Significance
The circadian clock mechanism is a daily rhythmic activation of circadian gene transcription by the CLOCK–BMAL1 heterodimer and repression by CRYs (CRY1 and CRY2) and PERs (PER1 and PER2). CRYs and PERs are highly expressed around predawn, when they begin repressing transcription of cry and per and other clock genes, leading to a nadir of CRYs and PERs in the afternoon. CRY represses by simply binding to the CLOCK–BMAL1 heterodimer on DNA, and PER represses by removing CLOCK–BMAL1 from DNA in a CRY-dependent manner. This work shows that removal of CLOCK–BMAL1 involves phosphorylation of CLOCK, which is accomplished by CK1δ when CRY and PER deliver CK1δ to the CLOCK–BMAL1 complex in the nucleus.
Keywords: cryptochrome, period, circadian clock, casein kinase, DNA binding proteins
Abstract
The mammalian circadian clock consists of a transcription–translation feedback loop (TTFL) composed of CLOCK–BMAL1 transcriptional activators and CRY–PER transcriptional repressors. Previous work showed that CRY inhibits CLOCK–BMAL1-activated transcription by a “blocking”-type mechanism and that CRY–PER inhibits CLOCK–BMAL1 by a “displacement”-type mechanism. While the mechanism of CRY-mediated repression was explained by both in vitro and in vivo experiments, the CRY–PER-mediated repression in vivo seemed in conflict with the in vitro data demonstrating PER removes CRY from the CLOCK–BMAL1–E-box complex. Here, we show that CRY–PER participates in the displacement-type repression by recruiting CK1δ to the nucleus and mediating an increased local concentration of CK1δ at CLOCK–BMAL1-bound promoters/enhancers and thus promoting the phosphorylation of CLOCK and dissociation of CLOCK–BMAL1 along with CRY from the E-box. Our findings bring clarity to the role of PER in the dynamic nature of the repressive phase of the TTFL.
In the canonical model for the mammalian circadian clock, CLOCK and BMAL1 make a heterodimer that activates the transcription of Cryptochrome (CRY) and Period (PER) genes, and the resulting CRY–PER complexes, after a time lag, act on CLOCK–BMAL1 and inhibit their transcriptional activator function, thus completing the transcription–translation feedback loop (TTFL) that constitutes the core molecular clock (1–7). Previous biochemical work has supported some key points of this model but has also revealed some facts that, at face value, contradict the model. First, it was found that CRY (CRY1 and CRY2) directly bind to the CLOCK–BMAL1–E-box complex and inhibit CLOCK–BMAL1-activated transcription even in the absence of PER proteins (6, 8–10). In addition, while PER (PER1 and PER2) is the primary repressor in the Drosophila TTFL (4), in mammals, in the absence of CRYs, it does not stably bind to the CLOCK–BMAL1–E-box complex and it cannot repress CLOCK–BMAL1 transcriptional activity (6, 11). Instead, in mammals, inhibition of CLOCK–BMAL1 by PER is CRY dependent, and in vivo, PER causes the removal of the entire CRY–CLOCK–BMAL1 ensemble from cognate promoters, leading to either inhibition or activation of the relevant genes (11, 12), depending on other transcription regulatory elements in the gene’s promoter and enhancer. Thus, two modes of repression in mammals were identified, “blocking” repression by CRY binding to the CLOCK–BMAL1–E-box complex, and “displacement” repression in which PER removes CLOCK–BMAL1 from the E-box in a CRY-dependent manner.
The data explaining blocking-type and displacement-type repression were based on the in vitro and in vivo behavior of CRY on CLOCK–BMAL1-controlled genes, and based on the in vivo effect of PER on the CRY–CLOCK–BMAL1 complex (11, 12). However, the data presented a paradox: In vitro data showed that PER removed only CRY from the CRY–CLOCK–BMAL1–E-box complex, while in vivo it removed the entire CRY–CLOCK–BMAL1 ensemble from E-boxes, with the consequent transcriptional inhibition or activation (12). We reasoned that since CK1δ/ε have been shown to play key roles in the clock mechanism (13–18), the discrepancy between our in vitro and vivo experiments was due to lack of these kinases in the in vitro reconstituted system. In support of this view, we found that PER mutants defective in casein kinase binding failed to remove the CRY–CLOCK–BMAL1 repressor complex in a reporter gene assay. Considering the limitations of reporter gene assays, we wished to test this model directly in vitro by using CK1δ/CK2 in the CLOCK–BMAL1–E-box binding experiment: Here, we report that under appropriate experimental conditions, CK1δ/CK2 phosphorylates CLOCK at multiple sites and this phosphorylation is associated with dissociation of CLOCK–BMAL1 from an E-box (17). Of special significance, the phosphorylation of CLOCK by CK1δ/CK2 is PER dependent in vivo but not CRY or PER dependent in vitro. This is consistent with the role of CRY–PER transporting CK1δ into the nucleus and mediating an increased local concentration of CK1δ at CLOCK–BMAL1-bound promoters/enhancers in vivo and provides an explanation for our in vitro observation of CK1δ-dependent but CRY–PER-independent removal of CLOCK–BMAL1 from an E-box.
Results
CK1δ Is Required for Displacement of BMAL1, CLOCK, and CRY1 from the Nr1d1 E-Box.
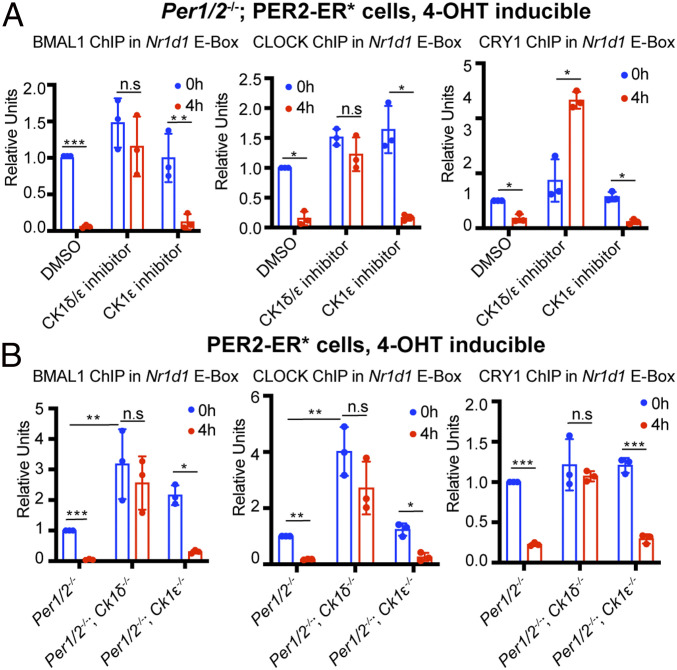
Nr1d1, which encodes the nuclear receptor NR1D1 (which is also an important component of the consolidating loop of the circadian clock), is controlled almost exclusively from an E-box in its promoter and thus constitutes a convenient system for investigating the working of the core clock (19). To test the suspected contributions of CK1 family kinases on the regulation of Nr1d1 by the core clock, we used the well-characterized CK1δ/ε inhibitor PF670462 (slightly selective for CK1δ), and as a control we used PF4800567, which is known to specifically inhibit CK1ε activity (20, 21). We tested these inhibitors in our “cell biochemical system” based on targeted delivery of the desired protein (PER2) into the nucleus by the 4-hydroxytamoxifen (4-OHT)/Estrogen Receptor* system. Per1/2−/−; PER2–ER* mouse embryo cells (Per1, Per2 double-knockout cells expressing PER2–Estrogen Receptor fusion protein) were incubated with DMSO (dimethyl sulfoxide) as a solvent control, or with PF670462/PF4800567, and then 4-OHT was added to the cultures to promote PER–ER* entry into the nucleus. At 0 and 4 h after addition of 4-OHT, we measured the binding of BMAL1, CLOCK, and CRY1 to the Nr1d1 E-box by ChIP (chromatin immunoprecipitation). Fig. 1A shows that entry of PER2–ER* into the nucleus strongly reduces binding of all three proteins (CLOCK, BMAL1, and CRY1) when DMSO or the control CK1ε kinase inhibitor are in the medium. In contrast, the binding of all three proteins does not diminish when the CK1δ/ε inhibitor is present in the medium, and for some reason the binding of CRY actually increases (Fig. 1A), indicating that CK1δ kinase plays an essential role in PER-mediated displacement-type repression in the core clock mechanism. A control experiment (SI Appendix, Fig. S1A) shows that, under the conditions employed, the CK1δ/ε inhibitor decreases PER2 and CLOCK phosphorylation levels but not BMAL1 phosphorylation.
