Significance
Here we report the identification of staple and exotic food remains in Bronze and Early Iron Age dental calculus from the Southern Levant. The analysis of dietary plant microremains and proteins sheds new light on consumed exotic foods from South and East Asia during the second millennium BCE. We provide the earliest direct evidence in the Mediterranean to date for the consumption of sesame, soybean, probable banana, and turmeric. The recovery and identification of diverse foodstuffs using molecular and microscopic techniques enables a new understanding of the complexity of early trade routes and nascent globalization in the ancient Near East and raises questions about the long-term maintenance and continuity of this trade system into later periods.
Keywords: proteomics, Bronze Age, Eastern Mediterranean, spice trade, early globalization
Abstract
Although the key role of long-distance trade in the transformation of cuisines worldwide has been well-documented since at least the Roman era, the prehistory of the Eurasian food trade is less visible. In order to shed light on the transformation of Eastern Mediterranean cuisines during the Bronze Age and Early Iron Age, we analyzed microremains and proteins preserved in the dental calculus of individuals who lived during the second millennium BCE in the Southern Levant. Our results provide clear evidence for the consumption of expected staple foods, such as cereals (Triticeae), sesame (Sesamum), and dates (Phoenix). We additionally report evidence for the consumption of soybean (Glycine), probable banana (Musa), and turmeric (Curcuma), which pushes back the earliest evidence of these foods in the Mediterranean by centuries (turmeric) or even millennia (soybean). We find that, from the early second millennium onwards, at least some people in the Eastern Mediterranean had access to food from distant locations, including South Asia, and such goods were likely consumed as oils, dried fruits, and spices. These insights force us to rethink the complexity and intensity of Indo-Mediterranean trade during the Bronze Age as well as the degree of globalization in early Eastern Mediterranean cuisine.
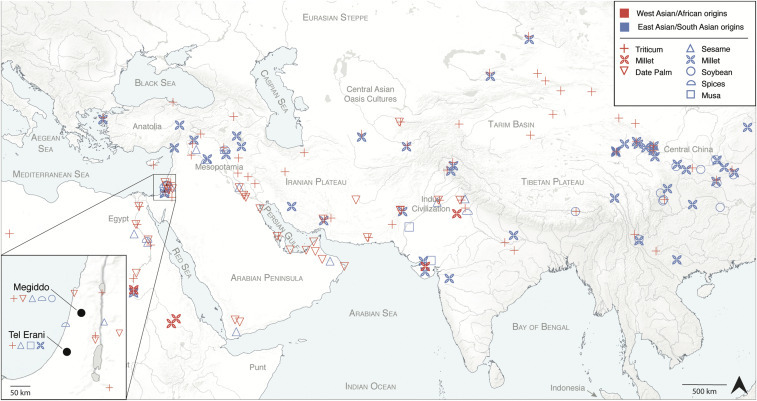
Long-distance trade across Eurasia has played a major role in connecting distant societies throughout recorded history, with the silk and spice trade being emblematic for early globalization (1–3). Recent decades of archaeological research have demonstrated the deep prehistory of these trans-Eurasian exchange networks and support a much earlier onset of globalization, known as “Bronzization” (4), which traces its roots to the Bronze Age during the third millennium BCE. The increasing importance of bronze served as a major impetus for the establishment of extensive trade contacts (5), which were largely driven by the uneven distribution of highly valued raw materials, such as tin, carnelian, and lapis lazuli (6–8). In addition to these raw materials, finished objects—as well as technologies, practices, and knowledge—were also conveyed over unprecedented distances. The major corridors of exchange connected Eurasia both by land across the Eurasian steppes and Iranian Plateau and by sea from India to the Near East via both the Persian Gulf and the Red Sea (9, 10). These networks served to link the major Bronze Age river valley societies of Egypt, Mesopotamia, the Indus Valley, and Central China to neighboring cultures in the Levant, the Arabian Peninsula, the Iranian Plateau, and the Central Asian oases (11–13). Such trade networks also further extended into Anatolia, the Aegean, and throughout South and East Asia. Despite periodic disruptions of some trade routes (14), the intensity of exchange gained momentum during the Middle and Late Bronze Ages of the second millennium BCE (15–17). During this period, bronze was produced on a large scale across Eurasia (4, 5, 18), and urban societies and early states linked by these trade routes developed a rapidly growing interest in exotic goods, including plant and animal products. In the early first millennium BCE, such trade networks had effectively linked West and East Asia, and several economically important crops had become widely dispersed throughout the continent (Fig. 1, and SI Appendix, Fig. S1).
Fig. 1.
Map of representative archaeobotanical evidence for the spread and trade of food crops prior to 500 BCE. See SI Appendix for data sources. Map Inset shows the location of the sites of Megiddo and Tel Erani on the southern Levantine coast; new dietary finds reported in this study are indicated for each site.
Evidence for Long-Distance Trade of Material Goods and Animals.
During the second millennium BCE, textual evidence in the Near East attests to a large amount of goods being transported over great distances. For example, cuneiform tablets from the Assyrian trade post of Kaneš (Kanesh) in Anatolia record caravans of hundreds of donkeys regularly transporting goods between the Mesopotamian city of Aššur (Assur) and Central Anatolia during the 19th and18th centuries BCE (19, 20). Starting with the expansion policy of Thutmosis III (15th century BCE) into the Levant, the flow of goods and people in West Asia intensified, which is documented most prominently in the famous Amarna letters (21). Ancient literary sources and illustrations also reference long-distance journeys to foreign lands to obtain exotic goods, such as ivory, ostrich eggshells, ebony, and frankincense. Among the most well-known of these accounts is an expedition initiated by the Egyptian queen Hatshepsut to the land of Punt (probably located in the Horn of Africa region) in the 15th century BCE (22, 23). In addition, evidence for long-distance trade between the Near East and the Indian subcontinent is growing for the second and third millennium BCE (8, 24), and includes written accounts (25–27), seals and stone weights (8, 28–30), shell, lapis lazuli, and carnelian jewelry (8, 24, 31, 32), and timber and ivory (33, 34).
Live animals were also transported long distances. Zooarchaeological and isotopic analyses have identified the movement of donkeys from Egypt to the Southern Levant during the third millennium BCE (35), and ancient DNA analysis has documented the transport of pigs from Italy to the southern Greek mainland (36) and from the Aegean to the Southern Levant during the second millennium BCE (37). In addition, depictions of zebu (Bos taurus indicus), a humped subspecies of cattle native to South Asia (38), have been found in Mesopotamia as early as the third millennium BCE (39), and a clear depiction of a zebu (Fig. 2) appears on a bichrome ceramic vessel from the southern Levant dating to 16th century BCE, the same period when depictions of zebu also become common in Egypt (39). Zooarchaeological study of faunal remains and genetic data further confirm the presence of zebu cattle in the Levant during the second millennium BCE (37, 40), and genetic evidence suggests possible taurine–zebu hybridization at the site of Megiddo at approximately 900 BCE (37). Monkeys depicted in 18th and 17th century BCE frescoes at Akrotiri on the Aegean island of Thera were recently identified as South Asian gray langurs (Semnopithecus sp.), with a probable origin in the Indus valley (41), and the identification of dermestid khapra beetles (Trogoderma granarium) that had infested a wheat deposit from a Middle Kingdom (2050 to 1710 BCE) Egyptian tomb at the site of el-Gebelein points in the same direction (42). At the same time, other animals, such as domestic chicken (Gallus gallus domesticus) were brought from East Asia to the Near East (possibly via South Asia) and displayed as exotic curiosities and used for cockfighting. Sporadic evidence of chicken occurs in Anatolia, Iran, Syria, and Egypt as early as the third millennium BCE, and by the late first millennium BCE some sites in the Levant appear to have economically specialized in chicken husbandry (43).
Fig. 2.
Iconographic representation of a zebu on a bichrome ceramic vessel from Tel Gerisa, Israel, 16th century BCE (photo courtesy of Zeev Herzog).
Evidence for Long-Distance Trade of Culinary and Economic Plants.
Although evidence for the movement of durable goods and animals is richly attested by historical and archaeological evidence, direct evidence for culinary and economic plants is more limited. Plant remains are highly perishable, and only certain conditions lead to macrobotanical preservation, which may be biased with respect to plant type or part (44). Nevertheless, plant remains, such as charred seeds, have documented the eastward spread of wheat and barley across Eurasia during and after the late fourth millennium BCE (45), as well as the westward spread of millet cultivation from East Asia during the third millennium BCE (9, 46, 47). Macrobotanical and microbotanical evidence also confirm the establishment of citron (Citrus medica), a fruit tree of South/Southeast Asian origin (48, 49), as an important crop in the Levant and Egypt by the first millennium BCE (50–52), although it may have been first introduced into the Eastern Mediterranean as early as the fourth millennium BCE (53, 54). Melon (Cucumis melo), another important crop of South Asian origin (55), was also cultivated in the Near East during the Bronze Age. Textual references to melons appear in third millennium BCE Sumerian texts, and melons and cucurbits are depicted in Egyptian tombs from the Old Kingdom onwards (54, 56, 57). Cucurbit seeds have been reported in Near Eastern archaeological contexts as early as the sixth century BCE (54), but finds prior to the first millennium BCE are less secure in their taxonomic assignment (56).
Beyond grains and fruits, there is also growing evidence for a spice trade between South Asia and the Eastern Mediterranean. This is supported by recent findings from organic residue analysis, including evidence for vanillin (58) and possibly also cinnamon (59), nutmeg, and jasmine (60), although evidence for the latter three spices requires further confirmation. Whereas many aspects of this early trade remain unknown, some extraordinary finds leave no doubt that an Indo-Mediterranean spice trade already existed during the Bronze Age (6, 9, 59). Peppercorns used in the mummification of Ramses II in 1213 BCE, for example, are native to southern India (61–63), and cloves, originally from Indonesia, were found at Terqa, Syria dating to 1720 BCE (64, 65), having likely followed an indirect route to Mesopotamia via South Asian trade routes (30, 66). Both of these examples highlight the extent of Indian Ocean trade during this period, despite the declining influence of the Indus Valley and the restructuring of political networks throughout the region (33).
