Abstract
We have been field observers of tropical insects on four continents and, since 1978, intense observers of caterpillars, their parasites, and their associates in the 1,260 km2 of dry, cloud, and rain forests of Área de Conservación Guanacaste (ACG) in northwestern Costa Rica. ACG’s natural ecosystem restoration began with its national park designation in 1971. As human biomonitors, or “insectometers,” we see that ACG’s insect species richness and density have gradually declined since the late 1970s, and more intensely since about 2005. The overarching perturbation is climate change. It has caused increasing ambient temperatures for all ecosystems; more erratic seasonal cues; reduced, erratic, and asynchronous rainfall; heated air masses sliding up the volcanoes and burning off the cloud forest; and dwindling biodiversity in all ACG terrestrial ecosystems. What then is the next step as climate change descends on ACG’s many small-scale successes in sustainable biodevelopment? Be kind to the survivors by stimulating and facilitating their owner societies to value them as legitimate members of a green sustainable nation. Encourage national bioliteracy, BioAlfa.
Keywords: climate change, BioAlfa, conservation by rewilding, biodevelopment, insect decline
As “insectometers,” also known as human biomonitors, we have watched the conspicuous ongoing decline of tropical insect abundance and species richness (1) here in northwestern Costa Rica (2–4) (see Figs. 3–5 and SI Appendix, Figs. S1–S5) for the past five decades of living, inventorying, and experimenting among their congruent dry forests, cloud forests, and rain forests. We, and other scientists, have also done this in other rain forest Caribbean and Pacific areas of Costa Rica (5).
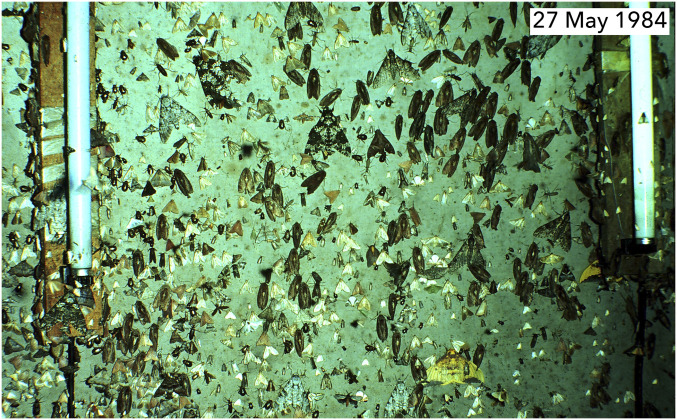
Fig. 3.
A normal 1980s assembly of moths at the Cliff Top light trap about 2 wk after the beginning of the rainy season, at the dark of the moon, 9 to 10 PM. The large black, gray, and white sphingid in the center, Manduca rustica, is a migrant from the Caribbean rain forest side of ACG, arriving with the rains, after a flight of perhaps 20 to 40 km. There are five local and univoltine Manduca dilucida on the sheet, easily recognized by their paired white shoulder patches. The elongate abundant dark moths on the right half of the sheet are local and largely univoltine Crinodes besckei and C. ritsemae (Notodontidae). All four of these species are now rare at this light trap station, as are now their caterpillars in the forest at any time of year.
Fig. 5.
The same light trap as viewed in Figs. 3 and 4, on 30 May 2019 at the same time in the moon cycle (dark) and 2 wk after the beginning of the rainy season. There are, as usual, two large yellow Eacles (as in SI Appendix, Fig. S2) on the ground in front of the white sheet. This dramatic change in moth density and species richness has now come to represent light trap catches in the ACG dry forest at the beginning of the rains (as repeated May 2020), and we have less extreme examples in the ACG cloud and rain forest as well, since about 2014 to 2015.
Dan has lived intensely with wild neotropical insects since 1953; Winnie has been doing the same in Costa Rica since 1978. In 1980, we set out to inventory the species of moths at light traps for the entire country. This produced 300,000+ mounted and labeled specimens of at least 10,000 species donated to Costa Rica’s Instituto Nacional de Biodiversidad (INBio) (6, 7) in 1997 (now in the Museo Nacional de Costa Rica), and to other international museums. We eventually focused into Área de Conservación Guanacaste (ACG) in northwestern Costa Rica (8–13) (Fig. 1) to demonstrate that there is truth to the then controversial concept that “once gone, a complex tropical forest can regenerate itself, if allowed and there are seed sources and seed dispersers” (13, 15).
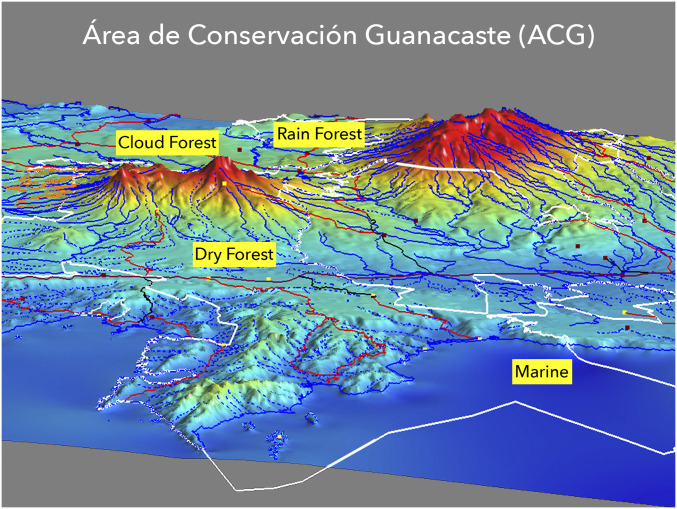
Fig. 1.
Área de Conservacion Guanacaste (ACG), northwestern Costa Rica, inside the white polygon covering ∼1,680 km2 from 6 km out into the Pacific Ocean, across the dry forest lowlands, over the cloud forested Cordillera Guanacaste (∼1,400- to 2,000-m elevation), and down into the Caribbean rain forest lowlands (∼1,260 km2 terrestrial). These are the three major tropical forested ecosystems. In ACG, they cover an area the size of New York City and its suburbs. We currently estimate that this terrestrial and freshwater area (approximate center at latitude 10.83764, longitude −85.61871, 300 m) contains at least a half million species of arthropods, based on our inventory and opportunistic sampling throughout ACG since 1978, and the ∼45,000 species collected by the first year (2014 to 2015) of intense sampling with Malaise traps. One of our standard Townes-type Malaise traps captured no fewer than 14,520 species of true insects during two consecutive years (14).
