Significance
A long-standing observation is that taste bud cell replenishment requires neuronal input. The molecular basis for neuronal regulation of taste tissue homeostasis is unclear. We demonstrate that Rspo2 is selectively expressed in sensory ganglion neurons. We further demonstrate that exogenous R-spondin can substitute for neuronal input to maintain taste tissue homeostasis by promoting generation of differentiated taste cells in vivo and that R-spondin is required for generation of differentiated taste cells in an ex vivo organoid culture system. We thus propose that Rspo2 may be the long-sought neuron-supplied niche factor for maintaining taste tissue homeostasis. The ability of R-spondin to promote generation of differentiated taste cells may provide a therapeutic approach for those who suffer from taste loss.
Keywords: R-spondin, nerve transection, taste bud cells, Znrf3/Rnf43, Lgr4/5/6
Abstract
Taste bud cells regenerate throughout life. Taste bud maintenance depends on continuous replacement of senescent taste cells with new ones generated by adult taste stem cells. More than a century ago it was shown that taste buds degenerate after their innervating nerves are transected and that they are not restored until after reinnervation by distant gustatory ganglion neurons. Thus, neuronal input, likely via neuron-supplied factors, is required for generation of differentiated taste cells and taste bud maintenance. However, the identity of such a neuron-supplied niche factor(s) remains unclear. Here, by mining a published RNA-sequencing dataset of geniculate ganglion neurons and by in situ hybridization, we demonstrate that R-spondin-2, the ligand of Lgr5 and its homologs Lgr4/6 and stem-cell-expressed E3 ligases Rnf43/Znrf3, is expressed in nodose-petrosal and geniculate ganglion neurons. Using the glossopharyngeal nerve transection model, we show that systemic delivery of R-spondin via adenovirus can promote generation of differentiated taste cells despite denervation. Thus, exogenous R-spondin can substitute for neuronal input for taste bud cell replenishment and taste bud maintenance. Using taste organoid cultures, we show that R-spondin is required for generation of differentiated taste cells and that, in the absence of R-spondin in culture medium, taste bud cells are not generated ex vivo. Thus, we propose that R-spondin-2 may be the long-sought neuronal factor that acts on taste stem cells for maintaining taste tissue homeostasis.
The sense of taste allows humans and other animals to test a food before ingesting it. It helps distinguish nutritional foods with an attractive taste (e.g., sweet, umami) from potentially harmful or toxic food items with an aversive taste (e.g., sour, bitter) (1–4). In the oral cavity, tastants (chemicals in food) are detected by specific receptors expressed in taste cells that are clustered in taste buds, onion-shaped structures found in the tongue and soft palate in the oral cavity (1–4). Taste buds are the basic units for processing taste information peripherally; each comprises a heterogeneous population of about 50 to 100 taste cells (1–4). Like the surrounding epithelial cells, taste bud cells undergo constant turnover throughout life; they are replenished by new cells generated from adult taste stem/progenitor cells, such as Lgr5+ and/or Lgr6+ cells (5–9).
Taste bud cells are innervated by gustatory neurons that relay taste information to the brain to initiate taste sensation/perception. Intact gustatory nerves are also essential for taste stem cells to generate new cells to maintain the integrity of taste buds. More than a century ago it was demonstrated that transection of the gustatory nerves leads to degeneration of the taste buds they innervate (10, 11). The restoration of taste buds occurs only after reinnervation by gustatory ganglion neurons (12, 13). Thus, neuronal input is required for taste bud cell replenishment and taste bud maintenance. This most likely involves a neuron-supplied niche factor(s) that regulates adult taste stem/progenitor cell activity, but the identity of such a factor(s) is unknown.
Recent work has suggested that sonic hedgehog (Shh) is a key neuron-supplied factor for direct patterning of taste organ regeneration (14). However, Shh is produced by both taste cells and gustatory neurons. Castillo-Azofeifa et al. (15) showed that disturbance of taste bud homeostasis occurs only when both sources of Shh, from gustatory neurons and from taste cells, are eliminated. Furthermore, in an ex vivo organoid culture system we developed, no exogenous Shh is required for taste stem/progenitor cells to produce mature taste cells. Thus, despite the requirement of Shh signaling for taste bud maintenance (16–19), Shh itself may not be the principal gustatory-neuron-produced factor for maintenance of taste buds and regeneration of taste bud cells.
To search for the principal gustatory-neuron-produced factor, we focused on the Lgr5 pathway because of the expression of Lgr4/5/6 in taste stem/progenitor cells (20). We asked if the ligand of Lgr4/5/6, R-spondin, may act as the principal gustatory-neuron-produced factor for regulating taste tissue homeostasis. Mechanistically, Lgr5 and its analogs (Lgr4/Lgr6) interact with all four R-spondin proteins promiscuously to regulate activity of stem cells (e.g., intestinal Lgr5+ stem cells) (21). Aside from Lgrs, all four R-spondin proteins also interact with the stem-cell-expressed E3 ligases Rnf43/Znrf3 to neutralize their ligase activity (21).
Here, by mining of the published single-cell RNA-sequencing (RNAseq) data of gustatory neurons (22), we show that Rspo2 is predominantly expressed in gustatory neurons, and we validate the expression of Rspo2 in gustatory neurons by in situ hybridization and qPCR. We demonstrate that systemic supply of exogenous Rspo1 and Rspo2 via adenovirus can maintain taste bud integrity after gustatory nerve (glossopharyngeal) transection and that cultured taste organoids require R-spondin (either Rspo1 or Rspo2) to produce taste cells. Our work suggests that R-spondin (primarily Rspo2) may be the neuron-produced factor that regulates Lgr5+- and/or Lgr6+-expressing adult taste stem/progenitor cell activity to maintain taste tissue homeostasis.
Results
Rspo2 Is Expressed in Gustatory Ganglion Neurons.
