Significance
Why some animals, but not others, respond to injury with extensive regeneration is an outstanding question and attracts considerable research interest. Planarian flatworms possess a polarity system throughout their anteroposterior axis that specifies the identity of missing tissues at wound sites. The regeneration of vertebrate appendages involves distal growth. However, whether vertebrate appendages can undergo bidirectional regeneration along the proximodistal axis remains unclear. Our study demonstrates that unidirectional blastema regeneration polarity occurs in zebrafish fins along the proximodistal axis. We identified calcineurin as a molecular switch that specifies the blastema of the PCE and controls regeneration polarity in zebrafish fins. Our results are of great importance for future regeneration polarity studies on vertebrates.
Keywords: calcineurin, polarity, fin regeneration, zebrafish
Abstract
Planarian flatworms regenerate their heads and tails from anterior or posterior wounds and this regenerative blastema polarity is controlled by Wnt/β-catenin signaling. It is well known that a regeneration blastema of appendages of vertebrates such as fish and amphibians grows distally. However, it remains unclear whether a regeneration blastema in vertebrate appendages can grow proximally. Here, we show that a regeneration blastema in zebrafish fins can grow proximally along the proximodistal axis by calcineurin inhibition. We used fin excavation in adult zebrafish to observe unidirectional regeneration from the anterior cut edge (ACE) to the posterior cut edge (PCE) of the cavity and this unidirectional regeneration polarity occurs as the PCE fails to build blastemas. Furthermore, we found that calcineurin activities in the ACE were greater than in the PCE. Calcineurin inhibition induced PCE blastemas, and calcineurin hyperactivation suppressed fin regeneration. Collectively, these findings identify calcineurin as a molecular switch to specify the PCE blastema of the proximodistal axis and regeneration polarity in zebrafish fin.
Regeneration widely occurs in the animal kingdom and varies with species (1). Planarian flatworms specify the identity of missing tissues to regenerate heads or tails at anterior or posterior wounds (2–6). This property is known as “regeneration polarity.” Although certain animals, such as Planaria and Hydra, undergo regeneration of the whole body, vertebrates are limited in their regenerative capacities. Nonetheless, some vertebrates, such as fish and amphibians, are capable of completely regenerating missing limbs (1). Directional polarity has been associated with the development of vertebrate limbs as they develop mainly from the proximal to distal direction. Although regenerative blastemas of some vertebrate appendages grow distally, it remains unclear whether they can grow proximally in vertebrate appendages.
Zebrafish have the ability to regenerate entire fins throughout their lives. Fins are well-organized structures that include nerves, vasculature, skin, mesenchyme, and a bone frame (7). Regeneration in zebrafish fins is accomplished by changes within preexisting tissues to form a regeneration blastema at the wound site. Blastemas are essential for the initial regeneration of limbs and eventual differentiation to produce missing structures (8, 9). Blastema formation requires many adult stem cells and a robust mechanism to specify missing tissue types. Many local factors such as connexins, adhesion molecules, and released ligands are involved in blastema formation and specification (10–12). Although many factors and mechanisms have been identified that regulate blastema cell formation and differentiation, it is still unclear what factors and mechanisms are involved in controlling blastema polarity during regeneration.
Calcineurin is a Ser/Thr phosphatase and a known regulator of proportional growth in zebrafish fins (13). The low activity level of calcineurin corresponds to high rates of fin regeneration, and calcineurin inhibition alters the fin positional information and enhances fin regeneration through the promotion of retinoic acid (RA) signaling (13, 14). Evidence shows that calcineurin regulates coordinated outgrowth of regenerating fins by controlling the average number of blastemal cell progeny divisions (15). Additionally, calcineurin has been associated with bioelectric signaling in the regulation of growth and proportion (16).
In this study, we constructed fin amputation patterns, which we designate as fin excavations, to produce a cavity in fins and found that zebrafish fins regenerated missing tissues only from the anterior cut edge (ACE) to the posterior cut edge (PCE) of the cavity along the proximodistal (P–D) axis. This unidirectional regeneration polarity occurs as PCE fails to build blastemas. Furthermore, we identified calcineurin to specify the PCE blastema. Calcineurin activities in the PCE were greater than in the ACE. Low calcineurin activities induced PCE blastema formation and regeneration, while calcineurin hyperactivation suppressed fin regeneration. These findings suggest that calcineurin specifies the PCE blastema of the P–D axis and regulates regeneration polarity in zebrafish fins.
Results
P–D Polarity Is Present in Zebrafish Fin Regeneration.
