Dear Editor,
One third of patients with severe brain injury develop lung complication that affect their prognosis. Prone positioning (PP) improves the outcome of patients with an acute respiratory distress syndrome (ARDS) [1], but its effect on patients with acute brain injury is still debated. While it improves oxygenation, the impact of PP on intracranial pressure (ICP) remains controversial: PP has been reported not to affect ICP [2] and conversely to increase ICP, thereby worsening brain injuries [3]. There is currently no consensus on criteria to identify patients who will safely benefit from PP [4]. Therefore, the aim of the present study was to evaluate the safety and efficacy of PP in patients with acute brain injury and moderate-to-severe ARDS.
A retrospective analysis in three French intensive care units was conducted. A query on digital medical records identified 27 patients with an ICP, moderate-to-severe ARDS (according to the Berlin criteria) and PP. Data were collected before and during the first PP. Patients who had at least one ICP measurement > 25 mmHg were considered as having intracranial hypertension (IH) as it is associated with a poor prognosis and used to consider a decompressive craniectomy [5].
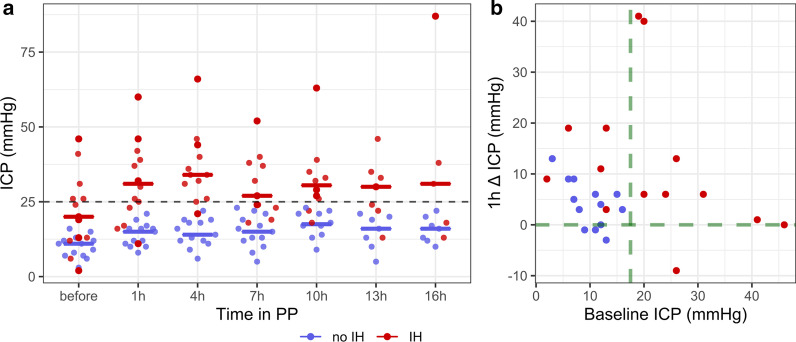
A total of 10 (37.0%) patients had traumatic brain injuries, 11 (40.7%) subarachnoid haemorrhage, and 7 (25.9%) haemorrhagic stroke (Table1). During PP, the median [IQR] PaO2/FiO2 increased significantly from 100 [89–126] before to 216 [171–257] after PP (Wilcoxon test, p < 0.001) and remained significantly higher back to supine position (146 [122–186], Wilcoxon test, p = 0.002). IH occurred in 14 (51.8%) patients. They had a significantly higher median [IQR] ICP before PP onset (20 [13–26] mmHg) compared to patients without IH (11 [7–12] mmHg, Mann–Whitney test, p = 0.005) and a greater ICP increase during PP (+ 19 mmHg [13–20] vs + 6 mmHg [3–8], Mann–Whitney test, p = 0.025), suggestive of a poorer brain compliance. PP was discontinued due to a sustain ICP increase in 5 patients (Fig. 1a).
Table 1.
