Cytoplasmic lipid droplets (LDs) store energy in the form of esterified fatty acids. Mobilization of those fatty acids can occur through cytosolic lipases that traffic to droplets, through lipophagy, or likely through LD contact sites with other organelles. Lipophagy, the degradation of LDs by lysosomes, has two forms: macrolipophagy, involving formation of autophagosomes, trapping LDs, that then fuse with lysosomes; and microlipophagy, in which LDs are directly engulfed by lysosomes. Macrolipophagy has been well described in liver, an organ in which LDs often accumulate in obesity and other disease states. The manuscript by Schulze et al. (1) in PNAS clearly shows that microlipophagy, a process well described in yeast, is a significant pathway in isolated hepatocytes and in liver in vivo. It is not difficult to imagine this is the tip of the iceberg and that the importance of microautophagic events in mammalian cells has been underappreciated until now.
LDs and Energy Homeostasis
LDs are composed of a neutral lipid core surrounded by a phospholipid monoleaflet into which are embedded proteins (2). The bulk of neutral lipids is usually triacyglycerols and steryl esters but may include acylceramides, retinyl esters, and other bioactive lipids. The LD surface is home to perilipins, which protect against adventitious lipolysis, and many enzymes of lipid metabolism. The surface also provides storage for histones, proteins of the innate immune system, and serves as a scaffold for the assembly of viral pathogens.
While LD are valuable, they are not always benign (3). Overextended adipose tissue invites inflammation. Fatty liver disease affects a growing fraction of the population of industrialized countries, as adipose stores become saturated upon chronic overeating. Foam cells develop and accumulate during atherogenesis as macrophages feast on available external lipid, filling up their cytoplasm with LDs.
Autophagic Pathways
Mobilization of fatty acids from LDs can quickly occur by cytosolic lipases that traffic to the organelle (4). They can also be released by lysosomal fatty acid lipase inside lysosomes following autophagy. Degradation of cytoplasmic contents by lysosomes not only allows recycling of metabolic building blocks but is essential for several other normal functions including tissue development and remodeling (5). Autophagy can be nonselective, in which the cargo is a small volume of cytoplasm, or highly selective for individual proteins (chaperone-mediated autophagy and cytoplasm to vacuole targeting) or organelles. Cargo of the latter group is trapped during mitophagy, pexophagy (peroxisomes as cargo), ER-phagy (endoplasmic reticulum as cargo), nucleophagy, and relevant to this discussion, lipophagy. For these targeted organelles, the core machinery recruits other components that can recognize receptors associated with cargo. Finally, autophagy can involve direct engulfment of cargo by lysosomes or initial trapping of cargo into a double-membraned intermediate.
Macroautophagy (often referred to simply as autophagy) involves more than 30 Atg proteins (the “core machinery”) in the basic process, which is largely conserved in fungi, animals, and plants (6). Briefly, a phagophore double-membrane structure binds to cargo, laterally expands, and finally seals, enveloping the cargo as a closed compartment, the phagosome. Membranes of the phagosome come from ER-derived Atg9-containing vesicles and probably several other locations. The phagosome is then recognized by lysosomes. The two organelles fuse, releasing single-membraned vesicles containing cargo into the lysosomes for degradation.
Microautophagy is the process by which a portion of cytoplasm is directly engulfed by lysosomes (7). [The term, coined by De Duve and Wattiaux (8) in 1966, does not signify that cargo captured is smaller than that enwrapped by macroautophagy.] In the simplest case, invaginations in the lysosomal membrane form until fusion of the edges occurs, resulting in fission of the trapped cargo, either nonselective or specific, from the lysosomal boundary membrane. This fusion–fission reaction involves the endosomal sorting complexes required for transport (ESCRT) machinery, especially ESCRT III, which brings the edges together. Lysosomal engulfment may also involve new extensions of the membrane that wrap around larger structures or may involve membranes that bind in trans to the cargo, grow, and then fuse to the lysosomes, again trapping the cargo, or both (7). In such cases, the core macroautophagic machinery may be involved in generating the lysosomal membrane extensions or the phagophore-like flattened double membrane acting in trans.