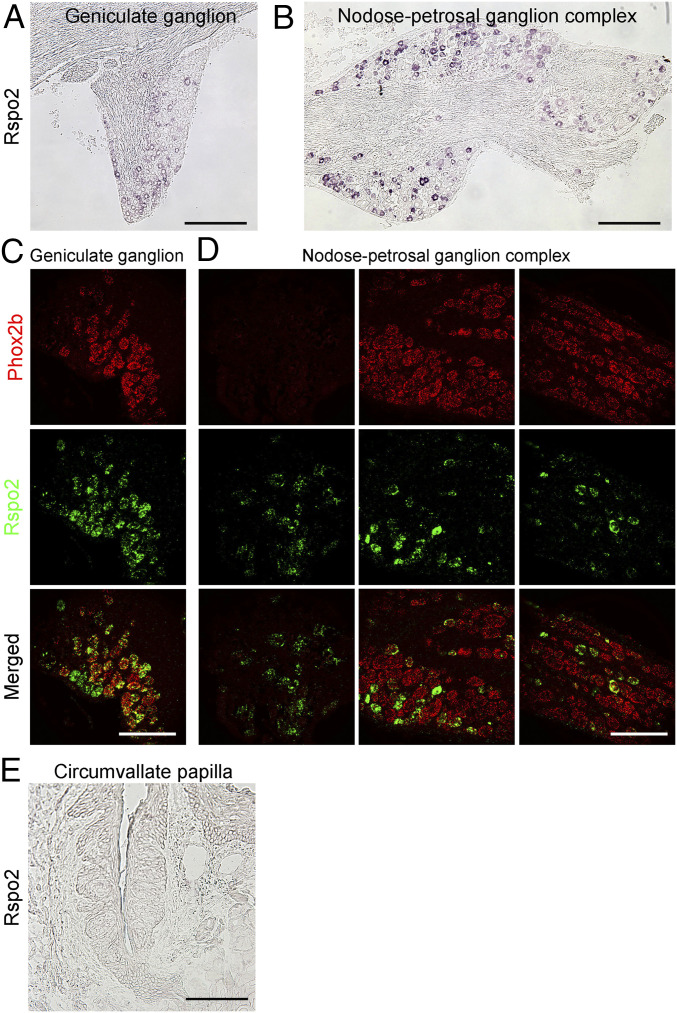
There are four R-spondin homologs in mice; all can bind to Lgr4/5/6 or Znrf3/Rnf43 promiscuously to regulate stem cell activity (21). To examine whether any of these R-spondins are expressed in gustatory ganglion neurons, we took advantage of a recently published single-cell RNAseq dataset of geniculate gustatory neurons (22). This dataset indicated that Rspo2 is expressed predominantly in Phox2b+ cells, which are believed to innervate taste tissues (SI Appendix, Fig. S1). To validate the RNAseq data, we performed in situ hybridization analyses using Rspo2 antisense probes. As expected, a subset of cells in the geniculate ganglion and the nodose-petrosal ganglion complex showed positive signals (Fig. 1 A and B). Double in situ hybridization using Rspo2 and Phox2b (Fig. 1 C and D) or P2rx2 (encoding P2X2, SI Appendix, Fig. S2) antisense probes confirmed their colocalization in a subset of neurons in these ganglia. P2X2 is expressed in gustatory ganglion neurons and forms a heterodimeric channel with P2X3 for detecting taste-cell-released ATP (23). Furthermore, we performed qPCR analyses of all four R-spondins using RNA extracted from the geniculate ganglion and nodose-petrosal ganglion complex, demonstrating that Rspo2 is strongly expressed in these ganglia (SI Appendix, Fig. S3). Slightly different from single-cell RNAseq data, other R-spondins can be detected at variable amounts by qPCR, suggesting they may be expressed in other types of cells in geniculate and nodose-petrosal ganglia (SI Appendix, Fig. S3).
Fig. 1.
Expression of Rspo2 in gustatory ganglion neurons. (A and B) In situ hybridization with Rspo2 antisense probes shows that Rspo2 is expressed in the geniculate ganglion (A) and in the nodose-petrosal ganglion complex (B). Images are Nomarski images. (C and D) Representative images of dual in situ hybridization with Rspo2 and Phox2b antisense probes show colocalization of Phox2b (red, Top) and Rspo2 (green, Middle) in a large set of neurons in the geniculate ganglion (C) and nodose-petrosal ganglion complex (D); three adjacent fluorescent images were taken to show the whole structure of the nodose-petrosal ganglion complex. (E) In situ hybridization with Rspo2 antisense probes shows there is no positive signal in any cells in the circumvallate papilla (Nomarski image). (Scale bars: 200 μm for A and B; 100 μm for C, D, and E.)
Shh is proposed to be a neuronal-derived factor that specifies taste bud patterning (14). However, Shh is supplied both locally by taste cells and remotely by ganglion neurons (14, 15). To address if Rspo2 could also be supplied locally by taste cells or the surrounding mesenchymal cells, we performed in situ hybridization analysis of the circumvallate papilla. No signal was detected in any cells in the circumvallate papilla (Fig. 1E). This is consistent with no reads of Rspo2 or no or very low expression of other R-spondins in the RNAseq data obtained from taste organoids or taste cells (24, 25). Therefore, unlike Shh, Rspo2 is principally supplied by gustatory neurons.
Recombinant Rspo1 Is Sufficient for Generation of Differentiated Taste Cells and Taste Bud Maintenance in the Glossopharyngeal Nerve Transection Model.
Glossopharyngeal nerve transection (GLx) leads to taste bud degeneration gradually (9, 10). No or few taste bud cells are present in the circumvallate papilla about 2 wk after nerve transection, presumably due to the inability to generate new taste cells from adult taste stem cells to replace senescent cells (9). We and others have shown that Lgr5 marks adult taste stem/progenitor cells (8, 9). Lgr5+ cells also contribute to taste cell regeneration after reinnervation in GLx models (9). Further, isolated Lgr5+ or Lgr6+ cells can give rise to taste bud cells in ex vivo taste organoid culture (20, 25, 26) and the Lgr4/5/6 ligand, Rspo2, is expressed in the gustatory neuron. Thus, we hypothesized that the Lgr4/5/6 ligand R-spondin could be the gustatory-neuron-produced factor that acts on taste stem-cell-expressed Lgrs to promote generation of differentiated taste cells. Nerve transection may lead to deprivation of gustatory-neuron-produced R-spondin.
