Significance
Mammals on landscapes within North America are now living in different climates than they did prior to major human expansions. Many smaller animals have expanded into climates now dominated by agricultural and urban regions; whereas large-bodied mammals were forced out of these climates and were relegated to colder, dryer regions. As a result, the distributions of modern mammals do not accurately reflect the range of climatological conditions where species can live. Rather, they reflect the marginalization or facilitation that those species have undergone following the expansion of major human activities. Failing to mitigate the ecological changes that mammals are experiencing can have unprecedented impacts on the future survival of the remaining large-bodied species in North America.
Keywords: mammals, realized niche, climatic niche, biogeography, paleoecology
Abstract
Cities and agricultural fields encroach on the most fertile, habitable terrestrial landscapes, fundamentally altering global ecosystems. Today, 75% of terrestrial ecosystems are considerably altered by human activities, and landscape transformation continues to accelerate. Human impacts are one of the major drivers of the current biodiversity crisis, and they have had unprecedented consequences on ecosystem function and rates of species extinctions for thousands of years. Here we use the fossil record to investigate whether changes in geographic range that could result from human impacts have altered the climatic niches of 46 species covering six mammal orders within the contiguous United States. Sixty-seven percent of the studied mammals have significantly different climatic niches today than they did before the onset of the Industrial Revolution. Niches changed the most in the portions of the range that overlap with human-impacted landscapes. Whether by forcible elimination/introduction or more indirect means, large-bodied dietary specialists have been extirpated from climatic envelopes that characterize human-impacted areas, whereas smaller, generalist mammals have been facilitated, colonizing these same areas of the climatic space. Importantly, the climates where we find mammals today do not necessarily represent their past habitats. Without mitigation, as we move further into the Anthropocene, we can anticipate a low standing biodiversity dominated by small, generalist mammals.
Worldwide, most mammals have lost at least 30% of their historical geographic ranges since 1900 AD due to increased anthropogenic pressure (1, 2). Urbanization and agricultural expansion can subsume and fragment habitats necessary for species’ survival (2–5), resulting in extinction (1, 3) or altering species associations (2, 4, 6). Range loss and fragmentation are especially detrimental for larger carnivores and grazers (>10 kg) that often require vast food resources acquired through long-range movement (2, 3, 7). These human-induced range changes affect the survival of mammals, not only by reducing their overall range sizes, but also by preventing them from accessing their preferred climates and habitats and impacting their survival as climates change (8).
Given today’s rapid climate change, it is crucial that we identify the climatic conditions in which mammals can and must live as well as the extent to which human-impacted landscapes prevent species from occupying them. We know that the modern geographic ranges of mammals have been encroached upon (2, 3, 5, 7). However, we do not understand the ecological implications of those range changes. Notably, we do not know whether there have been differential impacts across the distribution of climates occupied by mammals or for different species of mammals. Such changes would have important implications for our interpretations of species’ climatic tolerances and what landscapes must be preserved to facilitate their survival. Today’s mammal species might be living in very different climates than they did before major human expansions. Thus, an assessment of human impacts on mammalian climate niches must evaluate the dynamics of climatic niche change over centuries or millennia, particularly prior to the large-scale expansion of human land use.
Understanding the dynamics of climatic niche change over centuries or millennia can help formulate better predictions for the future of ecosystems in the current climate change crisis and design better conservation plans (9, 10). Previous studies have shown that certain species can change their ecological niches either globally or locally and that some populations displayed different niche preferences in the past than observed today (11–13). For example, we know that at least one population of Bison bison browsed in forested areas in eastern United States back in the Late Holocene, which differs from their modern niche distribution, exclusively grazing in open environments (11). Similarly, the chisel‐toothed kangaroo rat (Dipodomys microps) and the bushy-tailed woodrat (Neotoma cinerea) have shifted their climatic niche in response to Late Pleistocene and Holocene climatic fluctuations rather than tracking climate (12, 13). Incorporating data on past niche preferences from when anthropogenic impacts were low will help advance our understanding on species’ preferred niche (9, 10).
Here, we test whether modern mammal species have changed their realized climatic niche (hereafter climatic niche) while shifting their geographic ranges throughout the Holocene (the last 11,700 y) across the contiguous United States and evaluate how human activities have shaped these changes. The realized niche of a species is a snapshot of the environmental and ecological conditions in which a species lives at any time because of the prevailing biotic and abiotic conditions (14, 15). This differs from a fundamental niche, which is the full set of abiotic conditions in which a species can survive and maintain healthy populations (15–17). Conservation initiatives have traditionally used the inferred realized niche of a species for designing cost-effective implementation plans. However, modern mammal species may display different realized niches in the present than they did throughout the Holocene because human impacts have altered the geographic range of these species. Relying only on modern data to characterize the potential habitats where a species could live can hinder conservation efforts by focusing on marginal, rather than preferred, habitat for target species. Thus, it is imperative to characterize whether modern mammals could have also changed their realized niches and consider a (pre)historical perspective in conservation planning.
