Significance
Although catalysis by mineral surfaces has been considered to be important in prebiotic chemistry, the role of porous silica phases, with reactions taking place within specific confined environments, has not been explored. This paper proposes that structure direction through interaction of dissolved silica with organic species in aqueous solution produces porous silica catalysts for prebiotic organic reactions to form larger polymerized molecules, with possible control of chirality. This process may involve feedback and amplification if the organics produced by catalysis can also act as structure-directing agents for enhanced synthesis of the catalytic silica structures. To our knowledge, such structure direction and catalysis, though well known in materials science, have not been considered previously in the context of prebiotic chemistry.
Keywords: silica, zeolites, peptide synthesis, prebiotic chemistry
Abstract
Modern technology has perfected the synthesis of catalysts such as zeolites and mesoporous silicas using organic structure directing agents (SDA) and their industrial use to catalyze a large variety of organic reactions within their pores. We suggest that early in prebiotic evolution, synergistic interplay arose between organic species in aqueous solution and silica formed from rocks by dynamic dissolution–recrystallization. The natural organics, for example, amino acids, small peptides, and fatty acids, acted as SDA for assembly of functional porous silica structures that induced further polymerization of amino acids and peptides, as well as other organic reactions. Positive feedback between synthesis and catalysis in the silica–organic system may have accelerated the early stages of abiotic evolution by increasing the formation of polymerized species.
Abiotic organic reactions on the early Earth were the prelude to the origin of life. The transition from a world of small molecules (monomers) in solution to one of oligomers leading to the formation of proteins and other large solid molecular assemblies was critical to the development of life. A rich variety of small organic molecules is likely to have existed in a variety of settings on the prebiotic Earth, especially where silicate rock and aqueous solutions were in contact, particularly in shallow aqueous environments (1, 2) and in hydrothermal systems (3). Mineral surfaces, especially of phases containing ions of variable oxidation states (e.g., iron, manganese, and copper), have been suggested to play major roles in controlling and catalyzing reactions on the prebiotic Earth (4, 5).
Silica and silicates are pervasive in ancient and modern environments. Many form structures with porosity on different length scales, allowing the confinement of organic molecules in pores, cages, and channels. The dynamic interaction between organic molecules and various porous forms of solid silica continues to be investigated in the materials science community because of its technological importance. The goal of this paper is to bring together evidence that such tailored porous silica environments may be important for prebiotic organic transformations, especially from amino acids to peptides to proteins.
The crystallization of zeolites under the control of organic molecular structure directing agents (SDA) produces industrial-scale catalysts for the cracking of petroleum and other organic reactions (6, 7). Analogous synthesis, using surfactants as SDA, produces mesoporous silica phases for transformation of large organic molecules, catalysis of both organic and inorganic reactions, theranostics, and drug delivery (8–10). These technological processes and present-day silica biomineralization suggest that organic control of silica synthesis and ensuing catalytic reactions can occur under a wide variety of conditions.
We propose that analogous silica–organic interactions could have contributed to prebiotic synthesis of complex organics on the early Earth. Their mechanisms differ from catalytic reactions on mineral surfaces in several important ways. They involve organic-mediated synthesis of the catalysts themselves, either as separate phases or embedded in a less crystalline silica matrix. The catalytic sites are within the three-dimensional (3D) silica framework connected by pores and channels, rather than just on external surfaces. This 3D molecular confinement controls reactivity and may protect the products from degradation. Such silica–organic interactions are potentially a two-way street, where the organics direct the formation of specific porous structures and those structures in turn catalyze organic reactions. Indeed, one can envision a feedback loop in which the templated solid structure catalyzes the formation of additional SDA which enables the formation of more catalyst to produce more organic product. This feedback loop between synthesis and catalysis could amplify product formation and accumulation and accelerate the evolution toward chemical complexity.
We pose the following two conjectures (1). Early in prebiotic evolution, synergistic interplay arose between organic species in aqueous solution and silica formed from rocks by dynamic dissolution–recrystallization. The organics acted as SDA for assembly of functional silica structures that catalyzed polymerization of amino acids and other organic reactions.
Synergy and feedback between silica and organics may have been critical in the early stages of abiotic evolution, leading both to our complex protein world and to biomineralization (2). Organic–silica interactions at the heart of modern technology are useful models for abiotic synthesis on the early Earth. Our technology may be more geomimetic than we realize. Many aspects of prebiotic synthesis may be considered, from the vantage point of human endeavor, to be technomimetic.
In this paper we first describe the structures of porous silica phases, their synthesis using organic SDA, and their ability to catalyze organic reactions, including peptide formation. We give examples of the introduction of chirality at different stages in these processes. We then turn to the ancient geochemical environment. We briefly discuss possible prebiotic environments where silica–organic interactions could occur. We review evidence that reaction of mineral silica sources with aqueous solutions involves dissolution–reprecipitation mechanisms that put small amounts of dissolved silica in direct contact with organic molecules. We then identify possible natural organic SDA for prebiotic conditions. We propose that peptide synthesis and other organic reactions leading to larger molecular assemblies are catalyzed by the functional silica formed by such structure direction. We conclude with some opportunities for future research to test these hypotheses.
Porous Functional Silica Structures and Their Synthesis
The structure of crystalline and amorphous silicates (at ambient pressure) is controlled by the linkages of silicate; SiO4; and, if aluminum is present, aluminate, AlO4, tetrahedra. In low-pressure forms of silica, each SiO4 tetrahedron is corner shared to four others through oxygen atoms. Because the silicon–oxygen (Si–O) bond length is quite rigid, but the intertetrahedral angle (Si–O–Si) is quite flexible and can range from about 130 to 180°, a large variety of structures is possible. The natural silica minerals, with quartz the stable form under ambient conditions, are built of six-membered rings of tetrahedra and do not contain appreciable porosity and hence cannot absorb organic molecules. When silica is dissolved from rocks and then reprecipitates, it often forms an amorphous structure, similar to silica glass. This precipitated material may be nanoparticulate in size and heavily hydrated. Sometimes referred to as a gel, it is much more reactive than silica glass or crystalline silica and can interact strongly with organics.