Fig. 1.
PER2-mediated displacement of CLOCK, BMAL1, and CRY1 from an E-box in vivo is CK1δ dependent. (A) After treatment of Per1/2−/−; PER2–ER* cells for 24 h with 10 μM PF670462 (CK1δ/ε inhibitor) or 10 μM PF4800567 (CK1ε-selective inhibitor), 1 μM 4-OHT was added for 0 or 4 h to induce nuclear entry of PER2–ER*. CLOCK, BMAL1, and CRY1 binding to the Nr1d1 E-box was then measured by ChIP. (B) Experiments were then repeated (without inhibitors) to test cells with knocked-out CK1δ and CK1ε genes (Per1/2−/−; Ck1δ−/−; PER2–ER* and Per1/2−/−; Ck1ε−/−; PER2–ER* cells), using Per1/2−/−; PER2–ER* cells as a control. Results indicate a role of CK1δ but not CK1ε in removal of the CRY1–CLOCK–BMAL1 complex in vivo. Data for each panel were normalized to a value of 1 given to a control signal obtained with 0-h 4-OHT treatment (DMSO for A; PER2–ER* in B). Three biological repeats were used for quantification. Data are represented as dots for individual experiments and as columns for means. Error bars represent SDs. n.s, not significant; *P < 0.05, **P < 0.01, ***P < 0.001, as determined by t test between 0 and 4 h in same cell line and two-way ANOVA between different cell lines.
To follow up on the CK inhibition experiments, we constructed Per1/2−/−; Ck1δ−/− and Per1/2−/−; Ck1ε−/− cell lines using CRISPR/Cas9 technology to knock out the Ck1δ and Ck1ε genes (SI Appendix, Fig. S2). Then we tested the effect of 4-OHT on CLOCK–BMAL1 binding to the Nr1d1 E-box in these cell lines and the control Per1/2−/−; PER2–ER* cell line (Fig. 1B). Both BMAL1 and CLOCK dissociate from the Nr1d1 promoter upon addition of 4-OHT to Per1/2−/−; PER2–ER* and Per1/2−/−; Ck1ε−/−; PER2–ER* cell lines. However, the absence of CK1δ in the Per1/2−/−; Ck1δ−/−; PER2–ER* cell line produced two interesting results. First, prior to addition of 4-OHT, there are more CLOCK–BMAL1 complexes bound to the Nr1d1 E-box, and second, the entry of PER2–ER* into the nucleus after addition of 4-OHT does not significantly alter the amount of CLOCK–BMAL1 bound to the promoter. Interestingly, a change in overall CLOCK phosphorylation is not seen in the Ck1δ−/− versus Ck1δ+/+ fibroblasts following nuclear entry of PER2 (SI Appendix, Fig. S1C), suggesting that CLOCK possesses multiple potential phosphorylation sites (22–24), and only a subset of these sites is targeted by CK1δ and destabilize CLOCK–BMAL1 on DNA when phosphorylated.
Requirement for Casein Kinase Binding Domains of PER2 in Displacement of CLOCK and BMAL1 from Promoters.
Casein kinases bind to PER2 through two casein kinase binding domains (CKBDs), CKBDa (CK2 binding domain) and CKBDb (CK1ε binding domain), which are indicated in the schematic of PER2 protein in Fig. 2A (25–27). To examine the importance of a direct interaction between CK proteins and PER2 in displacement of BMAL1 and CLOCK from E-box promoters, we made constructs, shown in Fig. 2A, to express one mutant PER2–ER* protein with a deletion of the CK binding domain (CKBDb) and another protein with both the CKBDa and the CKBDb deleted (CKBDa/b). We also made a construct expressing PER2S659A, because it has been proposed that phosphorylation of PER2S659 by CK1 primes the phosphorylation of additional sites of PER2, which leads to increasing PER2 abundance (28, 29). We tested these constructs in our cell biochemical system based on targeted delivery of each PER2 construct into the nucleus by the OH-T/Estrogen Receptor* system (Fig. 2 B–D). As expected, entry of full-length (FL) PER2–ER* into the nucleus reduces BMAL1 binding to Nr1d1 E-boxes to background level. Importantly, the entry of PER2–ER* with CKBDa/b double deletions fails to reduce BMAL1 binding to the E-box (Fig. 2B and SI Appendix, Fig. S3). Similar effects were obtained with these PER2 mutants on CLOCK and CRY1 binding to the E-box (Fig. 2 C and D). Overall, this experiment and a prior report (11) showing efficient removal of CLOCK/BMAL1 by PER2 lacking amino acids 1 to 595 (CKBDa) reveal that deletion of CKBDa and CKBDb together but not singly eliminates CK1δ-mediated removal of CLOCK–BMAL1 from an E-box (summarized in Fig. 2A, Right).
Fig. 2.
Mapping PER2 domains required for removing CRY1–CLOCK–BMAL1 from an E-box in vivo. (A) Illustration of the PER2–ER* constructs expressed in the Per1/2−/− cell line. The numbers indicate amino acid residues bordering deletions made in the full-length, 1,257-amino acid-long PER2. CBD, CRY-binding domain; CKBDa, casein kinase 2 binding domain; CKBDb, casein kinase 1ε binding domain; ER*, estrogen receptor “tag”; PAS, PER–ARNT–SIM domain. The dashed lines indicate regions deleted (Δ) in the constructs. Locations of the CK binding domains a and b (CKBDa and CKBDb) are shown. (B−D) ChIP analyses of BMAL1, CLOCK, and CRY1 binding to an E-box in the Nr1d1 promoter in Per1/2−/− cell lines expressing different PER2–ER* proteins. Full-length PER2 (1–1,257), S659A PER2, and ΔCKBDb PER2 disrupt CRY1–CLOCK–BMAL1 binding to chromatin. Only the PER2 construct lacking both CKBDs (ΔCKBDa/b PER2) is unable to remove CRY1–CLOCK–BMAL1 from chromatin. S659A PER2, and ΔCKBDa PER2 appear to have only partial ability to remove CRY1 from the CLOCK–BMAL1–E-box complex. All data were normalized to a value of 1 for full-length PER2 (1–1,257) at 0-h 4-OHT. Three biological repeats were used for quantification. Data are represented as dots for individual experiments and as columns for means. Error bars represent SDs. n.s, not significant; *P < 0.05, **P < 0.01, ***P < 0.001, as determined by t test.
CLOCK Hyperphosphorylation and CK1δ Nuclear Localization Are PER and CRY Dependent in Vivo.
