Significance
Arthropods are excellent indicators for studying global change in the rapidly changing climate of the Arctic. We used the most comprehensive standardized dataset on Arctic arthropods to quantify diversity and abundance variation over 24 y in an area that is warming rapidly. Overall arthropod abundance and diversity showed opposing nonlinear trends, with a sharp increase in overall abundance in recent years. However, trends varied substantially among taxa and habitats and several groups declined in abundance. We found strong evidence of conditions outside the growing season and density-dependent feedbacks affecting abundance. Our results emphasize the need for a more integrated approach to investigating arthropod responses to environmental stressors at finer taxonomic resolution and by incorporating time-lagged effects.
Keywords: insects, long-term monitoring, spiders, temporal trend
Abstract
Time series data on arthropod populations are critical for understanding the magnitude, direction, and drivers of change. However, most arthropod monitoring programs are short-lived and restricted in taxonomic resolution. Monitoring data from the Arctic are especially underrepresented, yet critical to uncovering and understanding some of the earliest biological responses to rapid environmental change. Clear imprints of climate on the behavior and life history of some Arctic arthropods have been demonstrated, but a synthesis of population-level abundance changes across taxa is lacking. We utilized 24 y of abundance data from Zackenberg in High-Arctic Greenland to assess trends in abundance and diversity and identify potential climatic drivers of abundance changes. Unlike findings from temperate systems, we found a nonlinear pattern, with total arthropod abundance gradually declining during 1996 to 2014, followed by a sharp increase. Family-level diversity showed the opposite pattern, suggesting increasing dominance of a small number of taxa. Total abundance masked more complicated trajectories of family-level abundance, which also frequently varied among habitats. Contrary to expectation in this extreme polar environment, winter and fall conditions and positive density-dependent feedbacks were more common determinants of arthropod dynamics than summer temperature. Together, these data highlight the complexity of characterizing climate change responses even in relatively simple Arctic food webs. Our results underscore the need for data reporting beyond overall trends in biomass or abundance and for including basic research on life history and ecology to achieve a more nuanced understanding of the sensitivity of Arctic and other arthropods to global changes.
Long-term monitoring data, meta-analyses, and reviews indicate that the abundance and diversity of terrestrial arthropods are declining across many sites (1–5) and several anthropogenic drivers are likely involved (6). However, due to data limitations, each assessment has various strengths and weaknesses: For example, some studies are based on biological records requiring corrections for sampling effort (1, 4, 7), while others provide only a comparison of a few discrete points in time (7). These and other shortfalls have strengthened calls for more standardized long-term biological monitoring (8–11), especially from areas with reduced levels of direct human impacts (12–14). Previous work on arthropod communities points toward habitat fragmentation, habitat degradation and loss, and land-use intensification as primary drivers of species declines over the last decades (2), although climate changes will likely increase in importance (14–16). Teasing apart the effects of land-use change, climate change, biotic factors, and other stressors, however, remains a major challenge (14). For example, while Hallmann et al. (3) found a midsummer decline in flying insect biomass of 82% over 27 y in protected areas in Germany, this pattern could not be attributed to landscape or climate factors and, as few locations were sampled repeatedly, an assessment of the importance of biotic factors such as density dependence was not possible.
Arctic regions provide an important opportunity to isolate the ecological impacts of climate change because direct anthropogenic disturbances are largely absent (17). The region is also warming rapidly and snowmelt, which strongly affects invertebrate activity, is occurring earlier (18, 19). While long-term data are generally scarce in the Arctic (12), the arthropod monitoring program at Zackenberg, northeast Greenland, has been collecting standardized data since 1996, representing the longest-running terrestrial arthropod monitoring program in the Arctic (20). The climate trends for Zackenberg are in line with trends for the rest of Greenland, with significant warming trends of 0.1 to 0.3 °C⋅y−1 (21). These arthropod data offer a rare opportunity to detect population changes across a broad array of taxa from different habitats. Many long-term arthropod monitoring programs report only overall abundance, biomass, or trends from a single habitat type (3, 5, 22). Such studies may fail to detect important lineage-specific, spatial, and temporal patterns (23–25). For example, while Crossley et al. (26) found no net trend in abundance in their meta-analyses of insect populations across long-term ecological research sites in the United States, the authors acknowledged that focusing only on broad cross-taxon patterns likely masked important ecological effects at finer taxonomic levels.
Despite the simpler suite of drivers in the Arctic, predicting arthropod responses to climate change remains a challenge in part due to the various environmental changes occurring. For example, as elsewhere, Arctic organisms are limited by temperature and moisture (27). We might expect rapidly warming conditions to lead to increases in arthropod abundances while, on the other hand, melting permafrost and the associated declines in soil moisture would impose important constraints (15). Our previous work in the region has shown considerable heterogeneity in long-term changes and links to climate across invertebrate orders (28). For example, a subset of families exhibited declines in total abundance between 1996 and 2016 for all habitats studied (13), with habitat type playing an important role mediating the strength of species abundance trends for both spiders (Araneae) and muscid flies (Diptera) (13, 25, 29), while links to climate variables were taxon-specific and thus did not show a strong, consistent pattern. Conversely, we documented strong effects of climate on body size (30, 31), phenology (32–34), community composition (25, 28), and species interactions (35, 36). Previous work in Alaska has also indicated that density-dependent factors may play an important role in some Arctic arthropod populations (37), but rigorous statistical testing of effects of past population density requires long time series. Such complex responses are likely to be masked by overall trends in abundance or biomass and emphasize the urgent need for improved understanding of variation in population trends across arthropod taxa to predict the likely winners and losers as global climate continues to change.