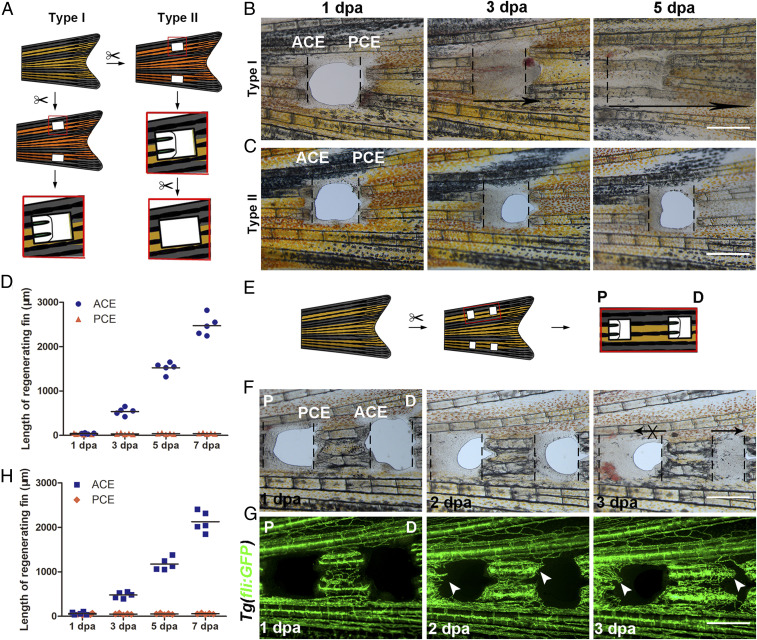
To investigate whether a regeneration blastema in zebrafish fins can grow proximally, two bony ray parts were removed and holes were made at specific positions along the P–D axis of the zebrafish caudal fins (Fig. 1A). Unidirectional regeneration of zebrafish fins was observed only from the ACE to the PCE (Fig. 1 A and B). The holes were crammed with regenerating tissues of the ACE at 3 and 5 days post amputation (dpa), but the PCE failed to regenerate, even when the regenerating tissues of the ACE were removed every day (Fig. 1 C and D). Similar results were observed in the anal and dorsal fins, which were also only from the ACE to the PCE (SI Appendix, Fig. S1A, arrow). These results indicate that unidirectional regeneration polarity occurs in zebrafish fins.
Fig. 1.
P–D regeneration polarity occurrs in zebrafish fins. (A) Schematic of two types of fin excavation. (B and C) Type I (n = 11/11) and II (n = 11/12) experimental strategies of unidirectional regeneration in zebrafish fins from ACE to the PCE. (D) Quantification of fin regenerative tissue lengths (in micrometers) of the ACE and PCE (n = 5). (E) Schematic of the two-hole excavation in the same two bony rays. (F and G) Images of bright fields and blood vessels in fin regeneration after two-hole excavations at 1, 2, and 3 dpa (n = 5/8). (H) Quantification of fin regenerative tissue lengths (in micrometers) with two-hole excavation (n = 5). P, proximal; D, distal. (Scale bar: 500 µM.)
To validate this result, the lepidotrichia between two holes in the same two bony rays were retained (Fig. 1E). Directional regeneration of the left lepidotrichia was observed only from the ACE to the PCE (Fig. 1 E, F, and H). Vascular sprouting also exhibited directional regeneration from the ACE to the PCE (Fig. 1G, arrow). Therefore, regeneration polarity was clearly present throughout the P–D axis in zebrafish fins.
The PCE Fails to Build Blastemas.
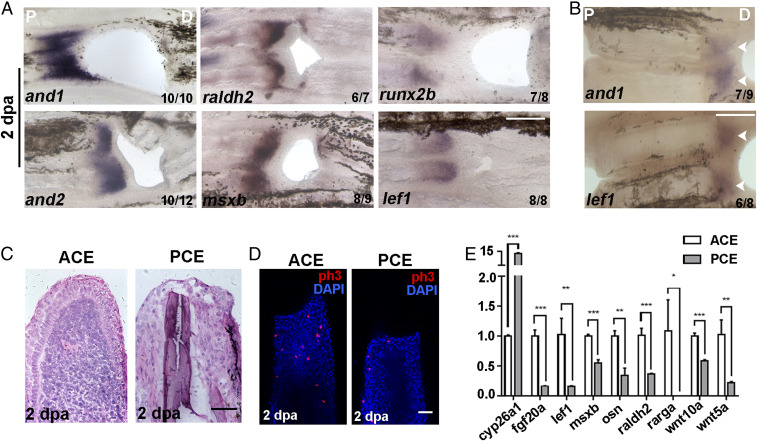
Blastemas are tissue structures formed at the beginning of regeneration and are essential for fin regeneration (17). To investigate whether the failure of PCE regeneration was due to nonestablished blastemas, the expression of blastema marker genes was assessed. Compared with the ACE, blastema marker genes and1/2, raldh2, msxb, runx2b, and lef1 were not expressed in the PCE at 2 dpa when blastemas had formed (Fig. 2A). Similar results were obtained for the PCE of lepidotrichia along the P–D axis (Fig. 2B, arrow). Additionally, histological analyses revealed that the structure of blastemas was absent and scar tissues were formed in the PCE (Fig. 2C). The amount of PCE cellular proliferation was significantly less than in the ACE (Fig. 2D).
Fig. 2.
The PCE failed to form blastemas. (A) In situ hybridization of and1/2, raldh2, msxb, runx2b, and lef1 in regenerative fins at 2 dpa showing that blastemas were not built in the PCE. (B) and1 and lef1 were not expressed in the PCE with two-hole excavations. (C) Histological analyses of ACE (n = 6/8) and PCE (n = 5/6). (D) H3P antibody staining (red) was used to examine cell proliferation in the ACE (n = 7/10) and PCE (n = 7/9) DAPI counterstaining (blue). (E) qRT-PCR results comparing the differences in the relative expression of regeneration-associated genes for the PCE over the ACE (Student’s t test, *P < 0.05, **P < 0.01, ***P < 0.001). Data are presented as mean ± SD. P, proximal; D, distal. (Scale bars: 200 µM in A and B; 100 µM in C and D.)
To determine whether nonestablished blastemas in the PCE were associated with the weakened expression of genes active during fin regeneration, the expression of candidate genes at 2 dpa was measured by quantitative real-time (qRT)-PCR. The transcriptional activity of the tested genes except for cyp26a1 in PCE groups were significantly lower than the ACE groups (Fig. 2E), indicating that nonestablished blastemas in the PCE were due to the absence of the proregenerative transcriptional program.