Emerging Picture of a Dynamic and Complex Exchange Network.
While the details of Bronze Age trade remain patchy, the overall evidence points toward the existence of a dynamic and complex exchange network connecting the Mediterranean with South Asia and beyond during the Middle Bronze Age (approximately 2000 to 1550 BCE), Late Bronze Age (approximately 1550 to 1200/1150 BCE), and Early Iron Age/Iron Age I (approximately 1200/1150 to 1000 BCE). Here we aim to explore the transformation of eastern Mediterranean cuisine as a consequence of Bronze Age globalization by analyzing microscopic and molecular traces of food remains in human dental calculus, a calcified form of dental plaque, from the Southern Levant during the second millennium BCE (Table 1). We focus on two sites, the Middle to Late Bronze Age urban center of Megiddo (17th to 15th centuries BCE) and the Early Iron Age site of Tel Erani (11th century BCE) (Fig. 1). In total, we analyze dental calculus from 16 individuals: 13 from Megiddo (SI Appendix, Fig. S2) and 3 from Tel Erani (SI Appendix, Fig. S3).
Table 1.
Overview of dietary findings
| Individual | Burial | Context* | Microremains | Dietary proteins |
| Megiddo, Middle Bronze Age III to Late Bronze Age I, n = 13 | ||||
| MGD001 | Tomb 50 | “King”; elite masonry chamber tomb (triple burial); ca. 1650–1550 BCE | Poaceae, cf. Triticum, Phoenix (date palm), Arecaceae unspecific (palm), bark, eudicot | None |
| MGD002 | Tomb 50 | “Queen”; elite masonry chamber tomb (triple burial); ca. 1650–1550 BCE | Poaceae, eudicot | None |
| MGD006 | Tomb 16/H/45 | Double pit burial; ca. 1550–1450 BCE | Poaceae, eudicot | None |
| MGD007 | Tomb 16/H/45 | Double pit burial; ca. 1550–1450 BCE | Poaceae | None |
| MGD008 | Tomb 12/K/89 | Pit burial; 1496–1320 BCE | Poaceae, eudicot | None |
| MGD009 | Tomb 12/K/96 | Double pit burial; ca. 1650–1400 BCE | Poaceae, cf. Triticum, Triticeae, eudicot | None |
| MGD010 | Tomb 12/K/96 | Double pit burial; 1638–1413 BCE | Poaceae, Triticeae, eudicot | None |
| MGD011 | Tomb 14/K/119, lower | Stone-lined cist, exotic grave goods; 1688–1535 BCE | Poaceae, cf. Triticum, Phoenix (date palm), eudicot | Sesamum, Triticum/Aegilops |
| MGD013 | Tomb 10/K/118 | Triple pithos burial; ca. 1650–1400 BCE | Poaceae, Phoenix (date palm), eudicot, bark | Not analyzed |
| MGD016 | Tomb 14/K/49 | Double brick-lined pit Burial; 1509–1432 BCE | Poaceae, eudicot | None |
| MGD017 | Tomb 100 | Masonry chamber tomb with corbelled roof, many commingled individuals; exotic grave goods; ca. 1650–1400 BCE | Poaceae, Cyperaceae, Phoenix (date palm), Arecaceae (palm), eudicot | None |
| MGD018 | Tomb 100 | Masonry chamber tomb with corbelled roof, many commingled individuals; exotic grave goods; ca. 1630–1550 BCE | Poaceae, eudicot, Triticeae | Curcuma, Glycine |
| MGD021 | Tomb 50 | Elite masonry chamber tomb (triple burial); ca. 1650–1550 BCE | Poaceae, Triticeae, Phoenix (date palm), eudicot | Not analyzed |
| Tel Erani, Early Iron Age, n = 3 | ||||
| ERA005 | Burial L2091 | Burial offering of one juglet; ca. 1100–1000 BCE | Poaceae | Sesamum |
| ERA017 | Burial L2160 | Burial offering of one Flask; ca. 1100–1000 BCE | Poaceae, Cyperaceae, eudicot, panicoid cf. millet | Musa |
| ERA023 | Burial L2181 | No burial offerings; ca. 1100–1000 BCE | Poaceae, eudicot | Sesamum |
Dates are provided as relative dates (marked with “ca.”) or calibrated radiocarbon dates (2σ). See Dataset S1.
During the Middle and Late Bronze Age, Megiddo was a major urban center in the Southern Levant, and it was embedded within long-distance networks and ruled by local kings. We selected Megiddo because it had already yielded suggestive evidence for exchange with South Asia in the form of both zebu genetic evidence (37) and vanillin residues (58). In this study, we analyze individuals from a variety of mortuary contexts, including pit burials, brick-lined pit burials, pithos burials, masonry-constructed collective tombs, and a recently excavated Middle Bronze Age royal tomb containing the so-called “king” (MGD001) and “queen” (MGD002) of Megiddo (SI Appendix).
Dating to ∼500 y later in time, the Tel Erani cemetery is one of the few Early Iron Age cemeteries excavated to date in Israel (SI Appendix). Although the related settlement is less understood, the cemetery has been associated with the “Philistine” occupation at the Southern Levant from the 12th century BCE onwards. In contrast to Megiddo, where several individuals sampled by us derived from high- or highest-status burial contexts, the individuals from Tel Erani instead appear to represent the rural general population of the Early Iron Age in this region.
By analyzing the two sites, we aim to gain a broader perspective on Levantine cuisines during the second millennium BCE, a period that witnessed the blossoming of Middle Bronze Age city states, periods of Egyptian domination and retreat during the Late Bronze Age, and the emergence of the so-called Philistines at the onset of the Early Iron Age during the 12th century BCE
Results
Plant remains were abundant in the Megiddo and Tel Erani dental calculus, and plant microremains and proteins were observed in all 16 dental calculus samples. Of these, probable dietary microremains were identified in all 16 analyzed samples (SI Appendix, Table S1), and included phytoliths consistent with wheat (Triticum), panicoid/millet (Panicoideae), and date palm (Phoenix sp.). Dietary proteins were observed in 5 of 14 analyzed specimens (SI Appendix, Table S2), and consisted of 19 dietary proteins from cereals (Triticum/Aegilops), oilseeds (Sesamum, Glycine), fruits (Musa), and spices (Curcuma).
Microremains.
Microremains were analyzed from all 16 individuals, including 2 individuals (MGD013 and MGD021) with insufficient calculus to conduct proteomic analysis (SI Appendix, Table S1 and Datasets S2 and S3). Representative examples of the dietary microremains observed in the Megiddo and Tel Erani dental calculus are provided in Fig. 3. Both the Megiddo and Tel Erani individuals produced microremains assemblages consisting of three main components: 1) Dietary microremains, most notably phytoliths (84 total morphotypes, 4,983 phytoliths), but also starches (333 granules); 2) nondietary remains, such as fibers; and 3) ambiguous microremains, such as fungal particles, charcoal particles, and other remains of unknown or ambiguous origin (Dataset S2). The abundance of microremains observed across individuals was highly variable (SI Appendix, Fig. S4), ranging from 9 (MGD007) to 1,795 (MGD001).
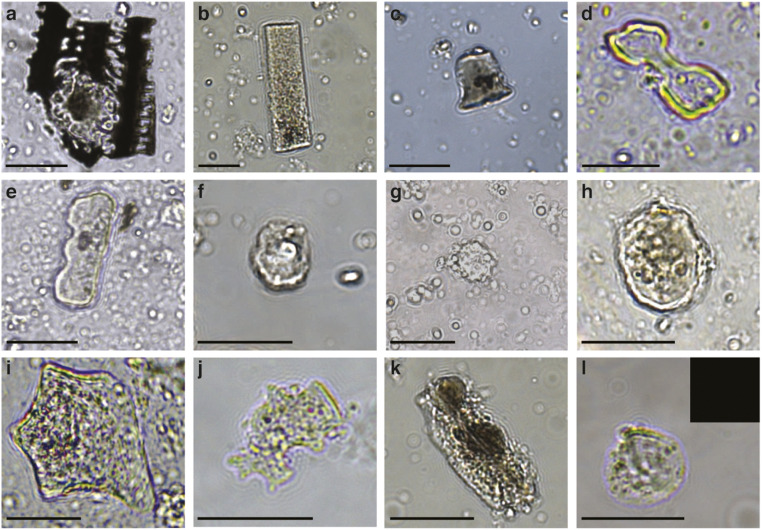
Fig. 3.
Microremains in Megiddo and Tel Erani dental calculus. (A) Articulated Poaceae husk phytolith, identified as wheat (MGD001). (B) Poaceae stem/leaf phytolith (MGD001). (C) Poaceae short cell rondel (MGD001). (D) Wide-lobed bilobate short cell identified as panicoid (ERA017). (E) Poaceae polylobate short cell (MGD001). (F) Cone phytolith identified as sedge leaf (MGD018). (G) Spheroid echinate identified as date palm (MGD001). (H) Spheroid echinate phytolith, identified as nondiagnostic palm (MGD001). (I) Polyhedral plate phytolith, identified as eudicot (MGD011). (J) Decorated jigsaw phytolith, likely from fruit (MGD001). (K) Spheroid psilate phytolith, identified as bark type (MGD001). (L) Damaged Triticeae starch in brightfield, with Inset showing an absence of birefringence in cross-polarized light (MGD010). (Scale bars, 20 μm.)