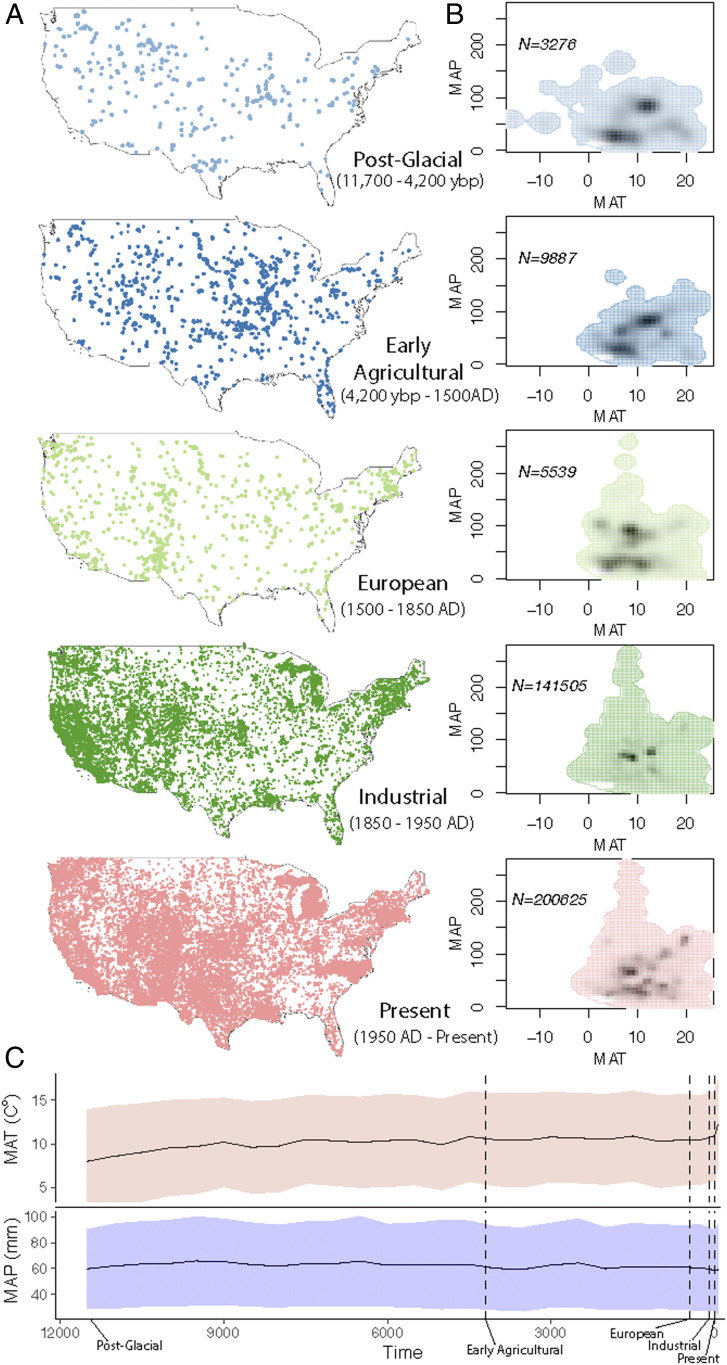
Here, we estimate fossil and modern mammal climatic niches at each of five anthropogenically relevant time periods covering the last 11,700 y in the contiguous United States (Fig. 1A) over the Holocene (11,700 to the present). These time intervals include: post-glacial (11,700 to 4,200 ybp), early agricultural (4,200 to 450 ybp [1500 AD]), European (450 to 100 ybp [1500 to 1850 AD]), industrial (100 to 0 ybp [1850 to 1950 AD]), and present (0 ybp [1950 AD] to the present).
Fig. 1.
Study data. (A) Geographical distribution of the individual species occurrences for the 46 species in the study and for five different time intervals (16). (B) Available niche for each time interval based on the MAT and MAP of all the occurrence data points. Colors represent the available background niche and shading represents the relative density of species in a particular MAT and MAP combination. N indicates number of occurrences. (C) MAT and MAP across the studied time interval from 11,700 ybp to the present for the contiguous United States. Black lines indicate average value from the background data for every 500 y (Materials and Methods). Shaded areas represent SD from the same data.
We compiled fossil and modern occurrences from Faunmap II database (18) and from Gbif (19), respectively, and associated climate variables for mammals in the contiguous United States (a total of 196 species). We separated the occurrences by time interval and retained species with at least 20 individual occurrences in every time interval, yielding 46 species covering six different taxonomic orders (Artiodactyla, Carnivora, Chiroptera, Insectivora, Lagomorpha, and Rodentia). We estimated mean annual temperature (MAT) and mean annual precipitation (MAP) (Materials and Methods) for each individual occurrence (Fig. 1B).
After estimating the climatic niche space for each species and time interval, we calculated niche overlap and equivalency (15, 17) (Materials and Methods) between time intervals to identify whether mammal climatic niche preferences shifted through time (SI Appendix, Fig. S1). This approach allows us to explore climatic niche change in mammals as it occurred concomitant with major human expansions. While other biotic and abiotic interactions could be contributing to these range changes, here we focus on the role of human expansion by evaluating the relationship between niche change and human land use.
We also investigate the role of mammal ecological traits (i.e., body mass and diet) on mediating climatic niche responses to human expansion across the Holocene. Previous research suggests that mammals with small body sizes and more generalist diets may be more likely to thrive in human-impacted areas such as urban settings and croplands (2, 20). However, did mammals with different body sizes and diet specializations have different niche preferences prior to major human expansions? Or, have human activities selectively extirpated larger mammals with more specialized diets from the climates where major human settlements occur? Laliberte and Ripple (2) explored the range sizes of carnivorans and ungulates in response to human activity over the last 300 y and found that larger carnivorous mammals had suffered the largest range contractions. Here, we explore these questions across a wide variety of mammal orders, including those with smaller body sizes, (i.e., rodents). We address these questions over a long time span, encompassing the time before major human expansions, and in the context of climatic niche preferences.
We test three hypotheses: 1) Modern mammals have changed their climatic niche over the last 11,700 y. 2) Mammal species have experienced different changes in their climatic niches based on their ecological traits (i.e., diet or body mass). 3) Changes occur in the portion of the climatic niche that corresponds with climates that are associated today with anthropogenic activities, particularly urbanization and agriculture. To test these hypotheses, we compare the climatic niche of each species across each of the five time periods (Fig. 1) to determine whether changes occur and the timing of those changes. We then evaluate whether there are significantly different climatic niche changes for different body size categories or dietary categories. Finally, we assess the amount of overlap between the climatic niches where human-impacted landscapes are found today and the climatic niche changes that occurred in each mammal species.
Results and Discussion
Changes in Mammalian Realized Niches.
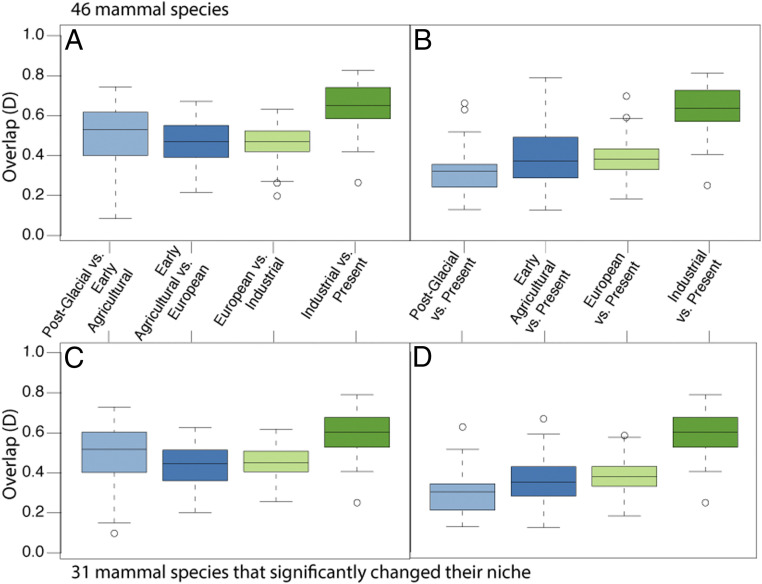
Mammals have changed the climates where they are found concurrent with the expansion of human settlements over the last 11,700 y, supporting our first hypothesis. Across all 46 species, present mammals display low average climatic niche overlap with the climatic niches they experienced during the postglacial (33%), early agricultural (41%), and European (39%) intervals, but higher average climatic niche overlap (64%) with the industrial interval (Fig. 2B). We also observe a lower average overlap between each subsequent time interval from postglacial to industrial (49%, 45%, and 45%, respectively; Fig. 2A) than from industrial to the present (64%; Fig. 2A), indicating that species’ climatic preferences today are more similar to what they were in the industrial time interval than any other past time interval. These findings demonstrate a consistent change in realized niche with increased human activity. Comparing the climatic niches of these 46 species before and after the Industrial Revolution in North America (1850 AD), 67% (n = 31) of the mammals in the study have significantly different niches today than they did before the onset of the Industrial Revolution (Fig. 2D and SI Appendix, Table S5).
