JGP study reveals that insufficient reuptake of calcium into the sarcoplasmic reticulum underlies arrhythmogenic variations in cardiac calcium transients.
Abstract
JGP study reveals that insufficient reuptake of calcium into the sarcoplasmic reticulum underlies arrhythmogenic variations in cardiac calcium transients.
Ca2+ alternans (Ca-Alts) are beat-to-beat changes in the amplitude of the Ca2+ transients evoked in cardiomyocytes, which can lead to arrhythmias and sudden cardiac death. Ca-Alts can be induced by an elevated heart rate (tachycardia) or metabolic impairments such as ischemia or hypothermia, but the molecular mechanisms underlying the phenomenon are unclear. In this issue of JGP, Millet et al. reveal that Ca-Alts arise when SERCA pumps are unable to fully replenish Ca2+ levels in the SR (1).
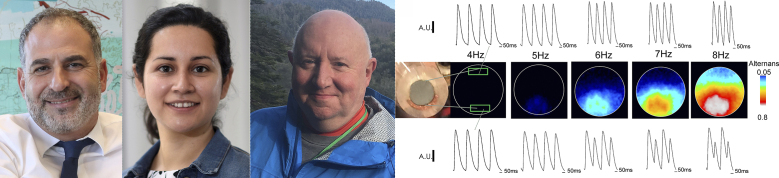
Jose Millet, Yuriana Aguilar-Sanchez, Ariel L. Escobar (left to right), and colleagues investigate the mechanisms underlying arrhythmogenic Ca-Alts. The FLOM technique shows how these beat-to-beat changes in Ca2+ transients can be induced in intact hearts by increased heart rate and local reductions in temperature produced by a cold finger. The researchers find that Ca-Alts result from insufficient replenishment of SR Ca2+ levels by SERCA pumps.
“Ca-Alts are very arrhythmogenic,” says Ariel L. Escobar, a professor at the University of California, Merced. “If you develop these alternans, you have a very high chance of suffering ventricular fibrillation.”
Yet the mechanisms underlying Ca-Alts remain unclear. Though they appear to involve changes in the amount of Ca2+ released from the SR (2,3,4), Ca-Alts could be triggered by variations in the duration of action potentials (APD-Alts) that stimulate calcium-induced calcium release, an incomplete recovery of the ryanodine receptor that releases Ca2+ from the SR, or incomplete refilling of the SR by SERCA ATPases.
To investigate the phenomenon in more detail, Escobar and colleagues, including co-first authors Jose Millet and Yuriana Aguilar-Sanchez, developed a new technique called fluorescence local field optical mapping (FLOM), which uses optical conduits containing >70,000 optical fibers to map the fluorescence of calcium-sensitive or potentiometric dyes in the epicardium of intact mouse hearts. “This approach allows us to study the spatiotemporal dynamics of calcium and membrane potential changes in a functional heart,” Escobar explains.
FLOM imaging confirmed that Ca-Alts can be induced by increased heart rate and/or global reductions in temperature, two conditions that also induce APD-Alts. More crucially, however, Escobar and colleagues used a small, crescent-shaped cold finger to show that local reductions in tissue temperature also induce Ca-Alts but do not cause APD-Alts, demonstrating that the two phenomena can be uncoupled and that Ca-Alts are not driven by changes in action potential duration.
Because the crescent-shaped cold finger creates a temperature gradient within the epicardium, Escobar and colleagues were able to carefully analyze the temperature dependence of Ca2+ dynamics. The relaxation of Ca2+ transients becomes gradually slower at lower temperatures, and a thermodynamic analysis of this process suggested that it involves not only active mechanisms—such as the ATPases that pump Ca2+ into the SR—but also passive mechanisms such as diffusion and binding to cytosolic buffers.
In contrast, the relatively steep temperature dependence of Ca-Alts indicated that they exclusively depend on an active process like SERCA-mediated Ca2+ reuptake into the SR. Indeed, Escobar and colleagues found that the Q10 temperature coefficient of Ca-Alts is remarkably similar to the Q10 of SERCA-mediated Ca2+ transport in vitro.
To confirm the importance of Ca2+ reuptake in Ca-Alts, Escobar and colleagues treated hearts with the SERCA inhibitor Thapsigargin. Partial blockade of SERCA-mediated reuptake enhanced the level of Ca-Alts, the researchers found, indicating that Ca-Alts are induced when SERCA pumps fail to fully replenish SR Ca2+ stores between heart beats. This could occur when the heart is beating particularly fast or when the metabolic activity of cardiomyocytes is impaired by, for example, low temperatures.
Escobar’s team is now developing a needle-shaped optical conduit that can be used to probe any layer within the ventricular wall. “We hope to measure Ca-Alts in each layer, including the endocardium where SERCA levels are lower and Ca-Alts tend to be initiated,” Escobar says.
References
- 1.Millet, J., et al. 2021. J. Gen. Physiol. 10.1085/jgp.202012568 [DOI] [Google Scholar]
- 2.Escobar, A.L., and Valdivia H.H.. 2014. Circ. Res. 10.1161/CIRCRESAHA.114.303823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Díaz, M.E., et al. 2004. Circ. Res. 10.1161/01.RES.0000119923.64774.72 [DOI] [Google Scholar]
- 4.Kornyeyev, D., et al. 2012. J. Mol. Cell. Cardiol. 10.1016/j.yjmcc.2011.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]