What we have seen and lived since the mid-1970s, unambiguously, in our Costa Rican tropical wild world, is that the biomass and species richness of insect individuals and species, and their interactions with everything, are decomposing. Since terrestrial arthropods as a whole are the great middle bulk of all terrestrial eukaryote food and interaction webs, their reduction, by whatever means, is to gut the terrestrial planet. In the ACG contiguous dry forests, rain forests, and cloud forests, and their many complex intergrades (4, 8–13, 16), the centuries of anthropogenic assaults have been and still are quite point-source specific. Forest clearing, fragmentation, monoculturing, logging, burning, hunting, insecticiding, herbiciding, droughts, flooding, and hurricanes have been obvious local depressants, although each hits a particular insect-rich interaction, ecosystem, or habitat, and recovers, in different ways. We have no logical or observational reason to feel that the 1,260 km2 protected and restoring terrestrial ACG (Fig. 1) is qualitatively different from any other species-rich and insect-rich, nonindustrial, large tropical agroscape, and especially one that is explicitly restoring (also known as rewilding) its three contiguous major ecosystems for more than 35 y. ACG is a huge ecologically diverse island in an ocean of increasingly industrializing agroscape. It will suffer some insularity effects, but they and 400 y of point-source assaults are not the overriding cause of the conspicuous insect decline since the mid-1980s when the initial national park was just 18% of ACG’s current area (3).
We conclude that the overall depression today of ACG insect species and individuals in its regenerating and old-growth forest ecosystems is attributable to the climate change excesses, speed, and erratic annual timing of temperature, wind, rain, cloud cover, and a plethora of combinations of these changes. We did not begin to notice this blanket of drier, hotter, more irregular, and unpredictable overall and point source impacts until about 1986, layered on the multitude and collage of small “normal” and “natural” point sources of perturbation: large, small, gradual, cryptic, ephemeral, and everywhere. These natural impacts are dotted all over ACG’s three major terrestrial ecosystems, all of which are now restoring/rewilding/recovering naturally, if idiosyncratically, from the elimination of nearly all of its 400+ years of helter-skelter European-style agroscape-forest manipulation and centuries of earlier patchy indigenous use (9).
All point source anthropogenic assaults influence insects and their interactive webs. These influences begin to vanish at variable rates when the assault is reversed and the adjacent ecosystems are allowed to reinvade their previous terrain and conditions, if they still exist. However, now, owing to climate change, to some degree this does not happen. An outstanding trait of climate change is that to an ever-increasing degree the “previous” no longer exists. A 200-y-old tree with all its attendant portions of thousands of ecosystem webs now and during its lifetime, now subject to climate change, no longer has the climate or interactors with which to reproduce itself the way its parents did. The herbivores, pollinators, seed dispersers, mycorrhizae, decomposers, diseases, competitors, commensals, mutualists, parents, parasites, and predators are all different from when it was a seed, seedling, and sapling.
The heterogeneous blanket of climate change is the overriding impact variable across the ACG landscape-level mosaic that began restoring to “intact” ecosystems in 1971 when the embryonic ACG comprised three small separate national parks in their preconservation state, a state characteristic of much of the less-polished tropics today (3, 9, 10). This gradual change in climate is so omnipresent and perturbing that only minimal and currently unknowable recovery is likely. Now, even natural perturbations extinguish populations because local populations are so reduced in number and microdistribution. When ACG formally embarked on its restoration in 1985 (3, 9, 10), it was still awash in insects and their conspicuous interactions. They were densely present at the lights, on the foliage, consuming a large although variable percent of annual leaf and seed production, plastered on windshields, in the metabolisms and feces of insectivorous vertebrates, fuel for the omnipresent army ants and spiders, inside and outside our dwelling in the forest edge, dormant while passing the seasons, and filling the air with their annual seasonal migrations back and forth (east–west) between ACG’s three major terrestrial ecosystems (e.g., refs. 17 and 18; SI Appendix, Fig. S1). We did not anticipate long-term serious decline beyond what happened seasonally and in ordinary drought/flood years. We were focused on restoration by giving the wild world back, not on its creeping decay.
Examples?
We can scratch out of our decades of insect and web observations and data, gathered for other purposes in pursuit of other patterns, some conspicuous recent impacts of climate change—temperatures, clouds, adult moths and caterpillars, and caterpillar parasitoids—extractable largely by serendipity. Our overall observation and research goals were, and still are, what actual species eat what, when, and where throughout ACG’s estimated 500,000 species of arthropods (19, 20). Beginning in 2004, we added DNA barcoding to the identification process (15, 19–24) so as to more accurately know and record the species. Here, we sift some fragments of examples out of the resulting information accumulated in the process of boosting ACG toward its truly sustainable survival through nondamaging biodevelopment within itself (20, 21) to be welcome to the societies surrounding it that must embrace its ownership if it is to survive (1–15, 16, 19–25).
Temperature and Rainfall
When Dan arrived in Liberia in 1963, 30 km south of ACG, an average of 116 d of the year reached temperatures of 32 °C or greater; when Winnie arrived in 1978 not much had changed. Today there are 193 d of the year with those temperatures (26). Not only is the dry season now 6+ mo rather than its centuries of being 4 mo, but the amount and timing of the annual rains are weeks to months more unpredictable in timing, duration, and amount. Traditionally, there were six vernacular yearly seasons in this dry forest ecosystem; now there are three, sloppily defined—rainy, long dry, short dry (“invierno,” “verano,” “veranillo”). Insects exhibit strong sensitivity to, and use of, weather cues and intensity (e.g., refs. 17, 18, and 27–34), be they resident or migrants [as expected of tropical species living “normally” in a relatively monotonous and predictable weather and climate regime (35, 36)]. All have been variously impacted by the mild to strong disruption of their cue patterns and intensities, and those of the plants and vertebrates with which they mutualize and trophically relate.