In this study, we used the Zackenberg dataset consisting of more than 1 million individual arthropods collected weekly throughout the growing season over the last 24 y to improve the understanding of long-term changes in a community of terrestrial and semiaquatic arthropods affected by climate change. Our first specific objective was to provide a detailed assessment of the temporal dynamics in overall site-level arthropod abundance and diversity, in abundance summed at the habitat level, and in abundance for all available family–habitat combinations. Second, we made a preliminary assessment of the relationships between arthropod abundance, climate variables, and abundance in the previous year (hereafter referred to as “density dependence”) for these time series. We fitted simple linear and segmented linear (hereafter referred to as “nonlinear”) models to all time series data to assess support for nonlinear dynamics. Based on previous results and our understanding of Arctic arthropods in general (12, 13, 38), we expected the 1996-to-2016 declines to continue and to be apparent in trends of summed abundance. In addition, we expected family-level diversity to be relatively stable over the study period due to a lack of direct habitat disturbance and a lack of documented species immigrations to this area. At the family level, we anticipated a range of trends in abundance as shown in other areas (14). Given previously documented weak links between abundance of some arthropod species and climate at Zackenberg, we did not expect substantial evidence that overall abundance trends would be climate-driven. However, as several groups are known to be especially sensitive to desiccation stress during the juvenile or larval stages, such as Collembola, Diptera, and some Hymenoptera (39), we did expect families within those groups to experience negative trends in association with warmer summer temperatures. Finally, as other authors have suggested the importance of climate conditions in other seasons (40), we included relevant climate variables across the full annual cycle to quantify their importance.
Results
Climatic Trends.
There were strong indications of warming at Zackenberg in the first decade (∼1996 to 2005) of the study period, including warming summers, increasing frequency of freeze–thaw events, and shorter winters (Fig. 1). This period was also characterized by decreasing fall and winter temperatures. However, since about 2005, the magnitude of these climate trends has reversed, albeit with increasing variability around trends in summer temperature and winter duration. We note that while we used soil temperature in this study, previous studies that have reported a strong warming trend at the site (e.g., ref. 25) were based on air temperatures, which are typically uncoupled from soil temperatures when the ground is snow-covered or waterlogged (41). Detailed climate results are contained in SI Appendix, Table S1.
Fig. 1.
Interannual variation and trends of key climate variables of relevance to arthropod abundance. Solid lines indicate significance for linear or segmented linear trends (see detailed results in SI Appendix, Table S1).
Trends in Total Abundance and Diversity.
Our analysis of trends in abundance revealed only limited evidence for clear, linear changes in abundances of insect, spider, and microarthropod populations at Zackenberg, a dataset that includes a total of 1,006,848 individuals collected across the entire 24-y time period (1996 to 2018). Instead, total arthropod abundance was best fitted by a segmented linear model showing a gradual decline until 2014, followed by a sharp increase (Fig. 2). This pattern was consistent for arid and mesic heath habitats but not for the wet fen, where no significant trend was found. We found an opposite pattern in diversity. Specifically, the exponential of Shannon’s entropy (Hill 1D) and the inverse Simpson’s concentration index (Hill 2D) computed from family-level data showed a gradual increase until 2015, followed by a sharp decrease. This pattern was consistent for separate analyses of arid and mesic heath habitats individually, which showed distinct change points in 2015 and 2014, respectively (Fig. 2). Overall family richness (Hill 0D) followed a somewhat different pattern, with an initial increase until 2005, followed by a gradual decline. This pattern was also found for mesic heath, whereas no trend was found in arid heath and wet fen. Detailed diversity results are presented in SI Appendix, Table S2.
Fig. 2.
Interannual variation and trends in total relative standardized arthropod abundance (Top) and arthropod diversity (Hill 0D, 1D, and 2D) for three habitat types extrapolated with individual-based rarefaction curves. Hill 0D, richness; Hill 1D, the exponential of Shannon’s entropy index; Hill 2D, the inverse of Simpson’s concentration index. Solid lines indicate significance for linear or segmented linear trends (see detailed results in SI Appendix, Table S2).
Trends in Abundance at Finer Taxonomic Resolution.
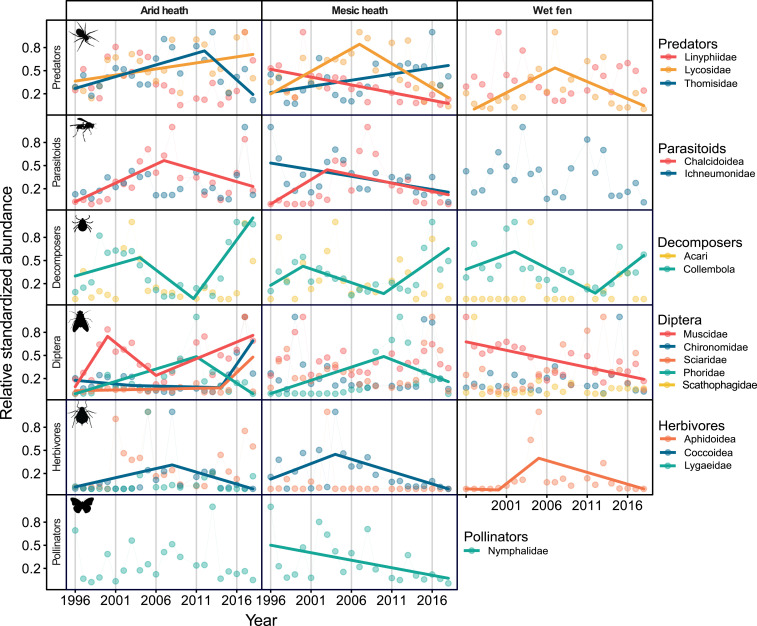
Despite the similar patterns in total arthropod abundance across habitats and for the arid and mesic heath sites individually, analyses of individual taxa revealed great variation. Some taxa such as Lycosidae and Muscidae even exhibited distinctly different patterns between habitats (Fig. 3). We found evidence of general linear declines in a few taxa, particularly in mesic heath (Linyphiidae, Ichneumonidae, and Nymphalidae), but nonlinear trends were much more common. This analysis also revealed distinct commonalities in the dynamics of several taxa. For example, the highest abundances of herbivores, most parasitoids, and certain predators were detected during the central part of the study period (∼2006 to 2012), while decomposers declined in abundance during the same period. Within the functionally diverse Diptera, we found contrasting trends and complex dynamics across groups. Explanations of taxonomic affiliations and broad functional groups of included taxa are presented in SI Appendix, Table S3, and detailed results from the segmented regression analyses of individual taxa from each habitat are presented in SI Appendix, Table S4.
