss is the most important factor. Relatively small losses of approximately 7-10% of body weight in 12 months seem to be effective in improving NAFLD already 11 , 20 .
Obesity and DM2, isolated or linked by metabolic syndrome, are the most important risk factors in the genesis of NAFLD. The role of bariatric surgery comes up in this context. Several consistent studies over the past few years, culminating in the publication of Philip Schauer in 2017 31 , have shown that surgical treatment is more effective in weight loss and in the control of glycemia and metabolic syndrome than intensive and optimized clinical treatment in the short, medium and long terms.
Although there are still no randomized clinical trials evaluating the role of bariatric surgery in the treatment of NAFLD, there are several retrospective and prospective studies in the literature, in addition to a few meta-analyzes, which evaluate the effect of surgical treatment on NAFLD 1 - 3 , 5 , 8 - 10 , 12 - 14 , 16 , 20 , 21 , 23 - 26 , 32 , 34 . The AASLD and EASL guidelines define bariatric surgery as a therapeutic option in NAFLD, mainly in those patients who did not respond to conservative clinical treatment 11 , 20 .
Roux-en-Y bypass (bypass) and vertical gastrectomy (sleeve) have been compared in several studies to determine which one has the greatest benefit in weight loss or in resolving obesity-related comorbidities. However, there is few knowledge about which of these techniques is associated with the best results on NAFLD. There are few comparative studies and these are small series with a large number of biases, such as heterogeneity of groups and assessment systems 7 , 15 , 28 .
In a group of obese patients, the main objective of the present study was to compare the bariatric sleeve and Roux-en-Y bypass techniques and their effects on NAFLD, also in epidemiological, demographic, clinical and laboratory parameters.
METHODS
The study was submitted to and approved by the Ethics Committee of the institution, where it was carried out. CAAE: 90475215500005335.
Patients selection
A retrospective study that evaluated patients undergoing bariatric surgical procedures by the same surgical team, between 2014 and 2016, at Irmandade da Santa Casa de Misericórdia de Porto Alegre, Porto Alegre, RS, Brazil.
Only patients with intraoperative and postoperative liver biopsy were included. The average time between biopsies was 12 months.
Demographic characteristics (gender and age), clinical parameters (weight, height, BMI, SAH, weight loss, excess weight lost) and laboratory (platelets, ALP, ferritin, GGT, AST, ALT), histopathological analysis and NAS score 16 were collected and analyzed.
Operative technique
All procedures were performed laparoscopically.
The sleeve surgery was made with the dissection of the greater gastric curvature 3 cm from the pylorus to the esophagogastric flexure. The gastric transection was calibrated with a 36F bougie and reinforcing the staple line with continuous suture with Caprofyl® 2.0.
In the bypass surgery, a 4 cm gastric pouch was done using a 36F bougie. The Roux limb used to be 100cm and the biliopancreatic limb used to be 150 cm. Gastroenteric anastomosis was performed laterally with a linear stapler calibrated by a 36F bougie.
Histopathological evaluation
All liver samples were analyzed in the hospital’s pathology laboratory by a single professional experienced in liver pathology. The NAFLD activity score (NAS) was used to assess and compare the stages of liver disease 18 . It proposes the characterization of NAFLD regarding the degree of steatosis, the presence of ballooning and inflammation activity. NAS scores the histological analysis from 0 to 3: degree of steatosis (0-3), lobular inflammation (0-3) and ballooning (0-2). The degree of fibrosis was assessed semi-quantitatively with a scale of 0 to 4.
Statistical analysis
Categorical results were presented using frequency and percentage and were analyzed using the X 2 test (chi-square). The likelihood-ratio test was performed in comparisons when there were more than 20% of the cells with an expected value below 20%. Quantitative results were displayed using means±standard deviations and when data followed normal distribution it was analyzed using Student’s t tests for paired and independent samples; when non-parametric, the Wilcoxon and Mann-Whitney tests were used. The normality of the data was verified using the Shapiro-Wilk test. The variation in the NAS score was calculated using the difference between the postoperative and the intraoperative score. The analyzes were performed using SPSS software, version 21 and significant results were considered when p <0.05.