As a specific example, Manduca dilucida (Sphingidae) is a common ACG dry forest, food plant specialist, large sphinx moth that has one annual larval generation in the first month of the rainy season (May–June). It spends the next 10 mo as a dormant pupa underground. However, with the now more intense dry/hot short dry season in the month of August being followed by the abrupt and cooler second peak of the rainy season in September, in some years it is “fooled” into eclosing then as if it were mid-May following the next 6 mo dry season. The September–October caterpillar offspring do not survive the second half of the long rainy season owing to presumed intense vertebrate predation; lower-quality, more mature, food plant leaves; or parasitoid build up. The consequence is many fewer adults eclosing at the beginning of the rains in the next May (see Fig. 4). This May–June reduction in caterpillars is likely the cause of the apparent disappearance of its once common, now apparently “extirpated,” endemic univoltine and host-specific parasitic ichneumonid wasp, Mokajoppa respinozai.
Fig. 4.
The same light trap as Fig. 3, 23 y later, same time in the rainy season and moon cycle, with the mass of moths normally present at this time of year since the 1970s. The cinnamon brown endemic Schausiella santarosensis (Saturniidae) are abundant on the right side (the UV-rich blacklight side), and there are the usual omnipresent, yellow, as-yet-undescribed species of Eacles (Saturniidae) (as also visible in SI Appendix, Fig. S2).
This large and conspicuous yellow-and-black wasp has been found only twice since 1993 (both in 2001) among 196 wild-caught M. dilucida caterpillars. Multiply this example by many hundreds of observed cases with other species of univoltine moths and their parasitoids. Such once-common species of univoltine insects may not, however, be locally extinct so much as have a new distribution and survive at very low densities, augmented by their abilities to find low-density mates and hosts by following aerial chemical cues.
It is a commonplace to comment to a visitor that we have not seen a conspicuous species of insect for one to two decades, and then turn around and encounter a single individual. For example, the leaf-eating weevil Phelypera distigma was a major univoltine defoliator of Guazuma ulmifolia new leaves in 1980 to 2000 (28). It is now “absent,” yet we found a single estivating adult in mid-rainy season in 2019. We could generate a list of such species hundreds long. Some of these are certainly on their way to local extinction or to local geographic displacements among ACG’s different ecosystems.
Climate change distorts much more than temperature and rainfall patterns. The entire temperature–rainfall blanket is sliding up the Pacific slopes of the volcanic 1,400- to 2,000-m Cordillera Guanacaste (Fig. 2) that bisects ACG northwest–southeast (Fig. 1).
Fig. 2.
Clouds of the cloud forest in ACG (red mountaintops in Fig. 1), Volcán Orosí (Left) and Volcán Cacao (Right) as viewed from the Pacific side of Costa Rica. In the 1980s, the standard view was a solid mass of clouds generated by the east-to-west trade winds pushing moist Caribbean air up and over the cordillera (Bottom image). When this air condenses, it creates a foggy and dripping cloud forest on the mountaintops. By the mid-1990s, a common view was a mix of 1980s views and many days with a fractured and much smaller cloud layer (Middle image). This was accompanied by drying forest litter, obvious reduction in epiphyte loads, and frequent reductions in stream flow. Today, there are often days with no clouds at all (Top image), mixed with days of a heavy cloud layer as in the 1980s, but the bottom of that layer is now 100 to 500 m higher in elevation. Heavy fog no longer swirls in through open doors and windows of the Cacao Biological Station at 1,100 m and ants are now a conspicuous part of the fauna at the station, whereas they were essentially absent when ACG was established in the mid-1980s (37, 38) because the soil and litter were too cold and wet for them.
As is the case with tropical mountains everywhere (e.g., ref. 39), the rising hot air mass is evaporating the clouds of the ACG cloud forest (Fig. 2). This subjects the cloud forest biota to hotter and drier environments and for more days each year. It also deprives the lower-elevation species of their mountaintop dry season clouds, the clouds in which many species of insects normally refrigerate themselves in a cold wet environment for the five to six dry season months of the year when their lowland rainy season food is missing (e.g., SI Appendix, Fig. S1). It also shrinks water flow for the stream-inhabiting insect and vertebrate fauna at lower elevations.
Adult Moths and Caterpillars
At the start of the rains in May 1978, Dan broke three ribs by jumping into a ravine and had to sit for a month in front of our open dwelling on the dry forest edge to recuperate. There were 2 h of light from a 25-W bulb at night. The front wall was literally plastered with adult moths (as in Figs. 3 and 4 and SI Appendix, Fig. S2). In the newly leafed-out forest, the ground was paved with caterpillar feces and many tens of species of trees were massively defoliated (40). It was then that we switched from the study of host specificity of seed-predator beetles (41) to caterpillar host specificity (e.g., 42–45). There has never been a such high-density caterpillar year since then, and there has been a continual decline after about 1990 that steepened about 2005–2006. There are gradually fewer individual defoliated trees and species, but frequently two to four caterpillar species still do 1- to 2-y defoliations of their particular species of food plant.
In 1978, we also began to survey moths from light traps to complement those reared from wild-caught and photographed caterpillars from their food plants (46). Our ACG light trap is on a cliff edge looking out over thousands of hectares of adjacent regenerating recently protected dry forest canopy ranging from three decades to centuries in age. We have selectively collected more than 3,000 species of moths from that single light trap. Serendipitously, we photographed the same light, sheet, bulbs, and view on 27 May 1984, 28 May 1995, 23 May 2007, and 20 May 2019 (Figs. 3–5 and SI Appendix, Fig. S2). These four photographs were taken at the dark of the moon, in the second week of the rainy season—the time of maximum moth attraction to light in species and numbers during the seasonal and monthly cycles of ACG forests. These four photographs emphatically capture what has happened to the ACG forest insects since the early 1990s.
Simultaneously, we have observed that major decline in all three ACG ecosystems, whether “viewing” the forest through the “footprints” of ovipositing moths or as through the presence of adults at our light traps. The adults’ footprints of variable duration are the caterpillars and their parasitoids (SI Appendix, Figs. S3 and S4). These two figures mirror what has just been reported (5) for caterpillars and their parasitoids in the Organization for Tropical Studies (OTS) station Finca La Selva (60-m elevation) that is about 150 km southeast of ACG in what was a relatively intact rain forest contiguous with ACG rain forest just five to six decades ago. While the ACG caterpillars are being collected daily since 1978, adults at light traps throughout ACG rain forest and cloud forest have been collected only monthly. However, these show the same general decline per light trap as displayed by Figs. 3–5.
