Abstract
The wide gap between the development of new healthcare technologies and their integration into clinical practice argues for a deeper understanding of how effective quality improvement can be designed to meet the needs of patients and their clinical teams. The COVID-19 pandemic has forced us to address this gap and create long-term strategies to bridge it. On the one hand, it has enabled the rapid implementation of telehealth. On the other hand, it has raised important questions about our preparedness to adopt and employ new digital tools as part of a new process of care. While healthcare organizations are seeking to improve the quality of care by integrating innovations in digital health, they must also address key issues such as patient experience, develop clinical decision support systems that analyze digital health data trends, and create efficient clinical workflows. Given the breadth of such requirements, embracing new technologies as a core competency of a modern healthcare system introduces a host of questions, such as “How best do patients participate in digital health programs that promote behavioral changes and mitigate risk?” and “What type of data analytics are required that enable a deeper understanding of disease phenotypes and corresponding treatment decisions?” This review presents the challenges in implementing digital health technology and discusses how patient-centered digital health programs are designed within real-world models of remote monitoring. It also provides a framework for developing new devices and wearables for the next generation of data-driven, technology-enabled cardiovascular care.
Keywords: digital health, remote patient monitoring, clinical decision support, electronic phenotyping, data analytics
THE DIGITAL TRANSFORMATION OF HEALTH CARE
Consider this scenario: A busy cardiovascular team is seeing an elderly female patient after a hospitalization for congestive heart failure. While she has experienced symptoms of heart failure, this is her first hospitalization. The care team collects pertinent information from the electronic health record such as comorbidities, medications, and diagnostic tests. They begin to formulate a plan to optimize treatment and suggest sensor-based management of pulmonary artery pressure. The patient asks several important questions, including: “How will the team use this information?” “How do I participate in this plan that uses a new technology?” and “Why did I develop symptoms of heart failure now?” As the cardiovascular team maps a strategy for using technology and data, these questions become the focal point for the design of a care coordination program that incorporates patient participation, remote patient monitoring, and clinical decision support in an effort to improve her outcomes. The team also considers what additional data would enable a deeper understanding of how the patient's cardiovascular function changed over time and how it could have better identified her cardiovascular risk.
Despite its ability to generate increasingly large quantities of electronic health data, health care continues to be reactive, treating a disease only after it is diagnosed. It is well recognized that this approach narrows our capacity to implement preventive measures and assumes that individuals mimic populations both in disease trajectories and in their response to treatments. Limited by the constraints of modern care delivery, healthcare organizations have yet to develop systematic methods for incorporating new digital health technologies into everyday practice and delivering care through virtual and remote techniques.1 Such technologies include wearable and wireless devices (activity monitors, sleep sensors, medication adherence devices), smartphone-connected technologies (single/multiple-lead ECG, handheld ultrasound), implantable sensors (pulmonary artery sensors, continuous glucose monitoring), and various lab-on-a-chip platforms.2 These digital devices are constantly expanding and becoming increasingly sophisticated in their ability to quantify physiologic measurements through advanced computational approaches, thus challenging our contemporary methods for how risk is measured and ultimately for how a disease is detected and monitored.3,4
Since 2015, the number of wearable and digital health devices that have been purchased in the United States has increased from 10 million devices to nearly 100 million, with a market size that has exponentially grown from $3 billion to nearly $25 billion.5 While this growth highlights the enthusiasm for the rapid deployment of digital healthcare technologies, it is also concerning in that we are beginning to adopt a new method for patient care without the requisite evidence to ensure that is effective and actually leads to greater healthcare efficiency.6,7 We do not yet understand the association between real-time digital health data and patient outcomes, nor do we understand how risk is measured longitudinally through the aggregation of data collected from home environments. These unknowns raise critical questions regarding which factors guide the effective use of digital health devices in patient care, how new digital technologies and data analytics should influence the patient-clinician interaction, and the type of infrastructure that is needed to deliver care that is virtual, timely, and effective.