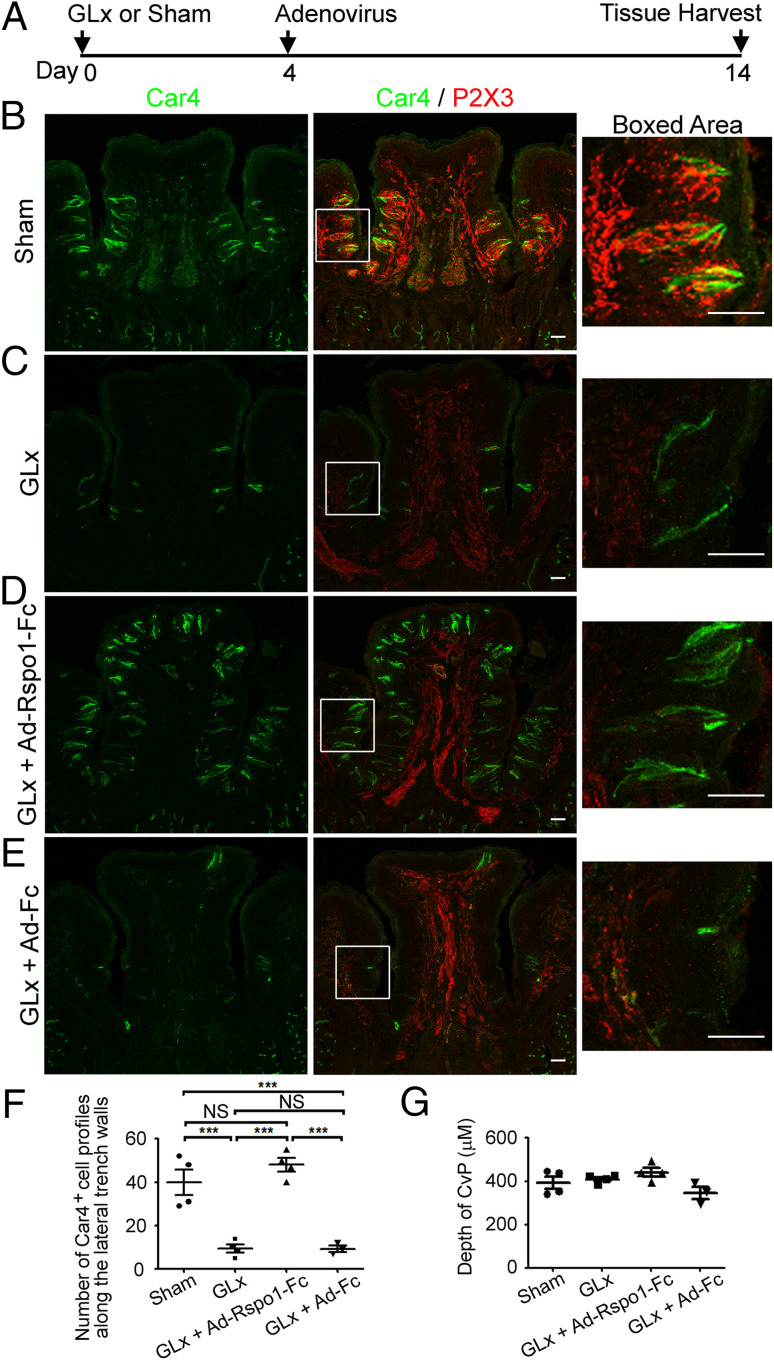
To test this hypothesis, we first established the GLx model in our laboratory (Fig. 2A and SI Appendix, Fig. S4A illustrate the experimental design). We performed immunostaining using antibodies against taste cell markers Car4, Krt8, and Plcβ2 to evaluate the number of remaining taste bud cells 2 wk after GLx. As expected, compared to sham-operated mice (Fig. 2B and SI Appendix, Fig. S4B), few taste bud cells were detected in the circumvallate papilla in the GLx mice (Fig. 2C, Car4+ cells, and SI Appendix, Fig. S4C, Krt8+ and Plcβ2+ cells). To directly validate nerve transection, we also stained circumvallate papilla sections with an antibody against P2X3 (23). P2X3+ signal with a typical nerve terminal staining pattern was readily detected in taste buds in the sham-operated mice (Fig. 2B, red). In contrast, in the GLx mice P2X3+ signal was detected only in the nerve-fiber-like structure in the mesenchymal core, with almost no signal detected in taste buds in the circumvallate papilla (Fig. 2C, red). This observation suggests that nerve terminal retraction or degradation occurred in the GLx mice. Thus, lack of P2X3 staining within taste buds in the circumvallate papilla is a direct indicator of GLx.
Fig. 2.
Exogenous R-spondin-1 promotes generation of differentiated taste cells and taste bud maintenance in the GLx model. (A) Schematic of the experiment design. Adenoviral infection was performed on day 4 after GLx. (B–E) Representative confocal images of immunostaining of circumvallate papilla sections. (Left) Car4 staining (green). (Middle) Merged images of Car4 staining (green) and P2X3 staining (red). (Right) The boxed areas in Middle. (B) Sections from sham-operated mice show typical Car4+ cells and P2X3+ nerve terminal web in taste buds. (C) Sections from GLx mice. Note the few Car4+ cells in the circumvallate papilla and no P2X3+ nerve terminal web in the circumvallate papilla but prominent P2X3-immunoreactive signals in the nerve trunk in the mesenchymal core. (D) Sections from GLx mice infected with Ad-Rspo1-Fc. Note the numerous Car4+ cells in taste buds in the circumvallate papilla and no P2X3+ staining in taste buds in the circumvallate papilla but prominent P2X3-immunoreactive signals in the nerve trunk in the mesenchymal core, a typical feature of GLx. (E) Sections from GLx mice infected with control adenovirus (Ad-Fc). The staining pattern is essentially the same as in mice with GLx alone. All images were acquired using the same settings. (Scale bars: 50 μm.) (F) Statistical analysis of Car4+ cell profiles along the lateral trench walls of the circumvallate papilla (CvP) from sham-operated, GLx, GLx + Ad-Rspo1-Fc, and GLx + Ad-Fc mice. Significant differences were noted among different treatment groups (***P < 0.001), with the exception of GLx vs. GLx + Ad-Fc and sham vs. GLx + Ad-Rspo1-Fc (NS, not significant). Each point represents a single mouse. Four mice were used for each experiment, except for control adenovirus Ad-Fc infection (three mice). (G) The depth of the CvP, representing the distance from the roof top to the bottom of the trench area. No significant differences were detected among four different treatment groups.
Using this model, we then examined the effects of providing exogenous R-spondin after nerve transection. Previously, it was shown that systemic delivery via adenovirus of mouse R-spondin-1 (or human R-SPONDIN-2) tagged with the Fc fragment (Rspo1-Fc) can promote intestinal epithelial tissue hyperplasia via its interaction with Lgr5 or Znrf3/Rnf43, suggesting the effectiveness of this approach for systemic delivery of exogenous R-spondin (27–29). Thus, we infected GLx mice with the same adenovirus encoding Rspo1-Fc (Ad-Rspo1-Fc) at a dose effective to promote gut hyperplasia (3 × 108 plaque-forming units [pfu] per mouse) or Fc alone (Ad-Fc as control, 3 × 108 pfu per mouse) on day 4 after GLx and examined the circumvallate papillae on day 14 after GLx. Consistent with previous observations, we observed intestinal hypertrophy after Ad-Rspo1-Fc infection but not after Ad-Fc infection (SI Appendix, Fig. S5), demonstrating the effectiveness of this adenovirus-based approach to produce exogenous R-spondin in our hands.
To examine whether exogenous Rspo1 can substitute for neuronal input to promote generation of differentiated taste bud cells, we used the taste bud cell markers Car4, Krt8, and Plcβ2, along with the taste nerve marker P2X3, to immunostain sections of circumvallate papilla from the GLx mice infected with Ad-Rspo1-Fc (3 × 108 pfu). We observed numerous taste bud cells immunoreactive for Car4 (Fig. 2D) or Krt8 or Plcβ2 (SI Appendix, Fig. S4D) in the circumvallate papilla of all the infected GLx mice. Notably, taste cells were less organized in these mice compared to sham-operated mice and also appeared in the upper cleft region and dorsal surface of the circumvallate papilla (Fig. 2D and SI Appendix, Fig. S4D), which normally lacks taste cells. In contrast, GLx mice receiving Ad-Fc (3 × 108 pfu) showed no or few taste cells in the circumvallate papilla (Fig. 2E and SI Appendix, Fig. S4E), similar to GLx mice. As expected, no or little P2X3 staining was found in taste epithelium of the circumvallate papilla in the GLx mice infected with either Ad-Rspo1-Fc or Ad-Fc (Fig. 2 D and E), suggesting that Rspo1 does not promote nerve regeneration.