Fig. 3.
Interannual variation and trends in relative standardized arthropod abundance at the family and higher taxonomic levels. Arthropod taxa are grouped into broad functional groups as outlined in SI Appendix, Table S3. Solid lines indicate where linear or segmented regression models were the best fit to the data (see detailed results in SI Appendix, Table S4).
Abundance Trends in Association with Climate Variables.
We used LASSO (least absolute shrinkage and selection operator) regression models to identify the explanatory climate variables that were the best predictors for overall abundance trends, as well as for trends across the different families (Fig. 4). We considered the importance of density dependence in these trends by including a first-order lagged abundance variable in each model. For the overall data, higher abundances were associated with warmer fall and winter temperatures and with lower summer temperatures in the year arthropods were sampled as well as the previous year. This pattern was relatively consistent for arid and mesic heath, although bootstrapped CIs around the coefficients suggest uncertainty around some of the predictors (SI Appendix, Table S5). There was also a strong density-dependent effect in the arid habitat, as indicated by the large coefficient for the lagged abundance term. Conversely, total abundance in wet fen was negatively affected by higher spring temperatures. When the analyses were carried out separately by family and habitat, we found that abundance was associated with summer temperatures in only 30% of the time series analyzed (SI Appendix, Tables S6–S8); in only one of these cases was previous summer temperature the only important driver (Linyphiidae in wet fen). Our results also identified consistent support for positive effects of density-dependent feedbacks across taxa.
Fig. 4.
LASSO regression coefficients for a subset of the models fitted at least 30% null deviance explained (1-residual deviance/null deviance). The families along the horizontal axis are response variables and metrics on the vertical axis formed the explanatory variable matrix. The size of circles indicates the size of standardized coefficients. Black outlines around circles indicate bootstrapped CIs that do not encompass 0. Blue circles, negative coefficient; red circles, positive coefficient. *, coefficients presented are from models with one or two outliers removed; §, coefficients are from models that violated assumptions of normally distributed residuals or homogeneous variance. These models are included for completeness. The full set of LASSO regression coefficients and % null deviance explained is shown in SI Appendix, Tables S5–S8.
Abundance was associated with temperature outside the summer season for many taxa, particularly the arid heath fauna, where conditions during the previous fall appear to be linked to abundances during the following summer. We found varying effects of other drivers, including precipitation in the previous year, winter duration, and winter freeze–thaw events. Taxa in mesic heath and wet fen in particular showed little fidelity to overall abundance patterns across habitats, with a complex array of largely negative climate effects, hampering our ability to generalize across habitats and taxa. There was high variability in model fit across taxa, with percentage deviance explained by the climate variables and density dependence ranging from 0 to 80.6% (SI Appendix, Tables S5–S8). Unsurprisingly, we found a high degree of variation among families in the importance of the various climate variables, and some families were even affected by different variables in each habitat (Fig. 4). For example, in wet fen and arid heath, Muscidae were positively influenced by previous fall temperatures. Yet, in mesic heath, winter freeze–thaw events and summer temperatures were the most important predictors.
Discussion
Recent commentaries of insect declines have called for standardized methodology and well-designed syntheses of population dynamics (42), balanced analysis (11), and realistic interpretation of results given the limitations of available data (43). With this study, we have met these goals by presenting and analyzing data from a long-term standardized monitoring program in the Arctic over 24 y. We observed a nonlinear pattern in overall arthropod abundance that included an initial decline followed by an abrupt recent increase. We also found arthropod populations to be highly dynamic. Many families show contrasting, and often nonlinear, patterns of abundance in comparison with the overall trends, a result that highlights how overall arthropod abundance patterns are inadequate proxies for abundance trends of individual taxa. While we did find support for strong associations between climate variables outside the growing season and density dependence (as measured by previous year abundances) in relation to overall abundance within and across habitats, the wide range of relationships at the family level suggests that generalization is not advisable. Overall, our findings suggest that, even for a “simple” food web, there are few straightforward relationships between arthropod abundance and climate variables.
We have previously reported linear declines in habitat-level arthropod abundance at Zackenberg for a subset of taxa between 1996 and 2016 (13), and we expected the pattern to continue, in line with other high-profile studies (2, 3, 44). However, with our complete dataset, we demonstrated that segmented regression techniques may be more appropriate for describing the dynamics of many arthropod time series. Had we used only linear regression for these data, a nonsignificant trend would have resulted in all but mesic heath (SI Appendix, Table S2). Increases in abundance in the last few years of the study period at our site could be part of natural variation cycles or a response to changes in climate. Even though the climatic change points are not coincident with those of abundance, there has been a period of consistent, relatively high fall and winter temperatures since 2014, so it is possible that less harsh conditions during these cold periods facilitated increases in abundances of some groups.
Interestingly, the change point in abundance did coincide with change points for the diversity indices 1D and 2D, which began to decrease dramatically around 2014 (Fig. 2). These results mirror findings from another recent long-term study showing opposing abundance and diversity trends (45). Together, our results indicate that recent abundance increases are driven by a small number of dominant taxa. In contrast, although overall family richness (Hill 0D) peaked in the central part of the study period, it showed little overall change (Fig. 2). This lack of overall change is consistent with other studies that have found overall species richness to be stable, even over extended periods, in the face of climate change (46). We speculate that the warmer summers during the central part of the study led to higher abundance of particularly temperature-limited taxa, but further study at the species level is necessary to understand which species benefited from warmer summer temperatures and why.