RESULTS
Twenty-four obese patients who underwent bariatric surgery were evaluated, of which 16 (66.7%) undergoing bypass and eight sleeve, with a mean segment time of 21.3±16 months and 15.5±12.5 months, respectively (p=0.380). 68.8% of patients undergoing bypass were women, on the sleeve group, 75% (p=0.571); 31.3% were hypertensive vs. 37.5% in the sleeve (p=0.553); in the bypass group, age and maximum weight were 38.6±11.3 years and 119.1±14.1 kg respectively vs. 36.7±8.4 years, p=0.692 and 119.1±13.5, p=0.999 in the sleeve group. Furthermore, the two techniques were also similar in terms of weight loss (44.2±15.1 kg vs. 37.9±7.4 kg, p=0.180, respectively) and the excess weight lost (84.4±29, 8 kg vs. 83.2±26.8 kg, p=0.904, respectively).
Table 1 shows the results of the clinical and laboratory parameters in the preoperative period and after the surgical procedures. In both periods and in all parameters analyzed, no difference was found between the techniques with statistical significance. However, there were significant reductions in BMI and glycemia in the two techniques analyzed, while reductions in alkaline phosphatase, ferritin, GGT and ALT were significant only in the bypass group.
TABLE 1. Pre and postoperative clinical and laboratory parameters according to the type of operation performed.
| Parameter | Technique | Pre-op | Post-op | p |
|---|---|---|---|---|
| BMI (kg/m²) | Bypass | 44.3±4.2 | 27.9±4.1 | <0.001 |
| Sleeve | 42.1±4.1 | 28.6±5.8 | <0.001 | |
| p | 0.239 | 0.730 | ||
| Platelets (x1000) | Bypass | 270±59 | 239±66 | 0.170 |
| Sleeve | 283±45 | 279±71 | 0.832 | |
| p | 0.606 | 0.191 | ||
| ALP (U/L) | Bypass | 92.2±30.7 | 72.8±16.8 | 0.010 |
| Sleeve | 105.5±20.6 | 103.0±70.3 | 0.910 | |
| p | 0.283 | 0.268 | ||
| Ferritin (ng/ml) | Bypass | 227.1±150.3 | 121.1±86.4 | 0.011 |
| Sleeve | 232.6±76.1 | 133.6±103.1 | 0.069 | |
| p | 0.854 | 0.976 | ||
| GGT (U/L) | Bypass | 55.9±31.1 | 19.1±10.8 | 0.000 |
| Sleeve | 111.0±151.8 | 64.5±116.3 | 0.123 | |
| p | 0.312 | 0.197 | ||
| Glycemia (mg/dl) | Bypass | 99.4±17.2 | 82.1±9.8 | 0.001 |
| Sleeve | 102.75±10.2 | 90.2±8.6 | 0.007 | |
| p | 0.616 | 0.059 | ||
| AST (U/L) | Bypass | 30.8±16.2 | 25.5±6.9 | 0.338 |
| Sleeve | 33.5±12.2 | 39.0±27.9 | 1.000 | |
| p | 0.408 | 0.358 | ||
| ALT (U/L) | Bypass | 42.1±20.6 | 28.3±10.6 | 0.023 |
| Sleeve | 39.9±12.5 | 31.6±13.2 | 0.207 | |
| p- | 0,806 | 0,602 |
BMI=body mass index; ALP=alkaline phosphatase; GGT=gamma glutamyl transpeptidase; AST=aspartate aminotransferase; ALT=alanine aminotransferase.
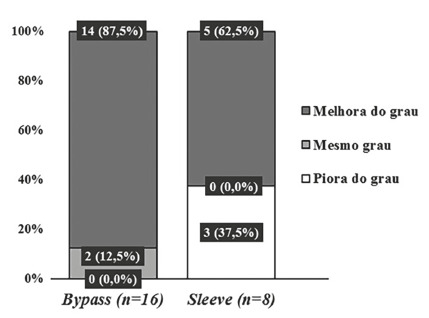
Evaluating the evolution of the NAFLD degree on the second biopsy compared to the first, 19 (79.2%) patients showed improvement, two (8.3%) remained in the same degree and three (12.5%) worsened. There was a significant association between the evolution of NAFLD and the surgical procedure performed (p=0.024). In the sleeve group, three (37.5%) worsened the level of NAFLD vs. no patient in the bypass group (Figure 1).
FIGURE 1. Evolution of NAFLD degree.