To quantify the differences in numbers of taste cells, we counted Car4+ cell profiles along the lateral trench walls (ectopic Car4+ cells in the dorsum of the circumvallate papilla were excluded from counting for a direct comparison of different groups); GLx mice treated with Ad-Rspo1-Fc had about the same number of Car4+ cell profiles as sham-operated mice and many more Car4+ cell profiles compared to untreated GLx mice or GLx mice infected with control adenovirus Ad-Fc (Fig. 2F). Despite significantly more taste bud cells in GLx mice treated with Ad-Rspo1-Fc, no differences were found in the depth of the circumvallate papilla among these four treatment groups (Fig. 2G), indicating that Rspo1 does not promote enlargement of the circumvallate papilla. The ability of Rspo1 to promote generation of differentiated taste cells in the absence of innervation suggests that R-spondin may be the neuron-produced factor that regulates taste tissue homeostasis.
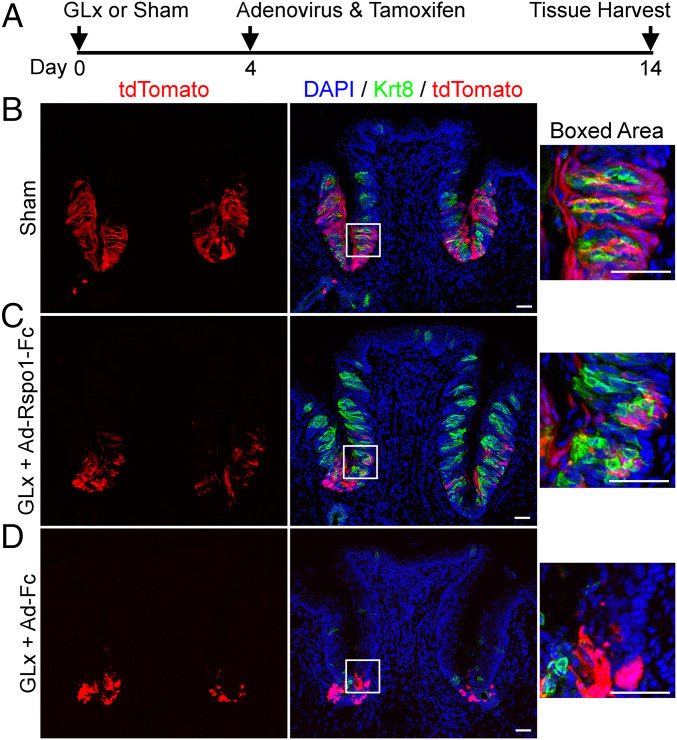
Similar results were obtained using Lgr5EGFP−IRES-CreERT2/+;Rosa26tdTomato mice (Fig. 3 and SI Appendix, Fig. S6). For those mice, we also performed a short-term lineage tracing of Lgr5+ cells (a single injection of tamoxifen at the same day [day 4] that mice were infected with adenovirus) (Fig. 3A and SI Appendix, Fig. S6A). Ten days later (day 14 after GLx) we examined cells positive for tdTomato, which identified Lgr5+ cells and their progeny in these Lgr5EGFP−IRES-CreERT2/+;Rosa26tdTomato mice. As reported previously (8, 9), many tdTomato+ cells were found in the circumvallate papilla in the sham-operated mice, including intragemmal cells [within taste buds, demarcated by Krt8 staining (Fig. 3B) or P2X3 staining (SI Appendix, Fig. S6B)]. Takeda et al. (9) showed that GLx leads to reduced expression of Lgr5-EGFP (enhanced green fluorescent protein). Consistent with this, we found fewer tdTomato+ cells in GLx mice infected with Ad-Rspo1-Fc using this dosing regimen. Nevertheless, tdTomato+ cells were also found to be intragemmal cells (Fig. 3C and SI Appendix, Fig. S6C), suggesting that Lgr5+ cells contribute to R-spondin-promoted taste tissue homeostasis after GLx. In contrast, many fewer cells were marked by tdTomato, and none of those appeared to be intragemmal cells (remnant taste buds revealed by Krt8 staining; Fig. 3D) in the circumvallate papilla of GLx + Ad-Fc mice (Fig. 3D and SI Appendix, Fig. S6D). The lack of tdTomato+ intragemmal cells in GLx + Ad-Fc mice is consistent with the fact that taste bud cells are not replenished and degeneration of taste buds occurs after nerve transection.
Fig. 3.
Lgr5+ cells contribute to R-spondin-promoted taste tissue homeostasis in GLx mice. (A) Schematic of the experimental design. Adenoviral infection and tamoxifen induction were performed on day 4 after GLx. (B–D) Lineage tracing of Lgr5+ cells in Lgr5EGFP−IRES-CreERT2/+;Rosa26tdTomato mice. The boxed areas (Right) highlight cells positive for tdTomato (red; Left) within a taste bud demarcated by Krt8 staining (green; Middle), counterstained with DAPI. (B) Sham-operated mice. Note the presence of tdTomato+ cells within taste buds and outside taste buds in the circumvallate papilla 10 d after tamoxifen induction, consistent with what was reported previously (8, 9). (C) GLx mice infected with Ad-Rspo1-Fc. Similar to sham-operated mice, tdTomato+ cells are visible both in and outside taste buds 10 d after tamoxifen induction. (D) GLx mice infected with control adenovirus (Ad-Fc). Few Krt8+ cells are found in the circumvallate papilla. A few tdTomato+ cells are visible in the trench area but do not show typical taste cell morphology and are not present in Krt8-demarcated remnant taste buds. Due to degeneration of taste bud cells, only residual Krt8 immunoreactivity can be detected in taste bud remnants. Images were acquired using the same setting. Each experiment was performed using two mice, except sham operated (three mice). (Scale bars: 50 μm.)
