Abstract
Background
Zinc uptake-regulator (Zur)-regulated lipoprotein A (ZrlA) plays a role in bacterial fitness and overcoming antimicrobial exposure in Acinetobacter baumannii. This study further characterized the zrlA gene and its encoded protein and investigated the roles of the zrlA gene in bacterial morphology, antimicrobial susceptibility, and production of outer membrane vesicles (OMVs) in A. baumannii ATCC 17978.
Results
In silico and polymerase chain reaction analyses showed that the zrlA gene was conserved among A. baumannii strains with 97–100% sequence homology. Recombinant ZrlA protein exhibited a specific enzymatic activity of D-alanine-D-alanine carboxypeptidase. Wild-type A. baumannii exhibited more morphological heterogeneity than a ΔzrlA mutant strain during stationary phase. The ΔzrlA mutant strain was more susceptible to gentamicin than the wild-type strain. Sizes and protein profiles of OMVs were similar between the wild-type and ΔzrlA mutant strains, but the ΔzrlA mutant strain produced 9.7 times more OMV particles than the wild-type strain. OMVs from the ΔzrlA mutant were more cytotoxic in cultured epithelial cells than OMVs from the wild-type strain.
Conclusions
The present study demonstrated that A. baumannii ZrlA contributes to bacterial morphogenesis and antimicrobial resistance, but its deletion increases OMV production and OMV-mediated host cell cytotoxicity.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12866-020-02083-0.
Keywords: Acinetobacter baumannii, Zur-regulated gene, ZrlA, Carboxypeptidase, Outer membrane vesicle
Background
Acinetobacter baumannii is a leading cause of nosocomial infections, including ventilator-associated pneumonia, skin and soft tissue infections, urinary tract infections, meningitis, and sepsis, particularly in intensive care units [1, 2]. A. baumannii is a member of the ‘ESKAPE’ pathogens that are potentially drug-resistant bacteria [3]. The World Health Organization listed carbapenem-resistant A. baumannii as the most critical pathogen for development of new therapeutic agents. Like other pathogens, A. baumannii acquires nutrient metals, including iron, zinc (Zn), copper, magnesium, nickel, and manganese, from the host for a variety of biological processes [4–6]. However, hosts can limit the availability of these metals in a process referred to as nutritional immunity [4]. The acquisition of Zn and its utilization are associated with pathogenesis in A. baumannii [7]. The Zn uptake-regulator (Zur) is a conserved repressor that controls the expression of Zur-regulated genes. Mortensen et al. [7] identified 144 genes that were significantly up-regulated or down-regulated in expression in the Δzur::Km mutant compared to that in A. baumannii ATCC 17978 using RNA-sequencing analysis [7]. The A1S_3412 gene encoding Zur-regulated lipoprotein A (ZrlA) is a significantly up-regulated (18.8-fold) during Zn starvation [7]. An ΔzrlA mutant exhibits increased envelope permeability and decreased membrane barrier function, which subsequently increases susceptibility to antimicrobial agents [8]. Moreover, this mutant strain exhibits reduced biofilm formation and surface motility, low adherence to epithelial cells, and low bacterial burden in the bloodstream compared to wild-type [9]. Thus, ZrlA contributes to antimicrobial resistance and pathogenicity in A. baumannii and is a potential target for anti-virulence agents against multidrug-resistant A. baumannii.
All bacterial cells, including gram-positive and gram-negative bacteria, produce extracellular vesicles (EVs) [10–12]. Bacteria-derived EVs are involved in biological processes such as nutrient acquisition, biofilm formation, horizontal gene transfer, and cell to cell communication [10, 13–18]. Also, bacterial EVs contribute to pathogenic events in host-pathogen interactions regarding the delivery of virulence factors and toxins, host cell death, and inflammatory responses [13, 19–21]. Little is known about mechanisms of EV biogenesis for gram-positive bacteria, whereas several models for outer membrane vesicle (OMV) biogenesis in gram-negative bacteria have been proposed, including a reduction in crosslinking between peptidoglycans and the outer membrane [21–24], deacylation of lipopolysaccharides [25], accumulation of phospholipids in the outer leaflet of outer membranes [18, 26], and localized membrane remodeling [27], suggesting that OMVs are likely to be produced by several pathways in gram-negative bacteria. OMV production is stimulated by harsh environments, such as presence of antimicrobial agents, and envelope and oxidative stresses [13, 14, 28]. Further, sequestration of divalent cations such as Mg2+ and Ca2+ increases OMV production [18]. However, the effect of Zn or Zur-regulated genes on OMV biogenesis has not been determined.
In this study, we explored the hypothesis that ZrlA plays a role in bacterial morphogenesis and OMV biogenesis, because ZrlA possesses peptidase activity for peptidoglycan remodeling [8], which may affect the crosslinking between peptidoglycans and outer membranes. Moreover, we further characterized the zrlA gene and its encoded protein, even though ZrlA is known to be a Zn-binding peptidase located in the inner membrane [8].