Fig. 2.
Boxplot of the overlap between different time intervals for the 46 mammal species in the study (A and B) and 31 mammal species that significantly changed their niche after the Industrial Revolution in North America (C and D). Overlap is calculated between subsequent time intervals (A and C) and for each time interval compared with the present (B and D). Overlap is average overlap from 100 subsampling iterations as described in Materials and Methods.
Most mammal species (74%) experience a major shift in realized niche after the arrival of European settlers or at the onset of the Industrial Revolution (SI Appendix, Figs. S3 and S4). For example, the American bison (B. bison) and the cougar (Puma concolor), suffer their major shift between European and Industrial time intervals (SI Appendix, Fig. S4). Bison was overhunted during the Industrial Revolution in North America (21). Cougars have been victims of habitat and prey reduction and active hunting since the expansion of modern cities (22). However, some shifts that occur at other time periods, coincide nonetheless with documented events in the literature. The elk (Cervus elaphus) and the North American river otter (Lontra canadensis) show major niche shifts between early agricultural and European time intervals. Both these species were overhunted by the first European settlers for food and fur, and later reintroduced in some protected areas (23). The fact that these specific timings are corroborated by known historic events, lends strength to the merit of our analyses.
In our study, the length of the time interval does not affect the breadth of the climatic niche. Older time intervals have longer extents than younger time intervals, which could result in a higher chance of accumulating unique climatic niches or displaying a wider range of climatic niches, particularly if species lag in response to changing climates. To test this, we calculated niche disparity for the 46 species in the study and across five time intervals (Materials and Methods and SI Appendix, Fig. S2). Disparity measures the spread of data points in climate space. If time span was affecting our analysis, the older time intervals, which have longer time spans, would demonstrate higher disparity on average. This is not the case; all time intervals have similar average disparity (SI Appendix, Fig. S2). In fact, disparity is slightly higher in the shorter, more modern time intervals (European, industrial, and present), indicating that increased time duration is not driving the observed results.
It is unlikely that dispersal lags following the deglaciation are causing the differences in average climatic niche overlap observed between the postglacial and early agricultural time intervals. We examine mammalian climate niches throughout the Holocene specifically because there was relatively little climate change during this time in North America (Fig. 1C). However, our first time interval examined, the postglacial interval (11,700 to 4,200 ybp), directly follows the relatively rapid climate change that occurred during deglaciation. Additionally, early in the postglacial interval, and before 9,000 ybp, MAT was almost 2 °C colder than for the rest of the interval and during the early agricultural (Fig. 1 B and C). These rapidly changing climates could have resulted in temporal lags between where mammals were found and the climates that they preferred either as a result of slow dispersal times or if the plants on which they depend failed to disperse rapidly. However, if these lags were the cause behind the observed climatic niche change, we would expect significantly higher climatic niche disparity during the postglacial interval compared to later intervals, which we did not see (Materials and Methods and SI Appendix, Fig. S2).
The Impact of Human Land Use on Species’ Changing Niches.
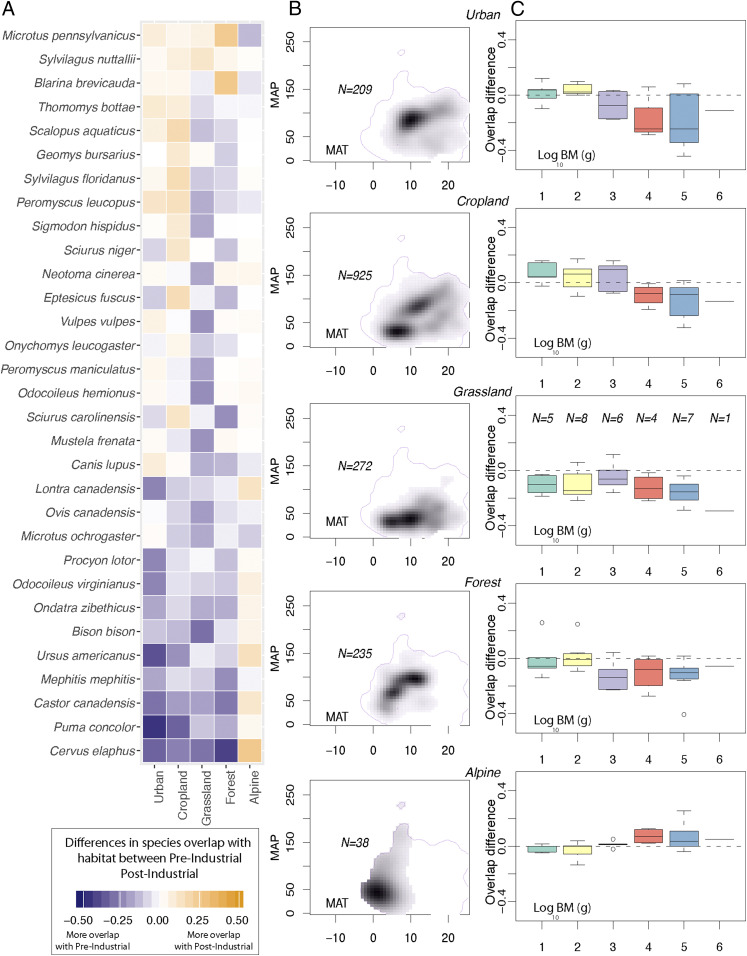
To test whether observed niche changes are affected by human land use changes, we calculated the present climatic niche of five modern land cover types in North America that we call “habitats”: urban areas, croplands, grasslands, forests, and alpine habitats (US Geological Survey, Department of the Interior [https://catalog.data.gov/]; see SI Appendix, Fig. S5). We calculate habitat niche change (HNC) by estimating climatic niche overlap between each of the 46 species and a particular habitat category for pre- and postindustrial time intervals (i.e., before and after 1850 AD; see Fig. 3), and between the postglacial and the present time intervals (SI Appendix, Fig. S7). Preindustrial includes postglacial, early agricultural, and European time intervals, and postindustrial includes industrial and present. We then calculate the difference between the overlap observed in the younger and the older time bin to see whether a species has moved from or into a particular climatic niche (Materials and Methods and SI Appendix, Fig. S6). By comparing the climate breadth of, for example, an urban habitat with the realized niche of a species in the preindustrial, we are not implying that urban habitats existed back in the preindustrial. Instead, we are calculating to what extent species occupied the urban climate niche before and after that type of habitat came into existence, (and thus to what extent the use of land for dense settlement has potentially changed the realized species niche). If a species overlaps more with a urban habitat in the preindustrial than it does in the present, and the realized niche space of this species has changed between the preindustrial and the present, then we can conclude that some biotic or abiotic interactions are preventing this species from living in its former climate niche space (SI Appendix, Fig. S7).