Chemical synthesis, taking advantage of the interaction of silica with organic SDA, has produced hundreds of new porous high-silica zeolitic materials, both emulating natural zeolites and having totally new structures (11). They contain (Fig. 1A) frameworks of linked silicate tetrahedra, but now these define much larger cages and tunnels which can absorb and perform catalytic chemistry on organic molecules converting them into value-added chemicals. Though much commercial zeolite synthesis uses high temperature and high pH to speed reaction and increase yield, a number of aluminosilicate zeolites can be made near room temperature under milder pH conditions.
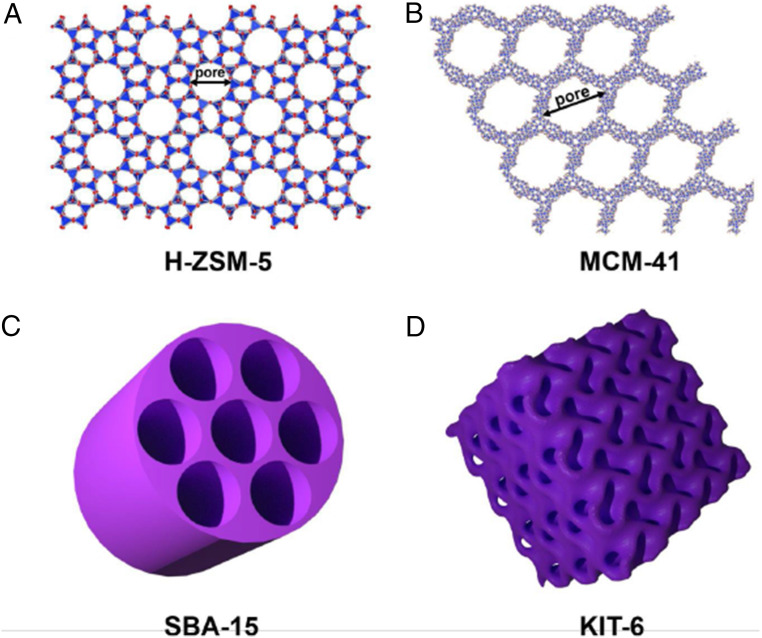
Fig. 1.
(A) Crystal structure of H-ZSM-5 zeolite in which the linkages of tetrahedra define large cages (pores) with 0.55 nm diameter connected by tunnels. It can incorporate molecules no larger than these cages. (B) Crystal structure of MCM-41 mesoporous SiO2 with periodically spaced unidimensional parallel channels typically 2 to 10 nm in diameter. (C) Schematic of SBA-15 mesoporous silica with hexagonally arranged large pores 5 to 15 nm in diameter, surrounded by largely amorphous silica walls. (D) Schematic of cubic mesoporous KIT-6 silica (pore size larger than 5 nm) with gyroid minimal surface and Ia3d symmetry composed of two interpenetrating chiral channels which result in an interpenetrating network of cylindrical mesopores.
Inasmuch as the silica framework is built around the organic, this is a classic ship in a bottle paradigm: the silica bottle assembles around the organic ship. Then, once the bottle forms, the ship can be disassembled and removed by chemical reaction or heating, leaving pores and channels ready to perform catalysis. The overall process requires a silica source; appropriate organic molecules; and control of pH, temperature, other ions in solution, and time. The SDA appears to perform at least two functions: curtailing the rapid growth of amorphous hydrated silica precipitates (gels) by keeping the particles small because of surface sorption and controlling pore size and geometry by the directed growth of the silica around the SDA. Calorimetric studies (12) of pure SiO2 zeolites have drawn the following conclusions (1). The difference in enthalpy and free energy between different zeolite polymorphs is less than about 15 kJ/mol (only 2 to 3 times the thermal energy available at synthesis conditions), with more open frameworks being energetically metastable relative to silica glass and less open frameworks somewhat more stable. This means that the role of the SDA is not to energetically stabilize an initially grossly metastable structure but to select among possible structures in a dense landscape of similar energies, enhancing the formation of a structure that is a good fit to the SDA (2). The interaction of the SDA and zeolite is also relatively weak (13). Thus, the structure selection may rely more on enhancing nucleation kinetics than on major differences in thermodynamic driving forces. This energy landscape is good news in the prebiotic context in that with a silica source and organic molecules present, the early Earth system is likely to sample many configurations and products, with some relevant to the origin of life.
Introduction of mesopores into an aluminum containing faujasite zeolite under mild conditions with little energetic cost has been observed by Linares et al. (14). Chawla et al. (15) have shown that the surfaces of faujasite can restructure rapidly at room temperature. Such postsynthesis modification confirms the chemical flexibility of porous silicate materials and, if true for silica, can provide further pathways for silica–organic interactions under prebiotic conditions.