To determine the mechanism by which CK1δ promotes CLOCK removal from E-boxes in vivo, we first tested the phosphorylation of CLOCK, BMAL1, and PER2 in vitro by the main members of the casein kinase family. Fig. 3 A and B utilized the constitutively active form of CK1δ, CK1δ ΔC, purchased from a commercial source, which lacks its autoinhibitory C terminus and retains 97% identity with CK1δ in the kinase domain. The autoradiograms in Fig. 3 A and B show that CLOCK, BMAL1, and PER2 are all substrates of CK1δ, CK1ε, and CK2 for labeling by [γ-32P]ATP. We next performed in vivo experiments to examine CLOCK–BMAL1 phosphorylation levels as a function of CT (circadian time) in wild-type (WT) as well as CRY and PER knockout mice. Phosphorylation levels were determined by examining bandshifts in immunoblots. The blots in Fig. 3 C and D clearly show circadian oscillations of CLOCK–BMAL1 phosphorylation levels in WT mouse liver nuclei. Notably, in the repressive phase when CLOCK–BMAL1 binding to the E-box becomes weak (CT12–22) (30, 31), CLOCK is hyperphosphorylated. In the active phase when CLOCK/BMAL1 binding to the E-box is strong (CT4–8) (30, 31), CLOCK is hypophosphorylated. CLOCK is also hypophosphorylated at all time points in the knockout mice lacking CRY and PER repressors. Thus, by analyzing both circadian expression and knockout mice, we see CLOCK–BMAL1 repression associated with hyperphosphorylation of CLOCK, and activation associated with hypophosphorylation. In contrast, in WT liver BMAL1 is hypophosphorylated between CT12 and CT24, and hyperphosphorylated between CT8 and CT12, and in the knockout mice, BMAL1 was hyperphosphorylated at all time points due to an unknown mechanism (Fig. 3 C and D).
Fig. 3.
CLOCK hyperphosphorylation and CK1δ nuclear translocation are PER and CRY-dependent in vivo. Preliminary experiments were done in vitro to assess phosphorylation of core clock proteins by CKs. Purified proteins were incubated with [γ-32P]ATP and separated by SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis), and reaction products were visualized by autoradiography. Autoradiograms show that PER2 (A) and CLOCK–BMAL (B) can be directly phosphorylated by CK1δ and CK1ε, and marginally phosphorylated by CK2. (C and D) CLOCK–BMAL1 phosphorylation levels in WT, Per1/2−/−, and Cry1/2−/− mice as detected by bandshifts in immunoblots. Nuclear protein extracts from mouse livers were prepared at six CT points for analysis. In WT mice, when CLOCK–BMAL1 binding to E-boxes is high at CT4–8, CLOCK is hypophosphorylated. However, when CLOCK–BMAL1 binding to E-boxes is low at CT12–22, CLOCK is hyperphosphorylated. BMAL1 is hyperphosphorylated between CT8 and CT12, and BMAL1 is hypophosphorylated between CT16 and CT24. In Per1/2−/− and Cry1/2−/− mice, CLOCK is hypophosphorylated but BMAL1 is hyperphosphorylated at all time points. (E) Nuclear (“Nuc”) and cytoplasmic (“Cyto”) temporal expression of core clock proteins in WT and Per1/2−/− mouse liver. (F) Nuclear (Nuc) and cytoplasmic (Cyto) temporal expression of core clock proteins in WT and Cry1/2−/− mouse liver. Equal amounts of cytoplasmic protein (120 μg) and nuclear protein (6 μg) were loaded in each protein lane. α-Tubulin (cytoplasmic) and Lamin B1 (nuclear) were probed to provide loading controls. All the immunoblots were performed with at least two biological repeats and some had three technical repeats. All data yielded similar results. Representative images are shown.
We next examined the apparent link between CK1δ and CLOCK phosphorylation by comparing the subcellular localization of CK1δ and the negative effectors CRY1 and PER2 in WT and knockout mouse liver as a function of CT. The immunoblot results in Fig. 3 E and F show that as expected, CRY1 and PER2 both oscillate in cytosol and nucleus of WT mice with peaks during the repressive phase at approximately CT20. The results from knockout mice show that CRY1 enters the nucleus in the absence of PER1/2, but PER2 requires CRY1/2 for nuclear entry (13). Importantly, nuclear entry of CK1δ directly requires PER1/2 and indirectly requires CRY1/2 since both CRY1/2 are necessary for PER2 entry in the nucleus. Also, nuclear levels of CK1δ oscillate with a pattern similar to the patterns of CRY1 and PER2. Of course, we cannot rule out the possibility that PER1/2 and CRY1/2 regulate CK1δ nuclear retention/nuclear export. Consequently, nuclear levels of CK1δ become elevated during the repressive phase of the clock when the substrate of CK1δ, CLOCK, becomes hyperphosphorylated and exhibits reduced activity. Taken together, these data are consistent with the idea that PER1/2 plays a role in the localization of CK1δ into the nucleus, which in turn phosphorylates CLOCK and causes the dissociation of the CLOCK–BMAL1 complex from the cognate promoter and ultimately ubiquitination and degradation by the proteasome (24, 32).
Displacement of CLOCK–BMAL1 from an E-Box by CK1δ in Vitro: Electrophoretic Mobility Shift Assay.
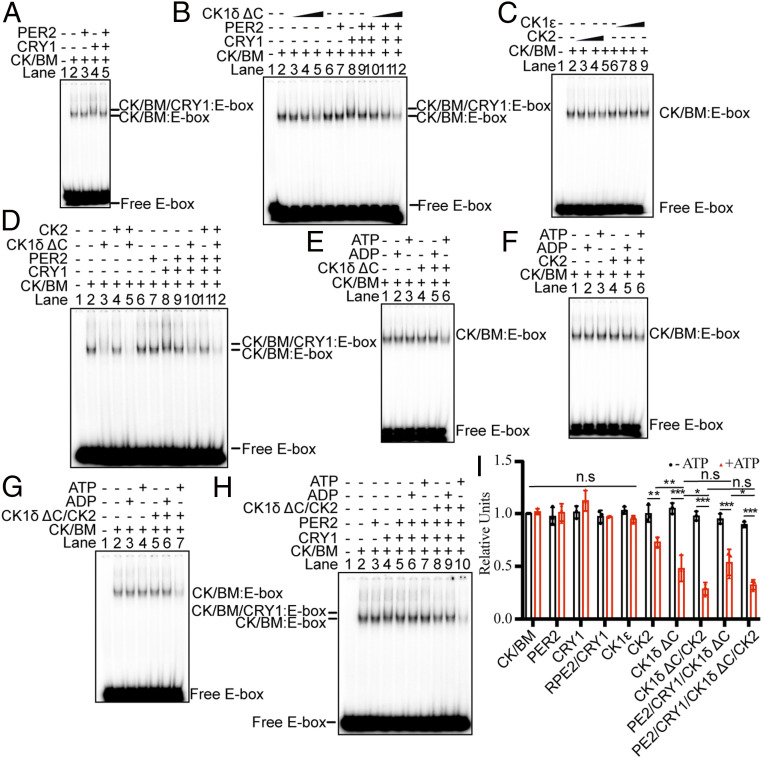
To further test the model of CRY1 and PER2-mediated recruitment of CK1δ to repress CLOCK–BMAL1–E-box complexes, we performed in vitro studies with purified CLOCK/BMAL1, PER2, CRY1, CK1δ, and CK1ε proteins (SI Appendix, Fig. S4). Since PER2 appears to be more important than PER1 for normal clock function in mice (33, 34), and CRY1 is a stronger repressor than CRY2 because of higher affinity to CLOCK/BMAL1 (35, 36), we used PER2 and CRY1 in our in vitro experiments. In electrophoretic mobility shift assays (EMSAs), the purified CLOCK/BMAL1 complex binds specifically to an E-box sequence in a 30-bp duplex, and binding is concentration dependent (SI Appendix, Fig. S5 A and B). Fig. 4A recapitulates our previous finding that CRY1 makes a CRY1–CLOCK–BMAL1–E-box complex (lane 4) and that addition of PER2 to this complex results in removal of CRY1 without affecting the level of CLOCK–BMAL1 at the E-box (lane 5), which, in vivo, depending on the types of regulatory elements in the promoter/enhancer may result in transcriptional repression or activation. Interestingly, Fig. 4B and SI Appendix, Fig. S6 show that above 40 nM, and in the presence of ATP, CK1δ ΔC reduces the amount of CLOCK–BMAL1 bound to the E-box independently of PER and CRY. Both full-length mCK1δ purified by us and hCK1δ purchased from commercial resource also remove CLOCK/BMAL1 from the E-box independently of PER2 and CRY1 (SI Appendix, Fig. S7). We wished to know whether the observed removal was due to nonspecific phosphorylation by kinases and thus tested CK1ε and CK2 kinases. At concentrations similar to those at which CK1δ was effective, CK2 has only a minor effect on a CLOCK–BMAL1–E-box complex and CK1ε has no effect (Fig. 4 C and I).