As expected, family-level abundance trends were highly variable and bore little resemblance to the overall patterns (except for the fly families Sciaridae and Chironomidae in arid heath). We observed concerning declines in abundance of some taxa, such as nymphalid butterflies. Spiders in the family Linyphiidae in mesic heath and flies in the family Muscidae in wet fen also suffered steady declines over our study period. In previous work at the same site, we have shown a range of positive, negative, and essentially unchanged population trends among individual species in these two families (12, 13), which further supports our general finding that analyses at coarse taxonomic levels mask responses at finer taxonomic resolution. Declines in some species of Muscidae may be driven by reduced soil moisture in warm years that negatively affects their aquatic or semiaquatic larvae (13). Given their functional importance as predators and pollinators (25) and numerical abundance at Zackenberg, further study on these taxa is particularly urgent.
Results from our penalized (LASSO) regression findings indicated that climate variables are especially important predictors of overall arthropod abundance (59% null deviance explained) and for summed abundance in wet fen (78%). Climate variables were also important for some individual taxa such as Collembola in arid heath (81%) and Thomisidae in mesic heath (69%) (Fig. 4). Our expectation that taxa particularly sensitive to changes in soil moisture, such as Collembola, would show declines linked to warming summers was not fully met. While Collembola were negatively associated with warmer summers in all habitats, both the abundance and summer temperature time series showed complex trends. Other variables such as fall temperature (arid and mesic heath), winter temperature (mesic heath), and summer precipitation (wet fen) were also important for this taxon. An additional finding from our analysis is the potential importance of density-dependent feedbacks on long-term patterns in abundance as suggested by lagged abundance variables. For most groups, density dependence was a more important predictor of abundance than any of the climate variables (Fig. 4). However, density-dependent feedbacks often cannot be fully understood through abundance data alone and warrant demographic and experimental study.
Our findings align with previous work at Zackenberg showing limited changes in abundance (13) and community composition (28) in the wet fen as compared with the heath sites, suggesting that communities in sites with high soil moisture are buffered from the effects of climate change. Specifically, we observed no overall abundance or diversity patterns in the wet fen habitat at Zackenberg, and there were few family-level trends. This habitat also exhibited a rather different set of relationships between abundance and climate variables. However, the negative impact of spring temperatures on overall abundance in this habitat warrants close monitoring, particularly as spring snowmelt timing is considered to be a key driver of population dynamics (47).
Another major finding from our results is that climate factors outside the growing season may be key drivers of arthropod abundance even in the harsh Arctic environment. The strong links between our climate variables and overall abundance (Fig. 4), combined with the opposing patterns of overall abundance and diversity (Fig. 2), suggest that conditions characterized by cool summers but warm “off-seasons” favor some taxa over others. For example, many of the taxa that have increased in recent years (e.g., Lycosidae, Collembola, Chironomidae, Muscidae, and Sciaridae in arid heath) are positively linked to fall temperature, while some declining groups (e.g., Thomisidae) are negatively associated with winter temperatures. Seasons outside of the summer are often neglected in Arctic climate change research (40, 48), and thus the reasons for these patterns are unclear. Warmer falls may provide fitness benefits to some groups via a delay in the onset of diapause or by allowing for additional generations (49); warmer winters may improve growth rates for some taxa (50) but could also increase mortality by reducing insulating properties of snow (40). Warmer winter temperatures can also differentially impact parasitoid species, with potential knock-on effects for prey species (51). Although we found few family-level effects of the number of winter freeze–thaw events, these are also thought to be an important factor in arthropod survival (40). Further studies are necessary to identify the combination of climate conditions outside the growing season, which have the strongest effects on Arctic arthropods, and the variation in critical climate conditions across taxa.
Recommendations for Future Work.
Our efforts at Zackenberg highlight that a number of challenges remain in linking temporal variation in environmental conditions to arthropod population dynamics. It is clear that the general assumption that arthropods will benefit from increasing summer temperatures is too simplified, and that identifying winners and losers of future Arctic climate conditions will instead require both whole-year and multiyear approaches to modeling climate impacts on arthropod abundance (28, 41). The first and arguably most important goal is to develop a better understanding of the combination of climate metrics most relevant for predicting responses to change in different arthropod lineages and guilds. For instance, desiccation stress is a key factor for arthropods inhabiting cold environments (52) and may interact with temperature stress (53). Soil moisture variation can influence arthropod communities (54, 55) even over small spatial scales (56). Data on snowmelt timing, soil temperature, and soil moisture should, therefore, be collected routinely at arthropod monitoring sites and at the localized scale of arthropod sampling and over all seasons (41). New efforts to compile and model such microclimate data will help in our understanding of relevant scales (57, 58).
Additionally, we suggest that future studies consider arthropod responses for different habitat types and at higher taxonomic resolution, because it is clear that responses often cannot be generalized at order and family levels (Figs. 3 and 4). In the Arctic, coordination and collaboration, such as provided by the Circumpolar Biodiversity Monitoring Program, could align plans for new monitoring programs with established programs to ensure intersite comparability (12, 42, 59). Arthropod trait databases that go beyond estimates of overall abundance or biomass would also contribute to a better understanding of which lineages and guilds are likely to exhibit correlated responses to climate factors (12, 13, 60) and allow for generalization beyond single study systems. For example, some environmental pressures impact soil-dwelling and semiaquatic larvae differently to flying adults that are more influenced by air temperatures and wind speed. Similarly, arthropod responses vary across life stages (e.g., juvenile vs. adult, feeding vs. nonfeeding, diapausing vs. reproductive) with variable long-term impacts on population dynamics. Our study provides a starting point for formulating hypotheses about how taxa with particular functional traits may respond to specific climate variables, which can be further tested in experiments and through modeling.