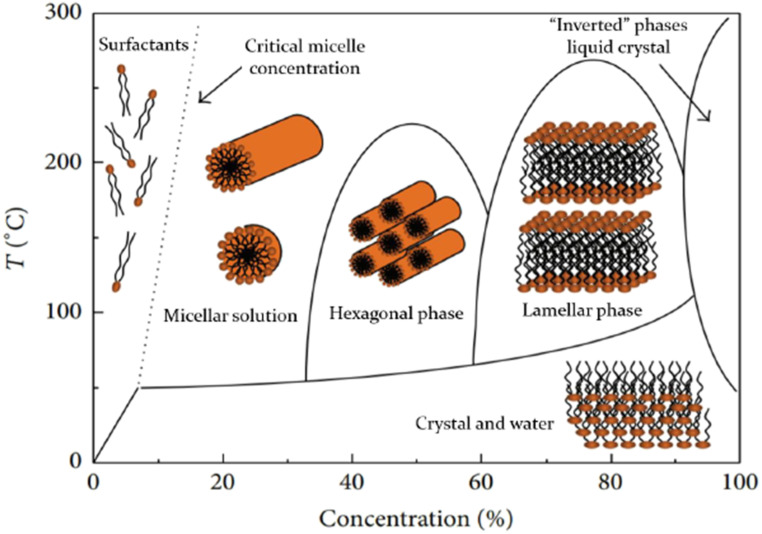
Mesoporous silica phases (Fig. 1 B–D) form a family of materials synthesized using common organic surfactants as SDA (9). These surfactants, rather than being uniformly dissolved in the aqueous solution, form micelles above a critical surfactant concentration. These are molecular aggregates in which the hydrophilic heads of the surfactant point into the aqueous phase while their long hydrophobic tails point into the center. At high surfactant concentrations the micelles self-assemble into aggregates, leading to formation of phases with different shape and size (Fig. 2), dictated by surfactant geometry as well as solution conditions. The dissolved silica source then hydrolyzes and condenses as an amorphous phase around the external surfaces of the micelles, which act as SDA to determine the final topology of the silica. The formation of this inorganic–organic composite is based on electrostatic interactions between the charged surfactants and the silicate species in solution. The surfactant can be removed by calcination, solvent extraction, UV irradiation, microwave digestion, or other means, leaving the mesoporous silica ready to absorb other molecules for catalysis or other reactions. Also, various postsynthetic modifications can alter pore size and/or functionalize the surfaces of the pores for specific binding to biomolecules or other substances. Mesoporous silica phases having various geometries greatly extend the realm of mesoporous materials, especially for catalysis and biomedical applications (17, 18). They can absorb and interact with large molecules, including peptides, proteins, fatty acids, and nucleic acids. Indeed, micelles undergoing self-assembly into larger structures may be models for, or progenitors of, vesicles that are incorporated in protocells leading to simple living cells (19, 20).
Fig. 2.
Schematic diagram representing the formation of micellar structures in a binary surfactant water system. Modified from ref. 16, which is licensed under CC BY 4.0.
This sequence of steps (surfactant to micelle to micelle aggregate to silica-coated micelle aggregate to organic-free mesoporous silica) is a general strategy applicable, with fine tuning, to syntheses using various surfactants and silica sources. The original synthesis of mesoporous silica MCM-41 (Mobil Composition of Matter No. 41) (21) via liquid crystal templating utilized sodium silicate as a silica source and quaternary ammonium salts as surfactants. Although most surfactants used to synthesize mesoporous silica are commercially available common organic chemicals, there have been successful syntheses using amino acid-related surfactants. Li et al. (22) reported fabrication of mesoporous silicas at room temperature by using tailor-made polymers (C16-l-His, C16-l-Pro, and C16-l-Trp) as SDA, which are derived from amino acids with ring structures. Xu et al. (23) reported the synthesis of ordered 2D hexagonal and parallel pore channel mesoporous silica materials with homogeneous size and spherical shape by using amino acid surfactant templating. Zhang et al. (24) used amino acids to introduce hierarchical mesoporosity into a microporous zeolite having the LTA topology. Thus, molecules based on amino acids and peptides, although somewhat different from those likely to occur in nature, have been demonstrated to serve as templates for the synthesis of specific porous silica forms.
The synthesis of chiral mesoporous silica which contains hexagonally ordered chiral channels transcribed from the organic SDA was first reported by Che et al. (25). Chiral ordered mesoporous silica was synthesized in the presence of the amino acid proline (26). Pure right- and left-handed chiral mesoporous silica and organosilica phases were produced using a chiral anionic surfactant in the presence of a chiral amino acid (l- or d-arginine) (27). Ordered mesoporous silica with chiral hexagonal pore structure was fabricated using folic acid as a template (28). Helical mesoporous silica can be synthesized from achiral surfactants (29–32). Properties of these materials can be manipulated further through functionalization of the organic molecules to produce mesoporous silica with large chiral internal surface area (33). These examples and others in a review by Cui et al. (34) show that chirality in mesoporous silica can be induced through a variety of synthetic pathways involving both chiral and achiral SDA.
Interaction of Mesoporous Silica with Amino Acids, Peptides, and Other Organics
The sorption of amino acids on porous silica has been studied by several investigators. Shir et al. (35) studied amino acids on mesoporous MCM-41 and SBA-15 to correlate internal surface structure and reactivity. Employing solid state NMR, the interfacial interactions and structural and dynamic states of bound glycine and l-alanine were revealed as functions of hydration and temperature. Goscianska et al. (36) studied sorption of l-phenylalanine on various mesoporous silica phases from solutions with different pH, while Gao et al. (37) studied the sorption of several amino acids (acidic, basic, and neutral) on four different mesoporous silicas. These studies show the binding is specific, strong, and dependent on the amino acid, the nature of the silica internal surface, pH, and temperature. Martra et al. (38) showed that silica surfaces were also able to sorb amino acids from the gas phase and catalyze their oligomerization.
There have also been studies of the sorption and binding of peptides on bulk and nanophase mesoporous silica. Brodrecht et al. (39, 40) investigated mesoporous silica modified through in-pore grafting of small peptides. The structure and surface functionalization were investigated by 2D solid state NMR combined with dynamic nuclear polarization and covalent binding was confirmed. Subra et al. (41) reported the use of Santa Barbara Amorphous-15 (SBA-15) mesoporous silica functionalized with aminopropyl groups for peptide oligomerization. MCM-41 silica containing initial SDA surfactant in the pores demonstrated excellent catalytic activity in condensation reactions (42, 43), and it was concluded that catalyst basicity originated from the silica and not the surfactant. Chen et al. (44) demonstrated flavanone synthesis through condensation over amino-functionalized SBA-15. A secondary amine immobilized mesoporous silica (FSM-16) showed remarkable catalytic activity for self-aldol condensation of unmodified aldehydes with higher activity than homogeneous amine catalysts (45). Thus, there is no doubt that various mesoporous silica phases are excellent catalysts for a variety of organic syntheses.