Phytoliths, a robust and abundant type of plant microfossil, allow the identification of vascular plants, and among angiosperms (flowering seed plants), they primarily form in monocots, particularly in members of the plant family Poaceae (grasses, including cereals). In contrast, magnoliids, eudicots, and other angiosperms (which include most fruits and vegetables) tend to form few phytoliths, while gymnosperms (nonflowering seed plants) produce even fewer (67). The dental calculus phytoliths observed in this study corresponded to these expected proportions (SI Appendix, Figs. S4 and S5) and were predominantly derived from the leaves, stems, and husks of wild or domestic Poaceae (SI Appendix, Fig. S6), totaling 2,551 phytoliths, plus an additional 916 unspecific Poaceae phytoliths (SI Appendix, Table S1). Smaller numbers of morphotypes from other monocots such as Cyperaceae (sedges) and Arecaceae (palms), as well as eudicot fruit/leaf phytoliths and nondiagnostic bark (angiosperm or gymnosperm), were identified in 13 individuals (SI Appendix, Fig. S7), totaling 245 phytoliths (SI Appendix, Table S1). Starch granules were rare in all samples, except ERA23 (SI Appendix, Fig. S4), even in samples not exposed to heat during protein extraction, and few could be identified. However, individual starch granules consistent with Triticeae were found in ERA023, MGD018, and MGD021 (SI Appendix, Table S1). In addition, we found 26 other types of microremains whose origins are either ambiguous (pollen, fungal particles, diatoms, foraminifera, sponge spicules, charcoal, fibers, insect fragments) or possible contaminants (skin scales) (Datasets S2 and S3). These microremains were not further analyzed.
Among phytoliths assigned to Poaceae, all morphotypes that form in grasses were represented, including those forming in epidermal short cells, long cells, bulliforms, hairs, papilla, and stoma (Dataset S2), in both single-cell and articulated forms. Grass phytoliths predominantly belonged to the pooid group (n = 432), but a smaller number of single-cell short cells could be assigned to the chloridoid (n = 51) and panicoid (n = 10) grass types (SI Appendix, Fig. S8). Although most phytoliths could not be assigned to specific wild or domesticated sources, the assemblage is consistent with grain consumption, and 105 articulated dendritic types were identified in 8 individuals (ERA017, MGD001, MGD002, MGD009, MGD010 and MGD011, MGD017, and MGD021). Among these, 10 articulated husk phytoliths consistent with Triticum (wheat) were identified in MGD001, MGD009, and MGD011, and 5 short cells (wide lobed bilobates) deriving from Panicoideae grasses (e.g., millets) were found in ERA017 (SI Appendix, Table S1 and Dataset S2).
Cones representing sedge (Cyperaceae) leaf were found in MGD001, MGD017, MGD021, and ERA017 (SI Appendix, Fig. S7 and Table S1). Such cones form in a variety of sedge cells, including sedge achenes and achene bracts cells. Palm (Arecaceae) phytoliths were identified in five individuals (SI Appendix, Table S1). A total of 12 globular rugulate/echinate phytoliths of date palm (Phoenix) were observed in MGD001, MGD011, MGD013, MGD017, and MGD021, and phytoliths from another unknown species of palm were also detected in MGD001 and MGD017 (Datasets S1 and S2). Globular echinate palm leaf phytoliths are known to be a particularly resilient morphotype (68). Finally, 217 plate, jigsaw, sclereid, tracheid, and related phytoliths from eudicot epidermal tissue were identified in 14 individuals, including a single Megiddo individual (MGD001) with 3 jigsaw morphotypes displaying protuberance decorations thought to originate from the eudicot epidermal tissue of fruit and seeds (SI Appendix, Fig. S7 and Datasets S2 and S3) (69).
Proteomics.
Total protein was extracted from the dental calculus of 14 individuals. Protein recovery was variable, but all samples yielded proteins typical of an oral microbiome (SI Appendix, Fig. S9 and Dataset S4) and contained damage-associated modifications (N,Q deamidation) (SI Appendix, Table S3) consistent with ancient samples. Dietary proteins were identified in 5 individuals and consisted of 19 proteins from 5 plants of known dietary importance: wheat (Triticum/Aegilops), sesame (Sesamum), soybean (Glycine), banana (probable Musa), and turmeric (Curcuma) (Fig. 4 and SI Appendix, Fig. S10 and Table S2). Detailed information regarding the identified peptides and peptide spectral matches (PSMs) for each protein is provided in Dataset S5.
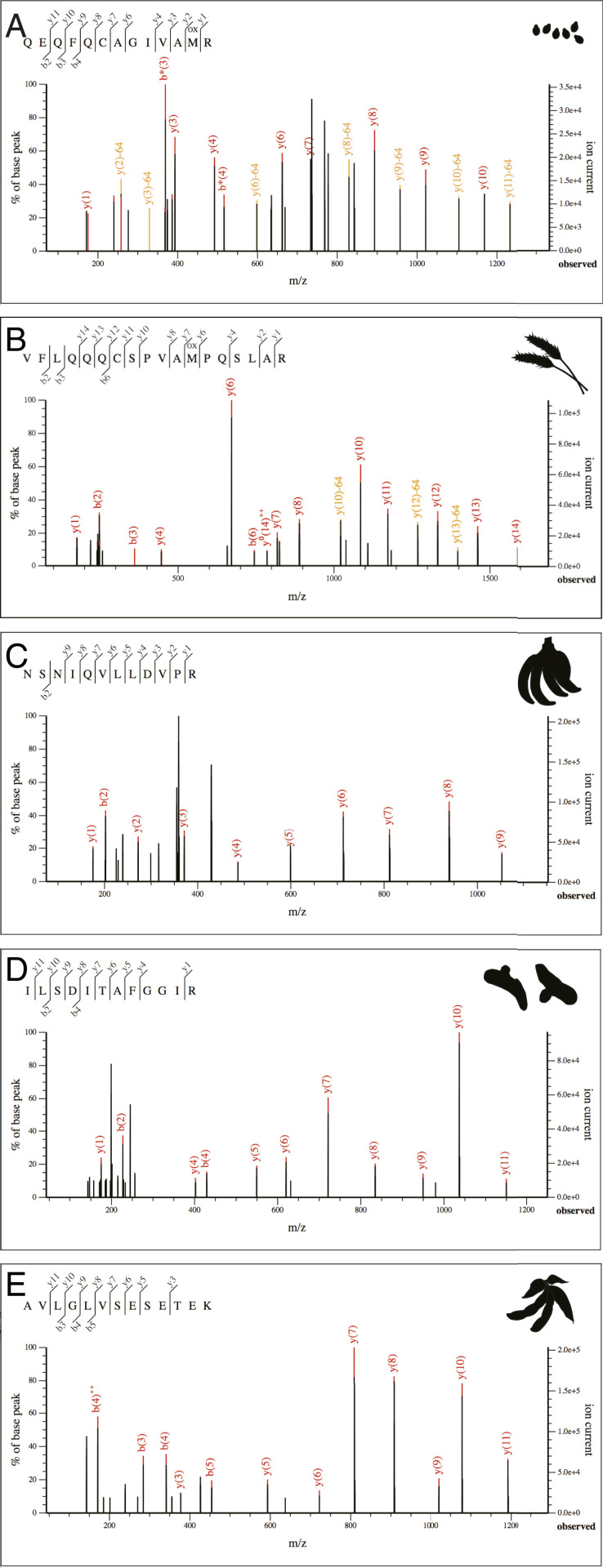
Fig. 4.
Representative MS/MS spectra of selected dietary peptides. (A) Sesamum, 11S globulin protein (MGD011). (B) Triticum/Aegilops, LMW glutenin (MGD011). (C) Musa, β-1,3 glucanase (ERA017). (D) Curcuma, turmerin (MGD018). (E) Glycine, sucrose-binding protein (MGD018).
Wheat proteins were identified in a single individual from Megiddo (MGD011) and consisted of two major seed proteins: α-amylase inhibitor (AAI) and low molecular weight (LMW) glutenin. AAI makes up ∼4% of the protein content of wheat and is highly resistant to heat or proteolytic digestion (70). The identification of AAI was supported by three PSMs, with two PSMs matching specifically to either Triticum aestivum or Aegilops tauschii (a wild progenitor of T. aestivum), while the third peptide was less specific and is also found in Hordeum (barley). LMW glutenin, a major gluten protein, was identified with the support of 3 peptides and 13 PSMs. Gluten proteins make up ∼80% of the total proteins in whole wheat flour, and LMW glutenin accounts for 20 to 35% of the gluten protein content (71).
Sesame proteins were identified in one individual from Megiddo (MGD011) and two from Tel Erani (ERA005, ERA023) and consisted of 2 proteins supported by a total of 29 peptides and 78 PSMs: 11S globulin and 2S albumin seed storage proteins. These two seed storage proteins are tissue-specific and expressed during seed maturation. Together, they make up most of the protein in sesame seeds, accounting for 60 to 70% and 15 to 25% of total seed protein, respectively (72).
Soybean was identified in a single Megiddo individual (MGD018) and is supported by 30 PSMs specific to the proteins glycinin, an 11S storage protein, and β-conglycinin, a 7S storage protein. These two proteins are the primary storage proteins in soybean seeds, and together they make up ∼65% of the total soybean protein content (73). In addition to these two seed storage proteins, we also identified two peptides supported by two PSMs specific to soybean sucrose-binding protein, another member of the seed storage protein superfamily.