Fig. 4.
CK1δ and CK2 reduce CLOCK/BMAL1 binding to an E-box in vitro. (A) Effect of CRY1 and PER2 on the mobility of the CLOCK–BMAL1–E-box complex. EMSA was performed with a 32P-labeled 30-bp duplex containing an E-box (1 nM) and CLOCK–BMAL1 (2 nM). A supershift was caused by CRY1 (15 nM) but not PER2 (15 nM) (lanes 3 and 4). PER2 removes only CRY1 from the CLOCK–BMAL1–CRY1–E-box complex; the CLOCK–BMAL1–E-box remains (lane 5). (B) Effect of CK1δ ΔC, CRY1, and PER2 on the mobility of the CLOCK–BMAL1–E-box complex. The E-box duplex (1 nM) was incubated with CLOCK–BMAL1 complex at 2 nM and increasing amounts of CK1δ ΔC (40, 100, and 200 nM). CRY1 and PER2 were added as in A. (C) Effect of CK2 and CK1ε on the amount of CLOCK–BMAL1–E-box complex. (D) Effect of CK1δ and CK2 on the entire circadian protein assembly binding to the E-box. MgCl2 and ATP were present in all reactions in Fig. 4 A–D. EMSA showing the effect of CK1δ (E), CK2 (F), and CK1δ and CK2 together (G) on the amount of CLOCK–BMAL1–E-box complexes in the presence of ADP, ATP, or no nucleotide. (H) Effect of both CK1δ and CK2 on the entire circadian protein assembly binding to the E-box with/without ATP. The EMSAs data are representative of three independent experiments. (I) Quantitative analysis of the amount of CLOCK–BMAL1–E-box complex when adding core clock proteins with/without ATP. Three biological repeats were used for quantification. Data are represented as dots for individual experiments and as columns for means. Error bars are SDs of triplicate data. n.s, not significant; *P < 0.05, **P < 0.01, ***P < 0.001 were determined by two-way ANOVA.
A more comprehensive analysis of CLOCK–BMAL1–E-box stability in the presence of kinases, CRY1, and PER2 is shown in Fig. 4D. Here, addition of CK1δ directly dissociates nearly the entire CLOCK–BMAL1–E-box complex, and the inclusion of CK2 in the reaction makes only a modest difference (Fig. 4D, lanes 1 to 5, and Fig. 4I). Binding results obtained under conditions that mimic the in vivo conditions necessary for displacement of CLOCK–BMAL1 from the E-box, shown in Fig. 4D (lanes 6 to 12) and Fig. 4I, confirm earlier observations of PER2 displacing CRY1 from a CRY–CLOCK–BMAL1–E-box complex (lanes 8 and lane 9). Lane 10 shows strong displacement of the entire CLOCK/BMAL1 complex by CK1δ even when PER2 and CRY are present in the binding reaction. CK2 has only a minor effect on its own (lane 11), but it seems to enhance the displacement effect of CK1δ (lane 12), suggesting that in addition to CK1δ, CK2 may also contribute toward phosphorylation and displacement of CLOCK/BMAL1 from the E-box. We further examined whether PER2 might assist CK1δ in displacing CLOCK–BMAL1, and found that it did not, even following prolonged reaction times or using high concentrations of PER2 in combination with a low concentration of CK1δ (SI Appendix, Fig. S8 A and B). To test whether the CK1δ effect observed was due to the phosphorylation of target proteins and not an artifact of the presence of highly negatively charged nucleotides in the binding reaction, we performed experiments with ATP or ADP in the binding reactions. Fig. 4 E and I shows that in a reaction containing only CLOCK–BMAL1 and CK1δ, ADP does not affect the amount of CLOCK/BMAL1–E-box (lane 5), but ATP significantly reduces the amount of bound E-box (lane 6). Fig. 4F shows that the marginal effect of CK2 on the complex is also ATP dependent. Importantly, Fig. 4G shows that the combination of CK1δ and CK2 in the presence of ATP dissociates virtually the entire complex (lane 7). Finally, when the effect of CK1δ and CK2 is tested in the presence of the entire circadian protein assembly, results similar to those observed with CLOCK–BMAL1–E-box alone are observed (compare Fig. 4G, lane 7, and Fig. 4H, lane 10), showing that CK1δ/CK2 dissociation of CLOCK–BMAL1 from an E-box is ATP-dependent and independent of the CRY1 and PER2 negative effectors in vitro. We note that only 70% of 2 nM CLOCK/BMAL1 was removed from the E-box even with the high concentration of CK1δ/CK2 (200 nM) in vitro (Fig. 4I). However, it is also noted that only a small fraction of CK1δ/CK2 is active and the reported activity of CK1δ/CK2 from the commercial source was established under high substrate concentration. Furthermore, the presence of a relatively low amount of substrate (CLOCK–BMAL1–E-box) relative to enzyme may recapitulate the in vivo condition (37). Taken as whole, the data in Fig. 4 show that CK1δ and CK2 are capable of disrupting the CLOCK–BMAL1–E-box complex unassisted, provided they are transported to the nucleus. This unexpected finding led us to investigate in more detail the role of phosphorylation in clock repression.
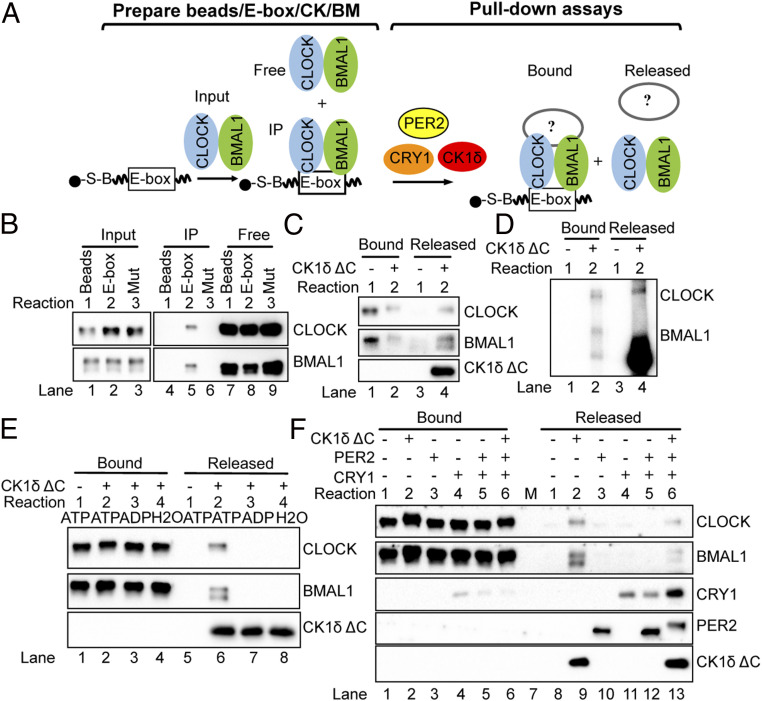
Release of the CLOCK–BMAL1 Complex from an E-Box by CK1δ in Vitro: Pull-Down Assay.