FK506 Induces Blastemal Formation and Regeneration of the PCE.
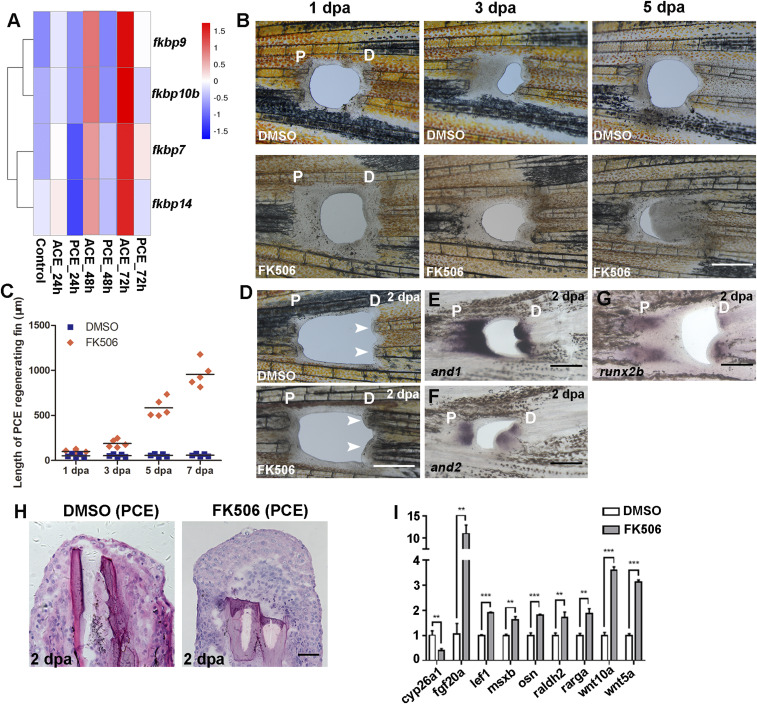
To identify the factors that regulate blastemal formation of the PCE, transcriptome sequencing of the ACE and PCE was performed. The three time points 24, 48, and 72 h postamputation (hpa) we selected were the key regeneration stages and represented mesenchymal disorganization, blastema formation, and regenerative outgrowth, respectively (9). The expression levels of FK506-binding proteins (FKBPs) fkbp7/9/10b/14 were lower in the PCE tissues compared with the ACE tissues (Fig. 3A). FK506 interacted with FKBPs, forming an FK506-FKBP complex that binds to calcineurin and inhibits its activity (18). Therefore, regenerative fins were subsequently treated with FK506 which induced regeneration and new osteoblast production in the PCE of caudal fins (Fig. 3 B and C and SI Appendix, Fig. S2 A and B). Similarly, the PCE regeneration of anal and dorsal fins also occurred after FK506 treatment (SI Appendix, Fig. S1B).
Fig. 3.
PCE blastemas are induced by calcineurin inhibition. (A) RNA-Seq course analysis of the ACE and PCE and the heat map of fragments per kilobase million (FPKM) values of the indicated genes. (B) Regenerative fins at 1, 3, and 5 dpa after dimethyl sulfoxide (DMSO) (n = 7/8) or FK506 treatment (n = 5/10). (C) Quantification of fin regenerative tissue lengths of the PCE (in micrometers) (n = 5). (D) Regenerative blastemas (indicated by arrows) occurred in zebrafish fins after FK506 treatment (n = 5/12) compared with DMSO (9/10) at 2 dpa. (E–G) and1, and2, and runx2b were expressed in the PCE after the FK506 treatment at 2 dpa. (H) Histological analyses of the PCE after DMSO (7/8) or FK506 treatment (5/8). (I) qRT-PCR results comparing the differences between the relative expression of regeneration-associated genes for FK506 or DMSO-treated PCE (Student’s t test, **P < 0.01, ***P < 0.001). Data are presented as mean ± SD. P, proximal; D, distal. (Scale bars: 500 µM in B–G; 100 µM in H.)
To validate this result, we utilized FK520, a derivative of FK506 (14), to treat regenerative fins and obtained similar results (SI Appendix, Fig. S3 A and B). In situ hybridization and histological analyses showed that the PCE blastemas were induced by FK506 treatment at 2 dpa (Fig. 3 D–H). Proregenerative transcriptional program genes were also up-regulated after FK506 treatment (Fig. 3I), indicating that the formation of PCE blastemas was due to the proregenerative transcriptional program activated by FK506 treatment. These data suggest that FK506 is able to induce blastema formation and regeneration of the PCE in zebrafish fins along the P–D axis.
FK506-Induced Blastemas Are Essential for Continuous PCE Regeneration.
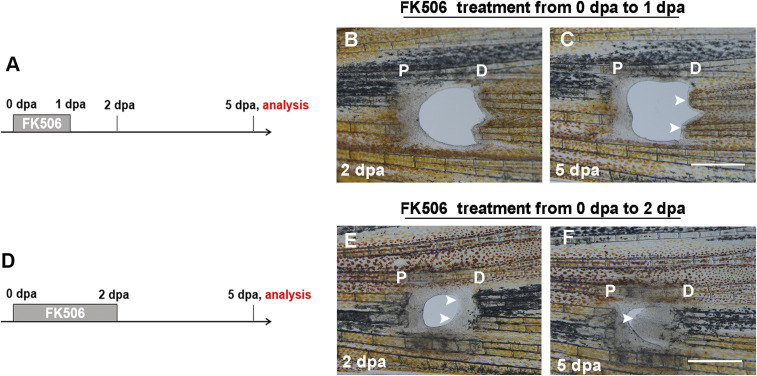
To determine the key time points of FK506 in playing its role in fin regeneration, we controlled the time of FK506 treatment during regeneration (Fig. 4 A and D). The FK506 treatment began at 0 dpa but stopped after 1 dpa when PCE blastemas did not form. The results revealed that PCE blastemas were not established at 2 dpa and PCE regeneration did not occur at 5 dpa (Fig. 4 B and C, arrow). However, the continuous treatment of FK506 until 2 dpa when PCE blastemas had formed led to the continuous regeneration of the PCE (Fig. 4 E and F, arrow). These results revealed that FK506-induced blastema formation was the key to the continuous regeneration of the PCE.