We identified endo-1,3-β-glucanase, an enzyme important in fruit ripening, in a single individual from Tel Erani (ERA017). This protein was supported by two peptides, of which one is highly specific to banana (Musa or Ensete), while the second is found in Musa and a number of other flowering plants, but not Ensete. Of the two plants, only members of Musa produce edible fruits, while Ensete (Abyssinian banana) is consumed for its starchy pseudostem and corm. The fruit of ripe bananas contains few proteins but relatively high concentrations of β-glucanases (74), which are highly resistant to heat and proteolytic degradation (75). Because this protein is expressed in fruit and peel and increases in abundance as the fruit ripens (76, 77), we tentatively identify this enzyme as originating from Musa. However, the enzyme is also expressed in generalized stress response, and thus is also present in diseased and injured plant tissues. Taken together, the identification of Musa is most strongly supported, but Ensete cannot be fully excluded.
Finally, we identified the turmeric protein turmerin, supported by three peptides and four PSMs, in a single individual from Megiddo (MGD018). Turmerin is an α-amylase/trypsin inhibitor that belongs to the leguminous kunitz-type serine inhibitor family of proteases. It has known antioxidant activity (78) and also plays a role in plant defense. Although making up only 0.1% of the dry-weight of turmeric, it is one of the most stable turmeric proteins, being resistant to heat, digestive enzymes, and UV irradiation (78).
Discussion
Among the identified dietary taxa, wheat and date palm were expected finds, as wheat has been a staple crop in the Levant since the seventh millennium BCE (79) and date palm fruits have been consumed and traded since at least the fifth millennium BCE (54, 57, 80). Moreover, date palm seeds and leaves have been previously recovered from Late Bronze II burials at Megiddo (81). Microfossil remains of both crops have been previously identified in the Bronze Age Levant (82–84), and here we identified both wheat and date palm phytoliths, as well as wheat proteins, including a major wheat gluten.
Sesame and millet, although not unexpected, are important new finds. These nonlocal domesticates from South and East Asia, respectively, spread to West Asia during the Bronze Age (85), but their arrival in the Levant is less well understood. To date, the oldest remains of sesame seeds (Sesamum indicum) have been found at Harappan sites in the Indus Valley (2500 to 2000 BCE), but charred and desiccated seeds have also been sporadically recovered at sites in the Near East since the late third millennium BCE (86, 87). The Akkadian word “šamaššammȗ,” which refers to an oil plant (possibly sesame), appears in cuneiform texts from 2400 BCE onwards (87–89), and although questioned in the past, the identification of sesame seeds in the tomb of Tutankhamun (14th century BCE) is now considered credible (90). We found robust evidence for multiple Sesamum proteins in individuals at both Megiddo and Tel Erani, suggesting that by the second millennium BCE, sesame had become a staple oil-bearing crop in the Levant.
We identified Panicoideae phytoliths consistent with dietary millets in a single individual at Tel Erani. Although not identifiable below the taxonomic level of subfamily, the Eurasian grasses Setaria and Panicum and the African grasses Sorghum and Pennisetum are possible candidates. Among these, Sorghum and Pennisetum are unlikely, as there is no evidence for the dispersal of sorghum into the Levant prior to the medieval period (54), and although African pearl millet (Pennisetum glaucum) had spread from East Africa to South Asia by the mid-second millennium BCE (91, 92), there is no evidence for its cultivation in the Near East. In contrast, Asian broomcorn (Panicum miliaceum) and foxtail (Setaria italica) millets are known to have reached western Eurasia via Central Asia (93, 94) by the second or possibly third millennium BCE (54, 95), although likely no earlier based on recent radiocarbon dating and collagen stable isotope studies (96). Within the Near East, current macrobotanical evidence suggests that S. italica and P. miliaceum millets functioned as minor crops from the first millennium onwards (54, 97–100), and this is consistent with our identification of the panicoid phytoliths at Iron Age Tel Erani.
In contrast to the crops above, the consumption of soybean (Glycine), probable banana (Musa), and turmeric (Curcuma longa) were unexpected finds. Soybean cultivation was unknown in this region before the 20th century CE and, like millet, its domestication center was near the Yellow River in Central China, where it was cultivated as early as 7000 to 6500 BCE (101). However, soybean, like sesame, is a major oil plant, and its oil could have been transported over long distances. Exotic oils are frequently mentioned in Old Babylonian, Akkadian, and Egyptian texts (102–104). However, many of the plants from which they derive remain untranslated or unknown; for example, it is still disputed whether Egyptian “baq oil” refers to olive oil, moringa oil, or to the oil of another plant (103). There was high demand for oils of different flavor and origin in ancient Mesopotamia and Egypt, where such oils played vital roles in cuisine, medication, bodycare, and illumination. Furthermore, they were part of a broad spectrum of daily and ritual practices, where they were used, for example, to anoint objects and embalm the dead (103–106). Recent X-ray computed tomography imaging of archaeological soybeans indicates that oil content was a major target of selection during domestication, and cultivars with high oil content were prevalent in China by approximately 2000 BCE (107). The relative scarcity of evidence for soybean in the archaeological record might be explained by its predominant use as an oil combined with a lack of organic residue analysis on relevant material and/or the difficulty of differentiating plant oils through lipid analysis. Proteomics has been previously shown to be a powerful tool for identifying archaeological oils and fats (108), as plant oils and rendered animal fats produced using preindustrial techniques generally contain residual proteins. By identifying strong evidence of soybean seed proteins in dental calculus, we confirm that Megiddo individual MGD018, who was also buried with other exotic grave goods (Table 1 and SI Appendix), likely had access to soybean oil, and we further demonstrate the utility of proteomics as a method for identifying exotic oils in the Near East that otherwise leave few archaeological traces.
Banana (Musa) was domesticated during the fifth millennium BCE in New Guinea (109) and by the first millennium BCE had dispersed under human cultivation as far west as Cameroon in West Africa (110, 111). However, reconstructing the intervening cultivation and trade of bananas has proven particularly difficult to trace through the archaeological record. Banana fruit is highly perishable and domesticated bananas are seedless (reproducing instead by cuttings), and thus there is a strong bias against the recovery of banana macrobotanical remains. Phytoliths represent the principle method employed to trace early banana use, but banana phytolith recovery from archaeological contexts is generally low, perhaps due to the limited number of phytoliths produced by the plant, the lack of phytoliths in the consumed mesocarp, and other taphonomic factors (112, 113). Direct evidence of banana, in the form of phytoliths, has been found at three Indus sites dating to the late third millennium (114–117), placing the crop in South Asia by that date. The earliest reported archaeobotanical evidence for banana in the Near East is desiccated fruit pulp recovered from a vessel in an Egyptian 18th Dynasty tomb (15th ct. BCE), but the identification is highly contested (118, 119). Other scholars have argued for an introduction of banana in the Arabian peninsula prior to the ninth century BCE (120), but the first secure identification of Musa in the Near East consists of a banana leaf found in an Egyptian tomb at Antinoë dating to the fifth century CE (121), long after the crop had already spread further west to become a staple in West Africa (110, 111). The prehistoric spread and use of banana in South and West Asia remains poorly known, in large part due to preservation biases. Our identification of a major banana fruit-ripening protein in the dental calculus of individual ERA017 at Tel Erani lends support for either the banana being present in the Levant by the first millennium BCE or a mobile individual (e.g., a merchant or seafarer) who consumed banana during his lifetime in South or East Asia before being buried at Tel Erani. This identification, although only supported by two peptides, is nevertheless specific, and within the broader banana family Musaceae the peptides are consistent only with Asian bananas (Musa), to the exclusion of other related plants, such as the African enset (Ensete), also known as the Abyssinian banana. The protein itself, which is abundant in ripening fruits, is also supportive of a Musa fruit identification, as the fruits of the Abyssinian banana are inedible and only the starchy portions of the pseudostem and corm are consumed.
The Asian tropical plant family Zingiberaceae contains numerous economically useful plants used for food, spices, medicines, dyes, and perfumes (122, 123). Among these, the rhizomes of the genus Curcuma are consumed as foods in South and Southeast Asia, of which domesticated turmeric (C. longa) is among the most important and widely used. Turmeric starch grains have been identified in both cattle dental calculus and pottery at the Harappan site of Farmana, dating to between 2600 and 2200 BCE (124–126). Turmeric has multiple uses as both a spice and a cloth dye (72), and early medical texts in China and India also report its use as a medicinal (127). Within the Near East, the earliest references to turmeric appear during the seventh century BCE in Assyrian cuneiform medical texts from Ashurbanipal’s library at Nineveh (128), but no archaeological evidence has been found prior to the Islamic period during the 11th to 13th centuries CE (1). Our identification of Curcuma protein at the site of Megiddo suggests that it was already present or accessible to individuals in the Levant as early as the mid-second millennium BCE. Interestingly, turmeric protein was identified at Megiddo in the same individual (MGD018) whose dental calculus contained soybean protein. This individual was buried in a wealthy collective tomb containing exotic goods (Table 1 and SI Appendix), suggesting that this individual was either well connected with trading activities or may have even been a merchant or trader themself. As such, the individual may have consumed foods seasoned with turmeric or prepared with soy oil in the Levant, in South Asia, or elsewhere.
Overall, the plant microremains and proteins identified in dental calculus from Megiddo and Tel Erani point toward the existence of a dynamic and complex exchange network connecting the Mediterranean with South Asia during the second millennium BCE that outlived the dramatic socio-political transformation and associated shift from centrally organized trade during the Bronze Age to diverse small-scale trade entrepreneurship from the Early Iron Age onwards. Historically, archaeologists and historians in the Near East have relied on texts, iconography, and macrobotanical remains as their primary sources of information in reconstructing the region’s cuisine and trade connections. However, these approaches alone can be limiting. Although the text base is rich, including both cuneiform texts and papyri, many of the attested plant-related terms cannot yet be translated or fully understood, despite being transliterated. Iconography, while providing vivid images of the past, can lack the botanical detail necessary to make conclusive identifications, and macrobotanical remains are generally strongly biased toward grains and other seeds, especially those that have been carbonized. While important, these methods can miss oils, fruits, and spices that are unlikely to carbonize and which may have been traded in small quantities or in already processed powdered or liquid forms.
