Abstract
Prosthetically driven workflows using CBCT, digital optical scanning, 3D‐printed molds and frameworks, and dental implant component attachments to osseointegrated fixtures can produce anatomically accurate, esthetic, durable silicone ear replacements.
Keywords: 3D imaging, 3D printing, auricular prosthesis, digital workflow, osseointegrated implants
Digital workflow designed to guide auricular implant placement for fixed retained prosthesis.
1. INTRODUCTION
The right ear of a 55‐year‐old man was replaced using a digital workflow. Osseointegrated implants in the right mastoid were fitted with abutments and snap attachments (indexed to a 3D‐printed chrome‐cobalt framework). The final silicone prosthesis was fabricated using optical scanning and a 3D‐printed resin mold, achieving durable and esthetic results.
The more widespread use of digitally driven additive manufacturing (AM; 3D printing) as a basic alternative to subtractive manufacturing (SM, ie, milling) has generated some interesting recently published research in prosthetic medical care overall. Its established and emerging applications in combination with tissue engineering, living cell constructs, and biomaterials for otorhinolaryngology, 1 , 2 its advantages and limitations for surgery, 3 and its emerging mainstream status in health care and medicine in general 4 have been recently reviewed. Specifically, AM has been recently reviewed in regard to implant dentistry by Katkar et al, who cite 3D printing of objects as a potential cost obstacle. 5 Eley et al cite uniform availability of 3D‐printing technology within a given system (ie, the United Kingdom's NHS), and the potential advantages of establishing a centralized system to manage access and costs. 6 Barazanchi et al (2017) reviewed the current status of the use of AM in dentistry, citing the advantages of reduced waste, flexibility of use with a wide array of build materials, and energy efficiency, as compared to conventional SM, specifically suggesting suitability of AM for printing chrome‐cobalt and other difficult‐to‐handle materials. 7
Recent systematic reviews (2016) by Tack et al 8 and Martelli et al 9 cite the need for more structured cost‐benefit analyses to gain a more practical perspective on cost management in 3D printing.
In the current treatment scenario, the implementation of digital protocols to plan and execute AM processes for production of an auricular prosthesis via combined digital diagnostic, image capture/measurement, and production workflows offered anatomic accuracy, streamlining of process, expanded access, and time reductions for the provider team and our patient, compared with conventional analog workflows.
While clinically accurate, reliance on analog technologies to fabricate auricular prostheses 10 , 11 , 12 to replace ears lost due to accident, congenital absence or malformation, or surgical removal secondary to oncology concerns, can also be time‐intensive and costly. The aim of this study was to produce an auricular replacement prosthesis that directly mirrored the patient's contralateral ear, using a completely digital workflow, with final casting of the platinum‐cured silicone portion of the prosthesis using a three‐dimensional (3D)‐printed mold. Widespread adoption of such workflows could help reduce mainstream use of analog‐dependent fabrication methods currently taught and used in clinical practice. Ultimately, this could accelerate completion of a final prosthesis, reduce the overall costs for both patients and surgical/prosthetic teams involved, and offer both groups the benefits and convenience of remote access made possible by an optimally coordinated digital workflow comprising scanning, data capture, and computer‐aided design and manufacture (CAD/CAM) that includes AM/3D printing. For the current treatment scenario, the workflow needed to be concise, easily duplicated, and safe for the patient, enabling a precise outcome with low incidence of error. A digitally enabled and practical approach offers expanded treatment options, especially in remote areas with limited access for patients to specialized prosthetic care.
1.1. Case Report
A 55‐year‐old Caucasian man was missing his right ear due to a work‐related accidental injury approximately 6 months prior to an initial dental consultation in the author's dental practice. Several attempts to reattach the ear failed. After evaluation for auricular replacement, he underwent 3D digital treatment planning using cone‐beam computed tomography (CBCT) imaging, followed by guided surgery using implant‐placement software and 3D‐printed surgical guides for the placement of four VistaFix implants into the mastoid portion of his right temporal bone[REF Domingue et al surgical paper submitted to Clinical Case Reports 2020], which healed without complications.
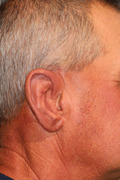
After 4 months' healing time, exposure of these implants was performed in the operating room, using sterile technique, endotracheal intubation, and general anesthesia. Local anesthesia (1% lidocaine with epinephrine) was administered in the area of the planned incision. The incision was made sharply, and the skin and subcutaneous tissue were elevated anteriorly toward the external auditory canal. The four previously placed implant cover screws were exposed (Figure 1A). All screws and implant fixtures were examined, and the fixtures were found to have osseointegrated well, without complication. Only three of the four implants were required for fixation of the final prosthesis; the posteriormost fixture and its cover screw were retained and covered again as a potential "backup," if needed in the future.
FIGURE 1.
A, Surgical reentry and exposure of implants. B, Primary closure with healing abutments
A single Vistafix healing abutment was placed over the superiormost implant (Figure 1B). The other two implants received Nobel BioCare healing abutments (all 5‐mm length) of the type used for dental implant healing (these were used initially due to a component supply issue). Primary closure was obtained with subcutaneous 3‐0 polyglactin and superficial 5‐0 plain gut for tension‐free primary closure.
After 2 weeks of healing, the patient was seen for follow‐up. The two Nobel BioCare healing abutments were submerged and surrounded by swollen soft tissue. Inflammation was present, likely due to insufficient height of the healing abutments. These were replaced by two Vistafix abutments (both 7.5‐mm length), each with a 14‐mm diameter healing cap, in order to prevent the tissues from swelling around the implant healing abutment again. The surgical site was allowed to heal for 8 weeks. In the interest of optimizing the healing process, time, and resources, no interim prosthesis was planned or constructed.