Caterpillar Parasitoids
Capturing and rearing ACG wild caterpillars of all instars from about 300 sites spread across all ACG ecosystems from sea level to 2,000 m blindly samples their parasitoid Diptera (Tachinidae) and Hymenoptera (many families) (e.g., refs. 47–54). Following DNA barcoding of more than 300,000 adult Lepidoptera and their parasitoids from the rearings, it has become clear that not only ACG caterpillar density, but also the percentage of caterpillars parasitized, has declined (SI Appendix, Fig. S4). There is a simultaneous reduction of overall parasitoid species richness per year and per species of host. This is visible in the 850,000-rearings ACG inventory database but not yet analyzed in detail because we are still DNA barcoding specimens to assure their identity, while continuing the year-round rearing program. The rearing of about 6,500 species of ACG caterpillars since 1978 has captured >3,000 species of parasitoids, and ongoing Malaise trapping more than doubles that count for ACG. Given the very high degree of host specificity of these parasites (e.g., refs. 47–54), it is no surprise that, as the caterpillar density and species richness decline, the percentages and species richness of their parasitoids declines (e.g., ref. 55; SI Appendix, Fig. S4). The loss of a host species does not result in a simple transfer to another host species. Such knock-on effects are rampant throughout the millions of interaction networks unavoidably influenced by climate change in these very species-rich forests (14). Even if a host plant is still present somewhere in ACG, a gravid female may well not be able to leave its “home ecosystem or habitat” to locate it; scientific presence is only variably related to ecological presence.
We cannot definitively state that the major and steep ACG caterpillar decline since about 2005 (ref. 5 and SI Appendix, Figs. S3 and S4) is due solely to climate change impact on caterpillars and their adults. However, we see no other generalized blanket impact variable across all ACG ecosystems and seasons that could trigger such a decline everywhere. Direct pesticide potential perturbations are decades gone and far from the ACG boundaries. There is nearly 100% natural forest restoration in motion on all ACG terrain. Forests have not been cleared since the 1960s, and the massive ACG insect density that we encountered on arrival in the 1970s had already suffered one to four centuries of frontier agricultural perturbation. No pathogen, natural enemy, or invasive species could have such a cross-taxon impact.
How to React to the Tropical Insect Decline in Species Richness and Abundance?
Measurement?
We have been impacted by climate change, and are being impacted, all of us, every species. How could we not be? How are we to react, as members of the global scientific guild, to the decline in ACG insect species and abundance, and to the same befalling tropical insect faunas elsewhere (5) in Costa Rica and the tropical world?
In 1985, faced with near disappearance of tropical dry forest in Central America (13, 15, 56), we, along with 15,000+ Costa Rican and international donors of cash and sweat equity in many currencies, took on the conspicuous immediate steps for ACG conservation with all of its contained biodiversity, no matter the taxon or assault (10). We and many others 1) knew from natural events that self-driven restoration is possible and a normal part of ecosystem dynamics [much as is today’s emphasis on optimism about both stopping and mitigating climate change (57)]; 2) enlarged ACG (from 30,000 to 169,000 ha) to where it could sustain most of its biodiversity and tolerate light footprints of its society, by straightforward open market purchase of about 350 properties (3); 3) facilitated ACG to be a government–non-governmental organization (NGO) hybrid to produce nondamaging goods and services indefinitely (also known as sustainably); 4) stimulated it to be self-sustaining in the many currencies it requires and can offer (2–4, 8–10); and 5) constructed it to be a pilot project meant to be transparent to everyone. ACG worked and is still working.
If the approximately $107 m USD raised to date for ACG had been spent in measuring the existence and impact of climate change over 35 y, instead of facilitating thousands of Costa Ricans and internationals to construct ACG for its multitude of goals, there would be no ACG to be impacted, mitigated, or saved. It would be a low-grade, industrial, agroforestry landscape populated by people with uncertain futures, and most of its goods and services flowing elsewhere. So, what is the next step as climate change descends on ACG’s many small-scale successes in sustainable biodevelopment (23)? Put most simply, expand the ACG concept to become a national attitude, both because if it is not possible to scale up, then it is likely to ultimately fail, and because a biodiversity-friendly country is more likely to invest in the perpetual survival of large wild areas through nondamaging biodevelopment instead of increased police forces.
Around 1999, the impacts and implications of climate change began to influence explicit strategies for ACG’s future. That led to increased fusing of unconnected major fragments (3) and to extension of ACG further into its rain-forested Caribbean side [which is the moist lifeboat for the dry-forested Pacific side that is being heated and severely dried by climate change (26, 37)]. ACG sought more size through inclusion of any fragments of old-growth or restorable second-growth forest. Also, and most importantly, ACG pushed for increasing direct involvement of neighboring and national societies to create a social climate that will accept and be kind to the survivors (BioAlfa). If ACG’s societies do not want to keep ACG terrain any more than its many other possible uses, climate change will not be the dominant threat to its sustainability as a conserved wildland. Realistically, all of ACG can be turned into intensely managed low-grade agroscape, mining, fishing to exhaustion, and uncontrolled tourism, via modern technologies and human expansion, rather than be the biodiversity cropland of natural capital that it is already, as it recovers from four centuries of heavy anthropogenic impacts.
At this point in time and budgets, ACG does not require more classical academic scientific study of climate change impacts. Rather, it calls for multipurpose designing of projects, protocols, and initiatives for social involvement, and for biodevelopment for its own survival. For one example among many, ACG insects can be biomonitored as a mine canary to measure climate change or industrial intrusion at a fine scale (14, 22). These designs may also be structured such that the data, involvement, and by-products are simultaneously useful for those who wish to study the temperature of our global house as it burns. Confronted with a metaphorical burning house today in the tropics, the critical priority is the complex of fire departments, fire codes, fire alarms, fire exits, emergency rooms for burn victims, and rules and views that prohibit candles in Christmas trees, rather than for more and fancier thermometers. Data, involvement, and by-products of scientific study are an essential part of this, but not the overriding purpose.