The immobilization of bioactive enzymes within a porous structure is one of the best strategies for increasing their activity, selectivity, and stability. Specifically, it has been reported that horseradish peroxidase enzyme immobilized in the mesopores of FSM-16 silica exhibits peak activity and excellent stability in organic solvents (46). Moreover, nature’s strategy of implementing multistep cascade reactions (two or more sequential reactions occurring in one reactor) for production of complex bioactive organic molecules has been successfully demonstrated using functionalized mesoporous silica catalysts (47, 48).
Saladino et al. (49) showed that metal silicate hydrates, produced by the dissolution of minerals, spontaneously self-assemble in aqueous solution at pH 12 to form tubular structures with remarkable catalytic activity. Such systems transformed formamide (NH2CHO) into a variety of organic compounds including amino acids. In addition, McKee (50) has shown that the reaction of amino acids and organic hydroxy acids to form oligomers with mixed peptide and carboxylate bonds (depsipeptides) may be an easier and lower-temperature route to peptide synthesis within mesoporous silica.
The above studies show that amino acids, peptides, and complex organic molecules bind selectively to mesoporous and zeolitic silica phases, and these materials can catalyze amino acid oligomerization. Furthermore, chiral silica pores can result in enantioselective sorption and polymerization of amino acids and peptides.
Such cooperative silica–peptide interactions may be the precursors for formation of more complex proteins (e.g., silicateins) and smaller silaffin-like peptides that control the formation of silica structures in diatoms, sponges, and even plants. Although the prebiotic conditions discussed here clearly predate the existence of complex proteins, silaffin peptides could arguably be prebiotic and could enhance silicification near pH 7 and at room temperature, perhaps greatly expanding the formation environments of mesoporous silica.
Implications for the Prebiotic Earth
For the above scenario to be applicable to the prebiotic Earth, several questions must be answered in the affirmative: 1) Were there suitable geochemical environments in terms of silica dissolution, organic molecules in aqueous solution, pH, temperature, and other factors? 2) What natural SDA might have been active? 3) Can the porous silica phases produced by these SDA catalyze peptide formation and other condensation reactions? 4) If the same molecules are involved as SDA and as products of polymerization, can a positive feedback loop be established to enhance the production of peptides?
We note at the outset that nature has time at its disposal, while technology does not, so the natural processes do not need the high throughput and efficiency required and enabled by engineering. Therefore, less efficient pathways, which utilize molecular species appropriate to prebiotic chemistry in specific local environments, may still lead to the needed synthesis and accumulation of complex products on the geologic timescale. Thus, silicate mineral dissolution through geologic processes, porous silica formation aided by natural SDA, and organic polymerization reactions within such silica phases should be viewed as continuous long-term processes.
Prebiotic Geochemical Environments.
Silica in contact with aqueous solutions may occur in a number of present-day and prebiotic environments. Hydrothermal systems have been suggested to be active settings for prebiotic reactions (51–53, 3). Their temperatures can vary spatially and temporally, from near room temperature to 500 °C, making them possible environments for forming and transforming silica phases, although not necessarily for achieving and maintaining equilibrium, and for promoting organic reactions. Rocks of the Hadean era are considered to be dominated by the products of a cooling magma ocean, solidifying to form olivine-rich basalts (komatiites) (54). Although no intact rocks with ages greater than about 4 billion y have been located, individual zircon (ZrSiO4) grains weathered out of rocks can be dated back to 4.4 Ga, well in the prebiotic regime. These incredibly durable zircons are intensely studied for radiometric age, trace element chemistry, and identification of trapped mineral inclusions that are protected from subsequent weathering. These data provide evidence (55) that the prebiotic Earth had an active rock cycle (weathering, sedimentation, burial, and melting) in a nonreduced surface environment (CO2 rather than CO or CH4) and that these melting events produced melts approaching granitic compositions. The alteration of rocks produced from such magmas could produce porous aluminosilicates and silica to participate in organic interactions.
Bolide impacts, prevalent on the early Earth, can fragment rocks; produce nanoparticles; and, in extreme cases, vaporize enough rock that then settles back to Earth forming a rich array of metastable phases and mixing organic and inorganic constituents. Volcanoes can produce hot water environments conducive to silica dissolution and reaction with dissolved organic and inorganic species. These various scenarios suggest that many prebiotic environments had the ingredients necessary for silica–organic interactions.
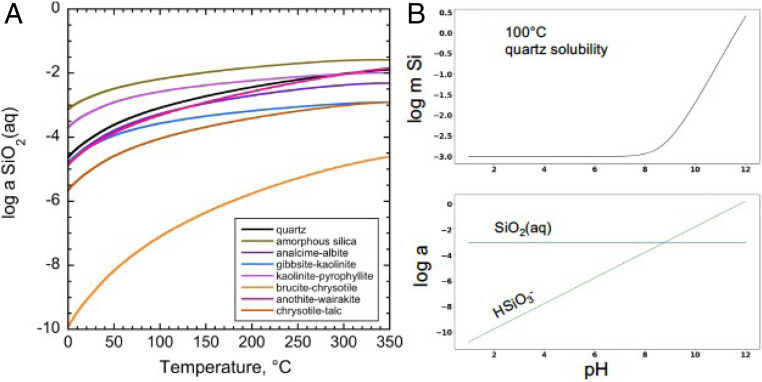
The amount of dissolved silica in natural solutions can be assessed relative to the stabilities and solubilities of rock-forming minerals. Examples in Fig. 3 provide a framework for quantifying silica abundances that hydrothermal and weathering processes can provide. Alteration of ultramafic rocks can be approximated by aqueous silica activities set by the brucite [Mg(OH)]–chrysotile [Mg3Si2O5(OH)4] assemblage that plot at lower values in Fig. 3A.
Fig. 3.
(A) Ranges of silica activities in aqueous solutions at equilibrium with quartz, amorphous silica and various mineral assemblages along the boiling curve for H2O. (B) pH dependence of quartz solubility and speciation of dissolved silica at 100 °C. Calculations are done with data and equations from refs. 56–59.