Fig. 3.
(A) HNC between pre- and postindustrial time intervals for the 31 mammal species in the study that significantly changed their niche after the Industrial Revolution in North America when compared with five habitat types. Species are ordered by average HNC value. (B) Climatic niche described from MAT and MAP for the five different land-cover types. (C) Average overlap difference (HNC) for each body mass category (log10 body mass pin grams) for the 46 species in the study and for each habitat type. Horizontal dashed lines were added as a reference for where no overlap difference occurs (overlap difference = 0).
A positive HNC value means that a species’ climatic niche overlaps more with the habitat’s climatic niche in the postindustrial than it did during the preindustrial interval. This suggests that the mammal’s climatic niche shifted into the climate associated with that habitat type (SI Appendix, Fig. S6 shows a graphical explanation of the method). Meanwhile, a negative HNC value indicates that a species’ climatic niche overlapped more with the habitat’s climatic niche in the preindustrial than it does during the postindustrial interval. This indicates that the mammal’s niche shifted away from the climate associated with that habitat type. Applying this method to human habitats (urban and croplands) provides an approximate anthropogenic tolerance index for each species. Additionally, species are grouped into log10 body mass and dietary categories to determine whether large and small mammals differ in their responses to human impacts.
We find differences in the responses of large and small mammals, which supports our second hypothesis that mammal species with different ecological traits have experienced different changes in their climatic niches. Large mammals have been extirpated from climates commonly used by humans, while small mammals have expanded their climatic ranges (Fig. 3C and see SI Appendix, Fig. S7). Present-day smaller mammals (<1 kg) generally occupy climates now found in human-impacted habitats (urban and croplands) more than they did in preindustrial times, and larger mammals generally occupy them less often (Fig. 3C and see SI Appendix, Fig. S7). Taken together, these findings support our third hypothesis: Changes in climatic niches correspond to climates associated today with anthropogenic activities. Past research also indicates that a combination of small body size, smaller shelter sizes, and flexible diet make small mammals more likely to thrive in human-impacted ecosystems (20). Additionally, body size differences among human-impacted habitats have also been observed. For instance, in Santini, et al. (20) the average body mass for urban dwellers is 5.6 kg, while mammals that only visit urbanized environments sporadically weigh an average of 39.4 kg.
Compared to the preindustrial interval, large mammals have shifted away from more moderate climates, where human disturbance is greater, into colder more alpine climates (Fig. 3A and see SI Appendix, Fig. S5). Larger mammals (>1 kg) with more specialized diets [e.g., herbivores and carnivores (24), see SI Appendix, Fig. S13], such as the cougar (P. concolor) or the elk (C. elaphus), are being displaced from more moderate climates—including forests and grasslands—and have only expanded in alpine climate regions that have colder and drier conditions on average (Fig. 3A and see SI Appendix, Fig. S5) (2). These findings align with previous research that found that the cougar and the North American river otter are losing a higher percentage of their historic range in areas with moderate climates than they are in colder environments, such as alpine areas (2). For instance, P. concolor is overlapping 40 and 32% less with urban and cropland habitats, respectively, in the postindustrial than it did in the preindustrial interval.
Conservation and Paleoecology.
Conservation practitioners often use correlative distribution models to identify the best regions for expanding protections, estimating the climatic conditions in which a species can survive (8). However, our results suggest that humans have altered mammal habitats, changing the climates they occupy today (Fig. 3A). Thus, the realized climatic niches that we observe today do not approximate mammals’ fundamental niches. In fact, they likely represent the margins of many species’ fundamental niches, which may have critical implications for niche-based conservation models. These models would underestimate the range of climates in which many mammals can survive if we only use the realized niches of modern occurrences, and therefore underpredict the potential suitability of habitats for those mammals in the future (5, 25). Meanwhile, merging fossil and modern data will not provide the whole fundamental niche of a species, but it will deliver a closer approximation. This mismatch between mammals’ predicted distributions and where they are actually found under very different climate conditions has previously been demonstrated (5, 26). A human-induced shift in the realized niche of these mammal species is likely one of the mechanisms for these poor predictions. One potential solution to this challenge is to approximate climatic niches by integrating species niches through time (5, 7, 27). However, accurately predicting precisely how a species is anticipated to shift its distribution in response to changing climates remains a difficult challenge for conservation practitioners going forward.
Rather than focus on the projected needs of individual species, some studies suggest that preserving half of Earth’s ecosystems by 2050 could mitigate the effects of human activities on wildlife (28). However, even considering the most optimistic half-Earth proposals, the areas likely to be conserved correspond to the most extreme climate ecoregions—hot, dry deserts and cold, dry boreal forests and alpine areas (1, 28). Thus, these conservation initiatives may only maintain species at the edge of where they can survive and into regions with low productivity (29). Our research suggests that this process has already begun. Larger mammals are shifting to environments where human activities are less pronounced, such as alpine areas. These same larger mammals, which usually have larger home ranges, are experiencing the most dramatic reduction of their available geographic and climatic space (29), making them more vulnerable to extinction in the near future (30).
One strategy for reducing this vulnerability is to build habitat corridors to connect remaining habitats, allowing species to move and forage freely while responding to climate change (8, 31, 32). Our results suggest that mammals have been affected differently by human activities (Fig. 3A), and thus species that have exhibited climatic niche contractions with respect to urban and croplands, such as cougars (P. concolor) or elk (C. elaphus), should be targeted for connectivity improvements. Meanwhile, other mammals such as the northern short-tailed shrew (Blarina brevicauda) or the meadow vole (Microtus pennsylvanicus) demonstrate a higher tolerance for anthropogenic activities and may not need increased protections (Fig. 3A) (20).