Population characteristics
| Total population (n = 27) | IH (n = 14) | No-IH (n = 13) | p* | |
|---|---|---|---|---|
| Age, median [IQR] | 46 [36–55] | 46 [36–50] | 46 [37–56] | 0.981 |
| Female, n (%) | 5 (18.5%) | 2 (14.3%) | 3 (23.1%) | 0.648 |
| BMI, median [IQR] | 26 [23–31] | 26 [22–30] | 26 [23–33] | 0.601 |
| Traumatic brain injury, n (%) | 10 (37.0%) | 6 (42.9%) | 4 (30.8%) | 0.694 |
| Subarachnoid haemorrhage, n (%) | 11 (40.7%) | 5 (35.7%) | 6 (46.1%) | 0.703 |
| Haemorrhagic stroke, n (%) | 7 (25.9%) | 4 (28.6%) | 3 (23.1%) | 1.000 |
| Ischemic stroke, n (%) | 1 (3.7%) | 0 (0%) | 1 (7.7%) | 0.481 |
| Aspiration pneumonia, n (%) | 13 (48.1%) | 7 (50.0%) | 6 (46.1%) | 1.000 |
| Ventilator-associated pneumonia, n (%) | 14 (51.8%) | 7 (50.0%) | 7 (53.8%) | 1.000 |
| Severity | ||||
| SAPSII, median [IQR] | 42 [34–53] | 47 [41–55] | 39 [30–46] | 0.076 |
| Glasgow Coma Scale at intubation, median [IQR] | 6 [4–8] | 6 [4–8] | 6 [4–7] | 0.769 |
| First ICP measure, median [IQR] | 22 [12–29] | 24 [19–37] | 16 [8–26] | 0.274 |
| IH treatment | ||||
| Craniectomy, n (%) | 3 (11.1%) | 1 (7.1%) | 2 (15.4%) | 0.595 |
| Hypothermia, n (%) | 5 (18.5%) | 3 (21.4%) | 2 (15.4%) | 1.000 |
| Osmotherapy, n (%) | 11 (40.7%) | 7 (50,0%) | 4 (30.8%) | 0.440 |
| Thiopental administration, n (%) | 10 (37.0%) | 6 (42.9%) | 4 (30.8%) | 0.694 |
| At least one of IH treatment, n (%) | 14 (51.8%) | 8 (57.1%) | 6 (46.1%) | 0.706 |
| EVD, n (%) | 14 (51.8%) | 7 (50.0%) | 7 (53.9%) | 1.000 |
| ARDS treatment | ||||
| Neuromuscular blockade, n (%) | 27 (100%) | 14 (100%) | 13 (100%) | |
| PP number, median [IQR] | 1 [1–2] | 1 [1–2] | 1 [1–3] | 0.295 |
| Duration of PP (hours), median [IQR] | 14 [9–19] | 13 [8–17] | 16 [11–20] | 0.305 |
| First PP delay, median days [IQR] | 5 [4–7] | 6 [5–7] | 5 [4–6] | 0.279 |
| Tidal volume mL/kg, median [IQR] | 6.8 [6.4–7.5] | 6.7 [6.4–7.5] | 6.9 [6.4–7.5] | 0.843 |
| Parameters before PP | ||||
| Initial ICP (mmHg), median [IQR] | 13 [8–20] | 20 [13–26] | 11 [7–12] | 0.005 |
| Initial CPP (mmHg), median [IQR] | 75 [66–82] | 67 [64–75] | 79 [77–87] | 0.041 |
| Initial PEEP (cmH20), median [IQR] | 10 [9–12] | 10 [9–11] | 10 [9–12] | 0.657 |
| Initial FiO2, median (%) [IQR] | 80 [60–89] | 80 [71–100] | 67 [60–81] | 0.231 |
| Initial plateau pressure (cmH20), median [IQR] | 23 [21–27] | 23 [21–29] | 23 [21–26] | 0.689 |
| Initial PaO2/FiO2, median [IQR] | 100 [89–126] | 99 [88–113] | 109 [93–142] | 0.481 |
| Initial PaO2 (mmHg), median [IQR] | 78 [74–95] | 78 [74–90] | 77 [74–99] | 0.903 |
| Initial PaCO2 (mmHg), median [IQR] | 43 [37–47] | 43 [38–46] | 44 [36–48] | 0.884 |
| Outcome | ||||
| Mechanical ventilation duration (days), median [IQR] | 23 [11–36] | 22 [7–35] | 23 [16–37] | 0.395 |
| Modified Rankin Scale at ICU discharge, median [IQR] | 4 [4–5] | 4 [4–6] | 4 [4–5] | 0.853 |
| Mortality, n (%) | 7 (25.9%) | 4 (28.6%) | 3 (23.1%) | 1.000 |
BMI Body mass index, CPP cerebral perfusion pressure, EVD external ventricular drainage, FiO2 inspiratory fraction of oxygen, ICP intracranial pressure, ICU intensive care unit, IH intracranial hypertension, PEEP positive end-expiratory pressure, PP prone positioning, SAPSII Simplified Acute Physiology Score 2
*p value IH group versus no-IH group (using the Mann–Whitney or the Fisher’s test)
Fig. 1.