Even with all of the aforementioned improvements in place, taxonomic identification will remain a hurdle: Too often it proves time-consuming, expensive, and, for many groups, unreliable. Luckily, current technological developments offer some promising outlooks for expanding basic monitoring work. Molecular identification tools can provide high taxonomic resolution, and new analytical approaches are emerging for deriving both identity and abundance data (e.g., ref. 61). Image-based solutions employing deep-learning models are an increasingly powerful tool for automated insect identification and biomass estimation, as well as for monitoring of species and species interactions in their natural environment (62, 63). Relevant modeling tools can be further developed to scale up paired arthropod and microclimate data. Such future advances to improve understanding of arthropod population dynamics will also require interdisciplinary efforts such as those stimulated by the Network for Arthropods of the Tundra (64).
Conclusions
Unlike findings on insect trends from parts of Europe and North America in the last decade, we have not found evidence of alarming overall population declines, although some taxa do exhibit strong negative trends in abundance at our site. Opposing trends in abundance and diversity also suggest a concerning increase in dominance of a smaller number of taxa. Importantly, we demonstrate that overall measures of abundance can give misleading information about long-term dynamics of individual taxa and the associated impacts of environmental factors. Our family-level trends depict widely differing and nonlinear patterns. Moreover, we found that associations between abundances and climatic variability were rarely generalizable across habitats. Finally, we found that abundances were more commonly associated with winter and fall conditions and possible within-population processes than with summer temperatures. We stress the need for biome-wide coordination of targeted monitoring efforts, high taxonomic resolution of abundance data, trait data improvements, continued development of efficient monitoring technologies, and collection of relevant climate (and microclimate) variables as equally important in the pursuit of a fuller understanding of biotic and abiotic impacts on arthropod population trends. The empirical findings we present here could have emerged only from long-term, family-level, standardized data. We argue that, by integrating such monitoring with hypothesis-driven experiments and modeling, the research community will be better positioned to advance mechanistic understanding of arthropod population dynamics (65).
Materials and Methods
Sampling and Specimens.
The Greenland Ecosystem Monitoring Program has been in operation at Zackenberg in northeast Greenland (74°28′N, 20°34′W) since 1996, and includes standardized pitfall trapping in five plots: one wet fen, two mesic heaths, and two arid heath plots (see ref. 20 for further details). Each plot consisted of eight yellow pitfall traps (1997 to 2006) or four pitfall traps (1996 and 2007 to 2018) (each trap 5 m from its nearest neighbor). The traps were in operation during the growing season starting at snowmelt in late May to early June and ending by late August to early September, and were emptied once a week. To compare across the years of sampling (1996 to 2018), we focused on arthropod data from only June, July, and August. Data from 2010 were lost during transportation between Greenland and Denmark. Arthropods were sorted to the family level for spiders and most insects, superfamily level for Aphidoidea, Chalcidoidea, and Coccoidea, and subclass for other arthropods, and counted. We note that juveniles were included in the family-level abundances for spiders. For some early years of the program, certain families of Diptera were not sorted, but one family strongly dominated samples from later years (32), so we pooled the Chironomidae and Ceratopogonidae (hereafter called “Chironomidae”), Anthomyiidae and Muscidae (hereafter called “Muscidae”), and Mycetophilidae and Sciaridae (hereafter called “Sciaridae”).
The wet fen plot is composed primarily of mosses, grasses (e.g., Eriophorum sp.), and sedges with scattered Arctic willow (Salix arctica). The mesic heath plots consist primarily of Arctic bell heather (Cassiope tetragona) and Arctic willow, grasses/sedges, and berry plants (Vaccinium), while the arid heath plots have a greater dominance of mountain avens (Dryas octopetala) (32). All plots are located within an area less than 1 km2. The wet fen and arid heath plots typically have snowmelt 2 wk earlier than the mesic heath plots and soil moisture is highest in the wet fen and lowest in the arid heath plots. Soil temperature (at 0-, 5-, and 10-cm depth) in mesic heath dominated by C. tetragona and precipitation (mm) were measured hourly by a meteorological station located centrally and with a distance of less than 1 km from each arthropod sampling plot (66).
Data and Analyses.
We standardized weekly abundance counts for each arthropod group by calculating the abundance per trap per day for each plot in each season. In subsequent abundance (but not diversity) analyses, we focused on taxonomic groups for which total abundance in each habitat across the time series was higher than 500 individuals in total to ensure robust estimates of trends even for taxa that do not occur in all years. We note that although we are omitting rare taxa, the likelihood of detecting trends is possibly lower for the less abundant taxa included in our analyses. We derived a range of climate variables of relevance to arthropod populations from the temperature and precipitation data. Summer precipitation was calculated from the sum of June, July, and August measurements. Most Arctic arthropods overwinter beneath the snow. Thus, in order to estimate average seasonal temperatures experienced by arthropods for the previous fall (Octobert−1 and Novembert−1), winter (Decembert−1 through Marcht), spring (Aprilt and Mayt), and summer (Junet, Julyt, and Augustt), we calculated the mean hourly temperature across these seasons using data collected at 0-, 5-, and 10-cm depth within the soil profile. We defined the length of winter as the number of days from the first day in fall with an average temperature below 2 °C to the last day in spring with an average temperature below 2 °C. The number of freeze–thaw cycles over the previous winter was calculated from the soil temperature data by counting the number of days with a maximum temperature above 0 °C and a minimum temperature below 0 °C. We also included lagged variables for summer precipitation (precipitationt−1) and summer temperature (summert−1), as conditions during the previous growing season could influence arthropod abundance.
With the iNEXT package, we measured diversity with Hill numbers qD, which quantify diversity in the number of taxa with increasing weight placed on more abundant taxa at higher orders of diversity q (67, 68). We estimated diversity for orders 0, 1, and 2, which correspond, respectively, to Hill 0D, the effective number of families in each habitat (richness), Hill 1D (the exponential of Shannon’s entropy), and Hill 2D (inverse Simpson’s concentration index). We used individual-based extrapolation curves for each year, family, and habitat to compute those three diversity metrics. The extrapolation end point in each habitat was set to the maximum annual number of individuals collected within a family. We compared models of monotonic temporal trends in climate, diversity, and abundance with segmented models [when breakpoints were detected (69)] built with the forecast package (70). Comparisons of models were based on Akaike information criterion corrected for small sample sizes and Bayesian information criterion values. Normality of residuals and other assumptions have been checked using the gvlma package (71). Detailed results are presented in SI Appendix, Tables S1, S2, and S4.