If we aim to make digital health a standard of care, it first requires an understanding of utilization factors and how patients and providers integrate new technologies and data analytics within clinical workflows. With this understanding, we can join the following three synergistic priorities to form the foundation upon which new digital innovations can advance healthcare delivery: (1) Develop a co-designed patient-centered digital health strategy that is efficient within the continuity of care; (2) implement digital health technologies within remote patient-monitoring programs centered on patient-generated data; and (3) implement “electronic phenotyping” through translational bioinformatics analyses of multidimensional datasets to produce individualized approaches to risk assessments and treatments. Developing a systematic approach that incorporates these three methods to integrate new digital health devices into cardiovascular care will best position our efforts to respond to the aforementioned questions.
PATIENT-CENTERED CO-DESIGNS
A technology acceptance model is a framework that models how users come to accept and use a new technology. Technology acceptance models based on the “Theory of Reasoned Action”—which explains the relationship between human attitudes and behaviors—state that key factors for new technology adoption in patient care are its perceived ease of use and usefulness.8 Patients and clinicians are frequently engaged in iterative phases of development that entail various activities aimed at increasing the success of a digital intervention. The key components for technology acceptance that are important for patients are fundamentally rooted within core principals of adoption, which include (1) identifying the determinants of adoption at the individual level (ie, functionality, operational efficiency), (2) testing the proposed adoption (ie, how digital information flows between the patient, caregiver, and clinician), and (3) developing a pre- and post-implementation strategy that can enhance a patient's use of a new technology (ie, iterative testing with user feedback). Rather than using usual deployment-evaluation cycles in which successive measures of adoption are collected over time, patient participatory models that employ an upfront period of engagement may be sufficient to solve key challenges for device utilization and identify those factors leading to long-term retention.9 It is therefore important to empirically define effective digital engagement and what constitutes a behavioral change.
In this context, the Telehealth Literacy Project evaluated upfront co-design prior to implementing a remote monitoring program for chronic diseases in elderly patients. Patient participation included mutual acceptance of the type of technologies selected to optimize its functionality and experience. This led to improvements in health literacy, health management skills, and access to health information related to a given patient's care.10 Similarly, Walsh and colleagues evaluated co-design as part of an eHealth platform for self-management of cardiovascular disease and cardiac rehabilitation.11 In their experience, organizing patient input upfront for the types of behavior and cognitive techniques needed—and using an iterative co-design process centered on education, enablement, and training—facilitated a better understanding of how an eHealth infrastructure can enable self-care. While co-design addresses key adoption challenges for a new technology, it can also be used to design a monitoring strategy to overcome common barriers (eg, form factors, physical limitations, and familiarity with Internet and Smartphone use) that would otherwise impede utilization and to identify which devices works best and in whom.
In this context, elderly patients, particularly high-risk elderly patients, represent a target population in whom the outcome of digital health and its derived benefit may be the greatest. Contrary to this assumption, the United Kingdom's Whole Systems Demonstrator trial aimed to determine the health outcomes, utilization, and barriers to the implementation of digital health monitoring in more than 3,000 elderly patients with diabetes, heart failure, or pulmonary disease. The study used a suite of devices for vital sign monitoring, oximetry, weight scales, and glucometry as well as ambient sensors in the patients' homes. At 12-months, while the outcomes of readmission and mortality were lower with digital health monitoring compared to standard care, several important determinants of adoption emerged.12,13 First, the patients who increased their digital health utilization may already have been motivated to make health and behavioral changes, especially when digital health monitoring was aligned with clinical decisions that resulted in benefit. This group may present an opportunity for health engagement with digital technologies. Second, those patients who did not use digital monitoring and withdrew from the trial largely did so due to the perceived threats towards their existing healthcare interactions. For example, patients perceived digital monitoring as a replacement for usual face-to-face visits, felt that it required self-care, had technical problems, and raised questions as to why digital monitoring was pursued in place of healthy aging. This study, executed in 2008-2009, has salient findings that present challenges for today, where the availability of devices are ubiquitous and the interest for patient care is ever increasing. Developing robust methodologies that integrate the who, how, and why within the different patterns of adoption and identifying how those patterns relate to health and disease trends will better position patients and their providers for more efficient technology utilization.