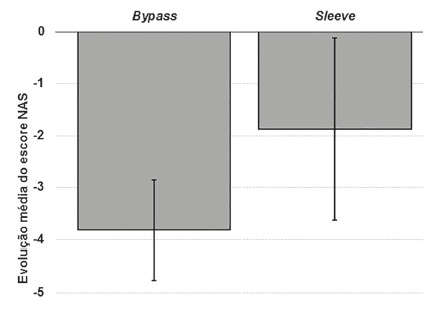
Table 2 shows the differences in the NAS 16 classification between the first and second biopsies. In all categories, a significant reduction was observed when the technique was bypass. However, in the sleeve group, only steatosis and the NAS score showed significant reductions.
TABLE 2. Comparison between the first and second liver biopsies.
| NAS classification | First | Second | Change | p |
|---|---|---|---|---|
| Bypass | ||||
| Steatosis | 2.0 ± 0.8 | 0.2 ± 0.4 | -1.81 ± 0.91 | <0.001 |
| Inflammation | 1.2 ± 0.7 | 0.5 ± 0.5 | -0.75 ± 0.77 | 0.005 |
| Ballooning | 1.5 ± 0.9 | 0.2 ± 0.7 | -1.25 ± 1.00 | 0.002 |
| Fibrosis | 0.9 ± 1.0 | 0.2 ± 0.7 | -0.69 ± 0.87 | 0.015 |
| NAS score | 4.8 ± 1.7 | 0.9 ± 1.3 | -3.81 ± 1.80 | <0.001 |
| Sleeve | ||||
| Steatosis | 1.5 ±0.7 | 0.2 ± 0.5 | -1.25 ± 0.89 | 0.014 |
| Inflammation | 0.7 ± 0.9 | 0.7 ± 0.5 | -0.13 ± 0.64 | 0.564 |
| Ballooning | 1.0 ± 1.1 | 0.5 ± 0.9 | -0.50 ± 1.41 | 0.317 |
| Fibrosis | 0.4 ± 0.7 | 0.6 ± 0.9 | 0.25 ± 1.39 | 0.581 |
| NAS score | 3.2 ± 1.8 | 1.4 ± 1.7 | -1.87 ± 2.10 | 0.048 |
The intensity of variations was compared between groups with no significant difference between them (p>0.05), except for the NAS score shown in Figure 2, whose reduction observed in the bypass was significantly higher than in the sleeve (-3,81±1,80 vs. -1,87±2,10, respectively; p=0,040).
FIGURE 2. Comparison of the mean variation of the NAS score.
DISCUSSION
In our country, bypass is still the most performed bariatric technique; but, in recent years, there has been an important increase in the number of vertical gastrectomy, which follows the worldwide trend of technical preference nowadays 4 .
Historically, patients with morbid obesity or those with clinical signs and symptoms of metabolic syndrome had in the operations of mixed character (bypass) - with restrictive and disabsorptive component -, their most frequent indication. Purely restrictive procedure - vertical gastrectomy - a relatively newer technique, was initially indicated for patients with grade II obesity or very high BMI levels greater than 60 kg/m², those in which the necessary weight loss was not too much or those that mixed operations has became impractical due to the great excess of weight. Thus, sometimes it becomes difficult to compare the results between the techniques, since the patients generally presented important clinical and epidemiological differences, making statistically different groups.
In the present study, evaluating the demographic characteristics of both groups - bypass and sleeve - it was noticed that there were no statistical differences between them. Gender, breed and age were similar. SAH was present in approximately 30% of patients. There were no diabetic patients and the mean blood glucose was practically the same in both groups, 100 mg/dl. Regarding weight, BMI, laboratory tests - platelets, alkaline phosphatase, ferritin, gamma-GT, glycemia, AST and ALT preoperatively - the groups also showed similarity. This parity between the groups contributes to a more reliable comparative analysis with a lower incidence of bias.
Already widely discussed and consolidated in the literature, these bariatric procedures have an important effect on weight loss. In the present study, in the mean follow-up of 12 months, the BMI showed an average reduction of 16.3±5.23 kg/m2 and 13.4±2.7 kg/m2 in the bypass and sleeve, respectively. This reduction represents the average loss of approximately 84% of excess body weight for both techniques. As previously published by several authors, and more recently by Peterli et al 27 , the tendency is not to have statistical differences between bypass and sleeve considering weight loss in the short, medium and long terms.