A chrome‐cobalt bar with integrated attachments was selected as the best option for rigidity and retention of the prosthesis with a strong, secure connection, as opposed to ferromagnetic components that would require removal prior to any MRI scanning. A chrome‐cobalt substructure was selected that could be laser‐sintered using metal 3D printing, and subsequently picked up in the silicone of the final ear prosthesis. Ample prosthetic space was present between the implants and replacement ear. The patient's motor skills were deemed compatible with insertion and removal of such a snap attachment prosthesis framework, further supporting the decision to avoid magnetic attachments.
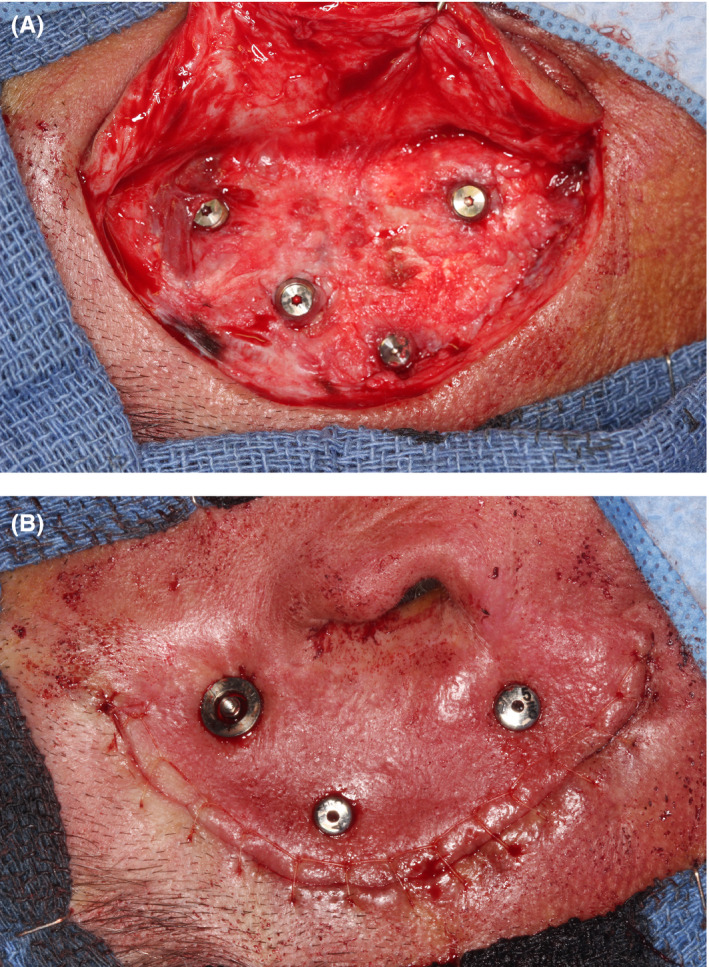
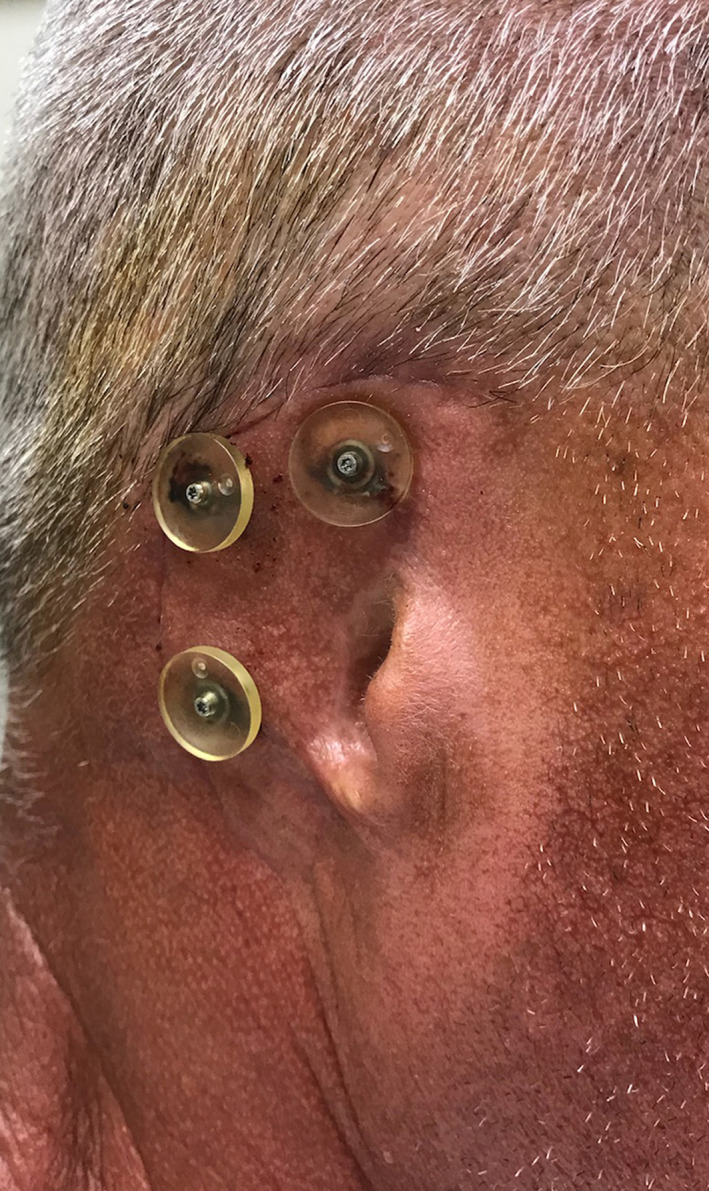
Ideal healing conditions were observed at 8 weeks postexposure (Figure 2); swelling resolution required 4 weeks. The healing abutments were removed, and gold‐hue titanium plasma‐sprayed Locator® overdenture attachments (Zest Anchors, LLC) were screwed into the implants and torqued to 35 Ncm. BIO | Locator‐compatible Super Snap attachment housings (BlueSkyBio) were placed over the Locator attachments (Figure 3), and an optical scan (3Shape) was obtained to document soft‐tissue healing and capture surface topography.