DIGITAL HEALTH REMOTE PATIENT MONITORING PROGRAMS
The integration of a self-managed remote patient monitoring (RPM) program for cardiovascular diseases using a digital health intervention is a primary focus because it is a contemporary phenomenon.14 Modern-day RPM programs harness patient-generated data—that is, any health-related data such as biometrics, symptoms, and patient-reported outcomes recorded by a patient. Based on the data they collect, patients can self-measure when desired and make self-informed decisions based on trends. Patient-generated data is distinguished from health data captured during a clinical visit, the latter of which often creates sporadic data trends. While there are concerns that patient-generated data may not be reliable because it is collected outside of a clinical setting,15 it is also valuable in that device measurements and their trends may result from lifestyle, environmental, and behavioral circumstances. These factors may or may not be immediately evident; however, they may be inferred when disease trajectories change from what would otherwise be expected, such as improvements in blood pressure or glycemic control that occur without a change in pharmacotherapy.16,17
The use of patient-generated digital health data supplemented by existing clinical information provides a more comprehensive picture of ongoing patient health. Through continuous data collection and analysis, patient-generated data offers opportunities to fill in information gaps for chronic disease management, utilize new workflows that enable clinical teams to participate in remote care, and develop a clinical decision support (CDS) system that allows clinicians and patients to communicate in real time. The implementation of patient-generated data specifically implies a model of care where patients actively acquire physiologic data on devices that are populated within electronic health records (EHRs), and the data is reviewed by clinicians in an ongoing basis (Figure 1). This type of model is cyclical in that additional clinical information is used to understand disease classifications with greater precision than single measurements alone.
Figure 1.
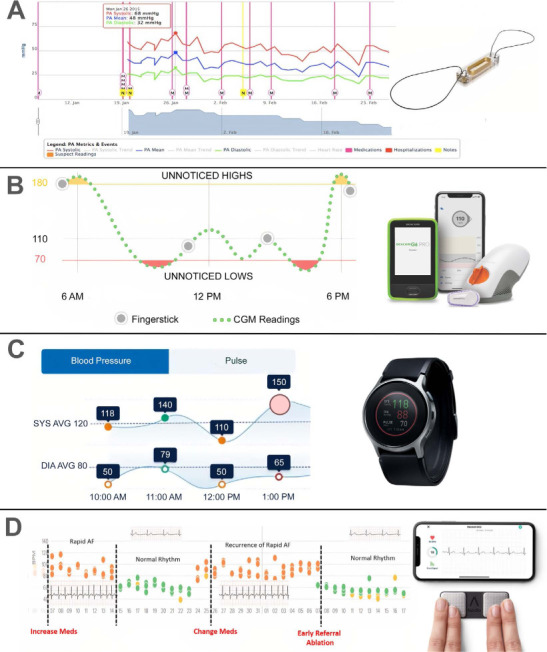
Examples of digital health monitoring devices used within real-world remote patient monitoring programs. (A) Implantable, wireless, pulmonary artery sensor for continuous pressure assessment in an 85 year old with heart failure with preserved ejection fraction and frequent hospitalizations. Red lines denote medication titration with subsequent reduction in pressure measurements leading to improved quality of life and for readmission risk reduction. (B) Continuous glucose monitor with patient-facing trends in a 64-year-old patient with type 2 diabetes and ischemic heart disease. Observations of AM hyperglycemia and PM hypoglycemia that require adjustments in medications to prevent further hypoglycemia and for lifestyle modification. (C) Wearable blood pressure monitor in a smartwatch form factor for patient-triggered blood pressure measurements in a 55 year old with hypertension and coronary disease. Tracings illustrate hypertensive episode predicted by an embedded algorithm analyzing sequential blood pressure trends. (D) Smartphone electrocardiogram (ECG) for patient-triggered single-lead ECGs in a 63 year old with paroxysmal atrial fibrillation and heart failure. Review of patient-generated data by clinical teams for medication adjustment (black lines) and to monitor recurrence of atrial fibrillation over time. Device images reprinted with permission from (A) Abbott, (B) Dexcom, Inc., (C) Omron Healthcare, Inc., and (D) AliveCor.