We complemented the above studies by using a pull-down assay to more directly monitor release of CLOCK–BMAL1 from an E-box in response to CKs, PER, and CRY. To do this, we first isolated CLOCK–BMAL1–E-box complexes using streptavidin beads bound to a biotin-tagged, E-box containing DNA duplex, as illustrated in the schematic in Fig. 5 A, Left. The immunoblot in Fig. 5B shows that the CLOCK–BMAL1 complex is pelleted (IP) with the streptavidin beads when the DNA duplex contains an E-box (lane 5), but not when the duplex has a mutant E-box (Mut) or when there is no DNA, demonstrating the binding specificity. For the pull-down assays (as outlined in the schematic, Fig. 5 A, Right) shown in Fig. 5 C–E, CLOCK–BMAL1–E-box complexes specifically bound to the E-box duplex and pelleted in this manner (Fig. 5 B, lane 5) were resuspended and used as input. (In Fig. 5F, the input for pull-down assays consisted of CLOCK–BMAL1 plus CRY1 bound to the E-box.) CLOCK–BMAL1–E-box complexes were incubated for 45 min at room temperature in the presence or absence of added ATP and clock proteins, and then pulled down to separate Bound and Released fractions.
Fig. 5.
Release of CLOCK–BMAL1 from an E-box by CK1δ in vitro. (A) Schematic showing the DNA pull-down assay to test the roles of PER2, CRY1, and CK1δ ΔC on CLOCK–BMAL1 release from an E-box. The E-box–containing duplex is tagged with biotin (“B”) and binds to and pellets with streptavidin (“S”) beads. CLOCK–BMAL1, bound to the E-box, also pellets with the beads. (In F, beads are prepared by adding CRY1 together with CLOCK–BMAL1 at this step, and in this case, the CLOCK–BMAL1–CRY1 complex pellets with the beads.) For pull-down assay, beads with CLOCK–BMAL1–E-box (or CLOCK–BMAL1–CRY1–E-box complexes) are then resuspended and incubated with PER2, CRY1, or CK1 δ, then pulled down to assess E-box bound and released proteins. (B) Preparation of CLOCK–BMAL1–E-box complexes (A, Left). CLOCK–BMAL1 (20 nM) binds to the DNA duplex (10 pmol) containing the E-box sequence and is pulled down (“IP”) by the streptavidin beads as shown in lane 5 of the immunoblot. When the duplex has a mutated E-box sequence, or in the absence of DNA, CLOCK–BMAL1 remain entirely in the “Free” fraction following pull-down, demonstrating specificity of binding. (C) For pull-down assay (A, Right), CLOCK–BMAL1–E-box complexes immunoprecipitated as in B, lane 5, were resuspended, incubated with CK1δ ΔC, and then pulled down again to separate Bound and Released fractions. The immunoblot analysis shows that CLOCK–BMAL1 is released from the E-box after adding 200 nM CK1δ ΔC. (D) SDS-PAGE autoradiogram showing that the bound and released CLOCK is hyperphosphorylated, but only bound BMAL1 is hyperphosphorylated after addition of 200 nM CK1δ ΔC. Proteins were radiolabeled by adding [γ-32P]ATP to the binding reactions. (E) Release of CLOCK–BMAL1 from the E-box by 200 nM CK1δ ΔC is ATP dependent in the pull-down assay. (F) Effect of 15 nM PER2, and 200 nM CK1δ ΔC on the CLOCK–BMAL1–CRY1–E-box complex. Note that the CRY1 amount associated with CLOCK–BMAL1 in the bound fraction (lanes 4 to 6) is lower than that observed in the EMSAs (Fig. 4 A, B, and D) because the multiple washes in the pull-downs causes dissociation of CRY from the CLOCK–BMAL1 complex. The DNA pull-down data are representative of three independent experiments.
Experimental results (Fig. 5 C, E, and F) consistently show CK1δ ΔC-dependent release of CLOCK–BMAL1 from the E-box. Most of the CLOCK and a portion of the BMAL1 are in phosphorylated form after incubation with CK1δ ΔC, as indicated by bandshifts in the immunoblots (Fig. 5 C, E, and F), and by 32P labeling when [γ-32P]ATP was included in binding reactions (Fig. 5D). Release is in fact ATP dependent (Fig. 5E). While both the bound and released CLOCK appear to be in phosphorylated form, a significant proportion of the released BMAL1 is nonphosphorylated; in fact, there appears to be a slight enrichment in nonphosphorylated BMAL1 in the released fractions compared to bound. These results support and extend our findings above in showing the CK1δ-dependent phosphorylation of CLOCK and consequent dissociation of the CLOCK–BMAL complex from an E-box.
The pull-down experiment in Fig. 5F, which included CRY1 and PER2 proteins, confirms earlier data in demonstrating the binding of CRY1 to the CLOCK–BMAL1–E-box complex (Reaction 4, “Bound”), and shows removal of the entire CRY1–CLOCK–BMAL1 complex from the E-box by CK1δ ΔC (Reaction 6, “Released”). Unexpectedly, removal by CK1δ ΔC is less efficient in the presence of PER2 (Reactions 6 versus 2, Released); this result may be unique to the experimental condition in which PER2 may compete with CLOCK for phosphorylation by CK1δ ΔC. More importantly, with respect to PER2, our results overall indicate an important role of PER2 in transporting CK1δ into the nucleus to promote hyperphosphorylation of CLOCK leading to dissociation of CLOCK–BMAL1 from E-boxes. Similar models have been proposed for PER-dependent phosphorylation of CLOCK leading to its displacement from cognate promoters in Drosophila (38–40), and FRQ–FRH-dependent hyperphosphorylation of WCC and its dissociation from target promoters in Neurospora (41, 42).
Nuclear Clock Protein Complexes Present during the Repressive Phase.
The dependence of CK1δ nuclear entry on CRYs and PERs described above suggests that these proteins are translocated into the nucleus as a complex. To examine possible clock protein–protein interactions in vivo during the repressive phase of the circadian cycle, we analyzed proteins in nuclear extract from WT mouse liver obtained at ZT19 (zeitgeber time) by glycerol gradient centrifugation. Fig. 6A and SI Appendix, Fig. S9A show that circadian proteins sedimented principally in two complexes; CRY, PER, and CK1δ comigrated at a position corresponding to ∼550 kDa (which is consistent with multimers of PER1, PER2, CRY1, CRY2, and CK1δ), and CLOCK and BMAL1 comigrated at a position corresponding to about 200 kDa, which is approximately the size of a CLOCK–BMAL1 heterodimer. Most of CRY1 is associated with PER in 550-kDa complexes in WT at ZT19; a small fraction of CRY1 is monomer (Fig. 6A and SI Appendix, Fig. S9A). Naturally, there is no high–molecular-weight PER complex in Per1/2−/− and Cry1/2−/− mice; CLOCK–BMAL1 migrate as a heterodimer. Most of CRY1 is monomer in Per1/2−/− mice (Fig. 6 and SI Appendix, Fig. S9 B and C). These results are consistent with the idea that a CRY–PER–CK1δ complex translocates into the nucleus and interacts transiently to phosphorylate CLOCK and inactivate the CLOCK–BMAL1 complex. These results also explain why PER and CRY are not necessary for removal of CLOCK–BMAL1 from E-boxes in vitro, where nuclear entry is not required. We should note, however, that our findings of a CLOCK–BMAL1 complex in the 200-kDa range separated from the CRY–PER–CK1δ complex in the 550-kDa range differs from reports of so-called PER megadalton complexes with 1.1- and 1.9-MDa size in the cytoplasm and nucleus, respectively (17). Moreover, these megadalton complexes reportedly contained the core clock proteins (CLOCK, BMAL1, CRY, PER, CK1δ) along with additional proteins in the nucleus (17). It must be noted, however, that these supercomplexes were detected by gel filtration chromatography or blue native-agarose polyacrylamide gel electrophoresis. We believe these isolation methods have some limitations and need to be supported by complementary biochemical approaches such as ultracentrifugation.