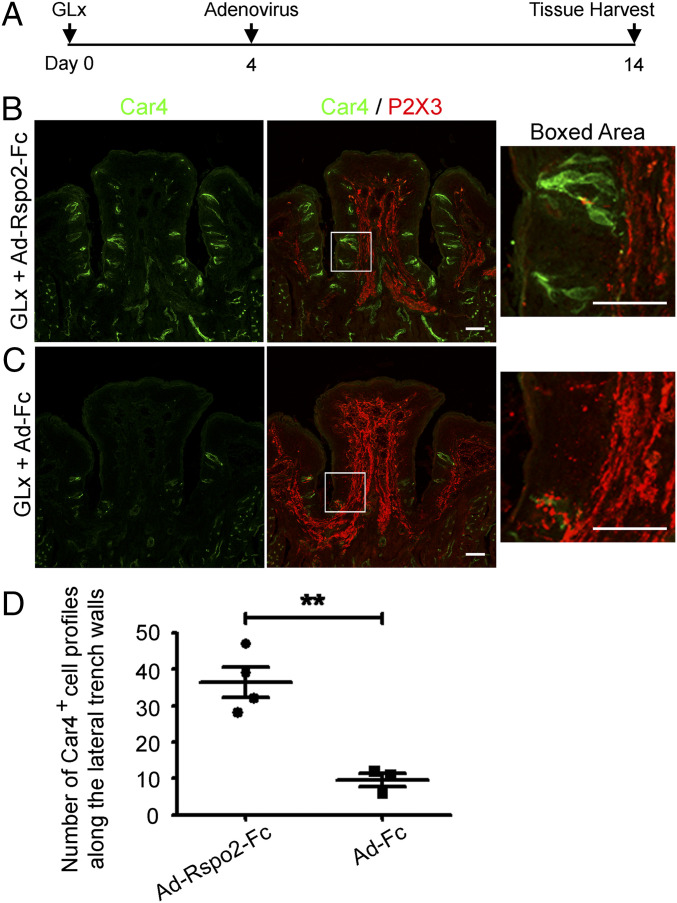
Exogenous Rspo2 Is Sufficient for Generation of Differentiated Taste Cells and Taste Bud Maintenance after GLx.
To determine whether Rspo2 can also substitute for neuronal input for generation of differentiated taste cells, we infected mice with adenovirus encoding mouse Rspo2-Fc (1 × 109 pfu) using the same procedure as for Ad-Rspo1-Fc (Fig. 4A and SI Appendix, Fig. S7A). Consistent with what we observed for Ad-Rspo1-Fc, we noted numerous taste bud cells immunoreactive for Car4 (Fig. 4B) or for Krt8 or Plcβ2 (SI Appendix, Fig. S7B) in the circumvallate papilla of all the GLx mice infected with Ad-Rspo2-Fc, suggesting Rspo2 can also promote generation of differentiated taste cells in the absence of neural input. As expected, GLx mice receiving control Ad-Fc at a high titer (1 × 109 pfu) showed taste bud degeneration (Fig. 4C and SI Appendix, Fig. S7C). No or little P2X3 staining was found in taste epithelium of the circumvallate papilla in the GLx mice infected with either Ad-Rspo2-Fc or Ad-Fc (Fig. 4 B and C). Counting of Car4+ cell profiles along the lateral trench walls of the circumvallate papilla showed a significant difference between these two groups (P < 0.01; Fig. 4D). This set of data further supports the idea that R-spondin promotes generation of differentiated taste cells.
Fig. 4.
Generation of differentiated taste cells by Ad-Rspo2-Fc in the GLx mouse model. (A) Schematic of the experiment design. Adenoviral infection was performed on day 4 after GLx. (B) Immunostaining of sections from GLx mice infected with Ad-Rspo2-Fc shows numerous Car4+ cells (green) but no P2X3+ nerve terminal web (red) in taste buds in the circumvallate papilla. (C) Immunostaining of sections from GLx mice infected with control adenovirus (Ad-Fc) shows few Car4+ cells and no P2X3+ nerve terminal web. Images were acquired using the same settings. (Scale bars: 50 μm.) (D) Statistical analysis of Car4+ cell profiles along the lateral trench walls of the CvP from GLx + Ad-Rspo2-Fc and from GLx + Ad-Fc mice. Significant differences were noted between these two groups (**P < 0.01). Each point represents a single mouse. Four mice were used for Ad-Rspo2-Fc infection, and three mice were uses for control Ad-Fc infection.
Sham-Operated Mice Treated with Ad-Rspo1-Fc or Ad-Rspo2-Fc Show Essentially the Same Pattern of Taste Cell Distribution as GLx Mice Infected with These Viruses.
We also infected sham-operated mice with Ad-Rspo1-Fc and Ad-Rspo2-Fc (SI Appendix, Figs. S8A and S9A). In both cases, numerous Car4+ taste cells (SI Appendix, Fig. S8 B and C) and Plcβ2+ and Krt8+ cells (SI Appendix, Fig. S9 B and C) were found in the lateral trench walls, upper cleft region, and dorsal surface of the circumvallate papilla, resembling the pattern observed in the GLx mice treated with the viruses but differing from the normal pattern observed in sham-operated mice infected with control adenovirus Ad-Fc (SI Appendix, Figs. S8D and S9D). As expected, strong P2X3+ nerve terminals were detected in taste buds in these sham-operated (no nerve transection) mice (SI Appendix, Fig. S8 B–D). These results provide additional evidence that R-spondin promotes generation of differentiated taste cells (e.g., ectopic taste cells in the dorsal surface of the circumvallate papilla).
R-Spondin Is Required for Generation of Differentiated Taste Cells in Cultured Taste Organoids.
We previously demonstrated that taste stem cells (e.g., Lgr5+ or Lgr6+ cells) give rise to mature taste cells in cultured organoids using the standard organoid culture medium, including epidermal growth factor, Noggin, and R-spondin (Rspo1 or Rspo2), for growing taste organoids (20, 25). More recently, we also supplemented the medium with Wnt3a-conditioned medium for culturing taste organoids (25). Despite the fact that Noggin and Wnt3a can affect generation of differentiated taste cells, we can always detect some taste cells even in taste organoids in the absence of Wnt3a or Noggin (25).
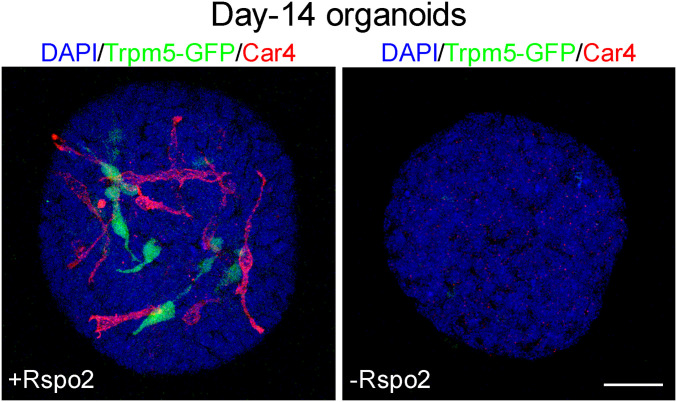
Because R-spondin can fully rescue taste cell replenishment in vivo in the absence of nerve input, we asked if generation of differentiated taste cells requires R-spondin. We used the ex vivo organoid culture system to address this question. We dissociated cells from circumvallate papilla and cultured them in the standard organoid culture medium in the presence or absence of Rspo2 (three independent experiments using Rspo2-conditional medium and wild-type and Trpm5-GFP mice and two independent experiments using a commercial Rspo2 recombinant protein and Trpm5-GFP mice). In the presence of Rspo2, we reproducibly detected Car4+ cells or Trpm5-GFP+ cells in cultured organoids (Rspo2-conditional medium and Car4+ organoids: 10 out of 107, 10 out 80, 12 out of 26 organoids; commercial Rspo2 and Car4+ organoids: 15 out 40 and 42 out of 75), as reported previously (25) (Fig. 5). In contrast, in the absence of R-spondin but with all other culture ingredients unchanged, none of the organoids from five independent experiments (out of 75, 81, 10, 22, and 30 organoids, respectively) contained any Car4+ cells or Trpm5-GFP+ cells (Fig. 5). A χ2 test showed a significant difference between the two groups (P < 0.001, χ2 = 70.6723, degrees of freedom = 1). Thus, R-spondin is required for generation of differentiated taste cells at least in the ex vivo condition.