The real-world benefits of this model can be seen with the implantable pulmonary artery sensor, which monitors ambulatory pressure in patients with heart failure. Clinical teams use patient-generated data (ie, pulmonary artery pressures) to titrate medications and make lifestyle recommendations and can see how these modifications affect future pressure readings. Through this approach, real-world registries have demonstrated a greater per-patient reduction in pressure readings compared to those changes observed in randomized trials.18 This example demonstrates how a data-driven, technology-enabled care program can modify a chronic disease, promote ongoing patient participation, and lead to improved outcomes.19
ELECTRONIC PHENOTYPING ANALYZING MULTIDIMENSIONAL DIGITAL DATASETS
Predicting patient outcomes is primarily based on standard demographic characteristics and diagnostic testing. While conventional risk models provide predictive information for populations, the aggregation of multidimensional data including EHR data, imaging, laboratory, and digital health trends can enhance risk stratification at the individual level. This process depends on robust development of data-driven CDS systems that can be used at the point of care.1 Although current CDS systems are static in that they lack generalizability, use simplistic logic (ie, yes/no), or use rule-based methods (ie, diagnosis and treatment codes), leveraging recent developments in data analysis such as electronic phenotyping can help overcome some of these challenges. Electronic phenotyping is a translational bioinformatics method that identifies patients with a certain characteristic of interest or outcome though multidimensional data analysis.20 This analytic framework characterizes patients by a set of encoded representations and categorizes structured digital information into vectors to identify patterns in the data that can be used to design CDS systems.21
The ongoing use of digital health devices perpetuates a cycle of data collection and analysis that generates larger and higher-definition datasets, ultimately creating an electronic phenotype of a specific disease and its severity. In contrast to current standards of care that use laboratory and clinical markers (eg, blood pressure, cholesterol levels, or signs/symptoms of heart failure) to identify the onset of disease and thresholds upon which to initiate therapy, the continuous streaming of digital health data into EHRs can provide a data-rich portrait of a patient at the point of care (Figure 2). Through supervised and unsupervised machine learning approaches, predictive modeling such as patient similarity networking,22 cluster analyses,23 and deep learning24 can be used to characterize those baseline factors that drive progression from health to subclinical disease to pathological phenotypes. For example, encoded representations can link echocardiographic measurements to define how heart failure progresses from one class to another20 or to identify which cluster of hypertensive patients within a heterogeneous population is at higher risk.21 This method can then be used to map where a specific patient is within a disease process and determine which type of digital health technology and monitoring strategy can alter future projections.
Figure 2.
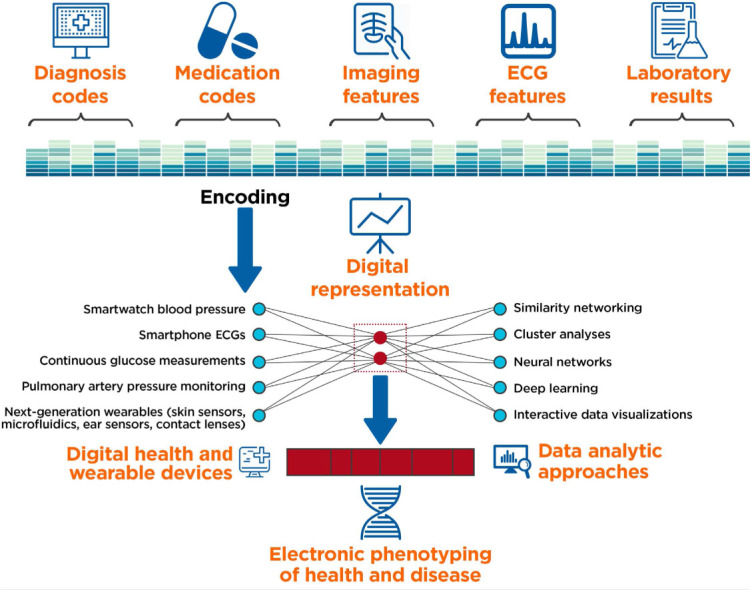
Electronic phenotyping through the analysis of multidimensional datasets (including electronic health record [HER] data, imaging, laboratory, echocardiography [ECG], and digital health device trends) can be used to define new patterns of health and disease.