Fig. 6.
Analysis of circadian complexes by glycerol gradient sedimentation. Mouse nuclear extract prepared from a mouse harvested at ZT19 and reference proteins were mixed together, and the extract and reference proteins were sedimented together through a 10 to 30% (wt/wt) glycerol gradient. Reference proteins included bovine thyroglobulin (669 kDa, 19 S), sweet potato β-amylase (222 kDa, 8.9 S), and chicken ovalbumin (43 kDa, 3.55 S). Fractions were collected starting at the bottom of the gradient and analyzed by loading samples to two SDS-PAGE gels. Gels showing gradient profiles for extracts from WT (A), Per1/2−/− (B), and Cry1/2−/− mice (C) at ZT19 were developed by immunoblot with PER1, PER2, CRY1, CRY2, CK1δ, CLOCK, and BMAL1 antibodies. “P” stands for pellet and indicates the sample obtained by washing the empty gradient tube with a small volume (240 μL) after collecting all fractions. The purpose of the P sample was to discover whether an insoluble pellet existed following centrifugation and to examine its composition. Three percent of the original sample was loaded directly to the SDS-PAGE gel as “Input.” The arrows indicate positions of the peak fraction of each reference protein as determined from the Coomassie Blue-stained SDS-PAGE gel (see SI Appendix, Fig. S9 for additional information related to this figure). Quantification of relative intensity is shown beside the immunoblot. Data for each protein were normalized to a value of 1 given to the peak fraction. Glycerol gradient experiments were performed with three biological repeats for WT and Cry1/2−/−, and two biological repeats for Per1/2−/− mice, and all data yielded essentially identical results. Representative images are shown in the figure.
Discussion
In our previous studies, we demonstrated that: 1) CRY (CRY1 or CRY2) bound to a CLOCK–BMAL1–E-box complex in vitro, 2) but PER (PER1 or PER2) did not bind to this complex. 3) Rather surprisingly, when PER2 was added to the CRY1–CLOCK–BMAL1–E-box complex, it caused displacement of CRY1 from the complex. 4) In an in vivo system where CRY1 or PER2 could be delivered to the nuclei of Cry1/2−/−; Per1/2−/− cells it was found that entry of CRY1 into the nucleus inhibited the expression of genes controlled solely by CLOCK–BMAL1 without removal of CLOCK–BMAL1 from the cognate E-box (blocking-type repression). 5) In the same quadruple mutant cell line, controlled entry of PER into the nucleus did not affect either the binding of CLOCK–BMAL1 to an E-box, or gene expression controlled by CLOCK–BMAL1 binding to an E-box. Most surprisingly, the controlled entry of PER into the nuclei of Cry1/2+/+; Per1/2−/− cells resulted in removal of the entire CRY–CLOCK–BMAL1 complex from the E-box (in contrast to the vitro experiments in which PER2 removes CRY1 but not CLOCK–BMAL1 from the E-box) resulting in inhibition of E-box controlled gene expression (displacement-type repression) (11). Furthermore, it was found that, in this in vivo system, removal of CLOCK–BMAL1 from the cognate promoter was dependent on intact CKBD (casein kinase binding domain) and CBD (CRY binding domain) regions of PER (11).
Taken in its entirety, the data presented above suggest the following displacement-type repression model: PER is bound to CK1δ through its CKBD and carries CK1δ to the nucleus with CRY and in the nucleus in a CRY-dependent manner increases local concentration of CK1δ at CLOCK–BMAL1 promoters/enhancers, phosphorylates (hyperphosphorylates) CLOCK and to a lesser degree BMAL1, and thereby causes the disruption and dissociation of the entire CRY–CLOCK–BMAL1 complex from the E-box. This interpretation of our data confirms and extends the models proposed by Kondratov et al. (24) on the role of CLOCK phosphorylation in the circadian clock and by Etchegaray et al. (15) on the role of CK1δ but not CK1ε on circadian period length. Furthermore, in this paper by introducing CK1δ into our in vitro system, we have tested the plausibility of the proposed model while at the same time taking into account the extensive studies that have addressed the role of CK1δ/ε in the mammalian circadian clock and those studies that have defined the roles of the functional orthologs of CK1δ/ε in the two other well-studied systems, Neurospora and Drosophila. Similar displacement-type repression models have been proposed for PER-dependent phosphorylation of CLOCK and its displacement from cognate promoters in Drosophila, as well as FRQ-dependent hyperphosphorylation of WCC and its dissociation from target promoters. However, there is no blocking-type repression reported in Neurospora. In Drosophila, PER has subsumed the role of the mammalian CRY as the primary repressor in on-DNA repression (43), indicating that increasingly more sophisticated regulation of repression evolved from Neurospora, to Drosophila, to mammals.
Cellular assays show that treatment with CK1δ/ε inhibitor and knockout of CK1δ can inhibit about 70% of CLOCK–BMAL1 displacement by PER2 (Fig. 1). However, CK1δ-mediated removal of CLOCK–BMAL1 from an E-box requires deletion of both CKBDa (CK2 binding domain) (26) and CKBDb (CK1ε binding domain) in PER2 (Fig. 2) (27). It is possible that CK1δ plays a primary role, and CK1δ/CK2 act synergistically in CLOCK–BMAL1 displacement from an E-box (Fig. 4). We cannot rule out the possibility that CK1δ binds both CKBDa (CK2 binding domain) and CKBDb (CK1ε binding domain) in PER2.
CLOCK and BMAL1 are substrates for phosphorylation at multiple sites by multiple kinases including PKG, PKC, and casein kinase family members (44–46). A prior study reported that phosphorylation specifically of Ser-38 and Ser-42 residues, located in the bHLH of CLOCK, reduces the transactivation activity of CLOCK (22, 23). In the present study, we find that CK1δ is the main kinase responsible for release of CLOCK–BMAL1 from an E-box; however, our data show that there is considerable “background” phosphorylation of CLOCK in vivo in addition to that produced by CK1δ and CK2 (Fig. 3). Thus, it is likely that phosphorylation of CLOCK by CK1δ/CK2 specifically at a defined subset of the potential CLOCK phosphorylation sites is responsible for reduced E-box binding activity of CLOCK–BMAL1. Further investigation is needed to identify amino acid residues important in mediating this inhibitory effect of CK1δ/CK2 and to further examine the roles of CLOCK residues Ser-38 and Ser-42.