Fig. 5.
R-spondin is required for generation of differentiated taste cells in cultured taste organoids. Organoids were grown from dissociated cells from the circumvallate papilla region of Trpm5-GFP mice in the presence (Left) or absence (Right) of R-spondin (Rspo2). Trpm5+ cells and Car4+ cells are visible in organoids cultured in the presence of Rspo2 (Left). Not a single Trpm5+ or Car4+ cell was detected in organoids cultured in the absence of Rspo2 (Right). Experiments were repeated five times using either Trpm5-GFP or wild-type B6 mice. (Scale bar: 25 μm.)
Discussion
Although the dependence of taste tissue homeostasis on gustatory neurons was noted more than a century ago, the exact gustatory-neuron-produced factor required for taste tissue maintenance remains unclear. In this study, we have demonstrated that Rspo2 is robustly expressed in gustatory ganglion neurons. Additionally, we have demonstrated that exogenous R-spondin promotes generation of differentiated taste cells in vivo in the circumvallate papilla in the GLx model and ex vivo in the taste organoid culture system derived from dissociated cells from the circumvallate papilla region. These data suggest that Rspo2 may be the principal neuron-produced factor for regulating taste tissue homeostasis.
An interesting observation of GLx mice with or without viral infection is that, despite a virtually complete loss of P2X3 staining in the taste epithelium on day 14 after GLx, there is strong P2X3 staining in the mesenchymal core of the circumvallate papilla, which we call a “nerve-fiber-like structure.” The exact nature of the structure is unclear. One possibility is that this nerve-fiber-like structure may represent regenerating gustatory nerve fibers yet to reach taste epithelium, as reinnervation occurs gradually and slowly in the GLx model. Indeed, after GLx at least two processes take place gradually: denervation on the taste bud side and reinnervation by gustatory neurons making new nerve fibers or extending the proximal end of the nerve fibers still connecting to the cellular body (9, 10, 12, 13, 30). Regardless, on day 14 after GLx the loss of taste cells was profound in the GLx and GLx + Ad-Fc mice, and there were virtually no P2X3+ nerve terminals in the taste epithelium in these mice, consistent with a predominant effect of denervation and very little effect of reinnervation at this stage.
The fact that a single recombinant protein can fully restore taste tissue homeostasis after denervation supports the idea that gustatory neurons produce R-spondin to promote generation of differentiated taste cells. Mechanistically, the ability of R-spondin to promote generation of differentiated taste cells appears to be somewhat different from the effect of R-spondin on intestinal stem cells. In the gut, infecting mice with Ad-Rspo1-Fc or Ad-RSPO2-Fc, which produce exogenous secreted Rspo1 and human RSPO2, respectively, promotes crypt hyperplasia, likely by actively driving and specifying the extent of stem cell expansion (29). In the taste system, it appears that R-spondin promotes taste stem/progenitor cells to generate mature taste cells.
The presence in taste buds of cells positive for tdTomato, which marks Lgr5+ stem/progenitor cells and their progeny, demonstrated that Lgr5+ cells may contribute to taste tissue homeostasis in the GLx model after Ad-Rspo1-Fc infection. However, we found fewer tdTomato+ cells in this model compared to mice without GLx. This is most likely due to reduced expression of Lgr5 after denervation (9), which may lead to reduced efficiency of lineage tracing in the GLx Lgr5EGFP−IRES-CreERT2 mice; for example, fewer Lgr5+ cells may be activated by tamoxifen, which was given at about the same time that Ad-Rspo1-Fc was administered.
In concordance with the finding that exogenous R-spondin is sufficient to maintain taste tissue homeostasis in GLx mice, our ex vivo data demonstrated that R-spondin is required for generation of differentiated taste cells. We showed that when cultured in the absence of R-spondin, organoids still grew but produced no taste cells. Therefore, at least in this ex vivo system, R-spondin is required for generation of differentiated taste cells. Interestingly, unlike taste organoids, intestinal organoids do not grow in the absence of R-spondin, suggesting somewhat distinct roles of R-spondin in regulating self-renewal and differentiation of taste stem cells and intestinal stem cells (27, 31).
R-spondin proteins (Rspo1 to Rspo4) engage Lgr4/5/6- and stem-cell-expressed Rnf43/Znrf3 promiscuously (21). The four secreted R-spondin proteins are members of a much larger family of proteins featuring the presence of two furin-like cysteine-rich domains (21). R-spondin interacts with Lgr4/5/6 via the furin-2 domain and with Rnf43/Znrf3 via the furin-1 domain. The ternary interactions enable R-spondins to potentiate Wnt signaling (21). Besides the expression of Lgrs in adult taste stem/progenitor cells (8, 9), transcriptome analysis of primate taste bud cells demonstrated the presence of both Rnf43 and Znrf3 in the taste tissue (24). Therefore, it is likely that, similar to other epithelial stem cells, the ability of R-spondin to promote generation of differentiated taste cells is mediated by tethering of its distinct receptors Lgr4/5/6 and Rnf43/Znrf3, thus allowing R-spondin–Lgr complexes to neutralize E3 ligases and to boost Wnt signaling (21, 32, 33). The expression of Lgr5-EGFP is reduced after nerve transection (9), suggesting that other Lgrs [all three Lgrs are found in Lgr5+ taste stem/progenitor cells (20)] may have redundant roles in mediating the effect of exogenous Rspo1 or Rspo2 to promote generation of differentiated taste cells in the GLx model.
Besides the presence of taste bud cells along the lateral trench walls of the circumvallate papilla in GLx or sham-operated mice receiving Ad-Rspo1-Fc or Ad-Rspo2-Fc, ectopic taste buds were frequently observed in the upper cleft region or dorsal surface of the circumvallate papilla as well. Lgr5-GFP+ stem/progenitor cells and their progeny had not been observed in these regions (8, 9, 20). For generation of those ectopic taste bud cells, systemically supplied R-spondin via adenovirus most likely acted on Lgr5– but potentially acted on Lgr4/6+ and Znrf3/Rnf43+ taste progenitor cells in those regions. In contrast, in sham-operated mice without treatment or treated with control adenovirus Ad-Fc, taste buds in the circumvallate papilla may be restricted to the lateral trench walls through activation of Lgr5+ stem/progenitor cells by neuronal delivery of Rspo2 from nerve terminals, which only occasionally innervate the upper cleft region or dorsal surface of the circumvallate papilla.