Results
Characterization of the zrlA gene and its encoded protein
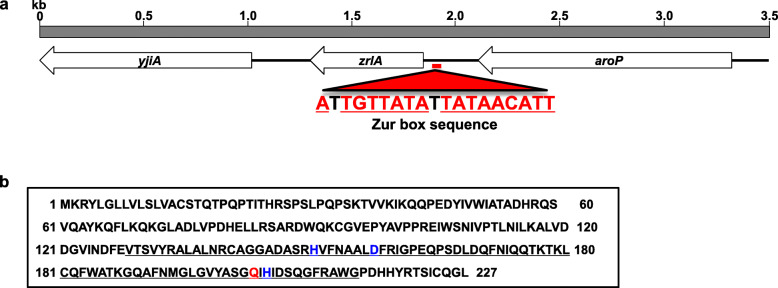
The complete sequence of the zrlA gene and surrounding genes in A. baumannii ATCC 17978 was analyzed (GenBank accession number NZ_CP018664.1). The zrlA gene (A1S_3412) is 684 bp long, and it is predicted to encode 227-amino acid protein. Two adjacent genes, yjiA (A1S_3411) and aroP (A1S_3413), were predicted to encode a putative GTPase and an APC family aromatic amino acid transporter, respectively (Fig. 1a). Sequence analysis showed a palindromic Zur box sequence in the promoter region of the zrlA gene. The zrlA gene was predicted to encode a peptidase of the M15 family (https://www.ebi.ac.uk/merops/), with an 86 residue peptidase domain between amino acids 129–214 (Fig. 1b). In enterococci, VanX is known to be a Zn-dependent D-alanine-D-alanine carboxypeptidase (DD-CPase) with H116, D123, and H184 being Zn-coordinated residues [29]. Three metal ligands (H150, D157, and H204) and an active site residue (Q202) were also present in the peptidase domain of ZrlA. These motifs, HXXXXXXD and QXH, were similar to the motifs HXXXXXXD and WXH found in peptidoglycan hydrolase of Burkholderia pseudomallei phage ST79 [30]. Sequence analysis indicated that the zrlA gene was conserved in all sequenced A. baumannii strains with 97–100% homology (https://blast.ncbi.nlm.nih.gov/). All amino acid variations were located outside of peptidase domains. Moreover, the zrlA gene was amplified in all tested clinical A. baumannii isolates from Korean hospitals, as well as in ATCC 19606T (Supplementary Fig. 1). Next, a recombinant ZrlA protein was expressed in Escherichia coli BL21 (Supplementary Fig. 2), and the enzymatic activity of recombinant ZrlA protein was assessed by the fluorimetric o-phthaldialdehyde (OPTA) method. The recombinant protein specifically cleaved the terminal D-alanine of Nα,Nε-diacetyl-L-Lys-D-Ala-D-Ala, but its enzymatic activity was lower for acetyl-L-Lys-D-Ala-D-Ala and D-Ala-D-Ala (Table 1), suggesting specific DD-CPase activity.
Fig. 1.
Physical map and sequence analysis of the zrlA gene in A. baumannii ATCC 17978. a Arrows indicate coding regions of yjiA, zrlA, and aroP. The figure was generated from the nucleotide sequence of A. baumannii (GenBank Accession No. NZ_CP018664.1). Predicted Zur box sequences are indicated in red. b Analysis of the predicted amino acid sequence of ZrlA using the MEROPS database (https://www.ebi.ac.uk/merops/). The peptidase domain is indicated with solid underlining. The active site (red) and metal ion ligands (blue) are also indicated
Table 1.
DD-CPase activity of the recombinant ZrlA protein
| Substrate | Enzymatic activity of rZrlA protein (U/mg)a |
|---|---|
| D-Ala-D-Ala | 9.6 ± 4.4 |
| Acetyl-L-Lys-D-Ala-D-Ala | 6.5 ± 4.2 |
| Nα,Nε-diacetyl-L-Lys-D-Ala-D-Ala | 34.9 ± 3.8 |
aEnzymatic activity (U/mg protein) of recombinant ZrlA proteins was determined using the fluorimetric o-phthaldialdehyde method. Recombinant ZrlA protein (40 μg) was added to 10 mM solutions of indicated substrates. The results are the mean ± SD. Assays were performed in triplicate
Role of ZrlA in bacterial morphology and antimicrobial susceptibility
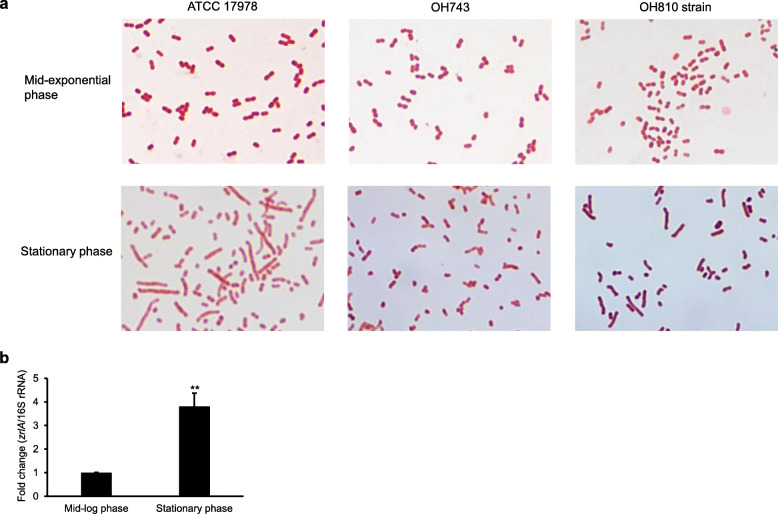
We investigated morphological differences between wild-type and ΔzrlA mutant OH743 strains because DD-CPases contribute to cell separation and peptidoglycan remodeling [31]. The wild-type and ΔzrlA mutant strains appeared as gram-negative coccobacilli at mid-exponential phase and no morphological difference was observed between the wild-type and OH743 strains at mid-exponential phase (Fig. 2a). The wild-type strain showed more morphological heterogeneity than the ΔzrlA mutant strain at stationary phase. Bacterial morphology of the zrlA-complemented OH810 strain was partially restored compared with that of the wild-type strain. Expression of the zrlA gene in A. baumannii ATCC 17978 was higher during stationary phase than during exponential phase (Fig. 2b). Minimum inhibitory concentrations (MICs) of 15 antimicrobial agents for the wild-type, OH743, and OH810 strains were determined to address the effects of ΔzrlA mutation on antimicrobial susceptibility. Gentamicin showed a 4-fold decrease in MICs for the OH743 strain, and colistin, tobramycin, and erythromycin showed a 2-fold decrease in MICs for the OH743 strain (Table 2). MICs for the remaining antimicrobial agents for the OH743 strain were the same or similar (< 2-fold change) to MICs for the wild-type strain. These results suggest that ZrlA contributes to bacterial morphogenesis and moderate resistance to several antimicrobial agents.