FIGURE 2.
Vistafix 7.5‐mm × 14‐mm healing abutments 8 wk postuncovering; optimal healing is evident
FIGURE 3.
Super Snap attachment housings positioned over Locator attachments
After removing the Super Snap attachment housings shown in Figure 3 and placing overdenture attachments (Blue Sky Bio) on the abutments on the patient, another digital scan was obtained with the Locator attachments in place (Figure 4A).
FIGURE 4.
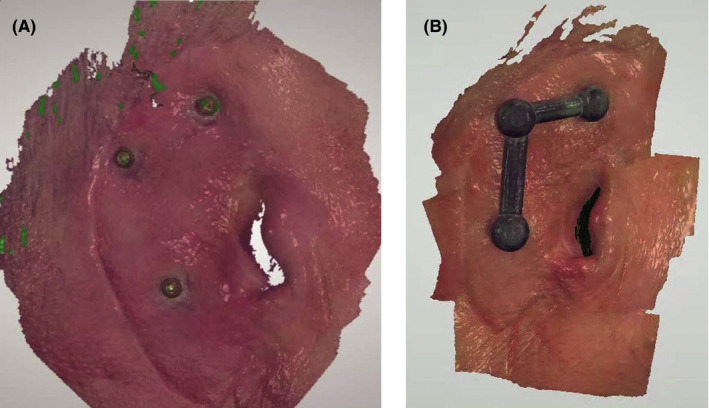
A, Locator attachments, on abutments on patient, as scanned into 3Shape. B, Virtual design of metal bar over Locators
Next, Super Snap housings (Blue Sky Bio) were virtually positioned onto the abutments using a free computer‐aided design (CAD) software (MeshMixer, Autodesk, Inc). These housings had to be picked up directly with heat‐processed indexing acrylic, which required at least 0.5 mm of space all around each housing. To accomplish this, the shape of the Super Snap housings was virtually extended in all directions by 0.5 mm to provide the required space; then, a further 1 mm of thickness was added virtually, to accommodate the minimum thickness for the metal 3D printer to be used to print the chrome‐cobalt framework (SLM® 280 dual‐laser 3D printer, SLM Solutions Group AG).
A portion of the mesh was digitally transformed, then virtually manipulated and pulled to the adjacent coping to connect it (Figure 4B). This was again scanned and captured using the 3Shape scanner (Figure 4B) for printing of the bar framework. The STL file for the resulting bar was sent for direct 3D‐printing in chrome‐cobalt metal. (Figure 5A).
FIGURE 5.

A, Metal framework design. B, Fixation of metal framework for indexing to Locators, prior to pickup of Super Snap attachments placed in framework
Because green (medium retention) Super Snaps have been shown to give 3 lbs of retentive force over 250 000 insertion‐removal cycles without losing retentive force, placement of these attachments within the framework was deemed the optimal solution for continued long‐term retention of the prosthesis.
Teflon was placed over the Locator attachments to prevent the framework from locking in during indexing (Figure 5B). Each Super Snap attachment in the framework was simultaneously picked up in acrylic to ensure passive seating and accurately index the Super Snap attachments in the framework with the Locator attachments on the abutments (as originally positioned on the patient, see Figures 3 and 4A,B.
One challenge in fabrication of this silicone auricular prosthesis was that the virtual ear created by mirroring the patient's opposite ear was too thin in many areas to fully encase the metal framework. To create adequate thickness of the silicone, areas of insufficient thickness were virtually "inflated" until there was a minimum of 3 mm of space around the bar in these areas. Once the shaping adjustments of the ear were complete, its final form was created by performing a virtual Boolean subtraction of the tissue surface within the MeshMixer software (Figure 6A).
FIGURE 6.
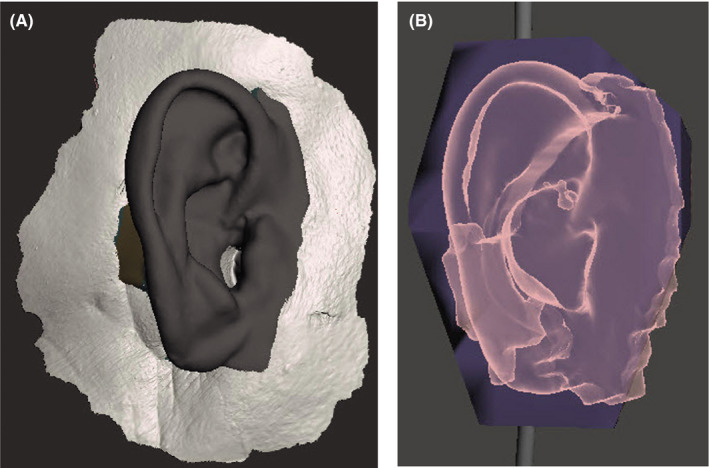
A, Virtual construction image of final ear for use in 3D printing of resin injection mold for production of final silicone prosthesis. B, Virtual pattern in MeshMixer for STL file used to 3D‐print digital mold
Direct 3D printing of custom‐colored silicone to accurately replicate the color variations of the human ear is not yet available.