Here we demonstrate the utility of combining microscopic and molecular techniques to reveal a broader range of foodstuffs that include both staple grains, as well as oils, fruits, and spices that otherwise leave behind little macrobotanical evidence. In particular, we found that plant phytoliths primarily reflected bulk dietary items, and were dominated by high levels of grass phytoliths, most likely deriving from wheat, but also including probable millets. We also identified palm phytoliths, almost certainly from date fruit. All of these resources are monocots, reflecting their tendency to be well represented by phytoliths. In contrast, protein analysis revealed a wider diversity of foods and is better at identifying plants with low levels of silicification and therefore few phytoliths, especially if they are protein-rich. Although the modes of protein preservation and incorporation in ancient dental calculus are still under study, most of the proteins we identified were either protease inhibitors or belonged to the seed storage protein superfamily. Both types of proteins are highly stable against proteolysis and thermal processing, traits that may increase their likelihood of survival in archaeological calculus. These same traits can also contribute to their potential allergenicity (129), and many of the identified proteins are also known allergens. In addition to being resilient, several of the proteins we identified, especially seed proteins, are also highly abundant in the foods from which they originate, and similar seed storage proteins have been previously identified in Neolithic pottery residues (130) and in dental calculus from the medieval and postmedieval periods (131–133).
The identification of sesame, soybean, banana, and turmeric proteins in Megiddo and Tel Erani dental calculus points to the need to reevaluate the current evidence for the second millennium BCE Indo-Mediterranean trade. Previous suggestions of such a trade network between India and Egypt were largely ignored due to the limited physical evidence (119, 134). However, such evidence is now growing, and thus earlier identifications of plants (e.g., jasmine, nutmeg, cinnamon), both from archaeological remains and from texts, should be reconsidered. The broader body of evidence for exotic goods, which also includes zebu cattle, chickens, citron, melon, cloves, millet, vanillin, peppercorns, monkeys, and beetles, points to a pattern of established trade. The individuals from Megiddo, tomb 100 (MGD017, MGD018) not only had access to exotic food, but were also buried with precious grave goods (Dataset S2), which might indicate a higher-status position or the collective burial of members of a trading house. Such traders and travelers may have transported cargoes of animals, spices, dried fruits, oils, and perfumes via different routes, either overland through Iran and the Central Asian oases or by sea across the Indian Ocean to either the Red Sea or Persian Gulf, or both.
This study highlights the potential of microscopic and molecular methods to reveal elements of trade and cuisine that otherwise leave few archaeological traces. As detection methods continue to improve, there will likely need to be a fundamental reconsideration of the dimensions and complexities of Bronze Age trans-Eurasian trade. Although named for a metal that is highly visible in the archaeological record, the process of Bronzization was likely a much broader phenomenon that also linked cuisines and economies across Eurasia.
Materials and Methods
Excavation.
All individuals analyzed in this study were excavated and documented in their archaeological context from the sites of Megiddo and Tel Erani within the last decade (Dataset S1). An archaeological and anthropological overview of each burial is provided in SI Appendix. Burials have been dated using radiometric methods and/or associated grave goods.
Sampling.
Dental calculus sampling was performed in a clean laboratory at the Tel Aviv University Megiddo excavation archives and at the Tel Erani excavation storage facility of the Israel Antiquity Authorities. Nitrile gloves were worn during collection, and calculus was sampled using dental curettes that were replaced or cleaned with isopropanol between samples. Calculus was collected onto weighing paper or aluminum foil and packaged individually. Samples were received at the Max Planck Institute for the Science of Human History ancient proteomics laboratory, where they were weighed and subsampled prior to protein extraction. Approximately 5 to 10 mg of dental calculus was used for protein analysis, and 0.5 to 2 mg of calculus was subsampled and transferred into 1.5-mg Eppendorf tubes for analysis by microscopy. These samples, along with nine pellets remaining after protein extraction were transported to the Max Planck Institute for Evolutionary Anthropology for analysis of microremains.
Microremains.
Prior to analysis, we first tested for the presence of surface contaminants in a subset of samples (n = 2; MGD002, MGD016) by performing a staged decalcification to compare the relative abundance and composition of surface and interior microremains (135, 136). We found surface contamination to be negligible (SI Appendix, Fig. S11), and so proceeded to process the remaining samples.
To extract the microremains we added ∼1 mL of 0.5 M EDTA to decalcify our preweighed dental calculus samples that were in 1.5-mL Eppendorf tubes under a Bio Air Aura Mini laminar flow in the Department of Primatology at the Max Planck Institute for Evolutionary Anthropology, Leipzig. Samples were left in EDTA until decalcification was complete, which varied from a few hours to a few days. In two samples (MGD002 and MGD016), we performed a predecalcification to separate microremains on the outside of the calculus pieces from the interior (staged decalcification) (SI Appendix). The samples were then centrifuged at 2,000 × g for 10 min (Roth Minicentrifuge) and EDTA was removed from the samples by pipetting the supernatant. This process was repeated three times. Then 25% glycerine for mounting was added to the tube. In addition to analyzing whole calculus pieces, cellular debris pellets left over after protein extraction (see next section) were also analyzed for ERA005, ERA017, ERA023, MGD001, MGD002, MGD009, MGD010, MGD011, and MGD017. For these pellets, 100 μL of 25% glycerine solution was slowly added to the tubes to avoid spillage loss due to foaming of residual SDS from protein extraction. For all samples, 20 µL of each sample were mounted on glass slides with 18 × 18- or 22 × 22-mm coverslips depending on volume. The mounting was performed in a laminar flow hood and examined under brightfield and cross-polarized light on a Zeiss Axioscope microscope at 400× magnification (Num. Aperture = 0.95). Microremains were analyzed by examining the whole slide and any encountered microremains were photographed, described, and documented using the procedures described in SI Appendix, Evidence of Long-Distance Trade in the Ancient World, and Procedures for Microremains Analysis.
Proteomics.
Protein extractions were performed on 14 dental calculus samples using a filter-aided sample preparation protocol modified for ancient proteins (see published protocol at https://www.protocols.io/view/ancient-proteins-extraction-protocol-7vwhn7e). Samples were extracted and digested alongside negative extraction blanks in order to monitor for potential laboratory contamination. Cellular debris pellets left over after protein extraction were set aside for microscopic examination of microremains (see above section). Extracted peptides were analyzed by LC-MS/MS using a Q-Exactive mass spectrometer (Thermo Scientific) coupled to an ACQUITY UPLC M-Class system (Waters) at the Functional Genomics Center Zurich of the University/Eidgenössiche Technische Hochschule Zurich (SI Appendix). Injection blanks were also run between each sample in order to identify and reduce potential carryover across samples. MS/MS spectra were converted to Mascot generic files by MSConvert v3.0.11781 using the 100 most-intense peaks in each spectra. All MS/MS samples were analyzed using Mascot (Matrix Science; v2.6.0). Mascot was set up to search the SwissProt Release 2019_08 database (560,823 entries) and Uniprot Trembl 2017_07 (88,032,926 sequences) assuming the digestion enzyme trypsin, with an automatic decoy option. Mascot was searched with a fragment ion mass tolerance of 0.050 Da and a parent ion tolerance of 10.0 PPM. Carbamidomethyl of cysteine was specified in Mascot as a fixed modification. Deamidation of asparagine and glutamine and oxidation of methionine and proline were specified in Mascot as variable modifications. All protein identifications were established at a protein false-discovery rate of less than 3.0% and peptide false-discovery rate of less than 1.0% using the Protein Prophet algorithm (137) implemented in the software program Scaffold (version Scaffold_4.8.9; Proteome Software). Dietary proteins were filtered at a minimum of 95% protein identification probability. Additionally, only proteins with a minimum of two unique peptides with at least one species-specific peptide were accepted (Dataset S5). Proteins that contained similar peptides that could not be differentiated on the basis of MS/MS analysis alone were grouped to satisfy the principles of parsimony. Proteins sharing significant peptide evidence were grouped into clusters. No dietary proteins were found in the extraction blanks, which contained only reagents, such as porcine trypsin, and known laboratory contaminants, such as collagen, keratin, or bacterial proteins. Representative MS/MS spectra of selected dietary proteins are provided in SI Appendix, Fig. S10. A complete list of all identified dietary spectra is provided in Dataset S5.
Supplementary Material
Acknowledgments
This study was funded by the European Research Council (ERC) under the European Union’s Horizon 2020 research innovation programme (ERC-2015-StG 678901-Food-Transforms) as part of P.W.S.’s ERC Starting Grant project “FoodTransforms: Transformations of Food in the Eastern Mediterranean Late Bronze Age.” Work at Megiddo is currently supported by the Shmunis Family Foundation, the Dan David Foundation, Jacques Chahine, Mark Weissman, as well as Norman and Vivian Belmonte. We thank Zeev Herzog and Lily Avitz Singer for giving us the permission to publish the pottery sherd photograph and for supplying information as to its context; and Chen Tao and Robert Spengler for helpful comments on the manuscript.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2014956117/-/DCSupplemental.
Data Availability.
Protein spectra have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository (https://www.ebi.ac.uk/pride) under the dataset identifier PXD021498.