In theory, such predictions can be used within CDS systems and at the patient-clinician interface where data analytics can augment the clinical interaction for shared decision making.25,26 Before techniques such as electronic phenotyping can be used within the process of care, it must first meet key criteria for reproducibility. On the one hand, bias assessment resulting from algorithm development is important as it can result in under- and overestimations as well as measurement error resulting from missing, incomplete, or misclassified data.27 On the other hand, it is vital that reproductivity ensures a similar outcome between real-world analyses and analyses upon which the initial analytic approach and algorithm were developed. In this context, generalizability is important to cross-validate electronic phenotyping and clustering within diverse patient populations, including cohorts of different age, race, and gender. Failure to ensure reproducibility can perpetuate an implicit bias propagating socioeconomic disparities and healthcare inequalities.28 While these do not result from machine learning methods themselves, they are inherent to the training and validation data sets upon which such computational approaches are developed.
THE IMPORTANCE OF COVID-19
The COVID-19 pandemic has necessitated a shift in healthcare delivery from a conventional approach of in-person and transactional care to one that is virtual, remote, and patient-generated. Telehealth and digital health technologies have enabled this transformation, and while virtual care will continue to find its place within new models of healthcare delivery, it has also magnified the barriers to equitable care in the United States.29,30 During the initial phases of the shelter-in-place order, nearly 3,000 telehealth visitations were scheduled between March and April 2020 at a University hospital in Philadelphia. In contrast to all types of telehealth visits, video visitations were less likely to be completed by black patients and those with the lowest median household income.31
Despite organizational shifts to telehealth and virtual care, tools designed to improve access to health care, socioeconomic disparities persist and are magnified. As such, equitable care and new models that include diverse patient populations and lower socioeconomic communities and drive greater and more efficient access are imperative within a changing healthcare landscape. The digital divide is defined as a lack of access to digital and communication technologies between distinct groups.6 In health care, the digital health divide not only identifies groups with limited access to new devices but also groups in which a higher burden of disease exists.32 This paradox between the need to risk stratify and monitor patients and the lack of access to available technologies to augment treatment approaches that mitigate risk is also primed for change. COVID-19 can potentially balance the scales towards equanimity.33 Patient participation, provider engagement, and data analytics will become increasingly important. Incorporating broad patient clusters within population health may overcome inherent biases and, in doing so, may drive a lower cost of care, particularly among those most vulnerable to high healthcare expenses.
NEXT-GENERATION TECHNOLOGY-ENABLED MODELS OF CARDIOVASCULAR CARE
Implementing new technologies in clinical settings should begin with patient participation and end with patient outcomes. As wearable devices become more miniaturized with new form factors ranging from epidermal microfluidics and smart contact lenses to stretchable skin sensors and ear-sensing technologies,34–37 passive biochemical and cardiovascular monitoring may emerge as standard approaches in health care. To maximize this potential, it is essential that we employ robust approaches for how new devices are used within remote monitoring programs and how advanced analytics on continuously streaming digital health data will be integrated into clinical decision support systems. By doing so, a new cardiovascular ontology of data-driven phenotypes can be targeted to uncover hidden features of health and disease and, ultimately, improve the quality of care across the cardiovascular care continuum.