This final ear could have been 3D‐printed as a burnout pattern from its scanned STL file, and invested for conventional casting. However, in keeping with our desire to maintain as complete a digital workflow as possible, we opted to design the actual mold itself digitally for 3D printing. This was accomplished by generating a virtual rectangular box shape around the virtual ear and then performing a second virtual Boolean subtraction of the ear from the box within the MeshMixer software (Figure 6B). This left a negative void inside the box in the shape of the ear that could be injected with silicone. In order to facilitate removal of the silicone ear, the virtual mold was cut into 3 parts. Addition of virtual small box and sphere forms aided in the indexing of the pieces of the mold into the proper position. The STLs for the 3‐part mold were printed in resin and sent to the anaplastologist (AV) for casting and shade characterization.
3D printing of the mold can expedite the otherwise traditional mold‐making process, which relies on the use of gypsum products. Furthermore, having a mold fabricated allows for exact duplicates to be fabricated and delivered when replacements are needed for the patient. This reduces overall time for the patient's future continuity of care.
The patient met with the anaplastologist to custom match and mix color samples to be used for the definitive prosthesis, which was made from a platinum‐cured silicone (2186Fast, Factor II, Inc). Five different colors were mixed in natural lighting conditions to match variations in the patient's natural anatomic auricular and facial structures and skin tones (Figure 7A). Reference photographs were taken for later use.
FIGURE 7.
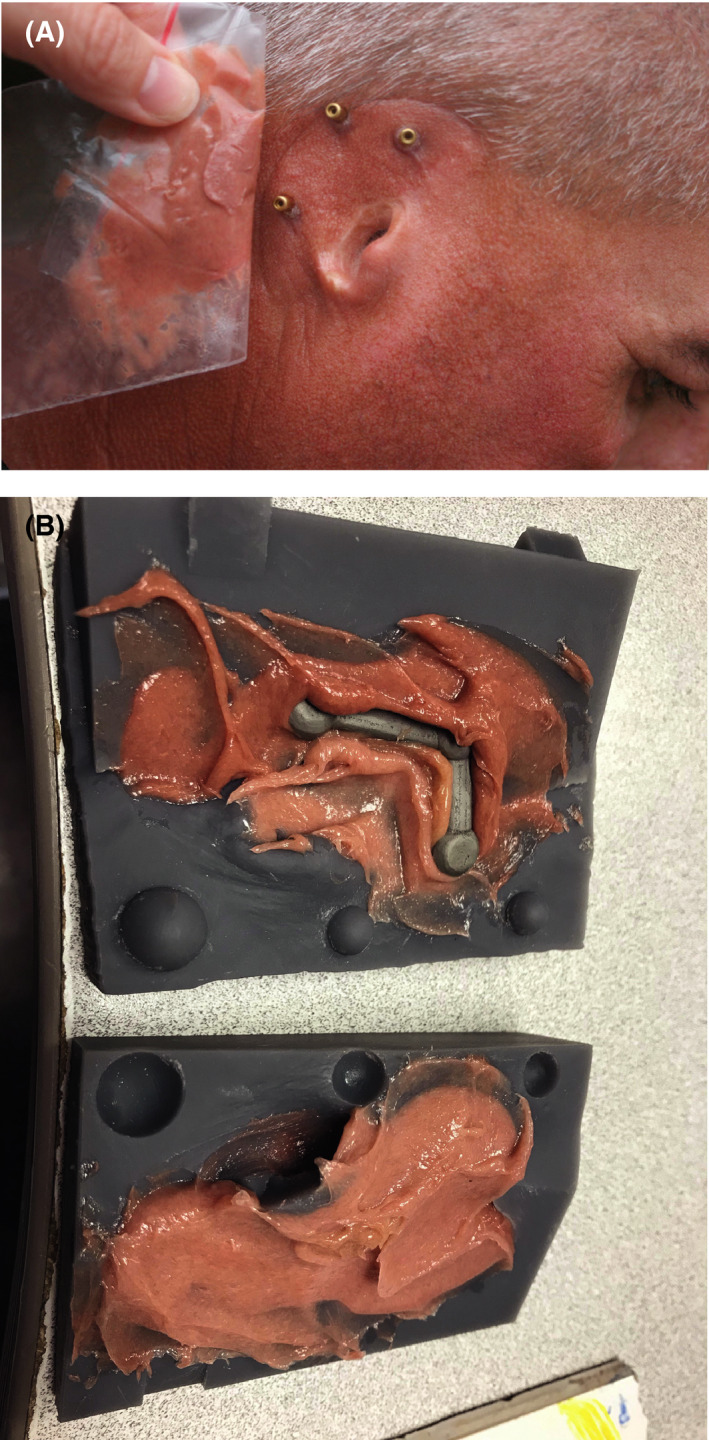
A, Custom color matching of silicone to patient for fabrication of prosthesis. B, Custom five‐color‐shaded silicone and chrome‐cobalt framework in 3D‐printed resin mold
Two versions of the designed mold were printed. The first version was printed in a high‐heat material (High Temp Resin, Formlabs, Inc). However, this version would not close, even with pressure clamps. A second version of the mold was printed in an engineering resin (GreyPro Resin, Formlabs, Inc; this mold version closed upon applying pressure. The metal bar was trial‐fitted into the GreyPro Resin 3D‐printed mold. Some areas in the bottom of the mold had to be relieved to allow space for the metal to fit passively. Approximately 2 mm of resin was ground out. The metal bar was cleaned and primed for silicone bonding. It was placed securely into the mold before painting intrinsic colors into the mold (Figure 7B). The 5 colors of silicone were carefully placed into the desired locations of the Grey Pro Resin mold. The mold was slowly clamped closed, allowing extra silicone to extrude from the vent holes. The silicone‐containing mold was slowly heated to the silicone's material safety data‐sheet specification of 180 degrees Fahrenheit. The mold was opened, the silicone ear trimmed, and its flashing removed (Figure 8).
FIGURE 8.