Mineral assemblages in more silica-rich rocks allow much higher aqueous silica activities indicated by the rest of the curves. Therefore, as mantle and crustal differentiation led to rocks enriched in silica, the silica content of fluids altering those rocks also increased. Since aqueous silica activities are higher at elevated temperatures, processes can supply supersaturated silica solutions that are primed for organic interactions. Over wide ranges of pH the changes in total dissolved Si contents of fluids are rather similar but are greatly enhanced at strongly basic conditions where the weak silicic acid dissociates. Fig. 3B shows the pH dependence of quartz solubility in water at 100 °C in the upper panel and the speciation of dissolved silica in the lower panel. In natural fluids the presence of other solutes, especially major rock-forming cations, can drive even greater total dissolved Si contents via the formation of cation–silicate complexes in solution (60).
Silica Dissolution and Precipitation Mechanisms.
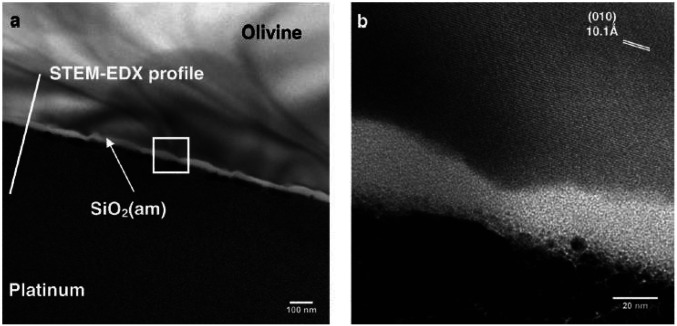
The alteration of minerals via reaction with solutions was initially considered to occur by solid-state volume interdiffusion, with cations from the mineral entering solution while protons replaced them, thus leaving behind silica-rich leached layers on mineral surfaces. However, the improvement of sample preparation techniques using focused ion beams and higher resolution and chemical specificity with electron microscopy has led to a rethinking of this process. As described in Hellmann et al. (61), dissolution of the mineral followed by precipitation of amorphous silica at the mineral/fluid interface was observed in many cases. High-resolution transmission electron microscopy (TEM) revealed 10- to 40-nm-thick rims of amorphous silica with no detectable porosity upon dissolution of olivine, (Mg,Fe)2SiO4 (62). Analogous rims were seen when dissolving other minerals, including wollastonite, anorthite, and garnet (61). In all cases, crystalline silicates were in sharp contact with amorphous silica with no evidence of a leached layer (Fig. 4).
Fig. 4.
(A) Bright-field TEM image of laboratory- weathered olivine showing amorphous silica contact. (B) Magnified version of box in A using high-resolution TEM. Note the sharp boundary between crystalline olivine and amorphous silica. Reprinted from ref. 62. Copyright (2011), with permission from Elsevier.
The strong conclusion drawn from these studies is that the amorphous silica layers form by a continuously occurring process of dissolution–precipitation rather than leaching. Thus, all the silicon atoms in the newly precipitated phase have spent some time in aqueous solution after leaving their original mineral phase. The concentration of dissolved silica at any time may be low, but all the SiO2 in the newly formed silica phase has come from the aqueous solution. The importance for prebiotic chemistry is that a steady-state, low concentration of dissolved silica species would be available to interact with organic SDA in the aqueous phase. Although the precipitated silica phase would look amorphous to cursory laboratory examination, it may contain local regions of more ordered structures that aid further organic transformations. Over the long term, any particular precipitated amorphous silica may be short lived, transforming to more stable phases such as quartz, but its continuous production may be sufficient to support the proposed scenario. A variety of silicate minerals, common in the prebiotic geologic environment, perhaps with olivine dominant, may be the sources of the transient dissolved silica, leading to amorphous and mesoporous silica with catalytic activity. Thus, the processes proposed here do not require rare and unique geochemical conditions but may be widespread, continuously producing small amounts of increasingly complex organic molecules.
Possible Structure Directing Agents in Prebiotic Environments.
Peptides containing 4 to 12 residues and a hydrophilic head (24) may function as prebiotic SDA. These structures undergo spontaneous assembly to form ordered arrangements including micelles, nanovesicles, and nanotubes. Small peptides based on glycine, alanine, and aspartic acid, among the chemically and structurally simplest amino acids, are of particular interest because of their presence in simulations of Earth’s prebiotic environment (63) and their detection in carbonaceous chondrites (64).
However, high alkalinity with pH above the point of zero charge leads to undesired μm-sized aggregates (65). To overcome this possible difficulty, dry-down conditions may force the self-assembly (66). Indeed, many prebiotic systems may require an evaporative environment to drive condensation reactions (67, 68).
Fatty acids form micelles and vesicles, and they have been considered as the fundamental building blocks of prebiotic membranes in that they are chemically simpler than phospholipids (69, 70). Amphiphilic molecules have been isolated from meteorites and synthesized under simulated prebiotic conditions (71, 72). Fatty acids with a saturated acyl chain are extremely unreactive compounds and therefore might have accumulated to significant levels, even with slow or episodic syntheses.
Aliphatic acids with chain length from C-8 to C-18 might act as SDA at pH up to 14. The most abundant amphiphilic species in these mixtures are short, saturated fatty acids (<C-12), which are also the primary species found in carbonaceous chondrite extracts (73, 74). Many of these fatty acids form micelles at pH > 9 (75), while their formation of vesicles is restricted to a rather narrow pH range (70, 76).
The evidence summarized above suggests that fatty acids naturally present in prebiotic waters may participate in micelle formation and help structure direct the formation of mesoporous silica with different pore geometries.
Prospects for Future Work
Experimental studies are needed to test whether peptides and/or fatty acids can function as structure-directing agents for various forms of mesoporous silica. Study of the extent and rate of mesoporous silica formation using peptide and fatty acid SDA as functions of temperature and pH is desirable.
The binding of SDA with zeolites and mesoporous silica after structure direction has been shown by calorimetry to be exothermic but small in magnitude, typically less than 5 kJ/mol (13, 77). However, the initial interaction of the SDA with hydrated silicate ions in aqueous solution may be stronger but has not been measured directly. Such measurements are possible, at least for higher dissolved silica concentrations.