Relationships between family abundances and local climate variables were assessed with a LASSO regression model. This procedure is useful in high-dimension situations with a large number of collinear predictors relative to the sample size, as the coefficients of less important predictors are reduced to 0. This helps to avoid overfitting and issues related to multicollinearity. A regularization parameter, λ (72), is estimated to control the amount of shrinkage and is optimized via cross-validation (73). The LASSO procedure is essentially a method designed for prediction rather than inference, and the estimation of statistical significance is problematic. However, some researchers have proposed the use of the bootstrap to estimate CIs around the coefficients (74), and we use this approach to provide information on uncertainty in the estimates of association between the climate variables and each arthropod family/taxon.
The LASSO models were fitted using the glmnet package (75). For each family, a lag variable was produced consisting of previous year abundance values (Nt−1). The inclusion of this variable in the explanatory variable matrix allowed for temporal autocorrelation to be addressed where necessary, but also meant that the first year was removed for each family (i.e., where the value for Nt−1 is unavailable). Year was also included in order to avoid overinterpreting linear trends. All explanatory variables were standardized during the model fitting. Models were initially cross-validated to obtain optimal λ values using the leave-one-out approach (76) due to the small sample size (n = 21). We also restricted the maximum number of explanatory variables that could be selected to 5, to avoid overfitting and favor ease of interpretation in the selected models. Values of λ that minimized the mean square error were taken as optimal and used to obtain predictions and deviance-explained values and subsequently assess residuals using the plotres function of the plotmo package (77). Where residuals displayed problems with model assumptions of normality or heteroscedasticity, attempts were made to address these issues by removing a maximum of two outliers or removing problematic predictor variables and rerunning the analysis. Models with persistent and extreme residual patterns were discarded as invalid, but results from models with moderate assumption violations are displayed and indicated for completeness. Finally, coefficients were bootstrapped with 999 replicates using the bootLasso function of the HDCI package (78). All statistical analyses were performed in the R 3.6.1 platform (79).
Supplementary Material
Acknowledgments
D. L. W. is gratefully thanked for convening the session “Insect Declines in the Anthropocene” at the Entomological Society of America annual meeting 2019 in St. Louis, MO, which brought the group of contributors to the special feature together. T. T. H. acknowledges funding from Independent Research Fund Denmark (Grant 8021-00423B). Data were kindly provided by the Greenland Ecosystem Monitoring program. We thank the Danish Environmental Protection Agency for funding over the years. Valuable inputs to the manuscript from Matthew Forister, D. L. W., M. R. B., and four anonymous reviewers are greatly appreciated.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. D.L.W. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2002557117/-/DCSupplemental.
Data Availability.
Species records and climate data reported in this paper have been retrieved from the Greenland Ecosystem Monitoring Database (https://doi.org/10.17897/V285-Z265).
References
- 1.Habel J. C., et al. , Butterfly community shifts over two centuries. Conserv. Biol. 30, 754–762 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Seibold S., et al. , Arthropod decline in grasslands and forests is associated with landscape-level drivers. Nature 574, 671–674 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Hallmann C. A., et al. , More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS One 12, e0185809 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas J. A., et al. , Comparative losses of British butterflies, birds, and plants and the global extinction crisis. Science 303, 1879–1881 (2004). [DOI] [PubMed] [Google Scholar]
- 5.Shortall C. R., et al. , Long-term changes in the abundance of flying insects. Insect Conserv. Divers. 2, 251–260 (2009). [Google Scholar]
- 6.Dirzo R., et al. , Defaunation in the Anthropocene. Science 345, 401–406 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Biesmeijer J. C., et al. , Parallel declines in pollinators and insect-pollinated plants in Britain and The Netherlands. Science 313, 351–354 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Kunin W. E., Robust evidence of insect declines. Nature 574, 641–642 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Komonen A., Halme P., Kotiaho J. S., Alarmist by bad design: Strongly popularized unsubstantiated claims undermine credibility of conservation science. Rethinking Ecol. 4, 17–19 (2019). [Google Scholar]
- 10.Willig M. R., et al. , Populations are not declining and food webs are not collapsing at the Luquillo Experimental Forest. Proc. Natl. Acad. Sci. U.S.A. 116, 12143–12144 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas C. D., Jones T. H., Hartley S. E., “Insectageddon”: A call for more robust data and rigorous analyses. Glob. Change Biol. 25, 1891–1892 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Gillespie M. A. K., et al. , Circumpolar terrestrial arthropod monitoring: A review of ongoing activities, opportunities and challenges, with a focus on spiders. Ambio 49, 704–717 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillespie M. A. K., et al. , Status and trends of terrestrial arthropod abundance and diversity in the North Atlantic region of the Arctic. Ambio 49, 718–731 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagner D. L., Insect declines in the Anthropocene. Annu. Rev. Entomol. 65, 457–480 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Høye T. T., Arthropods and climate change—Arctic challenges and opportunities. Curr. Opin. Insect Sci. 41, 40–45 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Halsch C. A. et al., Insects and recent climate change. Proc. Natl. Acad. Sci. U.S.A., 10.1073/pnas.2002543117 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conservation of Arctic Flora and Fauna , Arctic Biodiversity Assessment: Status and Trends of Arctic Biodiversity (Conservation of Arctic Flora and Fauna, Arctic Council, 2013). [Google Scholar]
- 18.Box J. E., et al. , Key indicators of Arctic climate change: 1971–2017. Environ. Res. Lett. 14, 045010 (2019). [Google Scholar]
- 19.Høye T. T., Post E., Schmidt N. M., Trøjelsgaard K., Forchhammer M. C., Shorter flowering seasons and declining abundance of flower visitors in a warmer Arctic. Nat. Clim. Chang. 3, 759–763 (2013). [Google Scholar]
- 20.Schmidt N. M., Hansen L. H., Hansen J., Berg T. B., Meltofte H., BioBasis—Conceptual design and sampling procedures of the biological monitoring programme within Zackenberg Basic. https://g-e-m.dk/fileadmin/g-e-m/Zackenberg/BioBasis_manual_2019.pdf. Accessed 4 December 2020.