Identifying efficient strategies to expand protected areas will help species expand into their preferred climates (33). In fact, national parks have been successful at preserving some grasslands and forests from direct human activities in North America (28). However, temperate-to-warm areas with easy access to water sources make the most productive land for farming and rangelands (1, 28). Unfortunately, these human-modified habitats are also usually located in areas of high diversity and primary productivity. Thus, human needs and biodiversity conservation often compete for the resources that these areas provide (31, 34). Moving forward, we must also assure that conservation measures balance socioeconomic needs (35).
Given rising human population sizes and agricultural needs, protected areas will not suffice to maintain viable populations of native mammals. Agriculture is one of the main anthropogenic activities causing changes in mammal ranges and climatic niches (36, 37). Industrial agriculture, particularly, can have profound impacts on ecosystems’ functioning and biodiversity (36, 37). Ecoagricultural practices propose a model in which the landscape is divided in a mosaic of natural habitats and productive areas (37, 38). These practices promote diversity of domesticated and wild species, as well as native ecosystems, integrated with agricultural landscapes. They also provide the habitat connectivity to allow species to track changing climates (8). These practices are especially necessary to protect species that are currently being extirpated by agricultural practices, particularly larger mammals. Further, biodiversity-friendly agricultural practices have the potential of making human-modified habitats more wildlife friendly to all species large and small (39–41).
Conclusions
Our results show that most mammals in the contiguous United States (67% of the studied species) are living in different climatic niches in the present than they did for the last 11,700 y, and that human impacts could be driving these niche shifts. Our research included the 23.6% of the mammals found in the contiguous United States for which enough quality fossil data exist to perform reliable niche analyses. These also represent the most common species in the contiguous United States. If human land use changes are shaping the climatic niche of these 46 species, which are common throughout the Holocene, then rare species that often have much more restricted niches are likely being affected to an even greater extent. Therefore, our study might only represent the tip of the iceberg for what might be happening across all other mammal species.
We find that most modern mammals have shifted their realized climatic niche throughout the Holocene. Human impacts are creating unprecedented ecological scenarios, and species might be forced to shift their niches in order to survive. Some of the largest species are being extirpated from their preferred habitats, while other opportunistic smaller species are thriving in human settlements. Thus, present climatic niches might not represent species’ whole range of niche preferences. Luckily, the study of the fossil record brings us important ecological information that we cannot discover by studying only the snapshot that modern ecosystems can provide. Moving forward, we need to incorporate fossil data into niche modeling, whenever available, in order to create more accurate predictions for ecological research and conservation strategies.
Materials and Methods
Datasets.
We used the R environment for data analysis and building figures (42).
We compiled fossil and modern occurrence data for North American terrestrial mammals. Fossil data were downloaded from the Faunmap II database (18). We calibrated all fossil ages estimated from radiocarbon dating using the R package Bchron and the calibration curve IntCal13 (43). Age for each occurrence was considered as the average between minimum and maximum age of the locality. We restricted our analysis to the Holocene (occurrences from 11,700 ybp or younger) (44). Modern occurrences were downloaded from GBIF (19) using the rgbif package in the R environment (45). We initially compiled all mammal species found in the contiguous United States (196 species) using the function occ_search in rgbif. For modern data, we excluded occurrences that were listed as being fossil specimens or unknown in the field “basisOfRecord,” limiting analyses to specimens based on human observation or preserved specimen. We were interested in testing how major historical events and changes in human settlements in the contiguous United States have affected niche preferences and availability, so the dates were chosen to correspond to the timing of these events. While these events did not occur synchronously across the whole continent, they represent a turning point to a steady change across human civilizations within the continent. Specifically, 11,700 ybp marks the end of the Pleistocene and beginning of the Holocene (44); 4,200 ybp marks the end of the mid-Holocene, coinciding with a widespread aridification event observed across all continents ∼4,299 ybp (44, 46, 47). The year 1500 AD marks the arrival of European settlements in North America (48); 1850 AD corresponds to the beginning of the Industrial Revolution in North America (48); 1950 AD marks the establishment of modern cities and road networks in the 1950s and when the present time begins (48). We obtained trait data for mammals from published databases (49, 50). Traits included diet and body mass. We qualitatively classified the diets of the 46 mammal species in the dataset following the classification criteria used by Pineda-Munoz and Alroy (24), which emphasizes the primary resource in a given diet. Species were classified as specialists if a single food resource made up 50% or more of its diet. If no food item comprised at least 50% of the diet, species were classified as a generalist. The specializations in our dataset included carnivory, herbivory, insectivory, and generalist.
Climate Estimates.
We extracted MAT and MAP for each species occurrence. For occurrences older than 1980 AD, we used the CCSM3 downscaled paleoclimate model (51, 52). For occurrences after 1980 AD, we estimated climate using the high-resolution gridded climatic research unit (CRU) data (53). In order to keep consistent data uncertainty between the two datasets, we estimated climate after 1980 AD with the CCSM3 downscaled paleoclimate model data in 1980 AD plus the difference between 1980 AD and the climate estimated from CRU data.
Initially, we tried seven climate variables including mean/min/max annual temperature/precipitation, and climate water deficit (potential evapotranspiration − actual evapotranspiration). Among the seven climate variables, min/max annual temperature/precipitation correspond to summer/winter temperature/precipitation for most sites, representing seasonal measures of climates. Wang et al. (54) did a correlation analysis between MAT, MAP, and all other variables across the same study period and found that the other five variables are strongly correlated with MAT and MAP (for details, please see the supplementary information in Wang et al.) (54). Thus, we used MAT or MAP for this work, as these two variables can catch most of the information of all climate variables, including the seasonal measures of climate. Additionally, limiting the analyses to MAT and MAP allows for a more intuitive interpretation of the results and figures.
Time Averaging and Climatic Ranges.
Some fossil localities had an age range that could span hundreds of years. Because climate can significantly change in that amount of time, this could result in an erroneous climate estimation. Thus, we performed a sensitivity analysis that calculated the possible climatic range within a fossil locality based on its age range (from the earliest to the latest estimated age for a locality). To perform the sensitivity analysis, we obtained climate estimates for each fossil locality at 10-y intervals from its minimum to maximum age using the CCSM3 downscaled paleoclimate model (51, 52). We then calculated the MAT and MAP range for each locality. We plotted temperature range against precipitation range in order to find a threshold for excluding localities that were more likely to result in erroneous climate estimations. We excluded all localities that had a MAT and MAP range that was higher than 3 °C and 25 mm, respectively, which represented the 82% percentile for MAT and the 85% percentile for MAP. These two thresholds limited our analysis to the samples with more robust climate estimations (SI Appendix, Fig. S8).
Background Data.
Niche models require climatic background data for each species/time period in an analysis. We obtained latitude and longitude values for every 0.5° for every 500 y from 11,700 ybp to the present for the contiguous United States. We then obtained MAT and MAP for every geographical point and time from the CCSM3 climate model (51, 52) before 1980 AD or high-resolution gridded CRU data (53) after 1980 AD using the same method described above. Background data were also classified between postglacial, early agricultural, European, industrial, and present.