Although the rate of peptide synthesis may be catalyzed by reactions within mesoporous silica, the feasibility of reaction and most stable final products are controlled by thermodynamics. There are a number of unknowns in looking at different possible peptides. Specific questions include the following: Do certain amino acids polymerize more readily than others, in both a thermodynamic and kinetic sense? Is the exclusion from biological systems of certain amino acids found in meteorites related to their ease of polymerization under prebiotic conditions or to later biological constraints? Is the ease of polymerization related to the energetics of forming the peptide bond? Is there a bottleneck in the energetics at some intermediate degree of polymerization for certain amino acids? Is the extent of polymerization related to the strength of sorption in the mesoporous silica? How is peptide formation affected by chirality in both the amino acid and growing peptide, as well as in the mesoporous silica?
Many geochemical processes and geologic events could provide environments for silica formation and its interaction with organics. Such interactions could occur in a variety of pressure, temperature, and pH conditions. To increase our understanding, these processes should be investigated by experiment and computation. Analogous processes could be occurring on the present Earth, although masked by biological activity.
We conclude that the hypotheses formulated above can be constrained by careful experimentation and computation. At the same time, we cannot constrain details of the prebiotic geochemical environment in more than a very general way. Indeed, its probable variability in space and time makes it all the more likely that the pathways proposed here have occurred somewhere sometime, perhaps many times or even continuously at some level. Therefore, even sporadic occurrence of the right conditions may be enough to enable silica-catalyzed organic synthesis since the Earth has time on its side.
Acknowledgments
The preparation of this paper was aided by funding from the US Department of Energy, Office of Science, Office of Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division under Award DE-FG02-97ER14749 and by staff support from the Navrotsky Eyring Center for Materials of the Universe at Arizona State University.
Footnotes
The authors declare no competing interest.
Data Availability.
All study data are included in the article and supporting information.
References
- 1.Hasterok D., Garda M., Coxa G., Hand M., A 4 Ga record of granitic heat production: Implications for geodynamic evolution and crustal composition of the early Earth. Precambrian Res. 331, 105375 (2019). [Google Scholar]
- 2.Hastie A. R., Fitton J. G., Bromiley G. D., Butler I. B., Odling N. W. A., The origin of Earth’s first continents and the onset of plate tectonics. Geology 44, 855–858 (2016). [Google Scholar]
- 3.Martin W., Baross J., Kelley D., Russell M.J., Hydrothermal vents and the origin of life. Nat. Rev. Microbiol. 6, 805–814 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Gillams R. J., Jia T. Z., Mineral surface-templated self-assembling systems: Case studies from nanoscience and surface science towards origins of life research. Life (Basel) 8, 10–28 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Preiner M., et al. , Serpentinization: Connecting geochemistry, ancient metabolism and industrial hydrogenation. Life (Basel) 8, 41–62 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newsam J. M., “Zeolites” in Solid State Chemistry: Compounds, Cheetham A. K., Day P., Eds. (Clarendon Press, 1992), vol. 2, pp. 234–280. [Google Scholar]
- 7.Rimer J. D., Rational design of zeolite catalysts. Nat. Catal. 1, 488–489 (2018). [Google Scholar]
- 8.Florek J., Guillet-Nicolas R., Kleitz F., “Ordered mesoporous silica: Synthesis and applications” in Functional Materials for Energy, Sustainable Development and Biomedical Sciences, Leclerc M., Gauvin R., Eds. (De Gruyter, 2014), pp. 61–100. [Google Scholar]
- 9.Chaudhary V., Sharma S., An overview of ordered mesoporous material SBA-15: Synthesis, functionalization and application in oxidation reactions. J. Porous Mater. 24, 741–749 (2017). [Google Scholar]
- 10.Zhao D., et al. , Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 279, 548–552 (1998). [DOI] [PubMed] [Google Scholar]
- 11.Davis M. E., Ordered porous materials for emerging applications. Nature 417, 813–821 (2002). [DOI] [PubMed] [Google Scholar]
- 12.Piccione P. M., et al. , Thermochemistry of pure-silica zeolites. J. Phys. Chem. B 104, 10001–10011 (2000). [Google Scholar]
- 13.Piccione P. M., Yang S. Y., Navrotsky A., Davis M.E., Thermodynamics of pure-silica molecular sieve synthesis. J. Phys. Chem. B 106, 3629–3638 (2002). [Google Scholar]
- 14.Linares N., et al. , Thermochemistry of surfactant-templating of USY zeolite. Chemistry 25, 10045–10048 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Chawla A., Linares N., Rimer J. D., García-Martínez J., Time-resolved dynamics of intracrystalline mesoporosity generation in zeolite USY. Chem. Mater. 31, 5005–5013 (2019). [Google Scholar]
- 16.Lombardo D., Kiselev M.A., Magazu S., Calandra P., Amphiphiles self-assembly: Basic concepts and future perspectives of supramolecular approaches. Adv. Cond. Matter Phys. 2015, 1–22 (2015). [Google Scholar]
- 17.Hartmann M., Ordered mesoporous materials for bioadsorption and biocatalysis. Chem. Mater. 17, 4577–4593 (2005). [Google Scholar]
- 18.Narayan R., Nayak U. Y., Raichur A. M., Garg S., Mesoporous silica nanoparticles: A comprehensive review on synthesis and recent advances. Pharmaceutics 10, 118–162 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luisi P. L., “Compartments” in The Emergence of Life, From Chemical Origins to Synthetic Biology (Cambridge University Press, 2006), pp. 182–213. [Google Scholar]