- 21.Abermann J., et al. , Hotspots and key periods of Greenland climate change during the past six decades. Ambio 46 (suppl. 1), 3–11 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melero Y., Stefanescu C., Pino J., General declines in Mediterranean butterflies over the last two decades are modulated by species traits. Biol. Conserv. 201, 336–342 (2016). [Google Scholar]
- 23.Brooks D. R., et al. , Large carabid beetle declines in a United Kingdom monitoring network increases evidence for a widespread loss in insect biodiversity. J. Appl. Ecol. 49, 1009–1019 (2012). [Google Scholar]
- 24.Van Dyck H., Van Strien A. J., Maes D., Van Swaay C. A. M., Declines in common, widespread butterflies in a landscape under intense human use. Conserv. Biol. 23, 957–965 (2009). [DOI] [PubMed] [Google Scholar]
- 25.Loboda S., Savage J., Buddle C. M., Schmidt N. M., Høye T. T., Declining diversity and abundance of High Arctic fly assemblages over two decades of rapid climate warming. Ecography 41, 265–277 (2018). [Google Scholar]
- 26.Crossley M. S., et al. , No net insect abundance and diversity declines across US long term ecological research sites. Nat. Ecol. Evol. 4, 1368–1376 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Danks H. V., Seasonal adaptations in Arctic insects. Integr. Comp. Biol. 44, 85–94 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Koltz A. M., Schmidt N. M., Høye T. T., Differential arthropod responses to warming are altering the structure of Arctic communities. R. Soc. Open Sci. 5, 171503 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bowden J. J., Hansen O. L. P., Olsen K., Schmidt N. M., Høye T. T., Drivers of inter-annual variation and long-term change in High-Arctic spider species abundances. Polar Biol. 41, 1635–1649 (2018). [Google Scholar]
- 30.Høye T. T., Hammel J. U., Fuchs T., Toft S., Climate change and sexual size dimorphism in an Arctic spider. Biol. Lett. 5, 542–544 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bowden J. J., et al. , High-Arctic butterflies become smaller with rising temperatures. Biol. Lett. 11, 20150574 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Høye T. T., Forchhammer M. C., Phenology of High-Arctic arthropods: Effects of climate on spatial, seasonal and inter-annual variation. Adv. Ecol. Res. 40, 299–324 (2008). [Google Scholar]
- 33.Høye T. T., et al. , Phenology of High-Arctic butterflies and their floral resources: Species-specific responses to climate change. Curr. Zool. 60, 243–251 (2014). [Google Scholar]
- 34.Høye T. T., Post E., Meltofte H., Schmidt N. M., Forchhammer M. C., Rapid advancement of spring in the High Arctic. Curr. Biol. 17, R449–R451 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Schmidt N. M., et al. , An ecological function in crisis? The temporal overlap between plant flowering and pollinator function shrinks as the Arctic warms. Ecography 39, 1250–1252 (2016). [Google Scholar]
- 36.Schmidt N. M., et al. , Interaction webs in Arctic ecosystems: Determinants of Arctic change? Ambio 46 (suppl. 1), 12–25 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koltz A. M., Wright J. P., Impacts of female body size on cannibalism and juvenile abundance in a dominant Arctic spider. J. Anim. Ecol. 89, 1788–1798 (2020). [DOI] [PubMed] [Google Scholar]
- 38.Hodkinson I., “Insect biodiversity in the Arctic” in Insect Biodiversity, Foottit R. G., Adler P. H., Eds. (Wiley-Blackwell, 2018), pp. 15–57. [Google Scholar]
- 39.Danks H. V., Kukal O., Ring R. A., Insect cold-hardiness: Insights from the Arctic. Arctic 47, 391–404 (1994). [Google Scholar]
- 40.Bale J. S., Hayward S. A. L., Insect overwintering in a changing climate. J. Exp. Biol. 213, 980–994 (2010). [DOI] [PubMed] [Google Scholar]
- 41.Convey P., Coulson S. J., Worland M. R., Sjoblom A., The importance of understanding annual and shorter-term temperature patterns and variation in the surface levels of polar soils for terrestrial biota. Polar Biol. 41, 1587–1605 (2018). [Google Scholar]
- 42.Montgomery G. A., et al. , Is the insect apocalypse upon us? How to find out. Biol. Conserv. 241, 108327 (2020). [Google Scholar]
- 43.Didham R. K., et al. , Interpreting insect declines: Seven challenges and a way forward. Insect Conserv. Divers. 13, 103–114 (2020). [Google Scholar]
- 44.Lister B. C., Garcia A., Climate-driven declines in arthropod abundance restructure a rainforest food web. Proc. Natl. Acad. Sci. U.S.A. 115, E10397–E10406 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Antão L. H., Pöyry J., Leinonen R., Roslin T., Contrasting latitudinal patterns in diversity and stability in a high-latitude species-rich moth community. Glob. Ecol. Biogeogr. 29, 896–907 (2020). [Google Scholar]
- 46.Antão L. H., et al. , Temperature-related biodiversity change across temperate marine and terrestrial systems. Nat. Ecol. Evol. 4, 927–933 (2020). [DOI] [PubMed] [Google Scholar]
- 47.Høye T. T., Kresse J.-C., Koltz A. M., Bowden J. J., Earlier springs enable High-Arctic wolf spiders to produce a second clutch. Proc. Biol. Sci. 287, 20200982 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gallinat A. S., Primack R. B., Wagner D. L., Autumn, the neglected season in climate change research. Trends Ecol. Evol. 30, 169–176 (2015). [DOI] [PubMed] [Google Scholar]
- 49.Bale J. S., et al. , Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Glob. Change Biol. 8, 1–16 (2002). [Google Scholar]