Fig. 2.
Morphological characteristics of the ΔzrlA mutant strain. a A. baumannii ATCC 17978, ΔzrlA mutant OH743, and zrlA-complemented OH810 strains were cultured in LB to mid-exponential or stationary phases, and cellular morphology was visualized after Gram staining. Magnification: 1000×. b Transcription levels of zrlA in A. baumannii ATCC 17978 were determined using qPCR. Data are presented as mean ± SD of three independent experiments. ** p < 0.01 comparing the expression of zrlA at mid-log phase
Table 2.
MICs of antimicrobial agents for A. baumannii strains used in this study
| Antimicrobial agent | MIC (μg/ml) | ||
|---|---|---|---|
| ATCC 17978 | OH743 | OH810 | |
| Aztreonama | 24 | 24 | 24 |
| Ceftazidimea | 3 | 3 | 3 |
| Cefoxitinb | 128 | 128 | 128 |
| Imipenema | 0.19 | 0.19 | 0.19 |
| Meropenemb | 0.5 | 0.5 | 0.5 |
| Colistinb | 0.25 | 0.125 | 0.25 |
| Ciprofloxacina | 0.125 | 0.19 | 0.125 |
| Levofloxacinb | 0.125 | 0.125 | 0.125 |
| Nalidixic acida | 4 | 3 | 3 |
| Gentamicina | 0.5 | 0.125 | 0.25 |
| Tobramycinb | 0.5 | 0.25 | 0.5 |
| Tetracyclinea | 1.5 | 1.5 | 1.5 |
| Tigecyclineb | 0.125 | 0.094 | 0.125 |
| Trimethoprima | > 32 | > 32 | > 32 |
| Erythromycinb | 16 | 8 | 16 |
a The MICs were determined by the Etest method
b The MICs were determined by broth microdilution
Role of zrlA in the production of OMVs
Bacteria were cultured in lysogeny broth (LB) and OMVs were isolated from culture supernatants. Sizes of OMVs from the wild-type, OH743, and OH810 strains were 197.8 ± 16.0 nm, 180.9 ± 25.9 nm, and 190.7 ± 18.2 nm, respectively, using nanoparticle tracking analysis (NTA) (Fig. 3a). OMV particles from 1 L culture of the wild-type, OH743, and OH810 strains contained 2.96 × 1012, 2.87 × 1013, and 2.58 × 1012 particles, respectively. The OH743 strain produced more OMV proteins (457.1 ± 10.5 μg/L) than the wild-type strain (52.1 ± 4.6 μg/L) (Fig. 3b). The OH743 strain produced 9.7 times more OMV particles and 8.8 times more OMV proteins than the wild-type strain, but the OH743 strain produced small sizes of OMVs as compared to the wild-type strain. Sodium-dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis showed similar protein profiles among the three OMV samples (Fig. 3c). These results suggest that ZrlA negatively affects OMV production in A. baumannii.
Fig. 3.
OMVs of A. baumannii ATCC 17978, ΔzrlA mutant OH743, and zrlA-complemented OH810 strains a Size and number of OMVs from A. baumannii strains were determined using NTA. Data are representative of three independent experiments with similar results. b Protein concentration of OMVs isolated from 1 L of bacterial culture was measured. Data are presented as mean ± SD of two independent experiments. ** p < 0.01 compared to wild-type ATCC 17978. c OMV proteins were resolved by SDS-PAGE in 12% gels. Lane M, molecular weight marker; 1, A. baumannii ATCC 17978; 2, ΔzrlA mutant OH743; 3, ΔzrlA-complemented OH810
Effect of zrlA on OMV-mediated cytotoxicity in epithelial cells
A549 cells were incubated with A. baumannii OMVs for 24 h and then cell viability was determined using the 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyltetrazolium bromide (MTT) assay. The wild-type strain OMVs triggered cytotoxicity at 5 μg/ml, whereas the ΔzrlA mutant OMVs triggered cytotoxicity at ≤0.625 μg/ml (Fig. 4). Cytotoxicity was significantly different between wild-type and OH743 mutant OMVs at concentrations ≥0.625 μg/ml. These results suggest that the zrlA gene negatively affects host cell cytotoxicity induced by A. baumannii OMVs.
Fig. 4.
Host cell cytotoxicity induced by A. baumannii OMVs. A549 cells were treated with OMVs from A. baumannii strains for 24 h and then cell viability was determined using the MTT assay. Data are presented as mean ± SD of three independent experiments. + p < 0.01 compared to untreated control cells. ** p < 0.01 comparing the same concentration of OMVs from A. baumannii ATCC 17978
Discussion
The zrlA gene is known to encode a Zn-binding DD-CPase located in the inner membrane [8]. Still, its presence and genetic variability among A. baumannii strains are not yet characterized. In silico and polymerase chain reaction (PCR) analyses indicated that the zrlA gene was conserved among A. baumannii strains with high sequence homology. Peptidase motifs typically found in DD-CPases were found in the predicted protein encoded by the zrlA gene. Recombinant ZrlA proteins specifically cleaved the terminal D-alanine of Nα,Nε-diacetyl-L-Lys-D-Ala-D-Ala, a substrate for penicillin-sensitive D-alanine CPase. Zur-regulated zrlA gene is thus conserved in A. baumannii strains, and its encoding protein ZrlA possesses a specific DD-CPase activity.