- 20.Schrum J. P., Zhu T. F., Szostak J. W., The origins of cellular life. Cold Spring Harb. Perspect. Biol. 2, a002212 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kresge C. T., Leonowicz M. E., Roth W. J., Vartuli J. C., Beck J. S., Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 359, 710–712 (1992). [Google Scholar]
- 22.Li H., et al. , Mesoporous silicas templated by heterocyclic amino acid derivatives: Biomimetic synthesis and drug release application. Mater. Sci. Eng. C 93, 407–418 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Xu H., Yang H., Xu G., Yang Y., Synthesis of 2D hexagonal mesoporous silica using amino acid-based surfactant templating. MATEC Web Conf. 67, 01006 (2016). [Google Scholar]
- 24.Zhang J., et al. , Influence of the nature of amino acids on the formation of mesoporous LTA-type zeolite. Microporous Mesoporous Mater. 252, 79–89 (2017). [Google Scholar]
- 25.Che S., et al. , Synthesis and characterization of chiral mesoporous silica. Nature 429, 281–284 (2004). [DOI] [PubMed] [Google Scholar]
- 26.Casado C., et al. , L- and D-proline adsorption by chiral ordered mesoporous silica. Langmuir 28, 6638–6644 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Yokoi T., et al. , Preparation of chiral mesoporous materials with helicity perfectly controlled. Chem. Mater. 23, 2014–2016 (2011). [Google Scholar]
- 28.Atluri R., Hedin N., Garcia-Bennett A. E., Nonsurfactant supramolecular synthesis of ordered mesoporous silica. J. Am. Chem. Soc. 131, 3189–3191 (2009). [DOI] [PubMed] [Google Scholar]
- 29.Wang B., et al. , Chiral mesostructured silica nanofibers of MCM-41. Angew. Chem. Int. Ed. Engl. 45, 2088–2090 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Wu X., et al. , Racemic helical mesoporous silica formation by achiral anionic surfactant. Chem. Mater. 18, 241–243 (2006). [Google Scholar]
- 31.Yang S., et al. , On the origin of helical mesostructures. J. Am. Chem. Soc. 128, 10460–10466 (2006). [DOI] [PubMed] [Google Scholar]
- 32.Fan J., Kotov N. A., Chiral nanoceramics. Adv. Mater. 32, 2070311 (2020). [DOI] [PubMed] [Google Scholar]
- 33.Kuschel A., Sievers H., Polarz S., Amino acid silica hybrid materials with mesoporous structure and enantiopure surfaces. Angew. Chem. Int. Ed. Engl. 47, 9513–9517 (2008). [DOI] [PubMed] [Google Scholar]
- 34.Cui M., Zhang W., Xie L., Chen L., Xu L., Chiral mesoporous silica materials: A review on synthetic strategies and applications. Molecules 25, 3899–3919 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shir I. B., Kababya S., Schmidt A., Molecular details of amorphous silica surfaces determine binding specificity to small amino acids. J. Phys. Chem. C 118, 7901–7909 (2014). [Google Scholar]
- 36.Goscianska J., Olejnik A., Pietrzak R., Adsorption of L-phenylalanine onto mesoporous silica. Mater. Chem. Phys. 142, 586–593 (2013). [Google Scholar]
- 37.Gao Q., Xu Y., Wu D., Sun Y., Adsorption of amino acids on SBA-15-type mesoporous materials. Stud. Surf. Sci. Catal. 170, 961–966 (2007). [Google Scholar]
- 38.Martra G., et al. , The formation and self-assembly of long prebiotic oligomers produced by the condensation of unactivated amino acids on oxide surfaces. Angew. Chem. Int. Ed. Engl. 53, 4671–4674 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Brodrecht M., Breitzke H., Gutmann T., Buntkowsky G., Biofunctionalization of nano channels by direct in-pore solid-phase peptide synthesis. Chemistry 24, 17814–17822 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Brodrecht M., et al. , Structural insights into peptides bound to the surface of silica nanopores. Chemistry 25, 5214–5221 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Subra G., et al. , Functionalized mesoporous silica: A good opportunity for controlled peptide oligomerization. J. Mater. Chem. 21, 6321–6326 (2011). [Google Scholar]
- 42.Kubota Y., et al. , Organic–silicate hybrid catalysts based on various defined structures for Knoevenagel condensation. Microporous Mesoporous Mater. 70, 135–149 (2004). [Google Scholar]
- 43.Sun L.-B., Liu X.-Q., Zhou H.-C., Design and fabrication of mesoporous heterogeneous basic catalysts. Chem. Soc. Rev. 44, 5092–5147 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Chen S.-Y., et al. , Synthesis and catalytic activity of amino-functionalized SBA-15 materials with controllable channel lengths and amino loadings. J. Mater. Chem. 22, 2233–2243 (2012). [Google Scholar]
- 45.Shimizu K., et al. , Self-aldol condensation of unmodified aldehydes catalysed by secondary-amine immobilised in FSM-16 silica. Tetrahedron Lett. 43, 9073–9075 (2002). [Google Scholar]
- 46.Takahashi H., et al. , Catalytic activity in organic solvents and stability of immobilized enzymes depend on the pore size and surface characteristics of mesoporous silica. Chem. Mater. 12, 3301–3305 (2000). [Google Scholar]
- 47.Shylesh S., Thiel W. R., Bifunctional acid–base cooperativity in heterogeneous catalytic reactions: Advances in silica supported organic functional groups. ChemCatChem 3, 278–287 (2011). [Google Scholar]
- 48.Parlett C. M. A., et al. , Spatially orthogonal chemical functionalization of a hierarchical pore network for catalytic cascade reactions. Nat. Mater. 15, 178–182 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Saladino R., Botta G., Bizzarri B. M., Di Mauro E., Garcia Ruiz J. M., A global scale scenario for prebiotic chemistry: Silica-based self-assembled mineral structures and formamide. Biochemistry 55, 2806–2811 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McKee A., “Prebiotic chemistry on mineral surfaces: Proto-oligopeptide formation on silica and other substrates within depsipeptide forming systems,” PhD thesis, Georgia Institute of Technology, Atlanta, GA: (2019). [Google Scholar]