KEY POINTS
Digital health is a rapidly growing field in health care that will eclipse 100 million connected devices in 2020.
Patient-centric co-designs is an important implementation factor for how new devices are effectively used by patients and their caregivers in home-based environments.
Remote patient monitoring and the acquisition of patient-generated data represents a new approach upon which physiologic trends are measured and assessed within the continuity of care.
Analytic approaches such as artificial intelligence and machine learning are under development to produce new “electronic phenotypes” of cardiovascular disease and to translate these phenotypes within an iterative process that merges digital health data and dynamic clinical decision support systems for individualized cardiovascular risk stratification.
Footnotes
Conflict of Interest Disclosure:
Dr. Bhavnani is a scientific advisor to Analytics 4 Life and Blumio; consultant to Bristol Meyers Squibb and Pfizer; and data safety monitoring board chair at Proteus Digital. He has received research support from Scripps Clinic and the Qualcomm Foundation and is a member of the innovation advisory boards at the American College of Cardiology, American Society of Echocardiography, and BIOCOM (all nonprofit institutions with all positions voluntary).
REFERENCES
- 1.Bhavnani SP, Sitapati AM. Virtual Care 2.0-a Vision for the Future of Data-Driven Technology-Enabled Healthcare. Curr Treat Options Cardiovasc Med. 2019 Apr 15;21(5):21. doi: 10.1007/s11936-019-0727-2. [DOI] [PubMed] [Google Scholar]
- 2.Bhavnani SP, Narula J, Sengupta PP. Mobile technology and the digitization of healthcare. Eur Heart J. 2016 May 7;37(18):1428–38. doi: 10.1093/eurheartj/ehv770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019 Jan;25(1):44–56. doi: 10.1038/s41591-018-0300-7. [DOI] [PubMed] [Google Scholar]
- 4.Sengupta PP, Adjeroh DA. AI tracks a beating heart's function over time. Nature. 2020 Apr;580(7802):192–4. doi: 10.1038/d41586-020-00819-6. [DOI] [PubMed] [Google Scholar]
- 5.Rock Health [Internet]. San Francisco, CA: Rock Health; c2020. 2019 Mid-year Digital Health Market Update: Exits are heating up; 2018 [cited 2020 Aug 3]. Available from: https://rockhealth.com/reports/2019-midyear-digital-health-market-update-exits-are-heating-up. [Google Scholar]
- 6.Bhavnani SP, Parakh K, Atreja A et al. 2017 Roadmap for Innovation-ACC Health Policy Statement on Healthcare Transformation in the Era of Digital Health, Big Data, and Precision Health: A Report of the American College of Cardiology Task Force on Health Policy Statements and Systems of Care. J Am Coll Cardiol. 2017 Nov 28;70(21):2696–718. doi: 10.1016/j.jacc.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 7.Bhavnani SP, Harzand A. From false-positives to technological Darwinism: controversies in digital health. Per Med. 2018 Jul 1;15(4):247–50. doi: 10.2217/pme-2018-0033. [DOI] [PubMed] [Google Scholar]
- 8.Venkatesh V. Technology acceptance model and a research agenda on interventions. Decis Sci. 2008 May;39(2):273–315. [Google Scholar]
- 9.Michie S, Yardley L, West R, Patrick K, Greaves F. Developing and Evaluating Digital Interventions to Promote Behavior Change in Health and Health Care: Recommendations Resulting From an International Workshop. J Med Internet Res. 2017 Jun 29;19(6):e232. doi: 10.2196/jmir.7126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banbury A, Nancarrow S, Dart J et al. Adding value to remote monitoring: Co-design of a health literacy intervention for older people with chronic disease delivered by telehealth - The telehealth literacy project. Patient Educ Couns. 2020 Mar;103(3):597–606. doi: 10.1016/j.pec.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Walsh DMJ, Moran K, Cornelissen V et al. The development and codesign of the PATHway intervention: a theory-driven eHealth platform for the self-management of cardiovascular disease. Transl Behav Med. 2019 Jan 1;9(1):76–98. doi: 10.1093/tbm/iby017. [DOI] [PubMed] [Google Scholar]