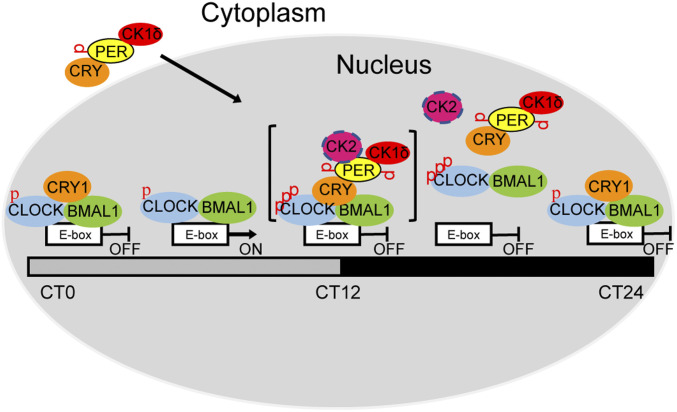
In Fig. 7, we present a model of the mammalian circadian clock that incorporates both blocking-type and displacement-type mechanisms. At CT4–8 (“active phase”), the CLOCK–BMAL1 heterodimer binds to an E-box and activates transcription and expression of PER and CRY. In the cytoplasm, PER, CRY, and CK1δ form a stable complex that enters the nucleus. In the nucleus, starting at about CT12, CRY bridges the interaction of PER with CLOCK–BMAL1–E-box. From CT12 to CT22, when the PER–CRY–CK1δ complex concentration increases in the nucleus, PER is hyperphosphorylated. At the same time, PER acts as scaffold to increase the local concentration of CK1δ/CK2 at CLOCK–BMAL1-bound promoters/enhancers and allow CK1δ/CK2 kinases to phosphorylate CLOCK, and then hyperphosphorylation of CLOCK leads to the release of the complexes from the E-box (CT12–22 displacement-type repression). Later, hyperphosphorylated PER is degraded by a ubiquitin-mediated proteasome pathway, so by CT0–4, CRY1, with the low concentration of PER, blocks CLOCK–BMAL1 in a poised state until the next cycle begins (CT0–4 blocking-type repression). This model is consistent with published ChIP data, which measured binding of clock proteins to E-box regions as a function of CT, and showed that at CT0–4 (when PER is degraded to very low levels) there is maximal mutual binding of CLOCK, BMAL1, and CRY1 (31).
Fig. 7.
Model showing the role of blocking-type and displacement-type repression in the mammalian circadian clock. At around CT4–8, CLOCK–BMAL1 binds to E-boxes to drive clock-controlled gene transcription. After protein synthesis in the cytoplasm, CRY recruits PER and PER recruits CK1δ/CK2 through its CKBD and then enters the nucleus and phosphorylates CLOCK, leading to CLOCK–BMAL1 dissociation from the E-box (CT12–22 displacement-type repression). At around CT0–4, PER levels are too low to be detected and only CRY1 binds to the CLOCK–BMAL1–E-box to block CLOCK–BMAL1 activity (blocking-type repression), which maintains CLOCK–BMAL1 in a repressed state until the next TTFL cycle begins. Note the CK2 is shown a circle with discontinuous circumference to indicate its partial contribution relative to CK1δ. In addition, the entire “repressor–activator complex” is shown in brackets to indicate that it must exist as a kinetic intermediate and not a stable megadalton complex.
The model in Fig. 7 uses brackets to denote the transience of the interaction between activator (CLOCK–BMAL1–E-box) and repressor (CRY–PER–CK1δ) complexes. The contrary notion of a stable activator–repressor interaction is implicit in reports that have described megadalton-sized, stable complexes composed of activator and repressor clock proteins together with varied combinations of the numerous clock protein-associated proteins and RNA that have been discovered since 2005 (17, 47–49). We also found that the entire clock protein ensemble comigrated in the megadalton range by gel filtration chromatography, as reported previously (17, 47–49). However, our sedimentation analysis found no PER supercomplexes at ZT19, as commonly invoked, but instead revealed apparent separate activator and repressor complexes of 200 kDa (containing CLOCK–BMAL1) and 550 kDa (containing CRY1/2, PER1/2, and CK1δ) (Fig. 6). In addition, we have been unable to detect PER binding to CLOCK–BMAL1–E-boxes in vitro (by EMSA or pull-down assays) (Figs. 4 and 5) or PER2 binding to E-boxes in vivo by standard ChIP assays (6, 11). Multidomain mosaic proteins, such as PERs are notorious for engaging in multiple protein–protein interactions (48). While some of these interactions are of functional significance, other interacting partners often are “fellow travelers rather than conjugal partners” (50). However, it must also be noted that PER binding to E-boxes was detected by ChIP using a sensitive dual–cross-linking approach (31), and clearly the activator–repressor complexes do interact, albeit transiently, to generate an ephemeral activator–repressor complex, thus reconciling our findings with the so-called nuclear megadalton PER complex. Phosphorylation of CLOCK by CK1δ recruited by the CRY–PER complex reduces activator–repressor affinity, paralleling the situation in Neurospora, in which hyperphosphorylation of FRQ in the repressive phase induces the rapid loss of interaction between FRQ and WCC (51, 52). Further work is needed to refine the mammalian clock model and better define the unique roles and dynamics of proteins in both the positive and negative arms of the molecular circadian clock.
Materials and Methods
For details on chemicals, animals, plasmids and cell lines, protein expression and purification, ChIP-qPCR, nuclear and cytoplasmic protein extraction, radioactive kinase assays, EMSAs, DNA pull-down assays, glycerol gradient centrifugation, and immunoblot analysis with antibodies, see SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
We thank Khagani Eynullazada for technical support and Dr. Laura A. Lindsey-Boltz for critical comments on the manuscript. This work was supported by NIH Grant GM118102 to A.S.
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2021174118/-/DCSupplemental.
Data Availability.
All study data are included in the article and SI Appendix.