DD-CPase, a member of the penicillin-binding proteins (PBPs), cleaves the terminal D-alanine from a muramyl pentapeptide. PBPs are classified into high molecular mass (HMM) and low molecular mass (LMM) PBPs based on amino acid sequence, molecular weight, and enzymatic activity [31–33]. HMM PBPs are responsible for polymerization of peptidoglycan and inter-strand crosslinking of adjacent peptidoglycan molecules [31, 34, 35]. HMM PBPs play a role in bacterial cell elongation, maintenance of cell morphology, and cell division. LMM PBPs, including DD-CPases and endopeptidases, contribute to cell separation and peptidoglycan remodeling. LMM PBPs are not essential for bacterial growth. The structure and function of HMM PBPs are well studied, but biological functions of LMM PBPs remain poorly investigated. In limited studies of A. baumannii, a LMM PBP 7/8, a D-alanine-D-alanine endopeptidase, was critical for survival in vitro and in vivo and contributed to serum resistance [36]. A deletion mutant of the PBP 7/8 gene in A. baumannii showed more coccobacillary forms than wild-type. Deletion of PBP7/8 seems to alter the structure of peptidoglycan, which in turn affects cell morphology and survival of A. baumannii both in vitro and in vivo. In the previous study, bacterial growth was not different between wild-type and ΔzrlA mutant strains cultured under shaking and static conditions [9], indicating that the zrlA gene is not essential for bacterial survival and growth. However, in the present study, wild-type A. baumannii grown to stationary phase showed more morphological heterogeneity than the ΔzrlA mutant strain. Morphological difference was not observed between wild-type and ΔzrlA mutant strains at mid-exponential phase, in contrast to a previous study that demonstrated more morphological heterogeneity in wild-type than ΔzrlA mutant strains cultured to mid- to late-exponential phase under Zn-replete conditions [8]. The expression of the zrlA gene was higher in sessile cells than planktonic cells [9]. Moreover, morphological heterogeneity is correlated with expression level of the zrlA gene among growth phases. ZrlA contributes to overcoming sub-MICs of antibiotic exposure in vitro and in vivo [8]. The ΔzrlA mutant exhibits increased envelope permeability. Mutant cells show increased susceptibility to antibiotics, including carbenicillin, vancomycin, tetracycline, and polymyxins B, and detergents, including SDS and ethylenediaminetetraacetic acid, in vitro compared to the wild-type strain [8]. The present study also showed that the ΔzrlA mutant was more susceptible to gentamicin, colistin, tobramycin, and erythromycin than the wild-type strain, but MICs were not greatly different between the two strains. Our results suggest that ZrlA is an LMM PBP possessing a specific DD-CPase activity involved in morphological plasticity and moderate antimicrobial resistance.
The production of OMVs increases in response to nutrient restriction and exposure to antibiotics or chemicals [13, 14, 28]. A reduction in crosslinking between the outer membrane and peptidoglycan also increases OMV production [21–24]. OmpA via the C-terminal OmpA-like domain interacts with diaminopimelate of peptidoglycans [37] and a ΔompA mutant of A. baumannii produced more OMVs than the wild-type strain [38]. In E. coli, peptidoglycans are covalently crosslinked to the outer membrane via short outer membrane-anchored lipoprotein Lpp. Levels of Lpp crosslinking to peptidoglycan negatively correlate with OMV production [23, 39]. Spr is a murein DD-endopeptidase located in the outer membrane of E. coli. Δspr mutants inhibit peptidoglycan turnover and other PBPs, such as PBP4, induce compensatory increases in peptidoglycan synthesis. This increase of peptidoglycans reduces the ability to form sufficient Lpp-outer membrane crosslinks [40]. This mutation may result in four times more OMV production in Δspr mutant than wild-type. The equilibrium between peptidoglycan synthesis and degradation may affect OMV production by altering the numbers of covalently crosslinked Lpp to peptidoglycan. In the present study, the ΔzrlA mutant produced 9.7-fold more OMV particles than the wild-type strain. However, sizes of OMVs from the ΔzrlA mutant were smaller than those from the wild-type strain. Regarding the number of OMV particles and their protein concentrations, each OMV particle from the ΔzrlA mutant strain may carry slightly less proteins than OMVs from the wild-type strain. ZrlA in A. baumannii displays peptidase activity like Spr in E. coli. Hence, the ΔzrlA mutant may inhibit peptidoglycan remodeling and decrease interactions between outer membrane proteins and peptidoglycans, resulting in increased envelope permeability and hyperproduction of OMVs.
Stress-inducing conditions alter both production and molecular composition of OMVs [28, 38]. Alterations in lipopolysaccharides, proteins, peptidoglycans, and pathogen-associated molecular patterns of OMVs likely trigger different host cell responses. OMVs from a ΔbfmS mutant were more cytotoxic in A549 cells than OMVs from the wild-type strain [41], yet OMVs from the ΔompA mutant were less cytotoxic than OMVs from the wild-type strain [19]. The present study showed that OMVs from the ΔzrlA mutant were more cytotoxic in A549 cells than the wild-type strain. OmpA was identified as a cytotoxic factor packaged in A. baumannii OMVs [19]. SDS-PAGE analysis of OMVs revealed that protein profiles were similar between the wild-type and ΔzrlA mutant strains. Even though SDS-PAGE images show similar profiles, differences in protein content between OMVs from the wild-type and ΔzrlA mutant strains cannot be excluded. SDS-PAGE analysis is less appropriate than two-dimensional gel electrophoresis. This study did not identify difference in protein content and cytotoxic factors in the ΔzrlA mutant OMVs, an issue that should be investigated in further studies.