Analyzing blood glucose separately, it was observed that both surgical techniques were effective, statistically reducing serum glucose levels by 17.3±15.7 mg/dl and 12.5±9.4 mg/dl, considering bypass and sleeve respectively. When comparing the final results between the techniques, there is a favorable trend towards bypass (p=0.059), probably depending on a larger sample. In recent years, it has been associated with techniques with intestinal deviation, a condition of metabolic change that would provide better results on glycemic control, compared to purely restrictive techniques.The increase in intestinal hormones such as glucagon-like peptide-1 (GLP-1) and peptideYY(PYY), the result of the food’s earlier contact with the ileum, would be responsible for this fact. However, more recently, although there is no established pathophysiological explanation, there are studies showing a similar result between the sleeve and bypass regarding glycemic control 30 .
Analyzing the other laboratory tests evaluated in the study, we observed that in the bypass group there was a significant improvement in the levels of alkaline phosphatase, ferritin, gamma-GT and ALT, which did not occur in the sleeve. AST and platelets showed no significant changes.
As previously mentioned, the mechanism by which bariatric surgery performs potential treatment for NAFLD is complex and has not been fully elucidated yet. It is probably related to improvement of factors such as insulin resistance, lipid profile, inflammation, weight loss and adipokines, which are directly involved in the development of metabolic syndrome and, consequently, in its hepatic phenotype, NAFLD. These changes are seen right after the surgical procedure, during the phase when weight loss is not yet significant. It is well established that weight loss plays a fundamental role in the control of metabolic abnormalities; however, endocrinophysiological changes resulting from the operation may have a more relevant effect in the long term on NAFLD. These findings have been confirmed by several studies published in the international literature. As in the present study, NAFLD in its several stages, simple steatosis, NASH and fibrosis, showed significant improvement after the bariatric procedure, be it bypass or sleeve 1 , 9 , 10 , 13 , 14 , 16 , 19 , 21 , 22 , 23 .
However, it can be noticed that part of the operated patients does not improve or could get a worse level of NAFLD, which ranges from 7-20% of patients 22 , 23 , 32 . In the present study, it was observed that five patients (20.8%) did not demonstrate complete resolution of NAFLD. Of these, three (12.5%) showed disease progression, all in the sleeve group.
In the quantitative analysis of NAFLD, the bypass group showed between the first and second biopsies, significant improvement in all NAS parameters (steatosis, inflammation and ballooning), in fibrosis and in the NAS score itself. In the sleeve group, statistical significance was observed only in the steatosis and in the NAS score. Comparing the change in the NAS score between the first and second biopsies, it was observed that this was significantly higher in the bypass group (-3.81±1.80 vs. -1.87±2.10; p=0.040).
In line with the literature, in the present study there was a significant improvement in NAFLD after bariatric procedures, both in the purely restrictive operation (sleeve) and in the mixed operation (bypass).
However, unlike the previous findings published by Froylich 15 , Billeter7 and Praveen 28 , who presented the operative results on NAFLD comparing the sleeve and bypass techniques and did not find statistical differences or tended to the sleeve as the best results procedure, in the present study, the bypass had better effects on NAFLD, including a statistical difference when considering the evolution of the NAS score. There is currently no clear explanation, but it is likely that anatomical changes, particularly duodenal exclusion, increased flow of nutrients to the distal small intestine and the resulting hormonal and metabolic changes related to bypass are responsible for these findings.
The retrospective nature of the study, the small number of patients in the groups and the possible selection biases are important limitations that must be considered when analyzing these results.
CONCLUSION
Sleeve and bypass were effective in restoring the liver histology of most operated patients. BMI and blood glucose showed significant improvement in both surgical techniques, with no significant difference in results between them. The levels of alkaline phosphatase, ferritin, gamma-GT and ALT decreased significantly only in the bypass group, but they did not differ from the valuesobserved in the sleeve. However, the bypass showed significantly better results in the change of the NAS score than the sleeve.
Footnotes
Financial source: none
Central message: When comparing the liver effects of the two most frequent bariatric procedures - sleeve and bypass - both were effective in promoting histological improvement of non-alcoholic fatty liver disease. When compared, bypass provided better results.
Perspectives: Non-alcoholic fatty liver disease is considered the hepatic manifestation of the metabolic syndrome and has bariatric surgery as a alternative treatment. Both most frequent techniques - sleeve and bypass - were effective in promoting liver histological improvement. When compared using the NAFLD activity score, bypass provided better results being the surgery of choice for this situation. A larger number of studies, mainly randomized control trials, are needed to confirm this hypothesis.