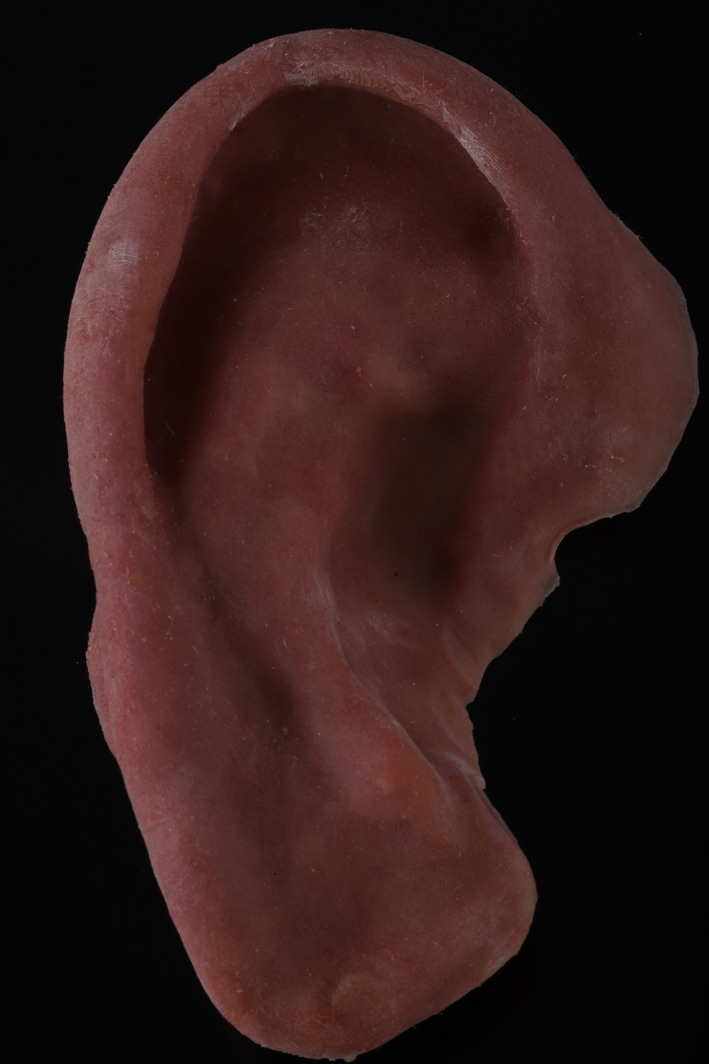
Final silicone prosthesis after finishing and shading adjustments
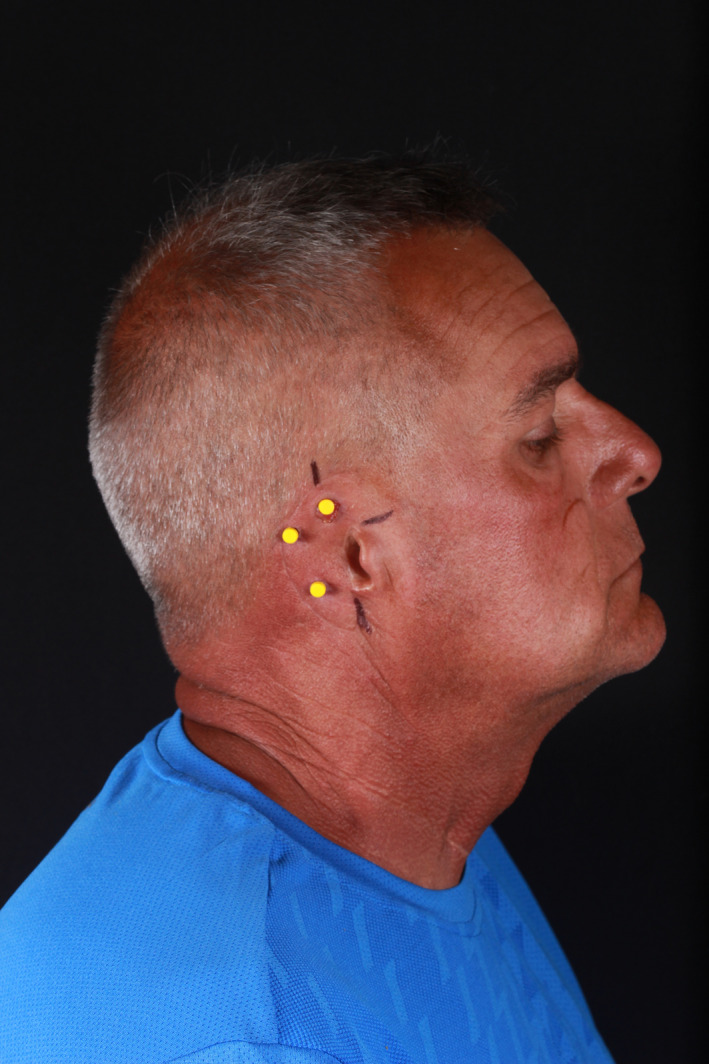
On the day of delivery, the prosthesis was tried on and trimmed again to provide a comfortable and esthetic result. An audible snap could be heard on each attachment, indicating a strong, nonmobile fit. The silicone ear was then extrinsically tinted (Figure 9A shows the prosthesis in place post‐tinting) for a more exact color match to the contralateral side (Figure 9B) using a condensation‐cured silicone. Figure 10 shows the patient's full profile with the finished prosthesis in place with natural lighting conditions.
FIGURE 9.