Minimum Sample Size and Species in the Study.
A minimum number of occurrences is required to obtain reliable data for niche analyses (15, 17, 55). Thus, we performed a sensitivity analysis that suggested a minimum number of 20 occurrences per species and time bin in order to have a statistically reliable analysis (Materials and Methods and SI Appendix, Figs. S9–S12 for justification and sensitivity analysis). Using our occurrence data for all terrestrial North American mammals, we first calculated niche overlap between two random samples of five occurrences from the present time interval for each species. We repeated this analysis 100 times and calculated the median value. We then increased the number of species occurrences from 5 to 80 occurrences per analysis, performing 100 repetitions each time. The closer we get to including all species occurrences in a sample, the closer the overlap value between two samples drawn from the same dataset will be to 1 (the two samples will end up identical). We plotted the results of this analysis for the 46 species in the study and found that overlap stabilizes between 20 and 40 occurrences (SI Appendix, Figs. S9–S12). While we analyzed datasets with more than 20 and less than 40 occurrences, the majority of the samples (91%) had 40 or more individual occurrences (SI Appendix, Table S2).
Based on the sensitivity analysis, we discarded species from our original set of 196 which did not have at least 20 occurrences per time interval, enough to perform a robust analysis. This left 46 species (23.6%), belonging to the taxonomic orders Artiodactyla (6 species), Carnivora (12 species), Chiroptera (1 species), Insectivora (2 species), Lagomorpha (5 species), and Rodentia (20 species).
Niche Overlap and Equivalency.
We used the R package ecospat for niche overlap analysis (15). To facilitate the interpretation of the results, we used only MAT and MAP values as the two dimensions for the climate niche space. First, we created a grid of 50 × 50 pixels within the climate-space graph for MAT and MAP data. The range of MAT was 56.49 °C and the range of MAP was 376.46 mm. We then calculated realized niche from occurrence densities for each species and time period using the function ecospat.grid.clim.dyn(). This function calculates climatic niche by measuring the frequency of occurrences of the species for each cell of the grid using a kernel smoother. We used the difference in occurrence densities to calculate niche overlap using the function ecospat.niche.overlap(), which calculates the proportion of cells that are common across two different time intervals. We used Schöner’s D metric (15–17, 56, 57), which varies between 0 (no overlap) and 1 (complete overlap). Schöner’s I metric was also calculated, but results did not differ and therefore are not discussed. We corrected for available niche space [option within the function ecospat.niche.overlap()] because different time periods could have different background available climate spaces. Thus, overlap was only measured among real available habitats, and habitats that do not exist in both time intervals being compared do not contribute to overlap calculations.
We also corrected for differences in sample size because some species had different numbers of occurrences per time bin that could vary by up to two orders of magnitude. We subsampled the time bin with the higher number of occurrences to be the same size to the time bin with the fewer occurrences. We then performed a niche overlap test between the two time bins as reported above. We repeated this operation 100 times and reported the average overlap as the “observed overlap” between the two time intervals (Fig. 2 and see SI Appendix, Table S4).
Niche Equivalency Test.
To investigate whether a species’ climatic niche was significantly different or similar across time periods, we calculated niche equivalency for each species and across time bins using a null model (17) (see SI Appendix, Fig. S1 for a graphic explanation of the method) and using the function ecospat.niche.equivalency.test() in ecospat (15). The function runs as follows: for a species in two different time bins T1 and T2 with NT1 and NT2 number of occurrences, respectively, it first calculates the observed overlap as described above. Then, it creates a pseudoreplicate dataset by randomly shuffling the time category while holding the number of pairs in each time interval constant. It then calculates the overlap D metric for this pseudoreplicated data. It repeats this process 100 times to create a null distribution of D values. The observed D value is compared to the null distribution. As described in Warren et al. (17) and Di Cola et al. (15), if the observed D value of a species falls below the lower 5th percentile, the species has a nonequivalent niche across two time periods. If it falls over the 95th percentile, the species is considered to have a higher equivalency between two time intervals than expected from the null model. The analyses were performed between: each consecutive pair of time intervals; between each time interval and the present; and between pre- and postindustrial time intervals. We are looking at the range of climatic conditions (climate niche) occupied by a species relative to its available climate across time intervals instead of at a given snapshot in time, which should minimize temporal autocorrelation. Given the dispersal capabilities of mammals, any lag in adjusting to climate can be minimized over the length of the time interval assessed.
Differences in Duration between Time Intervals.
We calculated niche disparity for each species and across time bins by measuring the average Euclidian distance between each data point (specimen) and the centroid for the climatic niche space in its time interval using the function centroid() from the R package dispRity (58) (SI Appendix, Table S6). This gave us a value for how dispersed data were for each species and time bin. If duration was affecting our analysis, we would observe that older time intervals, which have longer durations, would also have higher average disparity. This is not the case; all time intervals display similar average disparity (SI Appendix, Fig. S2).
Additionally, our goal is to calculate the realized niche of a species within a time frame with similar anthropogenic impacts, independent of how long these impacts persist. We estimate niche by applying a kernel density smoother (see niche overlap and equivalency section above) so that unique climatic conditions contribute less weight than most common ones in our analysis (15).
Habitat Niche Change Analysis.
We obtained land cover data for contiguous United States to test whether land use could explain the observed changes in the realized climatic niche of mammals. US Geological Survey land cover data divide the land surface of the contiguous US into polygons representing different land covers that we will refer to as “habitats” (e.g., urban, cropland, forest, or alpine habitat) (59). We used the land cover dataset from 1970 because the average date for present occurrence data were 1969 AD. Extracting occurrences for each grid cell within a habitat’s polygon would overfeed the dataset for habitats. Thus, in order to make the habitat occurrences more comparable in data size to the species’ occurrences, we limited habitat occurrences to one datapoint per polygon. We obtained the latitude and longitude for the centroid (center of a geometric object) of each landcover polygon in the dataset, using the R package sp version 1.3-1. Each centroid was treated as a locality or occurrence within its landcover category (e.g., the centroid of an urban polygon represents an occurrence data point for urban). To simplify the analysis and reduce ambiguity, some habitats were reclassified in a single category (e.g., category “woodland and forest with some cropland and pasture” was classified as cropland; see SI Appendix, Table S1), while others were excluded from the analysis. We also excluded landcovers represented by fewer than 15 polygons (SI Appendix, Table S1). Ambiguous definitions were excluded from the study because their definitions did not place them definitively in or out of one of our categories of interest. Analyzed categories included: urban, cropland, grassland, forest, and alpine.
| [1] |
where is the climatic niche overlap (D) between a particular habitat category and a species in the postindustrial (after 1850 AD) . And is the climatic niche overlap (D) between this habitat category in the present and this same species in the preindustrial interval (before 1850 AD) .