References
- 1.Reppert S. M., Weaver D. R., Coordination of circadian timing in mammals. Nature 418, 935–941 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Hastings M. H., Reddy A. B., Maywood E. S., A clockwork web: Circadian timing in brain and periphery, in health and disease. Nat. Rev. Neurosci. 4, 649–661 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Partch C. L., Green C. B., Takahashi J. S., Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 24, 90–99 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patke A., Young M. W., Axelrod S., Molecular mechanisms and physiological importance of circadian rhythms. Nat. Rev. Mol. Cell Biol. 21, 67–84 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Sancar A., Mechanisms of DNA repair by photolyase and excision nuclease (Nobel lecture). Angew. Chem. Int. Ed. Engl. 55, 8502–8527 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Ye R., Selby C. P., Ozturk N., Annayev Y., Sancar A., Biochemical analysis of the canonical model for the mammalian circadian clock. J. Biol. Chem. 286, 25891–25902 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cederroth C. R., et al. , Medicine in the fourth dimension. Cell Metab. 30, 238–250 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kondratov R. V., Shamanna R. K., Kondratova A. A., Gorbacheva V. Y., Antoch M. P., Dual role of the CLOCK/BMAL1 circadian complex in transcriptional regulation. FASEB J. 20, 530–532 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Shearman L. P., et al. , Interacting molecular loops in the mammalian circadian clock. Science 288, 1013–1019 (2000). [DOI] [PubMed] [Google Scholar]
- 10.Xu H., et al. , Cryptochrome 1 regulates the circadian clock through dynamic interactions with the BMAL1 C terminus. Nat. Struct. Mol. Biol. 22, 476–484 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ye R., et al. , Dual modes of CLOCK:BMAL1 inhibition mediated by cryptochrome and period proteins in the mammalian circadian clock. Genes Dev. 28, 1989–1998 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiou Y. Y., et al. , Mammalian period represses and de-represses transcription by displacing CLOCK–BMAL1 from promoters in a cryptochrome-dependent manner. Proc. Natl. Acad. Sci. U.S.A. 113, E6072–E6079 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee C., Etchegaray J. P., Cagampang F. R., Loudon A. S., Reppert S. M., Posttranslational mechanisms regulate the mammalian circadian clock. Cell 107, 855–867 (2001). [DOI] [PubMed] [Google Scholar]
- 14.Xu Y., et al. , Functional consequences of a CKIdelta mutation causing familial advanced sleep phase syndrome. Nature 434, 640–644 (2005). [DOI] [PubMed] [Google Scholar]
- 15.Etchegaray J. P., et al. , Casein kinase 1 delta regulates the pace of the mammalian circadian clock. Mol. Cell. Biol. 29, 3853–3866 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee H., Chen R., Lee Y., Yoo S., Lee C., Essential roles of CKIdelta and CKIepsilon in the mammalian circadian clock. Proc. Natl. Acad. Sci. U.S.A. 106, 21359–21364 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aryal R. P., et al. , Macromolecular assemblies of the mammalian circadian clock. Mol. Cell 67, 770–782.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Philpott J. M., et al. , Casein kinase 1 dynamics underlie substrate selectivity and the PER2 circadian phosphoswitch. eLife 9, e52343 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Preitner N., et al. , The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110, 251–260 (2002). [DOI] [PubMed] [Google Scholar]
- 20.Meng Q. J., et al. , Entrainment of disrupted circadian behavior through inhibition of casein kinase 1 (CK1) enzymes. Proc. Natl. Acad. Sci. U.S.A. 107, 15240–15245 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walton K. M., et al. , Selective inhibition of casein kinase 1 epsilon minimally alters circadian clock period. J. Pharmacol. Exp. Ther. 330, 430–439 (2009). [DOI] [PubMed] [Google Scholar]
- 22.Yoshitane H., et al. , Roles of CLOCK phosphorylation in suppression of E-box-dependent transcription. Mol. Cell. Biol. 29, 3675–3686 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robles M. S., Humphrey S. J., Mann M., Phosphorylation is a central mechanism for circadian control of metabolism and physiology. Cell Metab. 25, 118–127 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Kondratov R. V., et al. , BMAL1-dependent circadian oscillation of nuclear CLOCK: Posttranslational events induced by dimerization of transcriptional activators of the mammalian clock system. Genes Dev. 17, 1921–1932 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee C., Weaver D. R., Reppert S. M., Direct association between mouse PERIOD and CKIepsilon is critical for a functioning circadian clock. Mol. Cell. Biol. 24, 584–594 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsuchiya Y., et al. , Involvement of the protein kinase CK2 in the regulation of mammalian circadian rhythms. Sci. Signal. 2, ra26 (2009). [DOI] [PubMed] [Google Scholar]
- 27.Akashi M., Tsuchiya Y., Yoshino T., Nishida E., Control of intracellular dynamics of mammalian period proteins by casein kinase I epsilon (CKIepsilon) and CKIdelta in cultured cells. Mol. Cell. Biol. 22, 1693–1703 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Narasimamurthy R., et al. , CK1δ/ε protein kinase primes the PER2 circadian phosphoswitch. Proc. Natl. Acad. Sci. U.S.A. 115, 5986–5991 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu Y., et al. , Modeling of a human circadian mutation yields insights into clock regulation by PER2. Cell 128, 59–70 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rey G., et al. , Genome-wide and phase-specific DNA-binding rhythms of BMAL1 control circadian output functions in mouse liver. PLoS Biol. 9, e1000595 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koike N., et al. , Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science 338, 349–354 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spengler M. L., Kuropatwinski K. K., Schumer M., Antoch M. P., A serine cluster mediates BMAL1-dependent CLOCK phosphorylation and degradation. Cell Cycle 8, 4138–4146 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng B., et al. , Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell 105, 683–694 (2001). [DOI] [PubMed] [Google Scholar]
- 34.Bae K., et al. , Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron 30, 525–536 (2001). [DOI] [PubMed] [Google Scholar]
- 35.Fribourgh J. L., et al. , Dynamics at the serine loop underlie differential affinity of cryptochromes for CLOCK:BMAL1 to control circadian timing. eLife 9, e55275 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosensweig C., et al. , An evolutionary hotspot defines functional differences between CRYPTOCHROMES. Nat. Commun. 9, 1138 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Narumi R., et al. , Mass spectrometry-based absolute quantification reveals rhythmic variation of mouse circadian clock proteins. Proc. Natl. Acad. Sci. U.S.A. 113, E3461–E3467 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kloss B., et al. , The Drosophila clock gene double-time encodes a protein closely related to human casein kinase Iepsilon. Cell 94, 97–107 (1998). [DOI] [PubMed] [Google Scholar]
- 39.Yu W., Zheng H., Houl J. H., Dauwalder B., Hardin P. E., PER-dependent rhythms in CLK phosphorylation and E-box binding regulate circadian transcription. Genes Dev. 20, 723–733 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahesh G., et al. , Phosphorylation of the transcription activator CLOCK regulates progression through a ∼24-h feedback loop to influence the circadian period in Drosophila. J. Biol. Chem. 289, 19681–19693 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schafmeier T., et al. , Transcriptional feedback of Neurospora circadian clock gene by phosphorylation-dependent inactivation of its transcription factor. Cell 122, 235–246 (2005). [DOI] [PubMed] [Google Scholar]
- 42.He Q., et al. , CKI and CKII mediate the FREQUENCY-dependent phosphorylation of the WHITE COLLAR complex to close the Neurospora circadian negative feedback loop. Genes Dev. 20, 2552–2565 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Menet J. S., Abruzzi K. C., Desrochers J., Rodriguez J., Rosbash M., Dynamic PER repression mechanisms in the Drosophila circadian clock: From on-DNA to off-DNA. Genes Dev. 24, 358–367 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tischkau S. A., et al. , Protein kinase G type II is required for night-to-day progression of the mammalian circadian clock. Neuron 43, 539–549 (2004). [DOI] [PubMed] [Google Scholar]
- 45.Shim H. S., et al. , Rapid activation of CLOCK by Ca2+-dependent protein kinase C mediates resetting of the mammalian circadian clock. EMBO Rep. 8, 366–371 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eide E. J., Vielhaber E. L., Hinz W. A., Virshup D. M., The circadian regulatory proteins BMAL1 and cryptochromes are substrates of casein kinase Iepsilon. J. Biol. Chem. 277, 17248–17254 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown S. A., et al. , PERIOD1-associated proteins modulate the negative limb of the mammalian circadian oscillator. Science 308, 693–696 (2005). [DOI] [PubMed] [Google Scholar]
- 48.Partch C. L., Orchestration of circadian timing by macromolecular protein assemblies. J. Mol. Biol. 432, 3426–3448 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duong H. A., Robles M. S., Knutti D., Weitz C. J., A molecular mechanism for circadian clock negative feedback. Science 332, 1436–1439 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Doolittle R. F., The multiplicity of domains in proteins. Annu. Rev. Biochem. 64, 287–314 (1995). [DOI] [PubMed] [Google Scholar]
- 51.Baker C. L., Kettenbach A. N., Loros J. J., Gerber S. A., Dunlap J. C., Quantitative proteomics reveals a dynamic interactome and phase-specific phosphorylation in the Neurospora circadian clock. Mol. Cell 34, 354–363 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Larrondo L. F., Olivares-Yañez C., Baker C. L., Loros J. J., Dunlap J. C., Circadian rhythms. Decoupling circadian clock protein turnover from circadian period determination. Science 347, 1257277 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and SI Appendix.