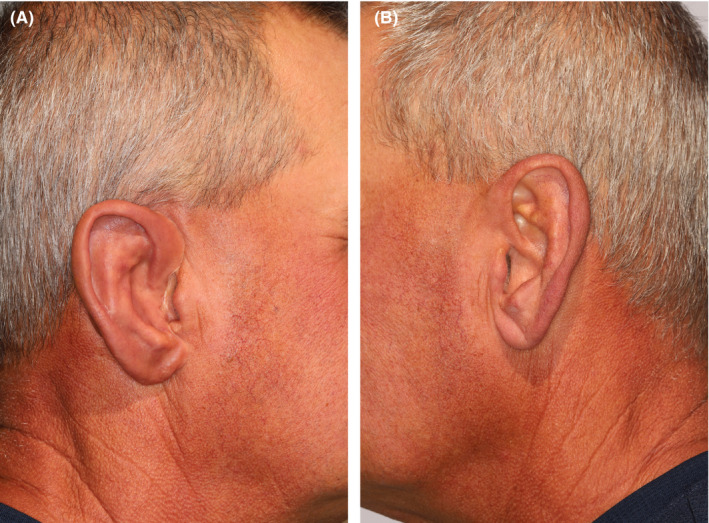
A, Right‐side patient profile with auricular prosthesis in place. B,Contralateral ear
FIGURE 10.
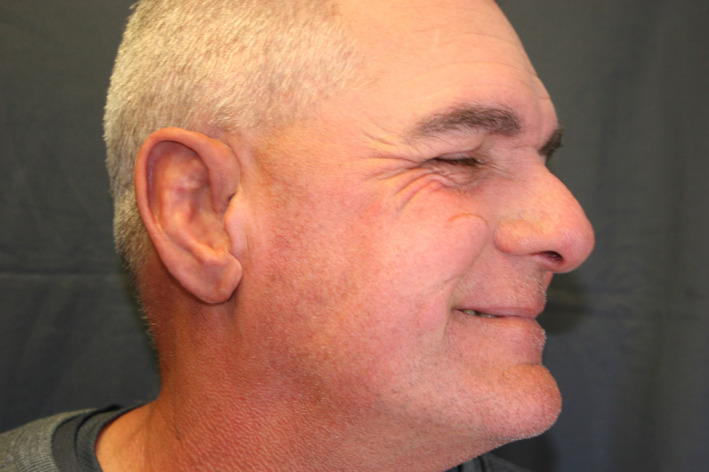
Prosthetic right ear in situ with patient's full profile
Of note, the 3D‐printed mold did not provide appreciable skin texture as compared to traditional molding techniques, a challenge explored from various angles in several recent studies. 13 , 14 , 15 , 16 To compensate, the anaplastologist extrinsically applied layers of silicone in desired locations to simulate skin texture. This provided a more natural appearance. The Super Snap attachments were distorted during the heating process, so they were replaced chairside with three new attachments. The patient was then instructed on how to remove and attach the ear himself. The patient was pleased with the appearance and fit of the final prosthesis.
2. DISCUSSION
Recently published research describes the use of various digital workflows to create auricular prostheses. 13 , 14 , 16 , 17 , 18 , 19 , 20 To the authors' knowledge, none specifically describes the process used in the current treatment scenario, in particular, the use of a 3D‐printed mold for injection of silicone for the final prosthesis (ie, a rapid‐tooling [RT] approach), subsequent to 3D CBCT‐guided implant placement in the initial surgical phase.[REF Domingue et al surgical paper, submitted to Clinical Case Reports 2020].
Overall, accurate reproduction of fine skin texture features on auricular and other craniofacial prostheses is acknowledged as challenging, and is an area of active research to refine digital workflows that use various AM/3D‐printing modalities to better reproduce such fine anatomic detail.
One recent study by Unkovskiy et al compared various additive manufacturing methods to directly 3D‐print auricular replicas based on in vivo scan data, and found forced deposition modeling to most accurately reproduce skin surface features. 14 In contrast with our RT workflow (ie, a directly 3D‐printed mold), that study used a rapid‐prototyping approach (ie, construction of a positive prototype prosthesis that was in turn used to construct the mold indirectly). These authors have also proposed a digital database containing numerous anatomic representations of such textures, to further simplify digital workflows. 13
Use of optical scanning of the contralateral ear (such as the detailed approach described by Ballo et al), 19 direct and indirect molding 16 have been reported as being clinically useful in creating anatomically and esthetically precise ear replacements, using digital workflows to varying degrees, with varying degrees of accuracy and consistency in regard to faithful reproduction of anatomic features and esthetics. 13 , 14 , 16
A specific comparison between conventional and digital workflows was published by Unkovskiy et al. 16 This case report compared three approaches to fabricate three separate prostheses: direct mold‐making (DMM; similar to our approach), indirect mold‐making (IMM), and the conventional use of thermoplastic wax in a three‐part stone mold. The authors concluded that IMM provided the best overall result (closest to that of conventional fabrication), and afforded the greatest opportunity for adjustment and color matching. They also emphasized the critical importance of involving a skilled anaplastologist, whose services were integral to all three approaches studied. They cited unpredictability of result and obviation of any try‐in of the prosthesis as the main drawbacks of the DMM approach, which we used in the current treatment scenario.
Such DMM injection molding into a 3D‐printed mold (ie, rapid tooling) was chosen over AM/3D‐printing of the ear itself because of the desired biocompatible properties of the silicone material that was finally chosen for the prosthesis. The chosen material (2186F) has a durometer of Shore A29, and its tear strength is 90 ppi, which provides a desirable combination of softness and durability.