Conclusions
The present study demonstrates the interplay between ZrlA, peptidoglycan dynamics, bacterial morphogenesis, and OMV production in A. baumannii. ZrlA contributes to overcoming antibiotic exposure and augments pathogenicity of A. baumannii both in vitro and in vivo [7–9]. The zrlA gene or its protein is a possible therapeutic target for treating A. baumannii infection. However, deletion of the zrlA gene increased the OMV production in A. baumannii. Moreover, OMVs produced by the ΔzrlA mutant were more cytotoxic to epithelial cells than OMVs from the wild-type strain. These observations provide opposing perspectives of ZrlA for anti-virulence strategies against A. baumannii [42].
Methods
Bacterial strains
A. baumannii ATCC 17978, ΔzrlA mutant OH743 strain (ATCC 17978 with ΔzrlA), and zrlA-complemented OH810 strain (OH743 with zrlA in chromosome) were used in this study [9]. Ten clinical A. baumannii isolates were obtained from the Kyungpook National University Hospital Culture Collection for Pathogens (KNUH-CCP). E. coli BL21 (DE3) star cells were used for production of recombinant ZrlA proteins. Bacteria were cultured in LB or blood agar plates at 37 °C.
Detection and cloning of the zrlA gene and recombinant protein production
PCR was performed to detect the zrlA gene using primers A1 (5′-GCT TTT ATA GTC CCT GAC A-3′) and A2 (5′-CTG TGG TTA AAA TCA AAC AA-3′). Genomic DNA was purified from A. baumannii strains using the SolGent™ Genomic DNA prep kit (SolGent, Daejeon, Korea). The full-length zrlA gene was amplified using primers C1 (5′- GGG CGG CGG TGG TGG CGG CAT GAA GCG TTA TTT AGG TTT A-3′) and C2 (5′- GTT CTT CTC CTT TGC GCC CTA TAG TCC CTG ACA AAT TGA GG-3′), designed for ligation-independent cloning [43]. A. baumannii ATCC 17978 genomic DNA was used as the PCR template. PCR products were treated with T4 DNA polymerase (New England Biolabs, Ipswich, MA) and inserted into the ligation-independent cloning expression vector pB4, a derivative of pET21a (Novagen, Madison, WI) [44]. DNA fragments and plasmid DNA were purified using the AccuPrep Gel Purification Kit (Bioneer, Daejeon, Korea) and the AccuPrep® Plasmid Extraction Kit (Bioneer), respectively. Plasmid construct pB4:zrlA was transformed into E. coli BL21 (DE3) star cells. ZrlA protein was purified using sequential chromatographic steps as previously described [45].
DD-CPase assays of recombinant ZrlA protein
Enzyme activity of recombinant ZrlA protein was assessed by measuring the release of D-Ala from Nα,Nε-diacetyl-L-Lys-D-Ala-D-Ala, acetyl-L-Lys-D-Ala-D-Ala, and D-Ala-D-Ala (Sigma-Aldrich, St. Louis, MO) using the OPTA method as previously described [46, 47]. Fluorescence intensity (λex = 340 nm: λem = 455 nm) was measured using a fluorescence microplate reader (Tecan Spark 10 M, Austria). Enzymatic activity was quantified using a standard curve with D-Ala. One unit of DD-CPase activity was defined as the amount of enzyme that produced 1 μM of D-Ala per min [46]. Assays were performed in triplicate.
Gram staining of bacteria
Bacteria were cultured in LB with shaking to optical density at 600 nm (OD600) of 1.2 (mid-exponential phase) or 1.8 (stationary phase). Bacteria were washed with phosphate-buffered saline (PBS). After centrifugation, bacterial pellets were stained with Gram’s reagents.
RNA isolation and quantitative real time PCR
Bacteria were cultured in LB with shaking to OD600 of 1.2 and 1.8 for mid-exponential and stationary phases, respectively. Total RNA was extracted using a RNeasy Mini Kit (Qiagen, Valencia, CA). cDNA was synthesized by reverse transcription with 1.5 μg of total RNA using random hexamer primer and RevertAid reverse transcriptase in a total reaction volume of 20 μl (Thermo Fisher Scientific, Waltham, MA). Specific primers for the zrlA gene, 5′-CCC AGC CGA CGA TTA CTC AT-3′ and 5′-GCG ATC CAA ACG ACA TAA TCT TC-3′, and 16S rRNA gene, 5′-GCA CAA GCG GTG GAG CAT-3′ and 5′-CGA AGG CAC CAA TCC ATC TC-3′, were used. Gene transcripts was quantified using a Thermal Cycler Dice TP850 (Takara Bio, Shiga, Japan) with SYBR Premix Ex Taq (Takara Bio) following the manufacturer’s instructions. Amplification specificity was evaluated using melting curve analysis. Gene expression was normalized to 16S rRNA expression in each sample, and fold change was analyzed using the ΔΔCt method.
Antimicrobial susceptibility test
The MICs of aztreonam, ceftazidime, nalidixic acid, ciprofloxacin, gentamicin, imipenem, tetracycline, and trimethoprim were determined by the Etest method following the manufacturer’s instructions (bioMe’rieux, Marcy-l’_Etoile, France). MICs of colistin, tigecycline, cefoxitin, meropenem, levofloxacin, erythromycin, and tobramycin were determined by broth microdilution following guidelines of the Clinical Laboratory Standards Institute (CLSI) [48]. E. coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 were used as quality control strains.