Fig. 4.
FK506-induced blastemas are essential to the continuous regeneration of the PCE. (A) Experimental scheme illustrating the FK506 treatment from 0 to 1 dpa and analysis at 5 dpa. (B and C) The FK506 treatment illustrated in A did not induce blastema formation and regeneration of the PCE (n = 6/7). (D) Experimental scheme illustrating the FK506 treatment from 0 to 2 dpa and analysis at 5 dpa. (E and F) The FK506 treatment was continuous up to 2 dpa when PCE blastemas formed (indicated by arrows), which led to the continuous regeneration of the PCE at 5 dpa (n = 5/9). P, proximal; D, distal. (Scale bars: 500 µM.)
FK506 is also an immunosuppressant and a previous report demonstrated that immunosuppression was able to promote Xenopus regenerative outgrowth (19). To determine whether immunosuppression is the mechanism for FK506-mediated PCE regeneration, fish were treated with other immunosuppressants after fin excavation. Rapamycin also binds to FKBPs and their complex functions as an immune response inhibitor (13, 20). However, the rapamycin treatment did not induce PCE regeneration (SI Appendix, Fig. S4 A–C), indicating that FK506 induces PCE regeneration by regulating a FK506-specific target, which was not due to immunosuppression.
Calcineurin Activity of the PCE Is Higher Than the ACE.
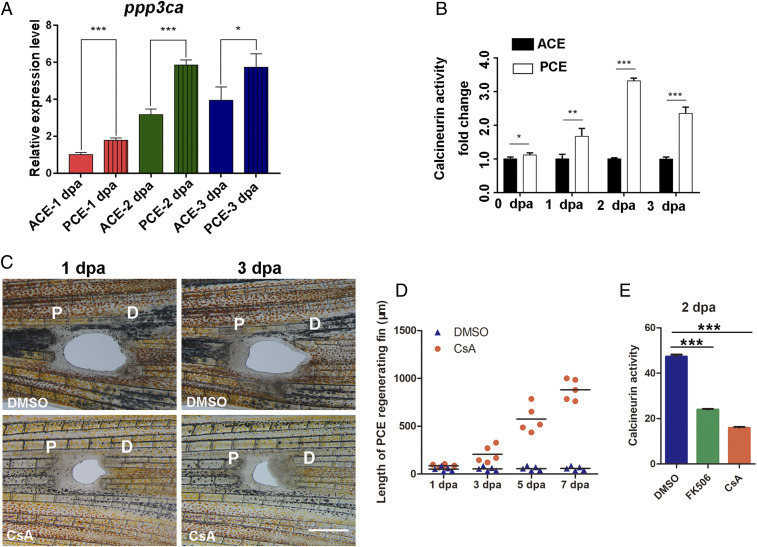
FK506 enhances fin regenerative outgrowth by inhibiting calcineurin activity (13). To determine how calcineurin induces the formation of PCE blastemas, we first examined the expression of calcineurin-related genes. The qRT-PCR analysis showed that the expression level of ppp3ca in the PCE, a main catalytic subunit of calcineurin, was higher than in the ACE at 1, 2, and 3 dpa (Fig. 5A). Moreover, the calcineurin activities of the PCE were also greater than those of the PCE, especially at 2 dpa (Fig. 5B). Thus, CsA, another inhibitor of calcineurin (18), also induced PCE regeneration (Fig. 5 C and D). Both FK506 and CsA induced regeneration of the PCE by inhibiting calcineurin activities (Fig. 5E). These results indicate that higher calcineurin activities explain why blastemas and regeneration of the PCE fail to form.
Fig. 5.
Obvious differences in calcineurin activity between the PCE and ACE. (A) The qRT-PCR analysis revealed that the ppp3ca expression of the PCE was higher than ACE (Student’s t test, *P < 0.05, ***P < 0.001). (B) Differences in calcineurin activity differences between the PCE and ACE at 0, 1, 2, and 3 dpa (Student’s t test, *P < 0.05, **P < 0.01, ***P < 0.001). Data are presented as mean ± SD. (C) PCE regeneration was induced by CsA treatment (n = 5/10) compared with DMSO treatment (n = 7/10). (D) Quantification of fin regenerative tissue lengths (in micrometers) of the PCE after FK506 or CsA treatment (n = 5). (E) Calcineurin activities of the PCE after FK506 or CsA treatment (n = 7, ***P < 0.001). P, proximal; D, distal. Data are presented as mean ± SD. (Scale bars: 500 µM.)
Low Endogenous Calcineurin Activity Is Essential for Inducing PCE Blastemas.