- 12.Steventon A, Bardsley M, Billings J, et al. Whole System Demonstrator Evaluation Team. Effect of telehealth on use of secondary care and mortality: findings from the Whole System Demonstrator cluster randomised trial. BMJ. 2012 Jun 21;344:e3874. doi: 10.1136/bmj.e3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanders C, Rogers A, Bowen R et al. Exploring barriers to participation and adoption of telehealth and telecare within the Whole System Demonstrator trial: a qualitative study. BMC Health Serv Res. 2012 Jul 26;12:220. doi: 10.1186/1472-6963-12-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cruz-Martínez RR, Wentzel J, Asbjørnsen RA, et al. Supporting Self-Management of Cardiovascular Diseases Through Remote Monitoring Technologies: aMetaethnography Review of Frameworks, Models, and Theories Used in Research and Development. J Med Internet Res. 2020 May 21;22(5):e16157. doi: 10.2196/16157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdolkhani R, Gray K, Borda A, DeSouza R. Patient-generated health data management and quality challenges in remote patient monitoring. JAMIA Open. 2019 Sep 20;2(4):471–8. doi: 10.1093/jamiaopen/ooz036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McManus RJ, Mant J, Franssen M et al. Efficacy of self-monitored blood pressure, with or without telemonitoring, for titration of antihypertensive medication (TASMINH4): an unmasked randomised controlled trial. Lancet. 2018 Mar 10;391(10124):949–59. doi: 10.1016/S0140-6736(18)30309-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puhr S, Calhoun P, Welsh JB, Walker TC. The Effect of Reduced Self-Monitored Blood Glucose Testing After Adoption of Continuous Glucose Monitoring on Hemoglobin A1c and Time in Range. Diabetes Technol Ther. 2018 Aug;20(8):557–60. doi: 10.1089/dia.2018.0134. [DOI] [PubMed] [Google Scholar]
- 18.Heywood JT, Jermyn R, Shavelle D et al. Impact of Practice-Based Management of Pulmonary Artery Pressures in 2000 Patients Implanted With the CardioMEMS Sensor. Circulation. 2017 Apr 18;135(16):1509–17. doi: 10.1161/CIRCULATIONAHA.116.026184. [DOI] [PubMed] [Google Scholar]
- 19.Desai AS, Bhimaraj A, Bharmi R et al. Ambulatory Hemodynamic Monitoring Reduces Heart Failure Hospitalizations in “Real-World” Clinical Practice. J Am Coll Cardiol. 2017 May 16;69(19):2357–65. doi: 10.1016/j.jacc.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Banda JM, Seneviratne M, Hernandez-Boussard T, Shah NH. Advances in Electronic Phenotyping: From Rule-Based Definitions to Machine Learning Models. Annu Rev Biomed Data Sci. 2018 Jul;1:53–68. doi: 10.1146/annurev-biodatasci-080917-013315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shickel B, Tighe PJ, Bihorac A, Rashidi P. Deep EHR: A Survey of Recent Advances in Deep Learning Techniques for Electronic Health Record (EHR) Analysis. IEEE J Biomed Health Inform. 2018 Sep;22(5):1589–604. doi: 10.1109/JBHI.2017.2767063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tokodi M, Shrestha S, Bianco C et al. Interpatient Similarities in Cardiac Function: A Platform for Personalized Cardiovascular Medicine. JACC Cardiovasc Imaging. 2020 May;13(5):1119–32. doi: 10.1016/j.jcmg.2019.