Lipophagy
The engulfment of LDs by a phagophore was first described in rat cultured hepatocytes as a response to starvation (9). Blocking autophagy resulted in an increase in fat content as well as in number and size of LDs. Strong evidence for this process in starved mice was also shown. The authors could find no evidence of microlipophagy.
Plin2, the most abundant perilipin in nonadipose tissue, is a barrier to macrolipophagy. Its removal by chaperone-mediated autophagy in mouse liver and cultured cells appears to be a prerequisite in mammalian cells for lipophagosome formation (10). There is no information yet on how denuded LDs are recognized by the autophagic machinery.
Macrolipophagy has been considered the dominant if not exclusive activity for selectively trafficking LDs to the lysosome in mammalian cells, including hepatocytes.
Meanwhile, direct uptake of LDs by lysosomes in yeast has been apparent for several decades (11). As cells approach stationary phase, LDs in each cell are easily seen in proximity to the large vacuole (the yeast lysosome) and often inside of it (12). Ultrastructural studies show the direct apposition of these two organelles. More recent studies have shown microlipophagy occurring under several conditions: two growth phases (the diauxic shift in which cells switch from glucose to ethanol as carbon source, and stationary phase), two specific starvation conditions (deprivation of carbon and nitrogen), and ER stress (13). There may be others yet to be determined. The dependence on the core autophagy machinery for successful microlipophagy varies among these conditions. For example, it is dispensable after the diauxic shift, whereas it is required during nitrogen starvation.
Under starvation conditions, the yeast vacuolar membrane becomes segregated into sterol-rich and sterol-poor domains in a soccer ball-like pattern. This structure is required for microlipophagy, which occurs only in the sterol-enriched membrane domains (14). Interestingly, in the early stage of nutrient starvation, LDs are not only consumed by the vacuole but also assembled there at nuclear–vacuolar junctions (15).
One argument to support the preference of yeast for microlipophagy and mammals for macrolipophagy is that the yeast vacuole is much larger than the mammalian lysosome and can therefore engulf large LDs. However, both autolipophagic pathways can involve nibbling of the juicy LD, extracting a small fraction of the organelle into an autophagophore or a lysosome.
Microlipophagy in Liver
The publication by Schulze et al. (1) in PNAS addresses the mechanism of autolipophagy in liver by demonstrating clearly that microlipophagy occurs if you know how to look for it, and it is not an insignificant mechanism for LD consumption (Fig. 1). [The group had recently published evidence that larger LDs are first subject to lipolysis by cytosolic enzymes before becoming cargo for lipophagy (16).] Using fluorescence microscopy in AML12 mouse liver cells or primary hepatocytes, they observed that LDs and lysosomes form transient contacts that usually lasted ∼30 s, although sometimes lasting 60 s or more, which were seen both in nutrient-replete and -starved conditions. These contacts did not depend on the core autophagic machinery. To test for exchange of material, they labeled droplets with BODIPY-C12 (a lipid probe of the fluorophore BODIPY conjugated with a C12 fatty acid) or with mono-red fluorescent protein-enhanced green fluorescent protein-Plin2, which fluoresces yellow in the cytosol and red in acidic compartments. It was clear by time-lapse microscopy that both BODIPY-C12 and Plin2 were entering lysosomes (which were also labeled). In most cases, only a fraction of the LD was transferred to the lysosome, but whole organelles were also consumed. Beautiful electron micrographs confirmed this activity, suggesting a “direct injection” of lipid from LDs. Sometimes more than one lysosome was caught in the process of nibbling at a single LD. Droplet-filled lysosomes were even more apparent after treatment with lalistat, an inhibitor of the lysosomal acid lipase. Finally, increasing the size of LDs by pretreatment with small interfering RNA against the cytosolic lipase adipocyte triglyceride lipase inhibited lipophagy, suggesting that it is more difficult for lysosomes to nibble at very large LDs. The authors suggested that the lysosome must exhibit, directly or indirectly, considerable force on the LD to allow lipophagy, and this may be harder with very large droplets.