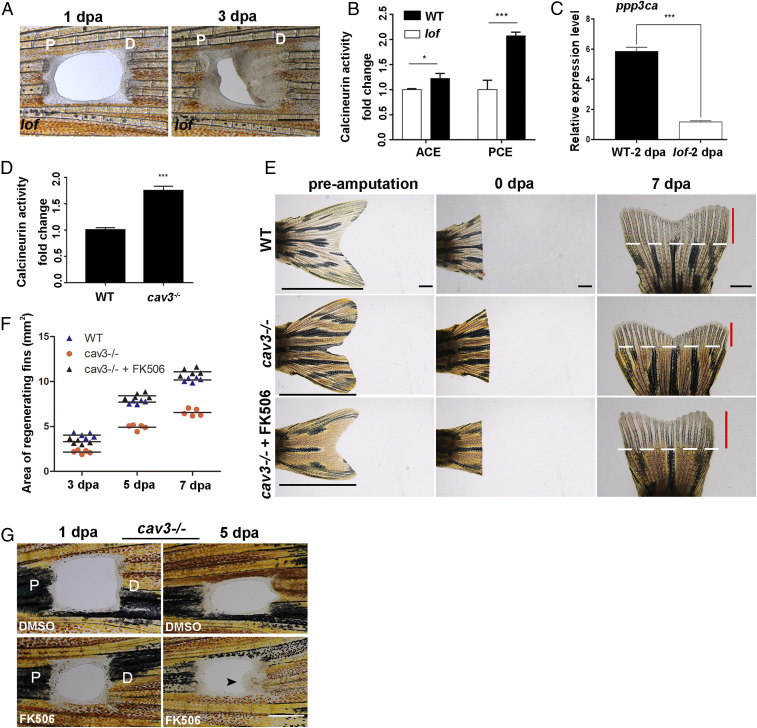
Zebrafish long fin (lof) mutants retain allometric fin growth (21), which is similar to the fish induced by FK506 results. Therefore, we amputated lof mutant fins and observed PCE regeneration in lof mutants (Fig. 6A). Calcineurin activities and ppp3ca expression in the PCE of lof mutants at 2 dpa were lower compared with the control (Fig. 6 B and C), indicating that low endogenous calcineurin activity induces PCE regeneration. Reductions in the scaffolding protein, caveolin 3 (cav3), resulted in hyperactivation of calcineurin (22). Therefore, fin regeneration of the cav3cq105 mutant was explored (SI Appendix, Fig. S5 A and B). Results revealed that the cav3 mutation led to up-regulated calcineurin activities and the inhibition of fin regeneration (Fig. 6 D–F). Moreover, FK506 was able to rescue fin regeneration and induce regeneration of the PCE in the cav3cq105 mutant (Fig. 6 E–G). Therefore, calcineurin activity is a key factor that induces blastemas and the regeneration of the PCE.
Fig. 6.
Endogenous calcineurin activity controls fin proximodistal regeneration. (A) PCE in the lof mutant regenerated (n = 5/6). (B) Fold change of calcineurin activities in lof mutant PCE or ACE normalized to control fins at 2 dpa (Student’s t test, *P < 0.05, ***P < 0.001). Data are presented as mean ± SD. (C) The expression of ppp3ca in the lof mutant at 2 dpa was lower compared with the control (Student’s t test, ***P < 0.001). Data are presented as mean ± SD. (D) Fold change of calcineurin activities in cav3 mutant regenerative fins normalized to control fins at 3 dpa (n = 7, Student’s t test, ***P < 0.001). Data are presented as mean ± SD. (E) Regeneration of the ACE was inhibited in cav3cq105 mutants (n = 8/8), while FK506 can rescue ACE regeneration at 7 dpa (n = 6/8) compared to WT (n = 7/8). White lines mark the amputation planes. Red lines show the extent of regenerative outgrowth, and black lines show the length of the entire uninjured caudal fin. (F) Quantification of the area of fin regenerative tissue (in square millimeters) (n = 5). (G) The regeneration of the PCE in cav3cq105 mutants was rescued by FK506 (n = 5/12) compared to control (n = 7/8). P, proximal; D, distal. (Scale bar: 500 µM in A and G; 2 mm in E.)
Retinoic Acid Signaling Does Not Induce the Blastema Formation in the PCE.
Calcineurin inhibition is also associated with the promotion of RA signaling (13). RA signaling is known to regulate appendage development and regeneration (23, 24). qRT-PCR analysis revealed that the expression level of cyp26a1, a RA antagonist gene, was higher in the PCE compared to the ACE (Fig. 2E). However, RA and adapalene, the accelerators of RA signaling, did not induce PCE regeneration (SI Appendix, Fig. S6 A–F). The RA inhibitor 13-c-RA also failed to induce PCE regeneration (SI Appendix, Fig. S6 G and H). These data indicate that the regeneration of the PCE induced by calcineurin inhibition occurs regardless of the promotion of RA signaling.
Additionally, some canonical signals, such as Fgf, Wnt, and Notch, which have been reported to regulate zebrafish fin regeneration (25–27), also failed to induce PCE regeneration (SI Appendix, Fig. S7 A–P). These data indicate that calcineurin regulates blastema polarity along the P–D axis by its specific targets.