References
- 1.Van der Veen M., Morales J., The Roman and Islamic spice trade: New archaeological evidence. J. Ethnopharmacol. 167, 54–63 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Freedman P., Out of the East: Spices and the Medieval Imagination (Yale University Press, 2008). [Google Scholar]
- 3.Wood F., The Silk Road: Two Thousand Years in the Heart of Asia (University of California Press, 2002). [Google Scholar]
- 4.Vandkilde H., Bronzization: The Bronze Age as pre-modern globalization. Praehist. Z. 91, 103–123 (2016). [Google Scholar]
- 5.Linduff K. M., Metallurgy in Ancient Eastern Eurasia from the Urals to the Yellow River (Edwin Mellen Press, 2004). [Google Scholar]
- 6.Kristiansen K., Suchowska-Ducke P., Connected histories: The dynamics of Bronze Age interaction and trade 1500–1100 BC. Proc. Prehist. Soc. 81, 361–392 (2015). [Google Scholar]
- 7.Pernicka E., “The expansion of tin bronze in the 3rd millennium” [in German] in Mensch und Umwelt in der Bronzezeit Europas, Hänsel B., Ed. (Oetker-Voges, 1998), pp. 135–147. [Google Scholar]
- 8.Kenoyer J. M., “Indus and Mesopotamian trade networks: New insights from shell and carnelian artifacts” in Intercultural Relations between South and Southwest Asia: Studies in Commemoration of E.C.L. During Caspers (1934–1996), Olijdam E., Spoor R. H., Eds. (Archaeopress, 2008), pp. 19–28. [Google Scholar]
- 9.Fuller D. Q., Boivin N., Hoogervorst T., Allaby R., Across the Indian Ocean: The prehistoric movement of plants and animals. Antiquity 85, 544–558 (2011). [Google Scholar]
- 10.Boivin N., Crowther A., Prendergast M., Fuller D. Q., Indian Ocean food globalisation and Africa. Afr. Archaeol. Rev. 31, 547–581 (2014). [Google Scholar]
- 11.Hall T. D., Comparing Globalizations: Historical and World-Systems Approaches (Springer, 2017). [Google Scholar]
- 12.Chew S. C., The Southeast Asia Connection: Trade and Polities in the Eurasian World Economy, 500 BC–AD 500 (Berghahn Books, 2018). [Google Scholar]
- 13.Rahmstorf L., “The use of bronze objects in the 3rd millennium BC—A survey between Atlantic and Indus” in Appropriating Innovations. Entangled Knowledgement in Eurasia, 5000–1500 BCE, Maran J., Stockhammer P., Eds. (Oxbow Books, 2017), pp. 184–210. [Google Scholar]
- 14.Chew S. C., The Recurring Dark Ages: Ecological Stress. Climate Changes, and System Transformation (Altamira Press, 2007). [Google Scholar]
- 15.Cline E. H., Sailing the Wine-Dark Sea: International Trade and the Late-Bronze Age Aegean. Tempus Reparatum (1994). [Google Scholar]
- 16.Monroe C. M., Scales of Fate: Trade, Tradition, and Transformation in the Eastern Mediterranean, Ca. 1350-1175 BCE (Ugarit-Verlag, 2009). [Google Scholar]
- 17.Aruz J., Graff S. B., Rakic Y., Cultures in Contact: From Mesopotamia to the Mediterranean in the Second Millennium B.C (Metropolitan Museum of Art, 2013). [Google Scholar]
- 18.Moorey P. R. S., Ancient Mesopotamian Materials and Industries: The Archaeological Evidence (Eisenbrauns, 1999). [Google Scholar]
- 19.Barjamovic G., “Interlocking commercial networks and the infrastructure of trade in Western Asia during the Bronze Age” in Trade and Civilisation: Economic Networks and Cultural Ties from Prehistory to the Early Modern Era, Kristiansen K., Lindkvist T., Myerdal J., Eds. (Cambridge University Press, 2018), pp. 113–142. [Google Scholar]
- 20.Barjamovic G., “The geography of trade. Assyrian colonies in Anatolia c. 1975-1725 BC and the study of early interregional networks of exchange” in Anatolia and the Jazira during the Old Assyrian Period, Derksen J. G., Ed. (Nederlands Instituutvoor het Nabije Osten, 2008), pp. 87–100. [Google Scholar]
- 21.Cohen R., Westbrook R., Eds., Amarna Diplomacy: The Beginnings of International Relations (The Johns Hopkins University Press, 2000). [Google Scholar]
- 22.Baumann S., Treasuries: Their Decoration and Spatial Conception in Egyptian Temples of the Greco-Roman Period [in German] (Harrassowitz Verlag, 2018).
- 23.Herzog R., Punt (Augustin, 1968). [Google Scholar]
- 24.Chakrabarti D. K., The External Trade of Indus Civilization (Munshiram Manoharlal Publishers, 1990). [Google Scholar]
- 25.Crawford H. E. W., Mesopotamia’s invisible exports in the third millennium BC. World Archaeol. 5, 232–241 (1973). [Google Scholar]
- 26.Possehl G. L., Seafaring merchants of Meluhha. South Asian Archaeol. 1995, 87–100 (1997). [Google Scholar]
- 27.Parpola S., Parpola A., Brunswig R. H., The Meluhha Village: Evidence of acculturation of Harappan traders in late third millennium Mesopotamia? J. Econ. Soc. Hist. Orie. 20, 129–165 (1977). [Google Scholar]
- 28.Possehl G. L., Shu-ilishu’s cylinder seal. Expedition 48, 42–43 (2006). [Google Scholar]
- 29.Gadd C. J., Seals of Ancient Indian Style Found at Ur (H. Milford, 1933). [Google Scholar]
- 30.Reade J., “The Indus-Mesopotamian relationship reconsidered” in Intercultural Relations between South and Southwest Asia: Studies in Commemoration of ECL during Caspers (1934-1996), Olijdam E., Spoor R. H., Eds. (Archaeopress, Oxford, 2008), pp. 12–18. [Google Scholar]
- 31.Jarrige J. F., Jarrige J. F., Quivron G., The Forgotten Cities of the Indus [in French] (Archéologie du Pakistan, 1988).
- 32.Reese D. S., The trade of Indo-Pacific shells into the Mediterranean Basin and Europe. Oxf. J. Archaeol. 10, 159–196 (1991). [Google Scholar]
- 33.McIntosh J., The Ancient Indus Valley: New Perspectives (ABC-CLIO, 2008). [Google Scholar]
- 34.Stein S. K., The Sea in World History: Exploration, Travel, and Trade [2 volumes] (ABC-CLIO, 2017). [Google Scholar]
- 35.Arnold E. R., et al. , Isotopic evidence for early trade in animals between Old Kingdom Egypt and Canaan. PLoS One 11, e0157650 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meiri M., Stockhammer P. W., Morgenstern P., Maran J., Mobility and trade in Mediterranean antiquity: Evidence for an “Italian connection” in Mycenaean Greece revealed by ancient DNA of livestock. J. Archaeol. Sci. Rep. 23, 98–103 (2019). [Google Scholar]
- 37.Meiri M., et al. , Eastern Mediterranean mobility in the Bronze and Early Iron Ages: Inferences from ancient DNA of pigs and cattle. Sci. Rep. 7, 701 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen S., et al. , Zebu cattle are an exclusive legacy of the South Asia Neolithic. Mol. Biol. Evol. 27, 1–6 (2010). [DOI] [PubMed] [Google Scholar]
- 39.Matthews R., Zebu: Harbingers of doom in Bronze Age Western Asia? Antiquity 76, 438–446 (2002). [Google Scholar]
- 40.Verdugo M. P., et al. , Ancient cattle genomics, origins, and rapid turnover in the Fertile Crescent. Science 365, 173–176 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Pareja M. N., et al. , A new identification of the monkeys depicted in a Bronze Age wall painting from Akrotiri, Thera. Primates 61, 159–168 (2020). [DOI] [PubMed] [Google Scholar]
- 42.Panagiotakopulu E., Insect remains from the collections in the Egyptian Museum of Turin. Archaeometry 45, 355–362 (2003). [Google Scholar]
- 43.Perry-Gal L., Erlich A., Gilboa A., Bar-Oz G., Earliest economic exploitation of chicken outside East Asia: Evidence from the Hellenistic Southern levant. Proc. Natl. Acad. Sci. U.S.A. 112, 9849–9854 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fritz G., Nesbitt M., “Laboratory analysis and identification of plant macroremains” in Method and Theory in Paleoethnobotany, Marston J. M., Guedes J. D. A., Warinner C., Eds. (University Press of Colorado Boulder, 2015), pp. 115–145. [Google Scholar]
- 45.Zhou X., et al. , 5,200-year-old cereal grains from the eastern Altai Mountains redate the trans-Eurasian crop exchange. Nat. Plants 6, 78–87 (2020). [DOI] [PubMed] [Google Scholar]
- 46.Liu X., Hunt H. V., Jones M. K., River valleys and foothills: Changing archaeological perceptions of North China’s earliest farms. Antiquity 83, 82–95 (2009). [Google Scholar]
- 47.Boivin N., Fuller D. Q., Shell middens, ships and seeds: Exploring coastal subsistence, maritime trade and the dispersal of domesticates in and around the ancient Arabian Peninsula. J. World Prehist. 22, 113–180 (2009). [Google Scholar]
- 48.Langgut D., The Citrus Route revealed: From Southeast Asia into the Mediterranean. HortScience 52, 814–822 (2017). [Google Scholar]
- 49.Wu G. A., et al. , Genomics of the origin and evolution of citrus. Nature 554, 311–316 (2018). [DOI] [PubMed] [Google Scholar]
- 50.Langgut D., Gadot Y., Porat N., Lipschits O., Fossil pollen reveals the secrets of the Royal Persian Garden at Ramat Rahel, Jerusalem. Palynology 37, 115–129 (2013). [Google Scholar]
- 51.Van der Veen M., “The botanical evidence” in Survey and Excavations at Mons Claudianus 1987-1993, Maxfield V. A., Peacock D. P. S., Eds. (Institut Français d'Archéologie Orientale du Cairo, 2001), pp. 174–247. [Google Scholar]
- 52.Van der Veen M., Tabinor H., “Food, fodder and fuel at Mons Porphyrites: The botanical evidence” in The Roman Imperial Quarries: Survey and Excavation at Mons Porphyrites 1994-1998, Peacock D., Maxfield V., Eds. (The Egypt Exploration Society, London, 2007), pp. 84–156. [Google Scholar]
- 53.Hjelmqvist H., “Some economic plants and weeds from the Bronze Age of Cyprus” in Hala Sultan Tekke 5, Studies in Mediterranean Archaeology, Öbrink U., Ed. (Åströms Förlag, 1979), vol. 45, pp. 110–133. [Google Scholar]
- 54.Zohary D., Hopf M., Weiss E., Domestication of Plants in the Old World: The Origin and Spread of Domesticated Plants in Southwest Asia, Europe, and the Mediterranean Basin (Oxford University Press, 2012). [Google Scholar]
- 55.Sebastian P., Schaefer H., Telford I. R., Renner S. S., Cucumber (Cucumis sativus) and melon (C. melo) have numerous wild relatives in Asia and Australia, and the sister species of melon is from Australia. Proc. Natl. Acad. Sci. U.S.A. 107, 14269–14273 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muthukumaran S., “An ecology of trade: Tropical Asian cultivars in the ancient Middle East and the eastern Mediterranean,” PhD thesis, University College London, London, United Kingdom, (2016).