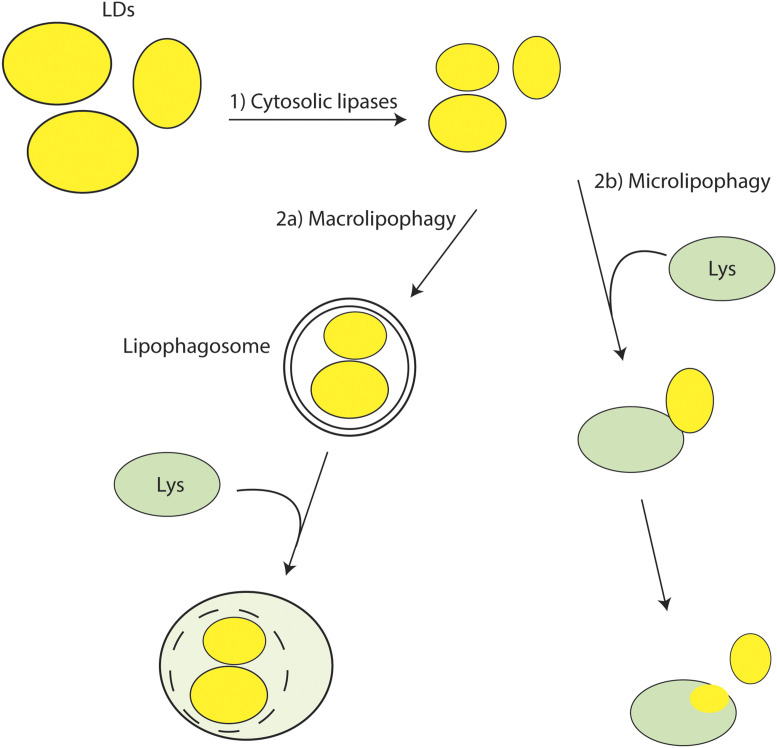
Fig. 1.
Pathways of LD digestion in liver cells. 1) Upon metabolic need, the size of LDs may first be reduced by cytosolic lipases initiated by adipocyte triglyceride lipase. They then are subjected to one of two autophagic pathways. 2a) Phagophore formation around LDs initiates macrolipophagy, resulting in fusion of the lipophagosome with a lysosome. 2b) Alternatively, as shown by Schulze et al. (1), an LD may be attracted directly to a lysosome, often resulting in macrolipophagy in which injection of LD contents into the lysosome occurs, or the entire LD is engulfed (not shown).
Questions Arising
Why have investigators in past studies not seen more evidence of microlipophagy in liver? Lipophagosomes are large and readily apparent, while the exchange of fluids between apposed lysosomes and LDs is likely difficult to detect. Perhaps microlipophagy of complete LDs, which would be easier to see, is rare except for small LDs. Finally, LDs are often larger than lysosomes, and the huge increase in vacuolar surface area seen when the yeast organelle engulfs several large peroxisomes (17) may be impossible for mammalian lysosomes to pull off.
With these observations, the authors have brought the relative roles of macro- vs. microautophagy in mammalian cells to center court. A key question is the joint regulation of these two activities, which certainly must occur. As the machinery of autophagosome generation has many more moving parts than that of simple microautophagy, perhaps under some conditions macroautophagy simply runs out of critical components, and the simpler system is used. It would be interesting to compare the dynamics of micro- and macrolipophagy in hepatocytes in the same experiment. Perhaps one predominates earlier or later (as starvation becomes more severe, for example) or depends on the type of metabolic or other stress.
LDs are unique among cellular organelles as they can form spontaneously without a need for proteaceous machinery if neutral lipid saturates membrane bilayers, while they require proteins such as seipin under physiological states to ensure proper lipid and protein composition and keep droplets on the ER (18). One wonders whether the addition of fatty acids, such as oleic, to cultured cells (as was done in the hepatocyte experiments) leads to a fraction of droplets that are normally not seen in vivo except under conditions such as a high-fat diet. Perhaps under such conditions, two sets of LDs are produced, and each set may prefer the macro- vs. the microlipophagic pathway.