Intracranial pressure changes during prone positioning. Each point represents a patient, in blue patients who did not experience intracranial hypertension (IH) during prone positioning (PP) and in red patients who did. a ICP changes over the PP session; solid lines indicate the median intracranial pressure (ICP) values of each group. b ICP changes 1 h after the PP onset according to the initial ICP before PP; when the initial ICP was below 17.5 mmHg and the ICP did not increase PP took place without IH (green lines), whereas an ICP above 17.5 mmHg or an ICP elevation over 10 mmHg were predictive of IH
All patients with an ICP > 17.5 mmHg prior to PP had an IH. Among patients with an ICP < 17.5 mmHg before PP onset, 13/18 (72%) had a safe PP session without IH. Rather than a single threshold of ICP changes, a grey zone approach was used to predict (i.e. sensitivity, Se > 90%) or exclude (i.e. specificity, Sp > 90%) a safe PP. The absence of ICP increase 1 h after the PP onset is suggestive of a preserved brain compliance and predicted a safe procedure (Sp = 93%), while an ICP elevation > 10 mmHg predicted the occurrence of IH (Sp = 93%). When the initial ICP was < 17.5 mmHg and did not increase 1 h after PP onset the manoeuvre took place without IH (Fig. 1b). Brain oxygen partial pressure was available for 4 patients and rose from 20.5 [18.8–23.5] mmHg to 28 [22–31] mmHg during PP.
The main limitations of this study are due to its retrospective design. The modalities for performing manually PP were not available although it can influence its tolerance [6]. In addition, the data collected during PP from the ICU software were sampled hourly at a specific time and may not reflect the average of the hour. Only 4 patients had an intracranial oxygenation probe improved during PP and suggested a preserved cerebral blood flow despite the ICP increase. Finally, the management of IH was not subject to protocol.
To conclude, we would argue for assessing the brain compliance before PP (e.g. transcranial Doppler), ICP, and the tolerance to an obstacle to venous return. Moreover, ICP changes within 1 h after PP onset could be useful to choose to pursue PP or not, as well as cerebral multimodal monitoring to evaluate PP tolerance. This strategy needs to be evaluated in a prospective clinical trial.
Acknowledgements
Not applicable.
Authors’ contributions
BP, LAC, DF and MS conceived and design the study. BP, MS and DG collected the data. BB, BP and LAC analysed and interpreted the data. BB and BP draft the article. All authors critically revised the article. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The datasets used during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the French Intensive Care Society (no IRB 00010254–2018–064).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Guérin C, Reignier J, Richard J-C, Beuret P, Gacouin A, Boulain T, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 2.Thelandersson A, Cider Å, Nellgård B. Prone position in mechanically ventilated patients with reduced intracranial compliance. Acta Anaesth Scand. 2006;50:937–941. doi: 10.1111/j.1399-6576.2006.01037.x. [DOI] [PubMed] [Google Scholar]
- 3.Roth C, Ferbert A, Deinsberger W, Kleffmann J, Kästner S, Godau J, et al. Does prone positioning increase intracranial pressure? A retrospective analysis of patients with acute brain injury and acute respiratory failure. Neurocrit Care. 2014;21:186–191. doi: 10.1007/s12028-014-0004-x. [DOI] [PubMed] [Google Scholar]
- 4.Robba C, Poole D, McNett M, Asehnoune K, Bösel J, Bruder N, et al. Mechanical ventilation in patients with acute brain injury: recommendations of the European Society of Intensive Care Medicine consensus. Intens Care Med. 2020;46:1–14. doi: 10.1007/s00134-020-06283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hutchinson PJ, Kolias AG, Timofeev IS, Corteen EA, Czosnyka M, Timothy J, et al. Trial of decompressive craniectomy for traumatic intracranial hypertension. N Engl J Med. 2016;375:1119–1130. doi: 10.1056/NEJMoa1605215. [DOI] [PubMed] [Google Scholar]
- 6.Højlund J, Sandmand M, Sonne M, Mantoni T, Jørgensen HL, Belhage B, et al. Effect of head rotation on cerebral blood velocity in the prone position. Anesthesiol Res Pract. 2012;2012:647258. doi: 10.1155/2012/647258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during the current study are available from the corresponding author on reasonable request.