Discussion
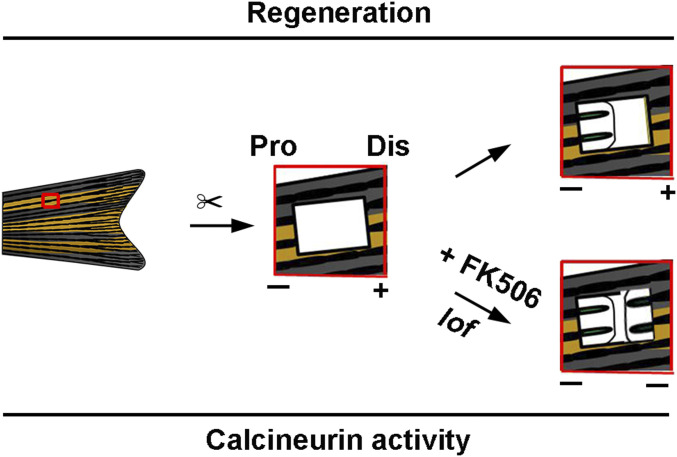
Collectively, the results of this study demonstrate the presence of blastema regeneration polarity in zebrafish fins along the P–D axis and the fundamental importance of calcineurin activities in the regulation of regeneration polarity (Fig. 7). These findings uncovered a unidirectional regeneration polarity model in zebrafish fins, which has been rarely observed during the progression of regeneration of other organs. Unidirectional regeneration polarity occurs in any fin position and is independent of the information position. Calcineurin activities differ between ACE and PCE after fin excavation, especially at the time of blastema formation. Low calcineurin activities are essential for blastema formation, as calcineurin inhibition shifts fin regeneration from a distal growth program to a proximal program (13) and activates the proregenerative transcriptional program in the PCE. Calcineurin inhibition promotes RA signaling, which plays an important role in the regulation of limb regeneration (23, 24). However, RA activation did not induce blastema formation of the PCE in this study. Additionally, Fgf, Wnt, and Notch failed to control PCE regeneration. Therefore, the mechanisms underlying the regulation of calcineurin activities during fin regeneration along the P–D axis remain to be clarified.
Fig. 7.
Proposed model of the functional roles of calcineurin activities in zebrafish fin regeneration along the P–D axis. The calcineurin activity of the PCE was higher than that of the ACE after fin excavation and calcineurin inhibition (FK506 treatment or lof mutant), which induced blastema formation and PCE regeneration.
Regeneration of fin hole punch injuries has been described for goldfish and Fundulus fins (28). Nabrit made fin holes at different sites and described anterior to posterior growth only from small (2 × 2 mm) injuries, but found that larger 4 × 4 mm injuries showed bidirectional growth (28). As a smaller fish, the size of the largest injuries we could generate in adult zebrafish caudal fins was 2.2 × 2 mm (SI Appendix, Fig. S8A), and fin breaks appeared beyond this size at the early regenerating stage. However, we found that the largest injuries still showed growth only from the ACE to the PCE (SI Appendix, Fig. S8 B and C), which differs from the results described by Nabrit in goldfish and killifish (28). It is possible that zebrafish fins have different regulatory properties than goldfish or killifish. However, we were unable to generate large 4 × 4 mm holes in zebrafish fins as Nabrit could in larger fish (28), and the largest injuries we generated in zebrafish were still only the size of the smallest injuries in goldfish/Fundulus (which also showed only anterior to posterior regeneration). Thus, we could not assess whether excavation size impacts the capacity to regenerate from the ACE to the PCE in this study.
Previous reports have demonstrated that limb regeneration depends on a nerve supply (29, 30). In this study, the amputation pattern of the lepidotrichia between two holes in the same two bony rays was retained (Fig. 1E), excluding neural differences between the ACE and PCE, which also confirmed the unidirectional regeneration of zebrafish fins, indicating that nerves are not dependent on the regeneration polarity of zebrafish fins. We also observed blood flow in the ACE and PCE and found that blood flowed toward the ACE and PCE, and minimal blood flow was observed in the ACE and PCE (Movie S1), indicating that blood flow has little effect on regeneration polarity. Although some immune cells such as macrophages and neutrophils are involved in the regulation of limb regeneration (31–33), immunosuppression failed to induce PCE regeneration, suggesting that the regeneration polarity of zebrafish fins does not depend on immunomodulation.
lof mutants retain allometric fin growth due to the positional information memory in lof mutant fins, which is altered along the P–D axis (34, 35). The regeneration of the PCE occurred in lof mutants due to the down-regulation of calcineurin activities compared with the control. Thus, lof may act upstream of calcineurin to inhibit its activity. However, it is necessary to confirm whether PCE regeneration is present in other zebrafish mutants that produce excessively long fins such as the another long fin (alf) and rapunzel (rap) mutants (21, 36) to verify these findings.
Materials and Methods
Fish Husbandry.
The Tg(osx:mCherry), Tg(fli1:GFP), Tg(hsp70l:fgf20a-mCherry), Tg(gata1:DesRed), and Tg(hsp70l:dnfgfr-GFP) (37) transgenic lines, and lof (from the China Zebrafish Resource Center) and cav3cq105 mutant lines were raised and maintained under standard laboratory feeding and breeding conditions as previously described (38). All fish were raised and maintained under standard laboratory conditions in accordance with the Guidelines of Experimental Animal Welfare of the Ministry of Science and Technology of the People’s Republic of China (2006) and the Institutional Animal Care and Use Committee protocols from Jinggangshan University (2016).
Fin Excavation.
Adult zebrafish were mildly anesthetized in tricaine and placed in a 1.5% agarose groove. Surgical blades were used to dig a hole in the two bony rays and remove two to three bony joints (0.6 × 0.6 mm), or six to seven bony joints in the six bony rays for regenerating the largest holes (2.2 × 2 mm). The regenerating tissues of the ACE were removed daily to prevent the hole from being filled. Fins were imaged using a SteREO Discovery V20 microscope (Carl Zeiss).
In Situ Hybridization and Antibody Staining.