Related to this is the question of recognition of droplets by either pathway. Thus far, no receptors have been identified for either arm of lipophagy. Does the removal of Plin2 in macrolipophagy expose a proteinaceous underlying receptor, or is the abnormal exposed surface of the phospholipid monoleaflet, with the underlying neutral lipids transiently exposed (19), itself a signal to induce the formation of a phagophore?
With regards to the liver, the independence of microlipophagy from the core Atg machinery suggests that the lysosomal membrane extensions, or the transphagophore-like structures in yeast, are not of significant importance in mammalian cells. Nonetheless, it seems likely that microautophagy in mammalian cells is more common than now believed.
Acknowledgments
This work was supported by NIH Grant GM084210.
Footnotes
The author declares no competing interest.
See companion article, “Direct lysosome-based autophagy of lipid droplets in hepatocytes,” 10.1073/pnas.2011442117.
References
- 1.Schulze R. J., et al. , Direct lysosome-based autophagy of lipid droplets in hepatocytes. Proc. Natl. Acad. Sci. U.S.A. 117, 32443–32452 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olzmann J. A., Carvalho P., Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 20, 137–155 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldberg I. J., et al. , Deciphering the role of lipid droplets in cardiovascular disease: A report from the 2017 National Heart, Lung, and Blood Institute Workshop. Circulation 138, 305–315 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lass A., Zimmermann R., Oberer M., Zechner R., Lipolysis - a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog. Lipid Res. 50, 14–27 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi A. M., Ryter S. W., Levine B., Autophagy in human health and disease. N. Engl. J. Med. 368, 651–662 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Feng Y., He D., Yao Z., Klionsky D. J., The machinery of macroautophagy. Cell Res. 24, 24–41 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuck S., Microautophagy - distinct molecular mechanisms handle cargoes of many sizes. J. Cell Sci. 133, jcs246322 (2020). [DOI] [PubMed] [Google Scholar]
- 8.De Duve C., Wattiaux R., Functions of lysosomes. Annu. Rev. Physiol. 28, 435–492 (1966). [DOI] [PubMed] [Google Scholar]
- 9.Singh R., et al. , Autophagy regulates lipid metabolism. Nature 458, 1131–1135 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaushik S., Cuervo A. M., Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis. Nat. Cell Biol. 17, 759–770 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogasawara Y., Tsuji T., Fujimoto T., Multifarious roles of lipid droplets in autophagy: Target, product, and what else? Semin. Cell Dev. Biol. 108, 47–54 (2020). [DOI] [PubMed] [Google Scholar]
- 12.van Zutphen T., et al. , Lipid droplet autophagy in the yeast Saccharomyces cerevisiae. Mol. Biol. Cell 25, 290–301 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia E. J., et al. , Membrane dynamics and protein targets of lipid droplet microautophagy during ER stress-induced proteostasis in the budding yeast, Saccharomyces cerevisiae. Autophagy, 10.1080/15548627.2020.1826691 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang C. W., Miao Y. H., Chang Y. S., A sterol-enriched vacuolar microdomain mediates stationary phase lipophagy in budding yeast. J. Cell Biol. 206, 357–366 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hariri H., et al. , Lipid droplet biogenesis is spatially coordinated at ER-vacuole contacts under nutritional stress. EMBO Rep. 19, 57–72 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schott M. B., et al. , Lipid droplet size directs lipolysis and lipophagy catabolism in hepatocytes. J. Cell Biol. 218, 3320–3335 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tuttle D. L., Lewin A. S., Dunn W. A. Jr, Selective autophagy of peroxisomes in methylotrophic yeasts. Eur. J. Cell Biol. 60, 283–290 (1993). [PubMed] [Google Scholar]
- 18.Cartwright B. R., et al. , Seipin performs dissectible functions in promoting lipid droplet biogenesis and regulating droplet morphology. Mol. Biol. Cell 26, 726–739 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prévost C., et al. , Mechanism and determinants of amphipathic helix-containing protein targeting to lipid droplets. Dev. Cell 44, 73–86.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]