- 51.Baross J. A., Hoffman S. E., Submarine hydrothermal vents and associated gradient environments as sites for the origin and evolution of life. Orig. Life Evol. Biosph. 15, 327–345 (1985). [Google Scholar]
- 52.Shock E. L., Geochemical constraints on the origin of organic compounds in hydrothermal systems. Orig. Life Evol. Biosph. 20, 331–367 (1990). [Google Scholar]
- 53.Shock E. L., Chemical environments of submarine hydrothermal systems. Orig. Life Evol. Biosph. 22, 67–107, 191–242 (1992). [DOI] [PubMed] [Google Scholar]
- 54.Shibuya T., et al. , Hydrogen-rich hydrothermal environments in the Hadean ocean inferred from serpentinization of komatiites at 300 °C and 500 bar. Prog. Earth Planet. Sci. 2, 1–11 (2015). [Google Scholar]
- 55.Trail D., et al. , Origin and significance of Si and O isotope heterogeneities in Phanerozoic, Archean, and Hadean zircon. Proc. Natl. Acad. Sci. U.S.A. 115, 10287–10292 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Helgeson H. C., Delaney J. M., Nesbitt H. W., Bird D. K., Summary and critique of the thermodynamic properties of rock-forming minerals. Am. J. Sci. 278A, 1–229 (1978). [Google Scholar]
- 57.Shock E. L., Helgeson H. C., Sverjensky D. A., Calculation of the thermodynamic and transport properties of aqueous species at high pressures and temperatures: Standard partial molal properties of inorganic neutral species. Geochim. Cosmochim. Acta 53, 2157–2183 (1989). [Google Scholar]
- 58.Shock E. L., Sassani D. C., Willis M., Sverjensky D. A., Inorganic species in geologic fluids: Correlations among standard molal thermodynamic properties of aqueous ions and hydroxide complexes. Geochim. Cosmochim. Acta 61, 907–950 (1997). [DOI] [PubMed] [Google Scholar]
- 59.Sverjensky D. A., Shock E. L., Helgeson H. C., Prediction of the thermodynamic properties of aqueous metal complexes to 1000 ° C and 5 kb. Geochim. Cosmochim. Acta 61, 1359–1412 (1997). [DOI] [PubMed] [Google Scholar]
- 60.Hunt J. D., Kavner A., Schauble E. A., Snyder D., Manning C., Polymerization of aqueous silica in H2O-K2O solutions at 25-200 °C and 1 bar to 20 kbar. Chem. Geol. 283, 161–170 (2011). [Google Scholar]
- 61.Hellmann R., et al. , Unifying natural and laboratory chemical weathering with interfacial dissolution-reprecipitation: A study based on the nanometer-scale chemistry of fluid-silicate interfaces. Chem. Geol. 294-295, 203–216 (2012). [Google Scholar]
- 62.Daval D., et al. , Influence of amorphous silica layer formation on the dissolution rate of olivine at 90 °C and elevated pCO2. Chem. Geol. 284, 193–209 (2011). [Google Scholar]
- 63.Leman L., Orgel L., Ghadiri M. R., Carbonyl sulfide-mediated prebiotic formation of peptides. Science 306, 283–286 (2004). [DOI] [PubMed] [Google Scholar]
- 64.Shimoyama A., Ogasawara R., Dipeptides and diketopiperazines in the Yamato-791198 and Murchison carbonaceous chondrites. Orig. Life Evol. Biosph. 32, 165–179 (2002). [DOI] [PubMed] [Google Scholar]
- 65.von Maltzahn G., Vauthey S., Santoso S., Zhang S., Positively charged surfactant-like peptides self-assemble into nanostructures. Langmuir 19, 4332–4337 (2003). [Google Scholar]
- 66.Fan H. Y., et al. , Hierarchically structured functional porous silica and composite produced by evaporation-induced self- assembly. Microporous Mesoporous Mater. 44, 625–637 (2001). [Google Scholar]
- 67.Lahav N., White D., Chang S., Peptide formation in the prebiotic era: Thermal condensation of glycine in fluctuating clay environments. Science 201, 67–69 (1978). [DOI] [PubMed] [Google Scholar]
- 68.Forsythe J. G., et al. , Ester-mediated amide bond formation driven by wet-dry cycles: A possible path to polypeptides on the prebiotic Earth. Angew. Chem. Int. Ed. 54, 9871–9875 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Egel R., Origins and emergent evolution of life: The colloid microsphere hypothesis revisited. Orig. Life Evol. Biosph. 44, 87–110 (2014). [DOI] [PubMed] [Google Scholar]
- 70.Ikari K., et al. , Dynamics of fatty acid vesicles in response to pH stimuli. Soft Matter 11, 6327–6334 (2015). [DOI] [PubMed] [Google Scholar]
- 71.Oro J., Chemical synthesis of lipids and the origin of life. J. Biol. Phys. 20, 135–147 (1994). [Google Scholar]
- 72.McCollom T. M., Ritter G., Simoneit B. R. T., Lipid synthesis under hydrothermal conditions by Fischer-Tropsch-type reactions. Orig. Life Evol. Biosph. 29, 153–166 (1999). [DOI] [PubMed] [Google Scholar]
- 73.Yuen G. U., Kvenvold K. A., Monocarboxylic acids in Murray and Murchison carbonaceous meteorites. Nature 246, 301–302 (1973). [Google Scholar]
- 74.Huang Y., et al. , Molecular and compound-specific isotopic characterization of monocarboxylic acids in carbonaceous chondrites. Geochim. Cosmochim. Acta 69, 1073–1084 (2005). [Google Scholar]
- 75.Cistola D. P., Atkinson D., Hamilton J. A., Small D. M., Ionization and phase behavior of fatty acids in water: Application of the Gibbs phase rule. Biochemistry 25, 2804–2812 (1986). [DOI] [PubMed] [Google Scholar]
- 76.Morigaki K., Walde P., Misran M., Robinson B. H., Thermodynamic and kinetic stability: Properties of micelles and-vesicles formed by the decanoic acid/decanoate system. Colloids Surf. A Physicochem. Eng. Asp. 213, 37–44 (2003). [Google Scholar]
- 77.Trofymluk O., Levchenko A. A., Navrotsky A., Mesoporous silica synthesis: Energetics of interaction between framework and structure directing agent. Microporous Mesoporous Mater. 149, 119–125 (2012). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All study data are included in the article and supporting information.