Regenerating fins were amputated and fixed overnight in 4% paraformaldehyde solution at 4 °C. Whole-mount in situ hybridizations were conducted as previously described (38). Antibody staining was performed following an earlier report (39). Antibodies against H3P (1:500) and Dsred fluorescent proteins (1:1,000) (Invitrogen) were used. Antibody-stained tissues were imaged using a TCS Sp8 confocal microscope (Leica).
qRT-PCR.
Total RNA was extracted from fish fins exposed to different treatments. RNA extraction and reverse transcription reactions were performed according to previous reports (40). qRT-PCR was performed on an ABI Step One Plus RT-PCR system (Applied Biosystems). The expression of fin regenerating-related genes was assessed. The results were calculated by the comparative Ct method formula. The primers used in the study are presented in SI Appendix, Table S2.
Chemical Treatment.
Zebrafish were treated with 3 µM FK506, 2 µM FK520, 4 µM CsA, 1 µM rapamycin, 1 µM RA, 1 µM adapalene, 1 µM 13-c-RA, 1 µM XAV939, 2 µM BML284, and 1 µM Notch intracellular domain (NICD) after fin excavation. All of the chemicals were purchased from MedChemExpress and changed every other day.
RNA Sequencing.
ACE and PCE fragment tissues at 0 (control), 24, 48, and 72 hpa were amputated and collected for transcriptome sequencing. The RNA-sequencing time courses included seven fin wound fragments and four time points 0 (control), 24, 48, and 72 hpa. Transcriptome sequencing and analysis were performed by Novogene Bioinformatics Technology Co., Ltd.
Histological Analyses.
Fin wound fragments were embedded in optimal cutting temperature compound (OCT), and 7-µm sections from fin wound fragments were prepared. Then, the fin sections were stained with hematoxylin/eosin as previously described (38).
Calcineurin Activity Assay.
Calcineurin phosphatase activity of the fin lysates was measured as previously described (13). A calcineurin phosphatase assay kit (ENZO, BML-AK804), which includes calcineurin, calmodulin, and phosphorylated RII peptide substrates, was used. After removal of free phosphates from the samples, phosphorylated RII peptides were added and the samples were incubated for 30 min at 30 °C. After incubation, the phosphate indicator, malachite green, was added and the amount of free phosphate in the samples was measured on a SpectraMax iD3 Multi-Mode Microplate Reader (Molecular Devices). Calcineurin activity was normalized to total protein concentration.
Heat Shock.
The transgenic fish Tg(hsp70l:fgf20a-mCherry) and Tg(hsp70l:dnfgfr-GFP) (38) were heat shocked after surgery at 39.5 °C once per day for 2 h.
Size Measurements and Statistical Analysis.
The length and area of regenerating fins were measured using a SteREO DiscoveryV20 microscope equipped with AxioVision Rel 4.8.2 software (Carl Zeiss). All statistical tests were performed with GraphPad Prism version 5.0. Statistical significance was determined using the Student’s t test. All values were shown as the mean ± SD. Differences with P values <0.05, 0.01, or 0.001 were deemed statistically significant or extremely significant.
Supplementary Material
Acknowledgments
We thank Atsushi Kawakami for providing the plasmid pTol2 (hsp70l:mCherry-fgf20a). This work was supported by National Key R&D Program of China (2018YFA0801000), the National Natural Science Foundation (31771606, 31900597, 81860282, and 81660087), and Natural Science Foundation Project of Jiangxi Province (20192ACB21013 and 2018ACB21033).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2009539118/-/DCSupplemental.
Data Availability.
The transcriptome sequencing data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi (accession no. GSE159560).
References
- 1.Brockes J. P., Kumar A., Comparative aspects of animal regeneration. Annu. Rev. Cell Dev. Biol. 24, 525–549 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Gurley K. A., Rink J. C., Sánchez Alvarado A., β-catenin defines head versus tail identity during planarian regeneration and homeostasis. Science 319, 323–327 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen C. P., Reddien P. W., Smed-betacatenin-1 is required for anteroposterior blastema polarity in planarian regeneration. Science 319, 327–330 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Umesono Y., et al. , The molecular logic for planarian regeneration along the anterior-posterior axis. Nature 500, 73–76 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Liu S. Y., et al. , Reactivating head regrowth in a regeneration-deficient planarian species. Nature 500, 81–84 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Sikes J. M., Newmark P. A., Restoration of anterior regeneration in a planarian with limited regenerative ability. Nature 500, 77–80 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becerra J., Montes G. S., Bexiga S. R. R., Junqueira L. C. U., Structure of the tail fin in teleosts. Cell Tissue Res. 230, 127–137 (1983). [DOI] [PubMed] [Google Scholar]
- 8.Nacu E., Gromberg E., Oliveira C. R., Drechsel D., Tanaka E. M., FGF8 and SHH substitute for anterior-posterior tissue interactions to induce limb regeneration. Nature 533, 407–410 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Whitehead G. G., Makino S., Lien C. L., Keating M. T., fgf20 is essential for initiating zebrafish fin regeneration. Science 310, 1957–1960 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Hoptak-Solga A. D., et al. , Connexin43 (GJA1) is required in the population of dividing cells during fin regeneration. Dev. Biol. 317, 541–548 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee Y., Grill S., Sanchez A., Murphy-Ryan M., Poss K. D., Fgf signaling instructs position-dependent growth rate during zebrafish fin regeneration. Development 132, 5173–5183 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Schwartz M. A., Assoian R. K., Integrins and cell proliferation: Regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. J. Cell Sci. 114, 2553–2560 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Kujawski S., et al. , Calcineurin regulates coordinated outgrowth of zebrafish regenerating fins. Dev. Cell 28, 573–587 (2014). [DOI] [PubMed] [Google Scholar]