REFERENCES
- 1.Algooneh A, Almazeedi S, Al-Sabah S. Nonalcoholic fatty liver disease resolution following Sleeve gastrectomy. Surg Endosc. 2016;30(5):1983–1987. doi: 10.1007/s00464-015-4426-0. [DOI] [PubMed] [Google Scholar]
- 2.Almogy G, Crookes PF, Anthone GJ. Longitudinal gastrectomy as a treatment for the high-risk super-obese patient. Obes Surg. 2004;14(4):492–497. doi: 10.1381/096089204323013479. [DOI] [PubMed] [Google Scholar]
- 3.Angrisani L, Santonicola A, Iovino P. Bariatric surgery and endoluminal procedures IFSO worldwide survey 2014. Obes Surg. 2017;27(9):2279–2289. doi: 10.1007/s11695-017-2666-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angrisani L, Santonicola A, Iovino P. Bariatric surgery and endoluminal procedures IFSO worldwide survey 2014. Obes Surg. 2017;27(9):2279–2289. doi: 10.1007/s11695-017-2666-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barros F, Negrão M G, Negrão G G. Weight loss comparison after Sleeve and Roux-en-Y Gastric Bypass systematic review. ABCD, Arq. Bras. Cir. Dig. 2019;32(4):e1474. doi: 10.1590/0102-672020190001e1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beymer C, Kowdley KV, Larson A. Prevalence and predictors of asymptomatic liver disease in patients undergoing gastric Bypass surgery. Arch Surg. 2003;138(111):1240–1244. doi: 10.1001/archsurg.138.11.1240. [DOI] [PubMed] [Google Scholar]
- 7.Billeter AT, Senft J, Gotthardt D. Combined non-alcoholic fatty liver disease and type 2 diabetes mellitus Sleeve gastrectomy or gastric Bypass? - a controlled matched pair study of 34 patients. Obes Surg. 2016;26(8):1867–1874. doi: 10.1007/s11695-015-2006-y. [DOI] [PubMed] [Google Scholar]
- 8.Bower G, Athanasiou T, Isla AM. Bariatric surgery and nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2015;27(7):755–768. doi: 10.1097/MEG.0000000000000375. [DOI] [PubMed] [Google Scholar]
- 9.Caiazzo R, Lassailly G, Leteurtre E. Roux-en-Y gastric Bypass versus adjustable gastric banding to reduce nonalcoholic fatty liver disease a 5-year controlled longitudinal study. Ann Surg. 2014;260(5):893–898. doi: 10.1097/SLA.0000000000000945. [DOI] [PubMed] [Google Scholar]
- 10.Cazzo E, Jimenez LS, Pareja JC, Chaim EA. Effect of Roux-en-Y gastric Bypass on nonalcoholic fatty liver disease evaluated through NAFLD fibrosis score a prospective study. Obes Surg. 2015;25(6):982–985. doi: 10.1007/s11695-014-1489-2. [DOI] [PubMed] [Google Scholar]
- 11.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Deseases. Hepatology. 2018;67(1) doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 12.Chavez-Tapia NC, Tellez-Avila FI, Barrientos-Gutierrez T. Bariatric surgery for non-alcoholic steatohepatitis in obese patients. Cochrane Detabase Syst Rev. 2010;20(1):CD007340–CD007340. doi: 10.1002/14651858.CD007340.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark JM, Alkhuraishi AR, Solga SF. Roux-en-Y gastric Bypass improves liver histology in patients with non-alcoholic fatty liver disease. Obes Res. 2005;13(7):1180–1186. doi: 10.1038/oby.2005.140. [DOI] [PubMed] [Google Scholar]
- 14.Deitel M, Gagner M, Erickson AL, Crosby RD. Third international summit current status of Sleeve gastrectomy. Surg Obes Relat Dis. 2011;7:749–759. doi: 10.1016/j.soard.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Froylich D, Corcelles R, Daigle C. Effect of Roux-en-Y gastric Bypass and Sleeve gastrectomy on nonalcoholic fatty liver disease a comparative study. Surg Obes Relat Dis. 2016;12(1):127–131. doi: 10.1016/j.soard.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Karcz WK, Krawczykowski D, Kuesters S. Influence of Sleeve gastrectomy on NASH and type 2 diabetes mellitus. J Obes. 2011;2011:765473–765473. doi: 10.1155/2011/765473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirkil C, Aygen E, Korkmaz M F, Bozan M B. Quality of life after laparoscopic Sleeve gastrectomy using baros system. ABCD, arq. bras. cir. dig. 2018;31(3):e1385. doi: 10.1590/0102-672020180001e1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kleiner DE, Brunt EM, Van Natta M. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 19.Lassailly G, Caiazzo R, Buob D. Bariatric surgery reduces features of non-alcoholic steatohepatitis in morbidly obese patients. Gastroenterology. 2015;149:377–388. doi: 10.1053/j.gastro.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 20.Marchesini G, Roden M, Vettor R, et al. The management of non-alcoholic fatty liver desease, EASL, EASD, EASO Clinical Practice Guidelines. The Journal of Hepatology. 2016;64 doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Mattar SG, Velcu LM, Rabinovitz M. Surgically-induced weight loss significantly improves nonalcoholic fatty liver disease and the metabolic syndrome. Ann Surg. 2005;242(4):610–617. doi: 10.1097/01.sla.0000179652.07502.3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moretto M, Kupski C, da Silva VD. Effect of bariatric surgery on liver fibrosis. Obes Surg. 2012;22(7):1044–1049. doi: 10.1007/s11695-011-0559-y. [DOI] [PubMed] [Google Scholar]
- 23.Mottin CC, Moretto M, Padoin AV. Histological behavior of hepatic steatosis in morbidly patients with obesity after weight loss induced by bariatric surgery. Obes Surg. 2005;15(6):788–793. doi: 10.1381/0960892054222830. [DOI] [PubMed] [Google Scholar]
- 24.Mummadi RR, Kasturi KS, Chennareddygari S, Sood GK. Effect of bariatric surgery on nonalcoholic fatty liver disease systematic review and meta- analysis. Clin Gastroenterol Hepatol. 2008;6(12):1396–1402. doi: 10.1016/j.cgh.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 25.NCD Risk Factor Collaboration (NCD-RisC) Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016;387:1377–1396. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palermo M, Serra E, Duza G. N-Sleeve gastrectomy an option for obesity and GERD. ABCD, arq. bras. cir. dig. 2019;32(4):e1482. doi: 10.1590/0102-672020190001e1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peterli R, Wölnerhanssen BK, Peters T. Effect of Laparoscopic Sleeve Gastrectomy vs Laparoscopic Roux-en-Y Gastric Bypass on Weight Loss in Patients With Morbid Obesity The SM-BOSS Randomized Clinical Trial. JAMA. 2018;319(3):255–265. doi: 10.1001/jama.2017.20897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Praveen Raj P, Gomes RM, Kumar S. The effect of surgically induced weight loss on nonalcoholic fatty liver disease in morbidly obese Indians "NASHOST" prospective observational trial. Surg Obes Relat Dis. 2015;11(6):1315–1322. doi: 10.1016/j.soard.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Saadeh S, Younossi ZM, Remer EM. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123(3):745–750. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- 30.Salminen P, Helmio M, Ovaska J. Effect of Laparoscopic Sleeve Gastrectomy vs Laparoscopic Roux-en-Y Gastric Bypass on Weight Loss at 5 year among patients with morbid obesity the Sleevepass randomized clinical trial. JAMA. 2018;319(3):241–254. doi: 10.1001/jama.2017.20313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schauer PR, Bhatt DL, Kirwan JP. Bariatric surgery versus intensive medical therapy for diabetes - 5 year outcomes. N Engl J Med. 2017;376:641–651. doi: 10.1056/NEJMoa1600869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vargas V, Allende H, Lecube A. Surgically induced weight loss by gastric Bypass improves non-alcoholic fatty liver disease in morbid obese patients. World J Hepatol. 2012;4(12):382–388. doi: 10.4254/wjh.v4.i12.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams CD, Stengel J, Asike MI. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy a prospective study. Gastroenterology. 2011;140(1):124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 34.Zilberstein B, Santo M A, Carvalho M H. Critical analysis of surgical treatment techniques of morbid obesity. ABCD, arq. bras. cir. dig. 2019;32(3):e1450. doi: 10.1590/0102-672020190001e1450. [DOI] [PMC free article] [PubMed] [Google Scholar]