Additionally, HNC was plotted against body mass and diet to explore whether a relationship existed between mammal traits and their niche change across the Holocene (SI Appendix, Fig. S13).
Spatial and Temporal Autocorrelation.
Our study is based on niche estimations across the Holocene based on geographical occurrence data. Thus, we needed to explore the role of temporal and spatial autocorrelation in our results. By calculating equivalency and HNC we are taking both spatial and temporal autocorrelation into account and asking how much change occurred given that we expect substantial overlap in geographic range and thus climatic niche through time and across space.
The existence of temporal autocorrelation makes our analyses more conservative with respect to the findings. Autocorrelation should increase the probability of finding no change in the climate niche because it should be similar to what it was in the previous time interval. What this means for our study is that 1) changes from one time interval to the next may appear smaller than they really are because time intervals span large temporal ranges and climatic niche is averaged over that time; 2) we can have more confidence that any changes we do find are real (because it’s in the opposite direction of the bias); and 3) our results are unlikely driven by issues with autocorrelation.
As with temporal autocorrelation, spatial autocorrelation should bias our results toward finding no change in climatic niche from one interval to the next. Thus, spatial autocorrelation also makes our analyses conservative with respect to our findings. While we could remove points in the same sample that are too close together, we are already subsampling occurrences heavily to calculate niche overlap, which should decrease the effects of closely sampled points.
Supplementary Material
Acknowledgments
We thank the Special Ecology and Paleontology Laboratory at Georgia Institute of Technology for helpful comments on the project and manuscript. We also thank Dr. Danielle Fraser for assisting with date calibrations in R and two anonymous reviewers for suggestions on how to improve this manuscript. Y.W. was funded under NSF Grant 1655898. J.M. and Y.W. were funded under NSF Grant 1945013. S.P.-M., A.B.T., and S.K.L. were supported, in part, by NSF Grant 1257625. This is the Evolution of Terrestrial Ecosystems program’s publication number 410.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1922859118/-/DCSupplemental.
Data Availability.
All study data are included in the article and supporting information.
References
- 1.Ceballos G., Ehrlich P. R., Dirzo R., Biological annihilation via the ongoing sixth mass extinction signaled by vertebrate population losses and declines. Proc. Natl. Acad. Sci. U.S.A. 114, E6089–E6096 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laliberte A. S., Ripple W. J., Range contractions of North American carnivores and ungulates. Bioscience 54, 123–138 (2004). [Google Scholar]
- 3.Crooks K. R., et al. , Quantification of habitat fragmentation reveals extinction risk in terrestrial mammals. Proc. Natl. Acad. Sci. U.S.A. 114, 7635–7640 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lyons S. K., et al. , Holocene shifts in the assembly of plant and animal communities implicate human impacts. Nature 529, 80–83 (2016). [DOI] [PubMed] [Google Scholar]
- 5.McGuire J. L., Davis E. B., Using the palaeontological record of Microtus to test species distribution models and reveal responses to climate change. J. Biogeogr. 40, 1490–1500 (2013). [Google Scholar]
- 6.Tóth A. B., et al. , Reorganization of surviving mammal communities after the end-Pleistocene megafaunal extinction. Science 365, 1305–1308 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Smith F. A., Smith R. E. E., Lyons S. K., Payne J. L., Villaseñor A., The accelerating influence of humans on mammalian macroecological patterns over the late Quaternary. Quat. Sci. Rev. 211, 1–16 (2019). [Google Scholar]
- 8.McGuire J. L., Lawler J. J., McRae B. H., Nuñez T. A., Theobald D. M., Achieving climate connectivity in a fragmented landscape. Proc. Natl. Acad. Sci. U.S.A. 113, 7195–7200 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terry R. C., Rowe R. J., Energy flow and functional compensation in Great Basin small mammals under natural and anthropogenic environmental change. Proc. Natl. Acad. Sci. U.S.A. 112, 9656–9661 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martínez-Meyer E., Townsend Peterson A., Hargrove W. W., Ecological niches as stable distributional constraints on mammal species, with implications for Pleistocene extinctions and climate change projections for biodiversity. Glob. Ecol. Biogeogr. 13, 305–314 (2004). [Google Scholar]
- 11.Widga C., Niche variability in late Holocene bison: A perspective from big bone lick, KY. J. Archaeol. Sci. 33, 1237–1255 (2006). [Google Scholar]
- 12.Jezkova T., Olah-Hemmings V., Riddle B. R., Niche shifting in response to warming climate after the last glacial maximum: Inference from genetic data and niche assessments in the chisel toothed kangaroo rat (Dipodomys microps). Glob. Change Biol. 17, 3486–3502 (2011). [Google Scholar]
- 13.Smith F. A., Betancourt J. L., The effect of Holocene temperature fluctuations on the evolution and ecology of Neotoma (woodrats) in Idaho and northwestern Utah. Quat. Res. 59, 160–171 (2003). [Google Scholar]
- 14.Levin S. A., et al. , The Princeton Guide to Ecology (Princeton University Press, 2009). [Google Scholar]
- 15.Di Cola V., et al. , ecospat: An R package to support spatial analyses and modeling of species niches and distributions. Ecography 40, 774–787 (2017). [Google Scholar]
- 16.Broennimann O., et al. , Measuring ecological niche overlap from occurrence and spatial environmental data. Glob. Ecol. Biogeogr. 21, 481–497 (2012). [Google Scholar]
- 17.Warren D. L., Glor R. E., Turelli M., Environmental niche equivalency versus conservatism: quantitative approaches to niche evolution. Evolution 62, 2868–2883 (2008). [DOI] [PubMed] [Google Scholar]
- 18.Graham R., Lundelius E. Jr, FAUNMAP II: New Data for North America with a Temporal Extension for the Blancan, Irvingtonian and Early Rancholabrean. FAUNMAP II Database. Version 1 (2010). Accessed 12 September 2018.
- 19.GBIF occurrence download. https://www.gbif.org/occurrence/download/0020247-190918142434337 (14 October 2019).