- 14.McMillan S. C., et al. , A regulatory pathway involving retinoic acid and calcineurin demarcates and maintains joint cells and osteoblasts in regenerating fin. Development 145, dev161158 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Tornini V. A., et al. , Live monitoring of blastemal cell contributions during appendage regeneration. Curr. Biol. 26, 2981–2991 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daane J. M., et al. , Bioelectric-calcineurin signaling module regulates allometric growth and size of the zebrafish fin. Sci. Rep. 8, 10391 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poss K. D., Nechiporuk A., Hillam A. M., Johnson S. L., Keating M. T., Mps1 defines a proximal blastemal proliferative compartment essential for zebrafish fin regeneration. Development 129, 5141–5149 (2002). [DOI] [PubMed] [Google Scholar]
- 18.Sieber M., Baumgrass R., Novel inhibitors of the calcineurin/NFATc hub - alternatives to CsA and FK506? Cell Commun. Signal. 7, 25 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bierer B. E., et al. , Two distinct signal transmission pathways in T lymphocytes are inhibited by complexes formed between an immunophilin and either FK506 or rapamycin. Proc. Natl. Acad. Sci. U.S.A. 87, 9231–9235 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiwad M., et al. , Comparative analysis of calcineurin inhibition by complexes of immunosuppressive drugs with human FK506 binding proteins. Biochemistry 45, 15776–15784 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Goldsmith M. I., Fisher S., Waterman R., Johnson S. L., Saltatory control of isometric growth in the zebrafish caudal fin is disrupted in long fin and rapunzel mutants. Dev. Biol. 259, 303–317 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Markandeya Y. S., et al. , Caveolin-3 overexpression attenuates cardiac hypertrophy via inhibition of t-type ca 2+ current modulated by pkcα in cardiomyocytes. J. Biol. Chem. 290, 22085–22100 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maden M., Vitamin A and pattern formation in the regenerating limb. Nature 295, 672–675 (1982). [DOI] [PubMed] [Google Scholar]
- 24.Blum N., Begemann G., Retinoic acid signaling spatially restricts osteoblasts and controls ray-interray organization during zebrafish fin regeneration. Development, dev.120212 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Poss K. D., Shen J., Keating M. T., Induction of lef1 during zebrafish fin regeneration. Dev. Dyn. 219, 282–286 (2000). [DOI] [PubMed] [Google Scholar]
- 26.Lee Y., Grill S., Sanchez A., Murphy-Ryan M., Poss K. D., Fgf signaling instructs position-dependent growth rate during zebrafish fin regeneration. Development 132, 5173–5183 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Grotek B., Wehner D., Weidinger G., Notch signaling coordinates cellular proliferation with differentiation during zebrafish fin regeneration. Development 140, 1412–1423 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Nabrit S. M., The role of the fin rays in the regeneration in the tail-fins of fishes. (In Fundulus and Goldfish). Biol. Bull. 56, 235–266 (1929). [Google Scholar]
- 29.Kumar A., Godwin J. W., Gates P. B., Garza-Garcia A. A., Brockes J. P., Molecular basis for the nerve dependence of limb regeneration in an adult vertebrate. Science 318, 772–777 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simões M. G., et al. , Denervation impairs regeneration of amputated zebrafish fins. BMC Dev. Biol. 14, 49 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petrie T. A., Strand N. S., Yang C. T., Rabinowitz J. S., Moon R. T., Macrophages modulate adult zebrafish tail fin regeneration. Development 141, 2581–2591 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li L., Yan B., Shi Y. Q., Zhang W. Q., Wen Z. L., Live imaging reveals differing roles of macrophages and neutrophils during zebrafish tail fin regeneration. J. Biol. Chem. 287, 25353–25360 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fukazawa T., Naora Y., Kunieda T., Kubo T., Suppression of the immune response potentiates tadpole tail regeneration during the refractory period. Development 136, 2323–2327 (2009). [DOI] [PubMed] [Google Scholar]
- 34.Iovine M. K., Johnson S. L., Genetic analysis of isometric growth control mechanisms in the zebrafish caudal Fin. Genetics 155, 1321–1329 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson S. L., Bennett P., Growth control in the ontogenetic and regenerating zebrafish fin. Methods Cell Biol. 59, 301–311 (1999). [DOI] [PubMed] [Google Scholar]
- 36.Perathoner S., et al. , Bioelectric signaling regulates size in zebrafish fins. PLoS Genet. 10, e1004080 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao Z., Mao X., Luo L., Germline stem cells drive ovary regeneration. Cell Rep. 26, 1709–1717.e3 (2019). [DOI] [PubMed] [Google Scholar]
- 38.Lu H., Ma J., Yang Y., Shi W., Luo L., EpCAM is an endoderm-specific Wnt derepressor that licenses hepatic development. Dev. Cell 24, 543–553 (2013). [DOI] [PubMed] [Google Scholar]
- 39.He J., Lu H., Zou Q., Luo L., Regeneration of liver after extreme hepatocyte loss occurs mainly via biliary transdifferentiation in zebrafish. Gastroenterology 146, 789–800.e8 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Cao Z., et al. , Exposure to diclofop-methyl induces immunotoxicity and behavioral abnormalities in zebrafish embryos. Aquat. Toxicol. 214, 105253 (2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The transcriptome sequencing data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi (accession no. GSE159560).