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo Q, Lu X, Gao Y et al. Cluster analysis: a new approach for identification of underlying risk factors for coronary artery disease in essential hypertensive patients. Sci Rep. 2017 Mar 7;7:43965. doi: 10.1038/srep43965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shameer K, Badgeley MA, Miotto R, Glicksberg BS, Morgan JW, Dudley JT. Translational bioinformatics in the era of real-time biomedical, health care and wellness data streams. Brief Bioinform. 2017 Jan;18(1):105–24. doi: 10.1093/bib/bbv118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miotto R, Li L, Kidd BA, Dudley JT. Deep Patient: An Unsupervised Representation to Predict the Future of Patients from the Electronic Health Records. Sci Rep. 2016 May 17;6:26094. doi: 10.1038/srep26094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Badgeley MA, Shameer K, Glicksberg BS et al. EHDViz: clinical dashboard development using open-source technologies. BMJ Open. 2016 Mar 24;6(3):e010579. doi: 10.1136/bmjopen-2015-010579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gianfrancesco MA, Tamang S, Yazdany J, Schmajuk G. Potential Biases in Machine Learning Algorithms Using Electronic Health Record Data. JAMA Intern Med. 2018 Nov 1;178(11):1544–7. doi: 10.1001/jamainternmed.2018.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Obermeyer Z, Powers B, Vogeli C, Mullainathan S. Dissecting racial bias in an algorithm used to manage the health of populations. Science. 2019 Oct 25;366(6464):447–53. doi: 10.1126/science.aax2342. [DOI] [PubMed] [Google Scholar]
- 29.Betancourt JA, Rosenberg MA, Zevallos A, Brown JR, Mileski M. The Impact of COVID-19 on Telemedicine Utilization Across Multiple Service Lines in the United States. Health care (Basel) 2020 Oct 1;8(4):E380. doi: 10.3390/healthcare8040380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yancy CW. COVID-19 and African Americans. JAMA. 2020 May 19;323(19):1891–2. doi: 10.1001/jama.2020.6548. [DOI] [PubMed] [Google Scholar]
- 31.Eberly LA, Khatana SAM, Nathan AS et al. Telemedicine Outpatient Cardiovascular Care during the COVID-19 Pandemic: Bridging or Opening the Digital Divide? Circulation. 2020 Aug 4;142(5):510–512. doi: 10.1161/CIRCULATIONAHA.120.048185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhavnani SP, Sola S, Adams D, Venkateshvaran A, Dash PK, Sengupta PP, ASEF-VALUES Investigators A Randomized Trial of Pocket-Echocardiography Integrated Mobile Health Device Assessments in Modern Structural Heart Disease Clinics. JACC Cardiovasc Imaging. 2018 Apr;11(4):546–57. doi: 10.1016/j.jcmg.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 33.Kichloo A, Albosta M, Dettloff K et al. Telemedicine, the current COVID-19 pandemic, and the future: a narrative review and perspectives moving forward in the USA. Fam Med Community Health. 2020 Aug;8(3):e000530. doi: 10.1136/fmch-2020-000530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bandodkar AJ, Wang J. Non-invasive wearable electrochemical sensors: a review. Trends Biotechnol. 2014 Jul;32(7):363–71. doi: 10.1016/j.tibtech.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Keum DH, Kim SK, Koo J et al. Wireless smart contact lens for diabetic diagnosis and therapy. Sci Adv. 2020 Apr 24;6(17):eaba3252. doi: 10.1126/sciadv.aba3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boutry CM, Nguyen A, Lawal QO, Chortos A, Rondeau-Gagné S, Bao Z. A Sensitive and Biodegradable Pressure Sensor Array for Cardiovascular Monitoring. Adv Mater. 2015 Nov 18;27(43):6954–61. doi: 10.1002/adma.201502535. [DOI] [PubMed] [Google Scholar]
- 37.Goverdovsky V, von Rosenberg W, Nakamura T et al. Hearables: Multimodal physiological in-ear sensing. Sci Rep. 2017 Jul 31;7(1):6948. doi: 10.1038/s41598-017-06925-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