Consistent with the idea of the redundant roles of Lgrs, deletion of Lgr5 in intestinal stem cells results in mice with no apparent altered phenotype, but simultaneous deletion of Lgr4 and Lgr5 can lead to dystrophy of the intestinal epithelium, suggesting functional redundancy of Lgrs (32). Furthermore, double deletion of Rnf43/Znrf3 in the intestinal epithelium of mice induces rapidly growing adenomas containing high numbers of Paneth and Lgr5+ stem cells, which were not noted in mice deficient for either Rnf43 or Znrf3 alone in the gut (34). Given the similarity between taste and gut stem cells, it would be of interest to determine how the ternary interaction among R-spondin, Lgr4/5/6, and Rnf43/Znrf3 contributes to taste tissue homeostasis mechanistically.
Even though all four R-spondin proteins can engage Lgr4/5/6 and Rnf43/Znrf3, they do not behave exactly the same (21). For instance, in vitro work demonstrated that human RSPO2 and RSPO3 can, but RSPO1 and RSPO4 cannot, potentiate WNT signaling without LGRs (35, 36). Regardless, the ability of both Ad-Rspo1-Fc and Ad-Rspo2-Fc to promote generation of differentiated taste cells in the absence of nerve input suggests that R-spondin is presumably the neuronal factor for maintaining taste tissue homeostasis.
Our mining of a published RNAseq dataset of geniculate ganglion neurons showed a near-complete match of Rspo2 and Phox2b, which marks gustatory sensory neurons (22). Our in situ hybridization work confirmed the expression of Rspo2 in neurons in both the nodose-petrosal ganglion complex and the geniculate ganglion but not in taste tissue or the underlying mesenchymal tissue in the circumvallate papilla. Our two-color in situ data showed that Rspo2 and Phox2b or P2X2 are largely colocalized. Thus, Rspo2 appears to be neuron specific and is expressed in gustatory sensory neurons in the geniculate ganglion and the nodose-petrosal ganglion complex. Furthermore, RNAseq data suggested that other R-spondins may not be expressed or are expressed at low levels in only a small set of sensory neurons in the geniculate ganglion (22). However, our qPCR analysis indicated that Rspo3 appears to be expressed at a level slightly less than Rspo2 in these ganglia and that transcripts for Rspo1 and Rspo4 are also detectable, albeit at a much lower level. Because qPCR used the entire geniculate ganglion or the nodose-petrosal ganglion complex, the exact cell type(s) for expressing these additional R-spondins cannot be determined. Given the results of the single-cell RNAseq analysis and our qPCR analysis of gustatory ganglia, it seems most likely that these other R-spondins may be expressed in cell types other than sensory neurons, such as glia. However, it is possible that single-cell RNAseq or other insensitive methods could fail to detect a low level of expression of other R-spondins in a large population of neurons in gustatory ganglia. Taken together, this set of data indicates that out of all four R-spondin proteins, Rspo2 is likely the long-sought neuron-produced factor that regulates taste tissue homeostasis. However, we cannot completely exclude the potential expression of other R-spondins in neurons and the possibility of their contribution to the maintenance of taste buds.
In a transgenic model that disrupts the expression of R-spondin-2, mice show perinatal lethality (37). Therefore, genetic ablation of Rspo2 in ganglion neurons would be required to determine the exact role of neuron-produced Rspo2 in regulating taste tissue homeostasis. Studies using this loss-of-function approach will complement our gain-of-function approach reported here (exogenous Rspo1 and Rspo2 produced by Ad-Rspo1-Fc and Ad-Rspo2-Fc infection) to elucidate the role of R-spondin, in particular neuron-produced Rspo2, in taste bud cell regeneration. We are actively pursuing this line of inquiry.
Recently, Lu et al. (14) proposed that neuronal delivery of Shh directs spatial patterning of taste organ regeneration. In contrast, Castillo-Azofeifa et al. (15) showed that disturbance of taste bud homeostasis occurs only when both sources of Shh, from gustatory neurons and from taste cells, are ablated. Regardless of the role of neuron-produced Shh in maintaining taste tissue homeostasis, it has been well established through the work of many others that the Shh signaling pathway is required for taste tissue homeostasis (17–19). For instance, conditional ablation of Gli2, the transcription factor for the Shh pathway, or Smo, the receptor for Shh, in taste progenitor cells (e.g., Krt5+, Krt14+) results in loss of taste bud cells (16). Small molecules developed to block this pathway can also affect taste in mice and in patients (14, 17). Additionally, genetically constitutive expression of Shh induced ectopic taste buds (18). This evidence indicates that the Shh pathway is essential for taste cell generation and maintenance. Nevertheless, that simply providing GLx mice with R-spondin is sufficient to maintain taste tissue homeostasis in the circumvallate papilla suggests the R-spondin–Lgr4/5/6–Rnf43/Znrf3 axis plays a key role for maintaining taste tissue homeostasis. Moreover, the facts that a rapid and great reduction of Shh signaling components such as Ptc and Shh has been noted in GLx mice (38) and that exogenous Shh is not required for culturing taste organoids (20, 25) suggest that the Shh pathway may act downstream of the R-spondin–Lgr4/5/6–Rnf43/Znrf3 axis. How these two pathways interact with each other merits further exploration.
Furthermore, our study focuses on neuronal regulation of taste tissue homeostasis in the circumvallate papilla; whether taste tissue homeostasis in other taste fields (e.g., fungiform papillae, taste buds in the soft palate, the upper esophagus, the cheek, and epiglottis) is regulated by the same mechanism remains to be characterized. However, given the expression of Rspo2 in the geniculate ganglion, it seems likely that R-spondin would play a similar role in other taste organs as well.
Materials and Methods
Virus Production, Packaging, Purification, and Titering.
Adenoviral vectors Ad-Rspo1-Fc and Ad-Fc were obtained from Calvin Kuo (Stanford University, Stanford, CA). A protocol from Clontech was followed for production and packaging of adenovirus (Takara Bio, PT5177-1). Purification of adenovirus was performed using the Add-N-Pure Purification Kit from Applied Biological Materials Inc. (catalog number A910). Adenoviral particles were dialyzed and then further concentrated using commercial filters (Millipore Sigma, Amicon Ultra-4, reference number UFC805024) to a degree sufficient for in vivo work (>2 × 109 pfu/mL). Adenovirus titering was performed using 5% low-melting agarose gel following a protocol from Kuo’s laboratory to determine the number of functional adenovirus plaques with serially diluted viral particles. Adenovirus encoding Ad-Rspo2-Fc (the entire coding region of Rspo2 fused with the Fc fragment of mIgG2a, which is used in Ad-Rspo1-Fc and Ad-Fc) was produced, packaged, and purified using the custom construction service offered by Vector Biolabs and further titered using the same procedure described for Ad-Rspo1-Fc and Ad-Fc.