To the authors' knowledge, direct 3D printing of this type of medical silicone is not being used clinically for auricular prostheses. Based on a 2018 case report by Unkovskiy et al that evaluated direct 3D printing of an interim nasal prosthesis, 15 this technology has not yet achieved a standard of clinical esthetic and skin‐feature reliability to be practically and consistently applicable to maxillofacial prosthetics, especially in regard to marginal adaptation and color matching. 15 In vitro studies by Jindal et al (2016 21 and 2018 22 ) have assessed certain aspects of the necessary physical properties of such a silicone prosthesis material, but did not address ear prosthesis printing, color, or esthetics in specific detail. 21 , 22
Importantly, the preoperative CBCT scans obtained in the treatment‐planning phase of our patient's auricular replacement provided a 3D representation of his anatomic structures prior to implant placement, and were essential in guiding the surgeon (JRW) to avoid large mastoid air cells in a highly pneumatized mastoid with few areas of cortical bone thick enough for housing the implants.[REF Domingue et al surgical paper, submitted to Clinical Case Reports 2020] This foundational step in the digital workflow process yielded diagnostic data preoperatively that proved critical to the implant‐placement process.
Although, in the authors' opinion, only two implants are necessary to retain an ear prosthesis, three of the four placed during surgery were used for this one in order to distribute insertion and removal forces more effectively. In parallel with this approach, the fourth implant was placed as a backup, as placing redundant implants permits erring on the side of caution and making optimal use of a single (ideally, the only) surgical procedure. In the event of implant failure, uncovering one of the redundant implants is far less challenging than having to place an additional one de novo, especially in view of the challenging osseous anatomy in this patient.
Continuing the digital workflow through optical scanning, mirror imaging of the contralateral ear, and 3D printing of the metal framework as well as the mold used to fabricate the final silicone prosthesis, and subsequent color and skin‐texture adjustments postproduction proved to be a simple straightforward process that produced an anatomically and esthetically precise and cost‐effective replacement ear.
Importantly, despite our desire to adhere as closely as possible to a digital workflow, this treatment scenario exemplifies the still‐critical need for the artistic skills of the experienced anaplastologist in achieving as esthetically undetectable a prosthesis as possible.
The digital workflow process used to produce this auricular prosthesis proved to be straightforward, accurate, easy to use, and faster compared with conventional analog fabrication, which is typically more technique‐sensitive and can be confounded by variables such as (a) impression pressure on the tissues; (b) shrinkage of material; (c) stone expansion; and (d) limitations of a two‐dimensional view throughout the process, which may not account accurately for implant positions.
Including the surgical implant‐placement phase, the entire time required to provide our patient with a finished prosthesis was between 5 and 6 months.
Continued research into the refinement, standardization, and centralization of digital workflows for 3D print fabrication of craniofacial prosthetics, and the use of centralized digital anatomic databases 13 is warranted.
Such an interdisciplinary case does require the coordination of multiple resources, materials, equipment, and skill sets, as well as collaboration among several healthcare disciplines, all of which carry potentially significant costs. However, standardizing such workflows to be as digitally driven and collaborative as possible could ultimately enable the coordination of such resources into a progressively reproducible, streamlined, and, overall, more cost‐effective care model as the technology continues to evolve. Most of the steps in the workflow described in this case report are at least partially reimbursable under Medicare, state‐based Medicaid programs, and some private insurance carriers. We believe the virtual planning protocols used for this case are in their infancy, as reflected by the ongoing and recently published research cited above. The workflow processes reported in this article can be improved upon by other clinicians and researchers who continue to help patients achieve optimal craniofacial prosthetics solutions.
CONFLICT OF INTEREST
NCG is a consultant for Blue Sky Bio, LLC, which produces components and software used in the placement and construction of the prosthesis described. All other authors declare no conflict of interest in connection with the current study.
AUTHOR CONTRIBUTIONS
DD: developed and oversaw the surgical and prosthetics treatment plan, patient was a patient of record in DD's restorative/implantology practice, produced initial draft of manuscript, attached final prosthetic ear on patient after AV finished customization, critically reviewed and provided input on all drafts, and approved final version of article manuscript; NCG: developed protocol for creating and 3D printing digital metal substructure bar for prosthesis and digital box to invest replacement ear, oversaw computer‐aided design of final prosthetic ear using various software programs in collaboration with AV, constructed 3D‐printed resin molds in collaboration with DD, drafted pertinent manuscript text in collaboration with AV, critically reviewed and provided input on all drafts, and approved final version of article manuscript; AV: performed custom shade matching of the silicone with the patient's skin tones, primed metal framework for silicone bonding, placed and heat‐cured initial custom‐shaded silicone within the engineering resin mold, performed final skin‐tone and skin‐texture characterization (post‐try‐on) of the final prosthesis using condensation silicone, drafted manuscript text (in collaboration with NCG) to describe fabrication steps, critically reviewed and provided input on all drafts, and approved final version of article manuscript; JRW: performed initial implant‐placement surgery and implant‐uncovering surgery on patient, in the operating room, drafted manuscript text to describe surgical implant‐uncovering procedure, critically reviewed and provided input on all drafts, and approved final version of article manuscript.
ETHICAL APPROVAL
There was no formal ethics committee approval for the current study, as it is a single patient case in a private dental practice. The patient's insurance company authorized the treatment described. The patient provided signed informed consent to undergo the procedure, in accordance with the currently amended Declaration of Helsinki.
DISCLOSURE
Dr Cory Glenn is a consultant for Blue Sky Bio, LLC, which manufactures components used in the treatment scenario presented in this article.
ACKNOWLEDGMENTS
The authors thank the patient for allowing his treatment process to be presented; Bertram Dental Laboratories (Neenah, WI) for 3D printing of the metal bar prosthesis substructure; FormLabs (Somerville MA) for 3D‐printing of the resin mold for the final ear prosthesis; and Scott A. Saunders, DDS, ELS of Dental and Medical Writing and Editing, LLC, Lancaster PA, for professional medical writing and editing services in preparation of the manuscript.