Isolation of OMVs
The OMVs derived from A. baumannii were purified from culture supernatants as previously described [19, 28]. In brief, bacteria were cultured in LB with shaking to reach OD600 of 1.5. Bacterial cells were removed by centrifugation at 8000 g for 20 min and then culture supernatants were filtered with a 0.22 μm membrane. The samples were concentrated using a QuixStand Benchtop System (GE Healthcare, Amersham, UK) with a 500 kDa hollow fiber membrane (GE Healthcare). OMVs were isolated by ultracentrifugation at 150,000 g at 4 °C for 3 h. OMVs were resuspended in PBS and protein contents were measured with a modified bicinchoninic acid (BCA) assay (Thermo Fisher Scientific). OMV proteins were separated on 12% SDS-PAGE gel and stained with Coomassie brilliant blue R-250 (Bio-Rad, Hercules, CA). The sterility of OMV samples were checked by streaking on blood agar plates.
Nanoparticle tracking analysis
Size and number of OMVs were measured by a NanoSight NS500 instrument with 488 nm laser and sCMOS camera modules (Malvern Instruments, Worcestershire, UK) [49]. Captured data were analyzed using NTA 3.1 software build 3.1.46. Experiments were performed in triplicate.
Cell culture and cell viability test
A549 cells derived from human lung carcinoma were obtained from the Korean Cell Line Bank (Seoul, Korea). Cells were grown in RPMI 1640 medium (HyClone, Logan, UT) supplemented with 10% heat inactivated fetal bovine serum (HyClone), 2.0 mM L-glutamine, and 100 U/ml penicillin at 37 °C in a 5% CO2. Cells were seeded at a concentration of 2 × 104/well in a 96-well microplate. Cell viability was measured using an MTT assay (Sigma-Aldrich). A549 cells were treated with A. baumannii OMVs for 24 h and then viability of cells was determined at 600 nm 3 h after treatment with MTT reagent. Assays were performed in triplicate in three independent experiments.
Statistical analysis
All data are presented as mean ± standard deviation (SD). Data were analyzed using R 3.6.3 (https://www.r-project.org/). The statistical significance of difference was calculated using nonparametric one-way ANOVA with Dunnett’s post hoc analysis or Student’s t-test. P values of < 0.05 were considered statistically significant.
Supplementary Information
Additional file 1: Table S1. Supplementary table shows the raw data in the manuscript.
Additional file 2: Figure S1. PCR amplification of the zrlA gene in A. baumannii strains. Amplicons of 579 bp were detected in all A. baumannii strains tested. Figure S2. Production of recombinant ZrlA proteins. SDS-PAGE was performed to detect recombinant proteins of ca. 24 kDa (arrow).
Acknowledgments
The abstract of this paper was presented at the e-Conference of 2020 Annual Meeting of the Microbiological Society of Korea, October 7–8, 2020, Gunsan, Korea, as a poster presentation: http://www.msk.or.kr/msk/2020/2020poster.pdf
Abbreviations
- EVs
Extracellular vesicles
- OMV
Outer membrane vesicle
- Zn
Zinc
- Zur
Zn uptake-regulator
- ZrlA
Zur-regulated lipoprotein A
- DD-Cpases
D-alanine-D-alanine carboxypeptidases
- OPTA
Fluorimetric o-phthaldialdehyde
- MICs
Minimum inhibitory concentrations
- NTA
Nanoparticle tracking analysis
- PBPs
Penicillin-binding proteins
- HMM
High molecular mass
- LMM
Low molecular mass
Authors’ contributions
Conceived and designed the experiments: MHO, SIK, MS, YCL, JCL; Performed the experiments: NK, HJK, SYK, MHK, JHS; Analyzed the data: MHO, SIK, MS, YCL, JCL; Wrote the paper: NK, HJK, MHO, JCL. All authors read and approved the final manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (NRF-2020R1A2B5B01002228). The funding agency had no role in the design of the study, collection, analysis or interpretation of data, or in the writing of the manuscript.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Nayeong Kim and Hyo Jeong Kim contributed equally to this work.