Details about nerve transection and viral infection, immunostaining and imaging, cell profile counting, organoid cultures, in situ hybridization, and qPCR analysis are described in SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
We thank all members of the P.J. laboratory for their input. Research reported in this publication was supported by NIH Grants DC010842 (to P.J.), DC018627 (to P.J.), DC015491 (to I.M.), and G20 OD020296. Imaging was performed at the Cell and Developmental Biology Core at the University of Pennsylvania and at the Monell Histology and Cellular Localization Core, which is supported in part by NIH National Institute on Deafness and Other Communication Disorders Core Grant DC011735 (to R.F.M.). We thank Dr. Calvin Kuo (Stanford University) for providing us with Ad-Rspo1-Fc and Ad-Fc adenoviral vectors, Dr. Jeffrey Whitsett (University of Cincinnati) for the Rspo2 stable cell line to produce Rspo2-conditioned medium, and Dr. Hans Clevers (Hubrecht Institute) for the Wnt3a stable cell line to produce Wnt3a-conditioned medium. We thank Dr. Karen Yee for assistance with figure preparation.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2001833118/-/DCSupplemental.
Data Availability.
All study data are included in the article and SI Appendix.
References
- 1.Bachmanov A. A., Beauchamp G. K., Taste receptor genes. Annu. Rev. Nutr. 27, 389–414 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaudhari N., Roper S. D., The cell biology of taste. J. Cell Biol. 190, 285–296 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liman E. R., Zhang Y. V., Montell C., Peripheral coding of taste. Neuron 81, 984–1000 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yarmolinsky D. A., Zuker C. S., Ryba N. J., Common sense about taste: From mammals to insects. Cell 139, 234–244 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beidler L. M., Smallman R. L., Renewal of cells within taste buds. J. Cell Biol. 27, 263–272 (1965). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamamichi R., Asano-Miyoshi M., Emori Y., Taste bud contains both short-lived and long-lived cell populations. Neuroscience 141, 2129–2138 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Perea-Martinez I., Nagai T., Chaudhari N., Functional cell types in taste buds have distinct longevities. PLoS One 8, e53399 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yee K. K., et al. , Lgr5-EGFP marks taste bud stem/progenitor cells in posterior tongue. Stem Cells 31, 992–1000 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takeda N., et al. , Lgr5 identifies progenitor cells capable of taste bud regeneration after injury. PLoS One 8, e66314 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vintschgau M. V., Hönigschmied J., Nervus Glossopharyngeus und Schmeckbecker. Arch. Ges. Physiol. 14, 443–448 (1876). [Google Scholar]
- 11.Olmsted J. M. D., Effects of cutting the lingual nerve of the dog. J. Comp. Neurol. 33, 149–154 (1921). [Google Scholar]
- 12.Guth L., Taste buds on the cat’s circumvallate papilla after reinnervation by glossopharyngeal, vagus, and hypoglossal nerves. Anat. Rec. 130, 25–37 (1958). [DOI] [PubMed] [Google Scholar]
- 13.Cheal M., Oakley B., Regeneration of fungiform taste buds: Temporal and spatial characteristics. J. Comp. Neurol. 172, 609–626 (1977). [DOI] [PubMed] [Google Scholar]
- 14.Lu W.-J., et al. , Neuronal delivery of Hedgehog directs spatial patterning of taste organ regeneration. Proc. Natl. Acad. Sci. U.S.A. 115, E200–E209 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castillo-Azofeifa D., et al. , Sonic hedgehog from both nerves and epithelium is a key trophic factor for taste bud maintenance. Development 144, 3054–3065 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ermilov A. N., et al. , Maintenance of taste organs is strictly dependent on epithelial hedgehog/GLI signaling. PLoS Genet. 12, e1006442 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mistretta C. M., Kumari A., Tongue and taste organ biology and function: Homeostasis maintained by hedgehog signaling. Annu. Rev. Physiol. 79, 335–356 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castillo D., et al. , Induction of ectopic taste buds by SHH reveals the competency and plasticity of adult lingual epithelium. Development 141, 2993–3002 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall J. M., Bell M. L., Finger T. E., Disruption of sonic hedgehog signaling alters growth and patterning of lingual taste papillae. Dev. Biol. 255, 263–277 (2003). [DOI] [PubMed] [Google Scholar]
- 20.Ren W., et al. , Single Lgr5- or Lgr6-expressing taste stem/progenitor cells generate taste bud cells ex vivo. Proc. Natl. Acad. Sci. U.S.A. 111, 16401–16406 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Lau W., Peng W. C., Gros P., Clevers H., The R-spondin/Lgr5/Rnf43 module: Regulator of Wnt signal strength. Genes Dev. 28, 305–316 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dvoryanchikov G., et al. , Transcriptomes and neurotransmitter profiles of classes of gustatory and somatosensory neurons in the geniculate ganglion. Nat. Commun. 8, 760 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finger T. E., et al. , ATP signaling is crucial for communication from taste buds to gustatory nerves. Science 310, 1495–1499 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Hevezi P., et al. , Genome-wide analysis of gene expression in primate taste buds reveals links to diverse processes. PLoS One 4, e6395 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren W., et al. , Transcriptome analyses of taste organoids reveal multiple pathways involved in taste cell generation. Sci. Rep. 7, 4004 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aihara E., et al. , Characterization of stem/progenitor cell cycle using murine circumvallate papilla taste bud organoid. Sci. Rep. 5, 17185 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ootani A., et al. , Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat. Med. 15, 701–706 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan K. S., et al. , The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc. Natl. Acad. Sci. U.S.A. 109, 466–471 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan K. S., et al. , Non-equivalence of Wnt and R-spondin ligands during Lgr5+ intestinal stem-cell self-renewal. Nature 545, 238–242 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.St John S. J., Garcea M., Spector A. C., The time course of taste bud regeneration after glossopharyngeal or greater superficial petrosal nerve transection in rats. Chem. Senses 28, 33–43 (2003). [DOI] [PubMed] [Google Scholar]
- 31.Sato T., et al. , Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009). [DOI] [PubMed] [Google Scholar]
- 32.de Lau W., et al. , Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 476, 293–297 (2011). [DOI] [PubMed] [Google Scholar]
- 33.Hao H. X., et al. , ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature 485, 195–200 (2012). [DOI] [PubMed] [Google Scholar]
- 34.Koo B. K., et al. , Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature 488, 665–669 (2012). [DOI] [PubMed] [Google Scholar]
- 35.Park S., et al. , Differential activities and mechanisms of the four R-spondins in potentiating Wnt/β-catenin signaling. J. Biol. Chem. 293, 9759–9769 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lebensohn A. M., Rohatgi R., R-spondins can potentiate WNT signaling without LGRs. eLife 7, e33126 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bell S. M., et al. , R-spondin 2 is required for normal laryngeal-tracheal, lung and limb morphogenesis. Development 135, 1049–1058 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Miura H., et al. , Shh and Ptc are associated with taste bud maintenance in the adult mouse. Mech. Dev. 106, 143–145 (2001). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and SI Appendix.