Domingue D, Glenn NC, Vest A, White JR. Osseointegrated implant‐retained auricular prosthesis constructed using cone‐beam computed tomography and a prosthetically driven digital workflow: a case report. Clin Case Rep 2021;9:37–45. 10.1002/ccr3.3386
REFERENCES
- 1. Zhong N, Zhao X. 3D printing for clinical application in otorhinolaryngology. Eur Arch Otorhinolaryngol. 2017;274(12):4079‐4089. [DOI] [PubMed] [Google Scholar]
- 2. Crafts TD, Ellsperman SE, Wannemuehler TJ, Bellicchi TD, Shipchandler TZ, Mantravadi AV. Three‐dimensional printing and its applications in otorhinolaryngology‐head and neck surgery. Otolaryngol Head Neck Surg. 2017;156(6):999‐1010. [DOI] [PubMed] [Google Scholar]
- 3. Li C, Cheung TF, Fan VC, Sin KM, Wong CW, Leung GK. Applications of three‐dimensional printing in surgery. Surgical innovation. 2017;24(1):82‐88. [DOI] [PubMed] [Google Scholar]
- 4. Liaw CY, Guvendiren M. Current and emerging applications of 3D printing in medicine. Biofabrication. 2017;9(2):24102. [DOI] [PubMed] [Google Scholar]
- 5. Katkar RA, Taft RM, Grant GT. 3D Volume rendering and 3D printing (Additive Manufacturing). Dent Clin North Am. 2018;62(3):393‐402. [DOI] [PubMed] [Google Scholar]
- 6. Eley KA. Centralised 3D printing in the NHS: a radiological review. Clin Radiol. 2017;72(4):269‐275. [DOI] [PubMed] [Google Scholar]
- 7. Barazanchi A, Li KC, Al‐Amleh B, Lyons K, Waddell JN. Additive technology: update on current materials and applications in dentistry. J Prosthodont. 2017;26(2):156‐163. [DOI] [PubMed] [Google Scholar]
- 8. Tack P, Victor J, Gemmel P, Annemans L. 3D‐printing techniques in a medical setting: a systematic literature review. Biomed Eng Online. 2016;15(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Martelli N, Serrano C, van den Brink H, et al. Advantages and disadvantages of 3‐dimensional printing in surgery: a systematic review. Surgery. 2016;159(6):1485‐1500. [DOI] [PubMed] [Google Scholar]
- 10. Anand V, Haribabu V, Gnanasamband V. A laboratory silicone for preclinical training in ear prosthesis. J Pharm Bioallied Sci. 2013;5(Suppl 2):S201‐S203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Demir N, Cevik P, Okutan Y, Ozturk AN, Colpan B. A different wax sculpture technique for implant‐retained auricular prosthesis. Eur J Dent. 2015;9(3):433‐437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arora A, Pasam N, Gilra S, Arora PC. Prosthetic rehabilitation of auricular defect: a clinical report. Prosthet Orthot Int. 2013;37(3):240‐244. [DOI] [PubMed] [Google Scholar]
- 13. Unkovskiy A, Roehler A, Huettig F, et al. Simplifying the digital workflow of facial prostheses manufacturing using a three‐dimensional (3D) database: setup, development, and aspects of virtual data validation for reproduction. J Prosthodont Res. 2019;63(3):313‐320. [DOI] [PubMed] [Google Scholar]
- 14. Unkovskiy A, Spintzyk S, Axmann D, Engel EM, Weber H, Huettig F. Additive manufacturing: a comparative analysis of dimensional accuracy and skin texture reproduction of auricular prostheses replicas. J Prosthodont. 2019;28(2):e460‐e468. [DOI] [PubMed] [Google Scholar]
- 15. Unkovskiy A, Spintzyk S, Brom J, Huettig F, Keutel C. Direct 3D printing of silicone facial prostheses: a preliminary experience in digital workflow. J Prosthet Dent. 2018;120(2):303‐308. [DOI] [PubMed] [Google Scholar]
- 16. Unkovskiy A, Brom J, Huettig F, Keutel C. Auricular prostheses produced by means of conventional and digital workflows: a clinical report on esthetic outcomes. Int J Prosthodont. 2018;31(1):63‐66. [DOI] [PubMed] [Google Scholar]
- 17. Hatamleh MM, Watson J. Construction of an implant‐retained auricular prosthesis with the aid of contemporary digital technologies: a clinical report. J Prosthodont. 2013;22(2):132‐136. [DOI] [PubMed] [Google Scholar]
- 18. Nuseir A, Hatamleh M, Watson J, Al‐Wahadni AM, Alzoubi F, Murad M. Improved construction of auricular prosthesis by digital technologies. J Craniofac Surg. 2015;26(6):e502‐e505. [DOI] [PubMed] [Google Scholar]
- 19. Ballo AM, Nguyen CT, Lee VSK. Digital workflow of auricular rehabilitation: a technical report using an intraoral scanner. J Prosthodont. 2019;28(5):596‐600. [DOI] [PubMed] [Google Scholar]
- 20. Watson J, Hatamleh MM. Complete integration of technology for improved reproduction of auricular prostheses. J Prosthet Dent. 2014;111(5):430‐436. [DOI] [PubMed] [Google Scholar]
- 21. Jindal SK, Sherriff M, Waters MG, Coward TJ. Development of a 3D printable maxillofacial silicone: part I. Optimization of polydimethylsiloxane chains and cross‐linker concentration. J Prosthet Dent. 2016;116(4):617‐622. [DOI] [PubMed] [Google Scholar]
- 22. Jindal SK, Sherriff M, Waters MG, Smay JE, Coward TJ. Development of a 3D printable maxillofacial silicone: Part II. Optimization of moderator and thixotropic agent. J Prosthet Dent. 2018;119(2):299‐304. [DOI] [PubMed] [Google Scholar]