References
- 1.Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008;21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dijkshoorn L, Nemec A, Seifert H. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol. 2007;5:939–951. doi: 10.1038/nrmicro1789. [DOI] [PubMed] [Google Scholar]
- 3.Pendleton JN, Gorman SP, Gilmore BF. Clinical relevance of the ESKAPE pathogens. Expert Rev Anti-Infect Ther. 2013;11:297–308. doi: 10.1586/eri.13.12. [DOI] [PubMed] [Google Scholar]
- 4.Hood MI, Skaar EP. Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol. 2012;10:525–537. doi: 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juttukonda LJ, Chazin WJ, Skaar EP. Acinetobacter baumannii coordinates urea metabolism with metal import to resist host-mediated metal limitation. mBio. 2016;7:e01475–e01416. doi: 10.1128/mBio.01475-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mortensen BL, Skaar EP. The contribution of nutrient metal acquisition and metabolism to Acinetobacter baumannii survival within the host. Front Cell Infect Microbiol. 2013;3:95. doi: 10.3389/fcimb.2013.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mortensen BL, Rathi S, Chazin WJ, Skaar EP. Acinetobacter baumannii response to host-mediated zinc limitation requires the transcriptional regulator Zur. J Bacteriol. 2014;196:2616–2626. doi: 10.1128/JB.01650-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lonergan ZR, Nairn BL, Wang J, Hsu YP, Hesse LE, Beavers WN, et al. An Acinetobacter baumannii, zinc-regulated peptidase maintains cell wall integrity during immune-mediated nutrient sequestration. Cell Rep. 2019;26:2009–2018. doi: 10.1016/j.celrep.2019.01.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee EK, Choi CH, Oh MH. Zur-regulated lipoprotein a contributes to the fitness of Acinetobacter baumannii. J Microbiol. 2020;58:67–77. doi: 10.1007/s12275-020-9531-7. [DOI] [PubMed] [Google Scholar]
- 10.Kim JH, Lee J, Park J, Gho YS. Gram-negative and gram-positive bacterial extracellular vesicles. Semin Cell Dev Biol. 2015;40:97–104. doi: 10.1016/j.semcdb.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Lee EY, Choi DY, Kim DK, Kim JW, Park JO, Kim S, et al. Gram-positive bacteria produce membrane vesicles: proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics. 2009;9:5425–5436. doi: 10.1002/pmic.200900338. [DOI] [PubMed] [Google Scholar]
- 12.Knox KW, Vesk M, Work E. Relation between excreted lipopolysaccharide complexes and surface structures of a lysine-limited culture of Escherichia coli. J Bacteriol. 1966;92:1206–1217. doi: 10.1128/JB.92.4.1206-1217.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacDonald IA, Kuehn MJ. Offense and defense: microbial membrane vesicles play both ways. Res Microbiol. 2012;163:607–618. doi: 10.1016/j.resmic.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kulp A, Kuehn MJ. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu Rev Microbiol. 2010;64:163–184. doi: 10.1146/annurev.micro.091208.073413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schooling SR, Beveridge TJ. Membrane vesicles: an overlooked component of the matrices of biofilms. J Bacteriol. 2006;188:5945–5957. doi: 10.1128/JB.00257-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nevot M, Deroncele V, Messner P, Guinea J, Mercade E. Characterization of outer membrane vesicles released by the psychrotolerant bacterium Pseudoalteromonas antarctica NF3. Environ Microbiol. 2006;8:1523–1533. doi: 10.1111/j.1462-2920.2006.01043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Domingues S, Nielsen KM. Membrane vesicles and horizontal gene transfer in prokaryotes. Curr Opin Microbiol. 2017;38:16–21. doi: 10.1016/j.mib.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 18.Mashburn LM, Whiteley M. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature. 2005;437:422–425. doi: 10.1038/nature03925. [DOI] [PubMed] [Google Scholar]
- 19.Jin JS, Kwon SO, Moon DC, Gurung M, Lee JH, Kim SI, et al. Acinetobacter baumannii secretes cytotoxic outer membrane protein a via outer membrane vesicles. PLoS One. 2011;6:e17027. doi: 10.1371/journal.pone.0017027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jun SH, Lee JH, Kim BR, Kim SI, Park TI, Lee JC, et al. Acinetobacter baumannii outer membrane vesicles elicit a potent innate immune response via membrane proteins. PLoS One. 2013;8:e71751. doi: 10.1371/journal.pone.0071751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jan AT. Outer membrane vesicles (OMVs) of gram-negative bacteria: a perspective update. Front Microbiol. 2017;8:1053. doi: 10.3389/fmicb.2017.01053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burdett ID, Murray RG. Electron microscope study of septum formation in Escherichia coli strains B and B/r during synchronous growth. J Bacteriol. 1974;119:1039–1056. doi: 10.1128/JB.119.3.1039-1056.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cascales E, Bernadac A, Gavioli M, Lazzaroni JC, Lloubes R. Pal lipoprotein of Escherichia coli plays a major role in outer membrane integrity. J Bacteriol. 2002;184:754–759. doi: 10.1128/JB.184.3.754-759.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rolhion N, Barnich N, Claret L, Darfeuille-Michaud A. Strong decrease in invasive ability and outer membrane vesicle release in Crohn’s disease-associated adherent-invasive Escherichia coli strain LF82 with the yfgL gene deleted. J Bacteriol. 2005;187:2286–2296. doi: 10.1128/JB.187.7.2286-2296.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elhenawy W, Bording-Jorgensen M, Valguarnera E, Haurat MF, Wine E, Feldman MF. LPS remodeling triggers formation of outer membrane vesicles in Salmonella. mBio. 2016;7:e00940–e00916. doi: 10.1128/mBio.00940-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roier S, Zingl FG, Cakar F, Durakovic S, Kohl P, Eichmann TO et al. A novel mechanism for the biogenesis of outer membrane vesicles in gram-negative bacteria. Nat Commun. 2016;7:10515. doi: 10.1038/ncomms10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schertzer JW, Whiteley M. A bilayer-couple model of bacterial outer membrane vesicle biogenesis. mBio. 2012;3:e00297–e00211. doi: 10.1128/mBio.00297-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yun SH, Park EC, Lee SY, Lee H, Choi CW, Yi YS, et al. Antibiotic treatment modulates protein components of cytotoxic outer membrane vesicles of multidrug-resistant clinical strain, Acinetobacter baumannii DU202. Clin Proteomics. 2018;15:28. doi: 10.1186/s12014-018-9204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCafferty DG, Lessard IAD, Walsh CT. Mutational analysis of potential zinc-binding residues in the active site of the enterococcal D-Ala-D-Ala dipeptidase VanX. Biochemistry. 1997;36:10498–10505. doi: 10.1021/bi970543u. [DOI] [PubMed] [Google Scholar]