A central path for wildland survival, largely ignored so far, is to facilitate human tropical societies to welcome biologically viable large tropical wild ecosystems as full members at their negotiating table, from locally to internationally. For tropical societies to begin down this path, be they local and small, full countries, or even multicountry collages, they need to know in ever-increasing detail what is in, and what are, their wild ecosystems. People around the world spend hundreds of millions of dollars “protecting” many kinds of tropical wildlands while having little knowledge of their hyperdiverse constituents, what they do, where they are, how to find them, and how to get that knowledge into the public domain. Adding biological literacy will give tropical biodiversity and its ecosystems a far greater chance to be partnered with their owner societies than will adding more fences and a military mindset. Charismatic vertebrates, tourist snapshots, and marketable big tree trunks are not even 0.001% of tropical biodiversity. The millions to billions of species, and billions of wild interactions still viable, are largely invisible without bioliteracy. There are as many species within 50 km of our dwelling in ACG in northwestern Costa Rica as in all of Europe or half of North America, and these species express vastly more and more complex interactions than are in the extratropics. Sweden reasonably estimates 33,000 species of insects (58); five Malaise traps for insects set for inventory in ACG can capture more than that in a year (14). With a second year, those trap catches will equal the 65,000 insect species estimated for the very well-studied United Kingdom. Yes, there are a few biodiversity specialists in the world who know a great deal about a minute slice of the 25+ million multicellular tropical terrestrial species, but this knowledge is not directed toward encouraging tropical societies to be eager to appreciate their own wild natural capital and allow it to be a sustainable member of its owner and caretaker society. Simultaneously, world markets and Holocene human behavior stand eager to monoculturalize the tropics to feed the extratropics as well as tropical large social aggregations.
A tropical bioliterate society can be facilitated to create itself the same way a literate society is created—an alphabet, a dictionary, libraries, printing, internet, literacy, and everyone being allowed and taught to read and write at a very early age—and for all of the same reasons. For wild tropical biodiversity, thanks to the invention of DNA barcoding in 2003 (59, 60) and the internet and all that implies, we now have the technical capacity to bring bioliteracy of the wild to all societies. Wild tropical biodiversity needs wide application of these tools to bring about biodiversity integration with society vastly more than it needs to only measure insect decline and wring our hands about it. Without a welcoming society, insect decline by climate change will be just one of the hundred cuts that will kill arthropod and other biodiversity roles and presence, clustered with all of the other ways that humans have been trashing wild tropical ecosystems for millennia. Note that Anthropocene began, and continues, not with the burning of coal and now other fossil fuels, but with extinction of the world’s megafauna (61). Local extinctions? Think about the former Iowa prairies or 100,000 km2 of soy, oil palm, or pasture carved out of tropical rain forest. A million species threatened with extinction? No, millions more. The real question is what have we learned from it, and what do we now do?
A tropical bioliterate society needs 1) the analogs to printing presses: personal, free DNA barcoding for wild biodiversity identification by anyone, anywhere, anytime; databases; websites; and the wireless internet; 2) the living libraries—today’s struggling surviving wildlands with their unread biodiversity; 3) the human and other brainpower as librarians, interpreters, publishers, and synthesizers: today’s taxonomists, literature, websites, apps, museums, natural historians, and others; and 4) disciplined open access: public unrestrained reading and probing of wild biodiversity for any sustainable purposes by any sector. In short, these are the ways and means to become as bioliterate as a modern society is literate, if not more so. Living wild biodiversity needs to be relevant to everyday agendas, some negative, many positive.
BioAlfa
The Costa Rican government has elected to embark on this path by officially and publicly labeling itself with the 10-y project BioAlfa (21, 62, 63). The name is derived from Spanish for bioliterate, as in “bioalfabetizado” (from “alfabetizado” = literate). Its socio-logistical technical goal is to find and DNA-barcode Costa Rica’s estimated one million multicellular (Eucaryota) species, thereby setting up a known platform for their sustainable (therefore relatively undamaging) biodevelopment by society. Costa Rica is doing it through inviting its own public, government, NGO, and commercial sectors of five million people to conduct an inventory of their own country, as well as facilitating international collaborations. This begins to self-educate its populace on broadscale and fine-scale levels about the multitude of ways all different sectors of society can make use of their bioliteracy to construct initiatives. BioAlfa is not meant to be an invitation for expeditionary exploration of Costa Rican biodiversity by the international community, but rather it is an autochthonous effort that encourages strong global collaborations to achieve common goals.
BioAlfa is envisioned to run for 10 y for $100 m USD: $50 m to combine with national and international sweat equity for the actual human and technical 10-y execution of the project, $25 m for permanent endowment for the process, and as data accumulate, $25 m to fund startups for motivated uses of the biodiversity platform: medical, educational, artistic, forensic, engineering inspirations, entertainment, agricultural, tourism and its service industry, bioremediation, environmental monitoring, aesthetics, psychological well-being, biomimicry, biodiversity prospecting, regulation, genome sourcing, biological control, carbon sequestration, soil restoration, and robotic bioengineering design, and more. While Costa Rica contributes the sweat equity, raw material, and biopolitical will, we call on the international community to be the cash partner for this global, biodiversity-based, biodevelopment public service initiative by a small tropical country attempting the survival of 4% of global wild terrestrial biodiversity.
Globally, BioAlfa is a deep dive through the thin layer of global biodiversity DNA barcoding envisioned by BIOSCAN (60), aimed at biodiversity barcoding for global public bioliteracy. Since insects are a major and very poorly known component of Costa Rica’s biodiversity, the early phases of BioAlfa include broad sampling by Malaise and other trapping methods. Costa Rica is nearly done with a first year of BioAlfa Malaise trap inventories of 10 national parks by their park staffs. These personnel have no advanced degrees in biodiversity management. They are learning on the job to be bioliterate, just as has been the case for 35 y with ACG and INBio parataxonomists (7, 19, 24).
BioAlfa has many roots (e.g., refs. 6, 7, 11, 15, 14, 19–24, 59, 60, and 62–71). The Costa Rican government has decreed that the species-level unique identifier, the DNA barcode, is public domain (67–69) and therefore can be shared nationally and internationally just as are the words in a dictionary. Biodevelopment of the genome attendant to the ID barcode will require an explicit contract between the source and user in accord with Costa Rica's 1998 Ley de Biodiversidad (62, 63), as is the case with any national crop and natural resource. In the 1990s, Costa Rica’s INBio initiated this process by legalizing and formalizing biodiversity prospecting (6, 7, 56, 69), which was part of the starter yeast for the Convention on Biological Diversity.