- 57.de Vartavan C., et al. , Codex of Ancient Egyptian Plant Remains (SAIS, 2010). [Google Scholar]
- 58.Linares V., et al. , First evidence for vanillin in the Old World: Its use as mortuary offering in Middle Bronze Canaan. J. Archaeol. Sci. Rep. 25, 77–84 (2019). [Google Scholar]
- 59.Gilboa A., Namdar D., On the beginnings of South Asian spice trade with the Mediterranean region: A review. Radiocarbon 57, 265–283 (2015). [Google Scholar]
- 60.Namdar D., Neumann R., Weiner S., “Residue analysis of chalices from the repository pit” in Yavneh I: The Excavation of the “Temple Hill” Repository Pit and the Cult Stands, Kletter R., Ziffer I., Zwickel W., Eds. (Academic Press Fribourg, 2010), pp. 167–173. [Google Scholar]
- 61.Lichtenberg R., Thuilliez A. C., On some unusual aspects of the radiology of Ramses II. [in French] B. Mem. Soc. Anthro. Par. 3, 323–330 (1981).
- 62.Plu A., “Wood and grains” in La Momie de Ramsès: Contribution Scientifique à l’Egyptologie, Balout L., Roubet C., Eds. (Éditions Recherche sur les Civilisations, 1985), pp. 166–174. [Google Scholar]
- 63.Asouti E., Fuller D. Q., Trees and Woodlands of South India: Archaeological Perspectives (Routledge, 2008). [Google Scholar]
- 64.Buccellati G., Buccellati M. K., Terqa: The first eight seasons. Les Ann. Archeol. Arabes Syriennes 32, 47–67 (1983). [Google Scholar]
- 65.Smith M., “The Terqa Cloves and the Archaeology of Aroma” in Between Syria and the Highlands; Studies in honor of Giorgio Buccellati & Marilyn Kelly-Buccellati, Valentini S., Ed. et al. (Arbor Sapientiae Editore, 2019), pp. 373–377. [Google Scholar]
- 66.Kingwell-Banham E., et al. , Spice and rice: Pepper, cloves and everyday cereal foods at the ancient port of Mantai, Sri Lanka. Antiquity 92, 1552–1570 (2018). [Google Scholar]
- 67.Piperno D. R., Phytoliths: A Comprehensive Guide for Archaeologists and Paleoecologists (Rowman Altamira, 2006). [Google Scholar]
- 68.Cabanes D., Shahack-Gross R., Understanding fossil phytolith preservation: The role of partial dissolution in paleoecology and archaeology. PLoS One 10, e0125532 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Albert R. M., Berna F., Goldberg P., Insights on Neanderthal fire use at Kebara Cave (Israel) through high resolution study of prehistoric combustion features: Evidence from phytoliths and thin sections. Quat. Int. 247, 278–293 (2012). [Google Scholar]
- 70.Rustgi S., Shewry P., Brouns F., Deleu L. J., Delcour J. A., Wheat seed Proteins: Factors influencing their content, composition, and technological properties, and strategies to reduce adverse reactions. Compr. Rev. Food Sci. Food Saf. 18, 1751–1769 (2019). [DOI] [PubMed] [Google Scholar]
- 71.Wang Z., et al. , New insight into the function of wheat glutenin proteins as investigated with two series of genetic mutants. Sci. Rep. 7, 3428 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Metheny K. B., Beaudry M. C., Archaeology of Food: An Encyclopedia (Rowman & Littlefield, 2015). [Google Scholar]
- 73.Colgrave M. L., Proteomics in Food Science: From Farm to Fork (Academic Press, 2017). [Google Scholar]
- 74.Peumans W. J., et al. , Purification, characterization and structural analysis of an abundant beta-1,3-glucanase from banana fruit. Eur. J. Biochem. 267, 1188–1195 (2000). [DOI] [PubMed] [Google Scholar]
- 75.Receveur-Bréchot V., et al. , Crystal structure at 1.45-A resolution of the major allergen endo-beta-1,3-glucanase of banana as a molecular basis for the latex-fruit syndrome. Proteins 63, 235–242 (2006). [DOI] [PubMed] [Google Scholar]
- 76.Thulasy G., Nair A. S., Comparative analysis of β-1,3-glucanase protein sequences and structure in two banana cultivars from Kerala. J. Food Biochem. 42, e12559 (2018). [Google Scholar]
- 77.Roy Choudhury S., Roy S., Singh S. K., Sengupta D. N., Molecular characterization and differential expression of β-1,3-glucanase during ripening in banana fruit in response to ethylene, auxin, ABA, wounding, cold and light-dark cycles. Plant Cell Rep. 29, 813–828 (2010). [DOI] [PubMed] [Google Scholar]
- 78.Srinivas L., Shalini V. K., Shylaja M., Turmerin: A water soluble antioxidant peptide from turmeric [Curcuma longa]. Arch. Biochem. Biophys. 292, 617–623 (1992). [DOI] [PubMed] [Google Scholar]
- 79.Fuller D. Q., Contrasting patterns in crop domestication and domestication rates: Recent archaeobotanical insights from the Old World. Ann. Bot. 100, 903–924 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gillet J. B., “Botanical samples” in Eridu, Safer F., et al., Eds. (Ministry of Culture and Information, State Organization of Antiquities and Heritage, Baghdad, 1981), pp. 317–318. [Google Scholar]
- 81.Liphschitz N., “Wood remains” in Megiddo V: The 2004–2008 Seasons Monograph Series No. 31, Finkelstein I., et al., Eds. (Institute of Archaeology, Tel Aviv University, 2013), pp. 1220–1236. [Google Scholar]
- 82.White C. E., Chesson M. S., Thomas Schaub R., A recipe for disaster: Emerging urbanism and unsustainable plant economies at Early Bronze Age Ras an-Numayra, Jordan. Antiquity 88, 363–377 (2014). [Google Scholar]
- 83.Fall P. L., Lines L., Falconer S. E., Seeds of civilization: Bronze Age rural economy and ecology in the Southern Levant. Ann. Assoc. Am. Geogr. 88, 107–125 (1998). [Google Scholar]
- 84.Damick A., The first identification of Phoenix dactylifera (date palm) from Early Bronze Age Lebanon. Veg. Hist. Archaeobot. 28, 583–589 (2019). [Google Scholar]
- 85.Hunt H. V., et al. , Millets across Eurasia: Chronology and context of early records of the genera Panicum and Setaria from archaeological sites in the Old World. Veg. Hist. Archaeobot. 17, 5–18 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zech-Matterne V., Tengberg M., Van Andringa W., Sesamum indicum L. (sesame) in 2nd century BC Pompeii, southwest Italy, and a review of early sesame finds in Asia and Europe. Veg. Hist. Archaeobot. 24, 673–681 (2015). [Google Scholar]
- 87.Bedigian D., History and lore of sesame in Southwest Asia. Econ. Bot. 58, 329–353 (2004). [Google Scholar]
- 88.Bedigian D., Harlan J. R., Evidence for cultivation of sesame in the ancient world. Econ. Bot. 40, 137–154 (1986). [Google Scholar]
- 89.Powell M. A., Epistemology and Sumerian agriculture: The strange case of sesame and linseed Aula Orientalis 9, 155–164 (1991). [Google Scholar]
- 90.Fuller D. Q., Further evidence on the prehistory of sesame. Asian Agrihist. 7, 127–137 (2003). [Google Scholar]
- 91.Manning K., Pelling R., Higham T., Schwenniger J.-L., Fuller D. Q., 4500-Year old domesticated pearl millet (Pennisetum glaucum) from the Tilemsi Valley, Mali: New insights into an alternative cereal domestication pathway. J. Archaeol. Sci. 38, 312–322 (2011). [Google Scholar]
- 92.Fuller D. Q., “African crops in prehistoric South Asia: a critical review” in Food, Fuel and Fields: Progress in African Archaeobotany, Neumann K., Butler A., Kahlheber S., Eds. (Africa Prehistorica 15, 2003), pp. 239–271. [Google Scholar]
- 93.Miller N., Rainfall seasonality and the spread of millet cultivation in Eurasia. Iranian. J. Archaeol. Stud. 5, 1–10 (2015). [Google Scholar]
- 94.Miller N. F., Spengler R. N., Frachetti M., Millet cultivation across Eurasia: Origins, spread, and the influence of seasonal climate. Holocene 26, 1566–1575 (2016). [Google Scholar]
- 95.Motuzaite-Matuzeviciute G., Staff R. A., Hunt H. V., Liu X., Jones M. K., The early chronology of broomcorn millet (Panicum miliaceum) in Europe. Antiquity 87, 1073–1085 (2013). [Google Scholar]
- 96.Tafuri M. A., Craig O. E., Canci A., Stable isotope evidence for the consumption of millet and other plants in Bronze Age Italy. Am. J. Phys. Anthropol. 139, 146–153 (2009). [DOI] [PubMed] [Google Scholar]
- 97.Kubiak-Martens L., “Plant remains from Tell Ashara (Terqa) and Tell Masaikh in the Middle Euphrates, south-eastern Syria” in Akh Purattim , Magueron J., Rouault O., Butterlin P., Lombard P., Eds. (Maison de l’Orient et de la Méditerranée & Ministère des Affaires étrangères et du Développement International, 2015), vol. 3, pp. 423–442. [Google Scholar]