- 30.Khakhum N, Yordpratum U, Boonmee A, Tattawasart U, Rodrigues JLM, Sermswan RW. Cloning, expression, and characterization of a peptidoglycan hydrolase from the Burkholderia pseudomallei phage ST79. AMB Express. 2016;6:77. doi: 10.1186/s13568-016-0251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sauvage E, Kerff F, Terrak M, Ayala JA, Charlier P. The penicillin binding proteins: structure and role in peptidoglycan biosynthesis. FEMS Microbiol Rev. 2008;32:234–258. doi: 10.1111/j.1574-6976.2008.00105.x. [DOI] [PubMed] [Google Scholar]
- 32.Pratt RF. Substrate specificity of bacterial DD-peptidases (penicillin-binding proteins) Cell Mol Life Sci. 2008;65:2138–2155. doi: 10.1007/s00018-008-7591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogawara H. Penicillin-binding proteins in Actinobacteria. J Antibiot. 2015;68:223–245. doi: 10.1038/ja.2014.148. [DOI] [PubMed] [Google Scholar]
- 34.Macheboeuf P, Contreras-Martel C, Job V, Dideberg O, Dessen A. Penicillin binding proteins: key players in bacterial cell cycle and drug resistance processes. FEMS Microbiol Rev. 2006;30:673–691. doi: 10.1111/j.1574-6976.2006.00024.x. [DOI] [PubMed] [Google Scholar]
- 35.Vollmer W, Joris B, Charlier P, Foster S. Bacterial peptidoglycan (murein) hydrolases. FEMS Microbiol Rev. 2008;32:259286. doi: 10.1111/j.1574-6976.2007.00099.x. [DOI] [PubMed] [Google Scholar]
- 36.Russo TA, MacDonald U, Beanan JM, Olson R, MacDonald IJ, Sauberan SL, et al. Penicillin-binding protein 7/8 contributes to the survival of Acinetobacter baumannii in vitro and in vivo. J Infect Dis. 2009;199:513–521. doi: 10.1086/596317. [DOI] [PubMed] [Google Scholar]
- 37.Park JS, Lee WC, Yeo KJ, Ryu KS, Kumarasiri M, Hesek D, et al. Mechanism of anchoring of OmpA protein to the cell wall peptidoglycan of the gram-negative bacterial outer membrane. FASEB J. 2012;26:219–228. doi: 10.1096/fj.11-188425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moon DC, Choi CH, Lee JH, Choi CW, Kim HY, Park JS, et al. Acinetobacter baumannii outer membrane protein a modulates the biogenesis of outer membrane vesicles. J Microbiol. 2012;50:155–160. doi: 10.1007/s12275-012-1589-4. [DOI] [PubMed] [Google Scholar]
- 39.Bernadac A, Gavioli M, Lazzaroni JC, Raina S, Lloubes R. Escherichia coli tol-pal mutants form outer membrane vesicles. J Bacteriol. 1998;180:4872–4878. doi: 10.1128/JB.180.18.4872-4878.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwechheimer C, Rodriguez DL, Kuehn MJ. NlpI-mediated modulation of outer membrane vesicle production through peptidoglycan dynamics in Escherichia coli. MicrobiologyOpen. 2015;4:375–389. doi: 10.1002/mbo3.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim SY, Kim MH, Kim SI, Son JH, Kim S, Lee YC, et al. The sensor kinase BfmS controls production of outer membrane vesicles in Acinetobacter baumannii. BMC Microbiol. 2019;19:301. doi: 10.1186/s12866-019-1679-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim HJ, Kim N, Jun SH, Ryu JH, Oh MH, Kim SI, et al. Contribution of Zur-regulated lipoprotein A to bacterial morphogenesis and production of outer membrane vesicles in Acinetobacter baumannii. 2020. [Google Scholar]
- 43.Aslanidis C, de Jong PJ. Ligation-independent cloning of PCR products (LIC-PCR) Nucleic Acids Res. 1990;18:6069–6074. doi: 10.1093/nar/18.20.6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun J, Jeon JH, Shin M, Shin HC, Oh BH, Kim JS. Crystal structure and CRISPR RNA-binding site of the Cmr1 subunit of the Cmr interference complex. Acta Crystallogr D Biol Crystallogr. 2014;70(Pt 2):535–543. doi: 10.1107/S1399004713030290. [DOI] [PubMed] [Google Scholar]
- 45.Kim BN, Shin M, Ha SC, Park SY, Seo PW, Hofmann A, et al. Crystal structure of an ASCH protein from Zymomonas mobilis and its ribonuclease activity specific for single-stranded RNA. Sci Rep. 2017;26:12303. doi: 10.1038/s41598-017-12186-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wright GD, Molinas C, Arthur M, Courvalin P, Walsh CT. Characterization of vanY, a D,D-carboxypeptidase from vancomycin-resistant Enterococcus faecium BM4147. Antimicrob Agents Chemother. 1992;36:1514–1518. doi: 10.1128/AAC.36.7.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Binda E, Marcone GL, Berini F, Pollegioni L, Marinelli F. Streptomyces spp. as efficient expression system for a D,D-peptidase/D,D-carboxypeptidase involved in glycopeptide antibiotic resistance. BMC Biotechnol. 2013;13:24. doi: 10.1186/1472-6750-13-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing: Twenty-seventh Informational Supplement M100-S28. Wayne: CLSI; 2018. [Google Scholar]
- 49.Gerritzen MJH, Martens DE, Wijffels RH, Stork M. High throughput nanoparticle tracking analysis for monitoring outer membrane vesicle production. J Extracell Vesicles. 2017;6:1333883. doi: 10.1080/20013078.2017.1333883. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Supplementary table shows the raw data in the manuscript.
Additional file 2: Figure S1. PCR amplification of the zrlA gene in A. baumannii strains. Amplicons of 579 bp were detected in all A. baumannii strains tested. Figure S2. Production of recombinant ZrlA proteins. SDS-PAGE was performed to detect recombinant proteins of ca. 24 kDa (arrow).
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.