Costa Rica is exploring synergisms nationally and internationally with the commercial sector, NGOs, private citizens, universities, and multiple government agencies in search of situations where particular projects will simultaneously create portions of the national biodiversity data platform through BioAlfa actions. For example, 7 y of Malaise trap biomonitoring of rain forest perturbation by a large government geothermal development has generated more than a million public insect specimen records of at least 50,000 species through deliberate collaboration between the Costa Rican national park system, industrial activity, the international taxasphere, and international aid (14) from Canada and Japan, simultaneously meeting objectives for each of their sectors. Costa Rica will, and already has, pioneered a culture of viewing wild natural capital as a sustainably usable resource (6, 7, 56, 69), just as the medical and agriculture industries view the human body and the agroscape. A surviving tropical wild world has to be desired by society. If it is only imposed by national and international forces, extracted for international markets for invasive exploitation, or a neglected one-time harvestable during a national emergency—such as cash for a war via clearcutting, feeding the international insatiable market, or appeasing restive creeping overpopulations—it will not survive. The Costa Rican BioAlfa goal vis-à-vis biodiversity and climate change is to reinforce a national attitude that is willing to keep its carbon out of the air, grow forest back on lands that once were cattle pastures, and keep its natural capital alive because of all of the things a million-plus species of eukaryotes and their microbial fellow travelers can offer.
The BioAlfa concept is to construct a biodiversity knowledge platform for a tropical country to achieve widespread bioliteracy among its citizenry, and thus build a mutualism between the wild and the nation. All members of a country can attain some level of proficiency as citizen scientists, if they are allowed and facilitated. This is the same process as learning to read early in grade school. Once started into literacy, a person continues their own individual or corporate life with that literacy. The most serious hurdle is the expression Dan grew up with in semirural Minnesota: “Keep ’em dumb and down on the farm.” The tropical agroscape and its adjacent remaining wildlands are heavily subject to centralization of knowledge and power away from the field and the wild areas. This is an enormous local barrier to really mitigating the impact of climate change on the tropical wild—as well as confronting the myriad of other challenges to the survival of tropical biodiversity.
Supplementary Material
Acknowledgments
We wish to recognize and thank the literally thousands of Costa Rican and international people, experiences, and institutions that have contributed and continue their thinking and support for the content of this essay. We are especially grateful for the staff and institutions that have created and maintained ACG since the beginning of its existence in 1966 and full germination in 1985. Without that, these ideas and reality would not exist, nor would there be the opportunity to notice what is happening to its biodiversity. We are equally appreciative of David Wagner, May Berenbaum, and David Stopak for their patient and positive editing of the manuscript.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. D.L.W. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2002546117/-/DCSupplemental.
Data Availability.
All study data are included in the article and SI Appendix.
References
- 1.Janzen D. H., Hallwachs W., Perspective: Where might be many tropical insects? Bio. Cons. 233, 102–108 (2019). [Google Scholar]
- 2.Janzen D. H., Hallwachs W., Área de Conservación Guanacaste, northwestern Costa Rica: Converting a tropical national park to conservation via biodevelopment. Biotropica 2020, 1–20 (2020). [Google Scholar]
- 3.Pringle R. M., Upgrading protected areas to conserve wild biodiversity. Nature 546, 91–99 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Janzen D. H., “Costa Rica’s Área de Conservación Guanacaste: A long march to survival through non-damaging biodiversity and ecosystem development” in Norway/UN Conference on the Ecosystem Approach for Sustainable Use of Biological Diversity., Schei P. J., Ed. (Norwegian Directorate for Nature Research; and Norwegian Institute for Nature Research, Trondheim, Norway, 2000), pp. 122–132. [Google Scholar]
- 5.Salcido D. M., Forister M. L., Garcia Lopez H., Dyer L. A., Loss of dominant caterpillar genera in a protected tropical forest. Sci. Rep. 10, 422 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gámez R., De Biodiversidad, Gentes y Utopías: Reflexiones en los 10 Años del INBio (INBio, Santo Domingo de Heredia, Costa Rica, 1999). [Google Scholar]
- 7.Janzen D. H., Hallwachs W., Jimenez J., Gámez R., “The role of the parataxonomists, inventory managers and taxonomists in Costa Rica’s national biodiversity inventory” in Biodiversity Prospecting, Reid W. V., et al., Eds. (World Resources Institute, Washington, DC, 1993), pp. 223–254. [Google Scholar]
- 8.Allen W., Green Phoenix: Restoring the Tropical Forests of Guanacaste, Costa Rica (Oxford University Press, New York, 2001), pp. 1–286. [Google Scholar]
- 9.Janzen D. H., Hallwachs W., “Biodiversity conservation history and future in Costa Rica: The case of Área de Conservación Guanacaste (ACG)” in Costa Rican Ecosystems, Kappelle M., Ed. (University of Chicago Press, Chicago, 2016), chap. 10, pp. 290–341. [Google Scholar]
- 10.Janzen D. H., “Guanacaste National Park: Tropical ecological and biocultural restoration” in Rehabilitating Damaged Ecosystems, Cairns J. J., Ed. (CRC Press, Boca Raton, FL, 1988), vol. II, pp. 143–192. [Google Scholar]
- 11.Janzen D. H., “Affirmative action for insects in tropical national parks” in The Evolutionary Ecology of Plants, Bock J. H., Linhart Y. B., Eds. (Westview Press, Boulder, CO, 1989), pp. 579–588. [Google Scholar]
- 12.Janzen D. H., Management of habitat fragments in a tropical dry forest: Growth. Ann. Mo. Bot. Gard. 75, 105–116 (1988). [Google Scholar]
- 13.Janzen D. H., “Tropical dry forest: Área de Conservación Guanacaste, northwestern Costa Rica” in Handbook of Ecological Restoration, Volume 2, Restoration in Practice, Perrow M. R., Davy A. J., Eds. (Cambridge University Press, Cambridge, UK, 2002), pp. 559–583. [Google Scholar]
- 14.Janzen D. H., et al. , Using DNA-barcoded Malaise trap samples to measure impact of a geothermal energy project on the biodiversity of a Costa Rican old-growth rain forest. Genome 63, 407–436 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Janzen D. H., Guanacaste National Park: Tropical Ecological and Cultural Restoration (Editorial Universidad Estatal a Distancia, San José, Costa Rica, 1986). [Google Scholar]
- 16.Área de Conservación Guanacaste, Área de Conservación Guanacaste: Fuente de Vida y Desarrollo https://www.acguanacaste.ac.cr/index.php. Accessed 7 March 2020.