- 20.Santini L., et al. , One strategy does not fit all: determinants of urban adaptation in mammals. Ecol. Lett. 22, 365–376 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hornaday W. T., The Extermination of the American Bison, with a Sketch of its Discovery and Life History (US National Museum, Washington, DC, 1889). [Google Scholar]
- 22.Barnhurst D., “Vulnerability of cougars to hunting” in All Graduate Theses and Dissertations (Utah State University, 1986), p. 6166. [Google Scholar]
- 23.Erb J., Roberts N. M., Chris Dwyer, An otterly successful restoration (The Wildlife Professional, May/June, 2018). [Google Scholar]
- 24.Pineda-Munoz S., Alroy J., Dietary characterization of terrestrial mammals. Proc. Biol. Sci. 281, 20141173 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elith J., Leathwick J. R., Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 40, 677–697 (2009). [Google Scholar]
- 26.Gavin D. G., et al. , Climate refugia: joint inference from fossil records, species distribution models and phylogeography. New Phytol. 204, 37–54 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Faurby S., Araújo M. B., Anthropogenic range contractions bias species climate change forecasts. Nat. Clim. Chang. 8, 252–256 (2018). [Google Scholar]
- 28.Dinerstein E., et al. , A global deal for nature: Guiding principles, milestones, and targets. Sci. Adv. 5, eaaw2869 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tucker M. A., et al. , Moving in the Anthropocene: Global reductions in terrestrial mammalian movements. Science 359, 466–469 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Urban M. C., Climate change. Accelerating extinction risk from climate change. Science 348, 571–573 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Kremen C., Reframing the land-sparing/land-sharing debate for biodiversity conservation. Ann. N. Y. Acad. Sci. 1355, 52–76 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Laurance W. F., et al. , Averting biodiversity collapse in tropical forest protected areas. Nature 489, 290–294 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Tallis H. M., et al. , An attainable global vision for conservation and human well-being. Front. Ecol. Environ. 16, 563–570 (2018). [Google Scholar]
- 34.Lambin E. F., Meyfroidt P., Global land use change, economic globalization, and the looming land scarcity. Proc. Natl. Acad. Sci. U.S.A. 108, 3465–3472 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phalan B., Balmford A., Green R. E., Scharlemann J. P., Minimising the harm to biodiversity of producing more food globally. Food Policy 36, S62–S71 (2011). [Google Scholar]
- 36.Frison E. A., From Uniformity to Diversity: A Paradigm Shift from Industrial Agriculture to Diversified Agroecological Systems (IPES, Louvain-la-Neuve, Belgium, 2016). [Google Scholar]
- 37.Scherr S. J., McNeely J. A., Biodiversity conservation and agricultural sustainability: towards a new paradigm of ‘ecoagriculture’ landscapes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363, 477–494 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scherr S. J., McNeely J. A., Farming with Nature: The Science and Practice of Ecoagriculture (Island Press, 2012). [Google Scholar]
- 39.Quinn J. E., Brandle J. R., Johnson R. J., The effects of land sparing and wildlife-friendly practices on grassland bird abundance within organic farmlands. Agric. Ecosyst. Environ. 161, 10–16 (2012). [Google Scholar]
- 40.Crespin S. J., García-Villalta J. E., Integration of land-sharing and land-sparing conservation strategies through regional networking: the Mesoamerican Biological Corridor as a lifeline for carnivores in El Salvador. Ambio 43, 820–824 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alvez J. P., Filho A. L. S., Farley J., Alarcon G., Fantini A. C., The potential for agroecosystems to restore ecological corridors and sustain farmer livelihoods: Evidence from Brazil. Ecol. Restor. 30, 288–290 (2012). [Google Scholar]
- 42.R Core Team , R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria, 2019). [Google Scholar]
- 43.Haslett J., Parnell A., A simple monotone process with application to radiocarbon-dated depth chronologies. J. R. Stat. Soc. Ser. C Appl. Stat. 57, 399–418 (2008). [Google Scholar]
- 44.Walker M. J., et al. , Formal subdivision of the Holocene Series/Epoch: a Discussion Paper by a Working Group of INTIMATE (Integration of ice-core, marine and terrestrial records) and the Subcommission on Quaternary Stratigraphy (International Commission on Stratigraphy). J. Quat. Sci. 27, 649–659 (2012). [Google Scholar]
- 45.Chamberlain S. A., Boettiger C., R Python, and Ruby clients for GBIF species occurrence data. PeerJ Prepr. 5, e3304v1 (2017). [Google Scholar]
- 46.Price T. D., Ancient farming in eastern North America. Proc. Natl. Acad. Sci. U.S.A. 106, 6427–6428 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grayson D. K., Mammalian responses to middle Holocene climatic change in the great basin of the western United States. J. Biogeogr. 27, 181–192 (2000). [Google Scholar]
- 48.Shi D. E., Tindall G. B., America: A Narrative History, Brief (W W NORTON & Company, ed. 10, 2016). [Google Scholar]
- 49.Smith F. A., et al. , Body mass of late quaternary mammals. Ecology 84, 3403 (2003). [Google Scholar]
- 50.Wilman H., et al. , EltonTraits 1.0: Species-level foraging attributes of the world’s birds and mammals. Ecology 95, 2027 (2014). [Google Scholar]
- 51.Lorenz D. J., Nieto-Lugilde D., Blois J. L., Fitzpatrick M. C., Williams J. W., Downscaled and debiased climate simulations for North America from 21,000 years ago to 2100AD. Sci. Data 3, 160048 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Z., et al. , Transient simulation of last deglaciation with a new mechanism for Bolling-Allerod warming. Science 325, 310–314 (2009). [DOI] [PubMed] [Google Scholar]
- 53.Harris I., Jones P. D., Osborn T. J., Lister D. H., Updated high-resolution grids of monthly climatic observations–the CRU TS3. 10 Dataset. Int. J. Climatol. 34, 623–642 (2014). [Google Scholar]
- 54.Wang Y., Shipley B. R., Lauer D. A., Pineau R. M., McGuire J. L., Plant biomes demonstrate that landscape resilience today is the lowest it has been since end-Pleistocene megafaunal extinctions. Glob. Change Biol. 26, 5914–5927 (2020). [DOI] [PubMed] [Google Scholar]
- 55.Gotelli N. J., Graves G. R., Null Models in Ecology (Smithsonian Institution Press, Washington, D.C., 1996), pp. 368. [Google Scholar]
- 56.Schoener T. W., The Anolis lizards of Bimini: Resource partitioning in a complex fauna. Ecology 49, 704–726 (1968). [Google Scholar]
- 57.Van der Vaart A. W., Asymptotic Statistics (Cambridge University Press, 2000). [Google Scholar]
- 58.Guillerme T., dispRity: A modular R package for measuring disparity. Methods Ecol. Evol. 9, 1755–1763 (2018). [Google Scholar]
- 59.Price C. V., Nakagaki N., Hitt K. J., Clawges R., Enhanced Historical Land-Use and Land-Cover Data Sets of the US Geological Survey (US Department of the Interior, US Geological Survey, 2007). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and supporting information.