- 50.Potts L. J., Koštál V., Simek P., Teets N. M., Energy balance and metabolic changes in an overwintering wolf spider, Schizocosa stridulans. J. Insect Physiol. 126, 104112 (2020). [DOI] [PubMed] [Google Scholar]
- 51.Kankaanpää T., et al. , Parasitoids indicate major climate-induced shifts in Arctic communities. Glob. Change Biol. 26, 6276–6295 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Convey P., Block W., Peat H. J., Soil arthropods as indicators of water stress in Antarctic terrestrial habitats? Glob. Change Biol. 9, 1718–1730 (2003). [Google Scholar]
- 53.Everatt M. J., Convey P., Bale J. S., Worland M. R., Hayward S. A. L., Responses of invertebrates to temperature and water stress: A polar perspective. J. Therm. Biol. 54, 118–132 (2015). [DOI] [PubMed] [Google Scholar]
- 54.Hansen R. R., et al. , Meter scale variation in shrub dominance and soil moisture structure Arctic arthropod communities. PeerJ 4, e2224 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Høye T. T., et al. , Elevation modulates how Arctic arthropod communities are structured along local environmental gradients. Polar Biol. 41, 1555–1565 (2018). [Google Scholar]
- 56.le Roux P. C., Aalto J., Luoto M., Soil moisture’s underestimated role in climate change impact modelling in low-energy systems. Glob. Change Biol. 19, 2965–2975 (2013). [DOI] [PubMed] [Google Scholar]
- 57.Bramer I., et al. , Advances in monitoring and modelling climate at ecologically relevant scales. Adv. Ecol. Res. 58, 101–161 (2018). [Google Scholar]
- 58.Lembrechts J. J., et al. , SoilTemp: A global database of near-surface temperature. Glob. Change Biol. 26, 6616–6629 (2020). [DOI] [PubMed] [Google Scholar]
- 59.Sikes D. S., et al. , The value of museums in the production, sharing, and use of entomological data to document hyperdiversity of the changing North. Arct. Sci. 3, 498–514 (2017). [Google Scholar]
- 60.Wong M. K. L., Guénard B., Lewis O. T., Trait-based ecology of terrestrial arthropods. Biol. Rev. Camb. Philos. Soc. 94, 999–1022 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ji Y., et al. , SPIKEPIPE: A metagenomic pipeline for the accurate quantification of eukaryotic species occurrences and intraspecific abundance change using DNA barcodes or mitogenomes. Mol. Ecol. Resour. 20, 256–267 (2020). [DOI] [PubMed] [Google Scholar]
- 62.Ärje J., et al. , Automatic image-based identification and biomass estimation of invertebrates. Methods Ecol. Evol. 11, 922–931 (2020). [Google Scholar]
- 63.Bjerge K., Nielsen J. B., Sepstrup M. V., Helsing-Nielsen F., Høye T. T., A light trap and computer vision system to detect and classify live moths (Lepidoptera) using tracking and deep learning. bioRxiv: 10.1101/2020.03.18.996447 (20 March 2020). [DOI]
- 64.Høye T. T., Culler L. E., Tundra arthropods provide key insights into ecological responses to environmental change. Polar Biol. 41, 1523–1529 (2018). [Google Scholar]
- 65.Schmidt N. M., Christensen T. R., Roslin T., A High Arctic experience of uniting research and monitoring. Earths Futur. 5, 650–654 (2017). [Google Scholar]
- 66.Kandrup N., Iversen K. M., Zackenberg ecological research operation—ClimateBasis manual. https://g-e-m.dk/fileadmin/Resources/DMU/GEM/Zackenberg/Nye_Zac_files/Zackenberg_Ecological_Research_Operation-_ClimateBasis_Manual_v1.pdf. Accessed 4 December 2020.
- 67.Chao A., et al. , Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 84, 45–67 (2014). [Google Scholar]
- 68.Hsieh T. C., Ma K. H., Chao A., iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 7, 1451–1456 (2016). [Google Scholar]
- 69.Killick R., Beaulieu C., Taylor S., Hullait H., EnvCpt: Detection of structural changes in climate and environment time series (R package, Version 1.1.2, 2020).
- 70.Hyndman R., et al. , forecast: Forecasting functions for time series and linear models (R package, Version 8.12, 2020).
- 71.Pena E. A., Slate E. H., gvlma: Global validation of linear models assumptions (R package, Version 1.0.0.3,2019). [DOI] [PMC free article] [PubMed]
- 72.Tibshirani R., Regression shrinkage and selection via the Lasso. J. R. Stat. Soc. Series B Methodol. 58, 267–288 (1996). [Google Scholar]
- 73.Hastie T., Tibshirani R., Friedman J., The Elements of Statistical Learning: Data Mining, Inference, and Prediction (Springer, New York, 2009). [Google Scholar]
- 74.Meinshausen N., Bühlmann P., Stability selection. J. R. Stat. Soc. Series B Stat. Methodol. 72, 417–473 (2010). [Google Scholar]
- 75.Friedman J. H., Hastie T., Tibshirani R., Regularization paths for generalized linear models via coordinate descent. J. Stat. Softw. 33, 22 (2010). [PMC free article] [PubMed] [Google Scholar]
- 76.Efron B., Stein C., The jackknife estimate of variance. Ann. Stat. 9, 586–596 (1981). [Google Scholar]
- 77.Milborrow S., plotmo: Plot a model’s residuals, response, and partial dependence plots (R package, Version 3.5.7, 2020).
- 78.Liu H., Xu X., Li J. J., HDCI: High dimensional confidence interval based on lasso and bootstrap (R package, Version 1.0-2, 2017).
- 79.R Core Team , R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria, 2019). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Species records and climate data reported in this paper have been retrieved from the Greenland Ecosystem Monitoring Database (https://doi.org/10.17897/V285-Z265).