- 17.Janzen D. H., How moths pass the dry season in a Costa Rican dry forest. Ins. Sci. App. 8, 489–500 (1987). [Google Scholar]
- 18.Hunt J. H., Brodie R. J., Carithers T. P., Goldstein P. Z., Janzen D. H., Dry season migration by Costa Rican lowland paper wasps to high elevation cold dormancy sites. Biotropica 31, 192–196 (1999). [Google Scholar]
- 19.Janzen D. H., Hallwachs W., Joining inventory by parataxonomists with DNA barcoding of a large complex tropical conserved wildland in northwestern Costa Rica. PLoS One 6, e18123 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janzen D. H., Hallwachs W., DNA barcoding the Lepidoptera inventory of a large complex tropical conserved wildland, Area de Conservacion Guanacaste, northwestern Costa Rica. Genome 59, 641–660 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Janzen D. H., Hallwachs W., How a tropical country can DNA barcode itself. iBOL Barcode Bull. 9, 1–6 (2019). [Google Scholar]
- 22.Holloway M., Democratizing taxonomy. Conserv. Pract. 7, 14–21 (2006). [Google Scholar]
- 23.Janzen D. H., et al. , Integration of DNA barcoding into an ongoing inventory of complex tropical biodiversity. Mol. Ecol. Res. 9, 1–26 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Janzen D. H., Setting up tropical biodiversity for conservation through non-damaging use: Participation by parataxonomists. J. Appl. Ecol. 41, 181–187 (2004). [Google Scholar]
- 25.Basurto X., Jimenez-Perez I., Institutional arrangements for adaptive governance of biodiversity conservation: The experience of the Área de Conservación Guanacaste, Costa Rica. J. Lat. Am. Geogr. 12, 111–134 (2013). [Google Scholar]
- 26.Anonymous, How much hotter is your hometown than when you were born? NY Times, 30 August 2018. https://www.nytimes.com/interactive/2018/08/30/climate/how-much-hotter-is-your-hometown.html. Accessed 6 March 2020.
- 27.Janzen D. H., Weather-related color polymorphism of Rothschildia lebeau (Saturniidae). Bull. Entomol. Soc. Am. 30, 16–20 (1984). [Google Scholar]
- 28.Janzen D. H., Natural history of Phelypera distigma (Boheman), Curculionidae, a Costa Rican defoliator of Guazuma ulmifolia Lam. (Sterculiaceae). Brenesia 16, 213–219 (1979). [Google Scholar]
- 29.Janzen D. H., Seasonal change in abundance of large nocturnal dung beetles (Scarabaeidae) in a Costa Rican deciduous forest and adjacent horse pasture. Oikos 41, 274–283 (1983). [Google Scholar]
- 30.Janzen D. H., “Caterpillar seasonality in a Costa Rican dry forest” in Caterpillars. Ecological and Evolutionary Constraints on Foraging, Stamp N. E., Casey T. M., Eds. (Chapman and Hall, New York, 1993), pp. 448–477. [Google Scholar]
- 31.Janzen D. H., Synchronization of sexual reproduction of trees with the dry season in Central America. Evolution 21, 620–637 (1967). [DOI] [PubMed] [Google Scholar]
- 32.Janzen D. H., Schoener T. W., Differences in insect abundance and diversity between wetter and drier sites during a tropical dry season. Ecology 49, 96–110 (1968). [Google Scholar]
- 33.Janzen D. H., Sweep samples of tropical foliage insects: Effects of seasons, vegetation types, elevation, time of day, and insularity. Ecology 54, 687–708 (1973). [Google Scholar]
- 34.Janzen D. H., Sweep samples of tropical deciduous forest foliage-inhabiting insects: Seasonal changes and inter-field differences in adult bugs and beetles. Rev. Biol. Trop. 24, 149–161 (1976). [Google Scholar]
- 35.Havird J. C., et al. , Distinguishing between active plasticity due to thermal acclimation and passive plasticity due to Q10 effects: Why methodology matters. Funct. Ecol. 00, 1–14 (2020). [Google Scholar]
- 36.Sheldon K. S., Huey R. B., Kaspari M., Sanders N. J., Fifty years of mountain passes: A perspective on Dan Janzen’s classic article. Am. Nat. 191, 553–565 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Warne C. P. K., Hallwachs W., Janzen D. H., Smith M. A., Functional and genetic diversity changes through time in a cloud forest ant assemblage. Biotropica, 10.1111/btp.12882 (30 October 2020). [DOI]
- 38.Smith M. A., Hallwachs W., Janzen D. H., Diversity and phylogenetic community structure of ants along a Costa Rican elevational gradient. Ecogeography 37, 720–731 (2014). [Google Scholar]
- 39.Moens J., Andes meltdown: New insights into rapidly retreating glaciers. Yale Environment 360 (2020). https://e360.yale.edu/features/andes-meltdown-new-insights-into-rapidly-retreating-glaciers. Accessed 20 January 2020.
- 40.Janzen D. H., Patterns of herbivory in a tropical deciduous forest. Biotropica 13, 271–282 (1981). [Google Scholar]
- 41.Janzen D. H., Specificity of seed-attacking beetles in a Costa Rican deciduous forest. J. Ecol. 68, 929–952 (1980). [Google Scholar]
- 42.Janzen D. H., Waterman P. G., A seasonal census of phenolics, fibre and alkaloids in foliage of forest trees in Costa Rica: Some factors influencing their distribution and relation to host selection by Sphingidae and Saturniidae. Bot. J. Linn. Soc. 21, 439–454 (1984). [Google Scholar]
- 43.Janzen D. H., Two ways to be a tropical big moth: Santa Rosa saturniids and sphingids. Oxf. Surv. Evol. Biol. 1, 85–140 (1984). [Google Scholar]
- 44.Janzen D. H., Natural history of Hylesia lineata (Saturniidae: Hemileucinae) in Santa Rosa National Park, Costa Rica. J. Kans. Entomol. Soc. 57, 490–514 (1984). [Google Scholar]
- 45.Janzen D. H., “How polyphagous are Costa Rican dry forest saturniid caterpillars?” in Arthropods of Tropical Forests. Spatio-Temporal Dynamics and Resource Use in the Canopy, Basset Y., Novotny V., Miller S. E., Kitching R. L., Eds. (Cambridge University Press, Cambridge, UK, 2003), pp. 369–379. [Google Scholar]
- 46.Janzen D. H., Hallwachs W., Philosophy, navigation and use of a dynamic database (“ACG Caterpillars SRNP”) for an inventory of the macrocaterpillar fauna, and its food plants and parasitoids, of Área de Conservación (ACG), northwestern Costa Rica. http://janzen.sas.upenn.edu. Accessed 20 October 2020.