- 98.Sołtysiak A., Schutkowski H., Stable isotopic evidence for land use patterns in the Middle Euphrates Valley, Syria. Am. J. Phys. Anthropol. 166, 861–874 (2018). [DOI] [PubMed] [Google Scholar]
- 99.Nesbitt M., Summers G. D., Some recent discoveries of millet (Panicum miliaceum and Setaria italica) at excavations in Turkey and Iran. Anatol. Stud. 38, 85–97 (1988). [Google Scholar]
- 100.Nesbitt M., Bates J., Hillman G., Mitchell S., The Archaeobotany of Aşvan: Environment and Cultivation in Eastern Anatolia from the Chalcolithic to the Medieval Period (British Institute at Ankara, 2017). [Google Scholar]
- 101.Lee G.-A., Crawford G. W., Liu L., Sasaki Y., Chen X., Archaeological soybean (Glycine max) in East Asia: Does size matter? PLoS One 6, e26720 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Middeke-Conlin R., The Scents of Larsa: A Study of the Aromatics Industry in an Old Babylonian Kingdom (Cuneiform Digital Library Journal, 2014), vol. 1, pp. 1–53. [Google Scholar]
- 103.Koura B., The “7 Sacred Oils” and Other Oil and Fat Names. A Lexicographical Study of the Names of Oils, Fats and Ointments Among the Ancient Egyptians from Early Dynastic Until the Beginning of the Ptolemaic Period (From 3000 BC–ca. 305 BC) [in German] (Shaker, 1999). [Google Scholar]
- 104.Görg M., Oils from abroad [in German]. Studien Altägyptischen Kultur 11, 219–226 (1984). [Google Scholar]
- 105.Eitam D., Heltzer M., Eds., Olive Oil in Antiquity: Israel and Neighboring Countries, from the Neolithic to the Early Arab Period (Sargon, 1996). [Google Scholar]
- 106.Serpico M., “Natural product technology in New Kingdom Egypt” in Invention and Innovation: The Social Context of Technological Change 2: Egypt, the Aegean and the Near East, 1650-1150 B.C, Bourriau J., Phillips J., Eds. (Oxbow Books, 2004), pp. 96–120. [Google Scholar]
- 107.Zong Y., et al. , Selection for oil content during soybean domestication revealed by X-ray tomography of ancient beans. Sci. Rep. 7, 43595 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shevchenko A., et al. , Open sesame: Identification of sesame oil and oil soot ink in organic deposits of Tang Dynasty lamps from Astana necropolis in China. PLoS One 12, e0158636 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Perrier X., et al. , Multidisciplinary perspectives on banana (Musa spp.) domestication. Proc. Natl. Acad. Sci. U.S.A. 108, 11311–11318 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mbida C. M., Van Neer W., Doutrelepont H., Vrydaghs L., Evidence for banana cultivation and animal husbandry during the first millennium BC in the forest of Southern Cameroon. J. Archaeol. Sci. 27, 151–162 (2000). [Google Scholar]
- 111.Mindzie C. M., Vrydaghs L., “Nkang: Early evidence for banana cultivation on the African continent” in Encyclopedia of Global Archaeology, Smith C., Ed. (Springer, New York, 2014), pp. 5293–5295. [Google Scholar]
- 112.Power R. C., Güldemann T., Crowther A., Boivin N., Asian crop dispersal in Africa and Late Holocene human adaptation to tropical environments. J. World Prehist. 32, 353–392 (2019). [Google Scholar]
- 113.Hodson M. J., White P. J., Mead A., Broadley M. R., Phylogenetic variation in the silicon composition of plants. Ann. Bot. 96, 1027–1046 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lawler A., European association for South Asian archaeology and art meeting. The ingredients for a 4000-year-old proto-curry. Science 337, 288 (2012). [DOI] [PubMed] [Google Scholar]
- 115.Farooqui A., Gaur A. S., Prasad V., Climate, vegetation and ecology during Harappan period: Excavations at Kanjetar and Kaj, mid-Saurashtra coast, Gujarat. J. Archaeol. Sci. 40, 2631–2647 (2013). [Google Scholar]
- 116.García-Granero J. J., Lancelotti C., Madella M., Ajithprasad P., “Plant processing activities at Loteshwar (North Gujarat, India): A micro-botanical approach” in Man and Environment in Prehistoric and Protohistoric South Asia: New Perspectives, Didier A., Mutin B., Eds. (Brepols, 2016), pp. 99–116. [Google Scholar]
- 117.Madella M., “Investigating agriculture and environment in South Asia: Present and future contributions from opal phytoliths” in Indus Ethnobiology: New Perspectives from the Field, Weber S., Belcher W., Eds. (Rowman & Littlefield Publishing Group, 2003) pp. 199–249. [Google Scholar]
- 118.Bernsen G., Pharmacognostic analysis of the content of a vessel from an Egyptian tomb of the 18th Dynasty. Arch. Pharm. Chem. (Kbh) 4, 65–70 (1976). [Google Scholar]
- 119.Germer R., Flora of Pharaonic Egypt [in German] (Zabern, 1985). [Google Scholar]
- 120.Al-Busaidi K. T. S., Banana domestication on the Arabian Peninsula: A review of their domestication history. J. Hortic. For. 5, 194–203 (2013). [Google Scholar]
- 121.Bonnet W., Ancient plants in the necropolis of Antinoë. J. Bot. 19, 5–12 (1905). [Google Scholar]
- 122.Harlan J. R., Crops and Man (American Society of Agronomy, 1975). [Google Scholar]
- 123.Sirirugsa P., “Thai Zingiberaceae: Species diversity and their uses,” invited lecture presented at the International Conference on Biodiversity and Bioresources: Conservation and Utilization, 23–27 November 1997, Phuket, Thailand. http://media.iupac.org/symposia/proceedings/phuket97/sirirugsa.pdf. Accessed: 15 November 2020.
- 124.Kashyap A., Weber S., Harappan plant use revealed by starch grains from Farmana, India. Antiquity 84, 326 (2010). [Google Scholar]
- 125.Weber S. A., Kashyap A., Mounce L., “Archaeobotany at Farmana: New insights into Harappan plant use strategies” in Excavations at Farmana, Indus Ethnobiology: New Perspectives from the Field, Weber S. A., Belcher B., Eds. (Lexington Books, 2011), pp. 808–823. [Google Scholar]
- 126.Kashyap A., Weber S., “Starch grain analysis and experiments provide insights into Harappan cooking practices” in Connections and Complexity, Abraham S. A., Gullapalli P., Raczek T., Rizvi U. Z., Eds. (Routledge, 2016), pp. 177–194. [Google Scholar]
- 127.Prasad S., Aggarwal B., “Turmeric, the golden spice” in Herbal Medicine: Biomolecular and Clinical Aspects, Benzie I. F. F., Wachtel-Galor S., Eds. (CRC Press/Taylor & Francis, ed. 2, 2011) chap 13, pp. 263–288. [Google Scholar]
- 128.Thompson R. C., A Dictionary of Assyrian Botany (British Academy, 1949). [Google Scholar]
- 129.Pekar J., Ret D., Untersmayr E., Stability of allergens. Mol. Immunol. 100, 14–20 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hendy J., et al. , Ancient proteins from ceramic vessels at Çatalhöyük West reveal the hidden cuisine of early farmers. Nat. Commun. 9, 4064 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hendy J., et al. , Proteomic evidence of dietary sources in ancient dental calculus. Proc. Biol. Sci. 285, 20180977 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jersie-Christensen R. R., et al. , Quantitative metaproteomics of medieval dental calculus reveals individual oral health status. Nat. Commun. 9, 4744 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cappellini E., et al. , A multidisciplinary study of archaeological grape seeds. Naturwissenschaften 97, 205–217 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lüchtrath A., tj-šps, the camphor tree of East Africa. [in German] Göttinger Miszellen 101, 43–48 (1988). [Google Scholar]
- 135.Hardy K., et al. , Dental calculus reveals potential respiratory irritants and ingestion of essential plant-based nutrients at Lower Palaeolithic Qesem Cave Israel. Quat. Int. 398, 129–135 (2015). [Google Scholar]
- 136.Warinner C., et al. , Pathogens and host immunity in the ancient human oral cavity. Nat. Genet. 46, 336–344 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nesvizhskii A. I., Keller A., Kolker E., Aebersold R., A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 75, 4646–4658 (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Protein spectra have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository (https://www.ebi.ac.uk/pride) under the dataset identifier PXD021498.