- 47.Fernandez-Triana J. L., et al. , Review of Apanteles sensu strictu (Hymenoptera, Braconidae, Microgastrinae) from Área de Conservación Guanacaste, northwestern Costa Rica, with keys to all described species from Mesoamerica. ZooKeys 383, 1–565 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hansson C., Smith M. A., Janzen D. H., Hallwachs W., Integrative taxonomy of new World Euplectrus Westwood (Hymenoptera, Eulophidae), with focus on 55 new species from Area de Conservación Guanacaste, northwestern Costa Rica. ZooKeys 485, 1–236 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharkey M. J., et al. , Alabagrus Enderlein (Hymenoptera, Braconidae, Agathidinae) species of Costa Rica, with an emphasis on specimens reared from caterpillars in Área de Conservación Guanacaste. Cont. Sci. Nat. Hist. Mus. L. A. 526, 31–180 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alex Smith M., et al. , DNA barcoding and the taxonomy of microgastrinae wasps (Hymenoptera, Braconidae): Impacts after 8 years and nearly 20 000 sequences. Mol. Ecol. Resour. 13, 168–176 (2013). [DOI] [PubMed] [Google Scholar]
- 51.Arias-Penna D. C., et al. , A species-level taxonomic review and host associations of Glyptapanteles (Hymenoptera, Braconidae, Microgastrinae) with an emphasis on 136 new reared species from Costa Rica and Ecuador. ZooKeys 890, 1–685 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith M. A., Woodley N. E., Janzen D. H., Hallwachs W., Hebert P. D. N., DNA barcodes reveal cryptic host-specificity within the presumed polyphagous members of a genus of parasitoid flies (Diptera: Tachinidae). Proc. Natl. Acad. Sci. U.S.A. 103, 3657–3662 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith M. A., Wood D. M., Janzen D. H., Hallwachs W., Hebert P. D. N., DNA barcodes affirm that 16 species of apparently generalist tropical parasitoid flies (Diptera, Tachinidae) are not all generalists. Proc. Natl. Acad. Sci. U.S.A. 104, 4967–4972 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fleming A. J., Wood D. M., Smith M. A., Hallwachs W., Janzen D. H., Revision of the New World species of Houghia Coquillett (Diptera, Tachinidae) reared from caterpillars in Area de Conservación Guanacaste, Costa Rica. Zootaxa 3858, 001–090 (2014). [DOI] [PubMed] [Google Scholar]
- 55.Stireman J. O., III, et al. , Climatic unpredictability and parasitism of caterpillars: Implications of global warming. Proc. Natl. Acad. Sci. U.S.A. 102, 17384–17387 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Janzen D. H., “Tropical dry forests: The most endangered major tropical ecosystem” in Biodiversity, Wilson E. O., Ed. (National Academy Press, Washington, DC, 1988), pp. 130–137. [Google Scholar]
- 57.Figueres C., Rivett-Carnac T., The Future We Choose: Surviving the Climate Crisis (Manilla Press, 2020). [Google Scholar]
- 58.Ronquist F., et al. , Completing Linnaeus’s inventory of the Swedish insect fauna: Only 5,000 species left? PLoS One 15, e0228561 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hebert P. D. N., et al. , Counting animal species with DNA barcodes: Canadian insects. Philos. Trans. R. Soc. Lond. B Biol. Sci. 371, 20150333 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.International Barcode of Life Consortium , BIOSCAN. http://ibol.org. Accessed 7 March 2020.
- 61.Janzen D. H., Martin P. S., Neotropical anachronisms: The fruits the gomphotheres ate. Science 215, 19–27 (1982). [DOI] [PubMed] [Google Scholar]
- 62.Greenfield P., Counting the species: How DNA barcoding is rewriting the book of life. The Guardian, 7 October 2020. https://www.theguardian.com/environment/2020/oct/07/counting-the-species-how-dna-barcoding-is-rewriting-the-book-of-life-aoe. Accessed 7 October 2020.
- 63.Hance J., Bold project hopes to DNA barcode every species in Costa Rica. Mongabay 27, 1–8 (2020). [Google Scholar]
- 64.Treuer T. L. H., et al. , Low-cost agricultural waste accelerates tropical forest regeneration. Rest. Ecol. 26, 275–283 (2017). [Google Scholar]
- 65.Reid W. V., et al. , Biodiversity Prospecting (World Resources Institute, Washington, DC, 1993). [Google Scholar]
- 66.Gaston K. J., Gauld I. D., Hanson P., The size and composition of the hymenopteran fauna of Costa Rica. J. Biog. 23, 105–113 (1996). [Google Scholar]
- 67.Government of Costa Rica, El Presidente de la República y el Ministro de Ambiente y Energía Decretan: Declaratoria de Interés Público del Proyecto BioAlfa. La Gaceta Diario Oficial (Gobierno de Costa Rica, San Jose, Costa Rica, 2019), no. 143, p. 6.
- 68.Government of Costa Rica, Ley de Biodiversidad No. 7788 de 23 Abril 1998. Registro Nacional (Government of Costa Rica, San Jose, Costa Rica, 1998). http://www.registronacional.go.cr/propiedad_industrial/documentos/pi_normativa/leyes/Ley%20biodiversidad.pdf. Accessed 9 March 2020.
- 69.Government of Costa Rica , Comisión Nacional para la Gestion de la Biodiversidad (CONAGEBIO) (1998). https://www.conagebio.go.cr/Conagebio/public/. Accessed 9 March 2020.
- 70.Daily G., Ellison K., The New Economy of Nature. The Quest to Make Conservation Profitable (Island Press, Washington, 2002). [Google Scholar]
- 71.Moreno M. L., Hidalgo H. G., Alfaro E. J., Cambio climático y su efecto sobre los servicios ecosistémicos en dos parques nacionales de Costa Rica, América Central. Revista Iberoamericana de Economía Ecológica 30, 16–38 (2019). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and SI Appendix.