Abstract
Background and Aims
Retinopathy of prematurity (ROP) is a severe disease in preterm infants. It is seen more frequently in Low‐Middle Income Countries (LMIC) like Indonesia compared to High‐Income Countries (HIC). Risk factors for ROP development are ‐extreme‐ preterm birth, use of oxygen, neonatal infections, respiratory problems, inadequate nutrition, and blood and exchange transfusions. In this paper, we give an overview of steps that can be taken in LMIC to prevent ROP and provide guidelines for screening and treating ROP.
Methods
Based on the literature search and data obtained by us in Indonesia's studies, we propose guidelines for the prevention, screening, and treatment of ROP in preterm infants in LMIC.
Results
Prevention of ROP starts before birth with preventing preterm labor, transferring a mother who might deliver <32 weeks to a perinatal center and giving corticosteroids to mothers that might deliver <34 weeks. Newborn resuscitation must be done using room air or, in the case of very preterm infants (<29‐32 weeks) by using 30% oxygen. Respiratory problems must be prevented by starting continuous positive airway pressure (CPAP) in all preterm infants <32 weeks and in case of respiratory problems in more mature infants. If needed, the surfactant should be given in a minimally invasive manner, as ROP's lower incidence was found using this technique. The use of oxygen must be strictly regulated with a saturation monitor of 91‐95%. Infections must be prevented as much as possible. Both oral and parenteral nutrition should be started in all preterm infants on day one of life with preferably mothers' milk. Blood transfusions can be prevented by reducing the amount of blood needed for laboratory analysis.
Discussion
Preterm babies should be born in facilities able to care for them optimally. The use of oxygen must be strictly regulated. ROP screening is mandatory in infants born <34 weeks, and infants who received supplemental oxygen for a prolonged period. In case of progression of ROP, immediate mandatory treatment is required.
Conclusion
Concerted action is needed to reduce the incidence of ROP in LMIC. "STOP ‐ R1O2P3" is an acronym that can help implement standard practices in all neonatal intensive care units in LMIC to prevent development and progression.
Keywords: low‐middle income countries, predisposing factors, recommendation, ROP, screening
1. INTRODUCTION
Retinopathy of prematurity (ROP) is a severe disease that might result in blindness. It is seen in preterm infants with increasing incidence alongside the shorter duration of pregnancy. 1 , 2 To a great extent, the disease is preventable via strict control of oxygen delivery to the newborn infant. ROP is seen more frequently in Indonesia as compared to high‐income countries (HIC). It is also seen in infants with gestational age above 32 weeks, a group of infants where ROP is not seen in HIC. In Indonesia, the reported incidence of all stages of ROP in infants <32 weeks ranged from 18% to 30%. 3 We studied the incidence in the NICU of Harapan Kita Women and Children Hospital, Jakarta, as one of NICUs' role models in Indonesia. We found that 40% of infants with a GA of <28 weeks had ROP all stages, and 21% showed stage >3 ROP. Also, concerning is the incidence of ROP in our patients with a GA of 28 to 32 weeks where any stage ROP was found in 29%, and stage >3 ROP in 3% of the screened infants. 4 In comparison, the incidence of any stage ROP in developed countries (NMR <5) is approximately 21.8% in infants with a gestational age of less than 32 weeks 5 and the incidence of ROP III‐V in infants with a gestational age of less than 26 weeks is 10%. 6 Many studies have shown that there are three main risk factors for the development of ROP. First, the shorter the gestational age, the higher the risk. Second, the lower the birthweight, the higher the risk. Finally, there is a clear correlation between the oxygen concentration in inspired air in the first weeks of life and the development of ROP.
Preventing ROP already starts before birth by preventing preterm labor and intrauterine growth retardation. After birth, strict control of oxygen delivery is needed, and neonatal disease should be prevented as much as possible. In this article, we give recommendations on reducing the high ROP incidence in an low‐middle income countries (LMIC) like Indonesia.
2. SEARCH MAJOR RISK FACTORS OF ROP
2.1. Gestational age at birth and birth weight
2.1.1. Background
Gestational age at birth (GA) and birth weight (BW) are the most substantial known risk factors for the development of ROP. The most effective way to reduce the incidence of ROP is to prevent preterm delivery. A 100 g increase in BW decreases the odds of severe ROP with 27%, and each week increase in GA decreases the incidence of severe ROP with 19%. 7 Although preterm birth cannot always be prevented, optimal antenatal care can reduce the incidence. Early recognition and treatment of pregnancy complications, such as hypertension, pre‐eclampsia, maternal malnutrition, maternal diabetes, and the mother's infections, will reduce prematurity incidence. The mother's smoking habits are also another risk factor for the development of ROP as smoking will result in intrauterine growth retardation. 8
2.1.2. Clinical management
Pregnant women with complications in pregnancy before 32 weeks must be referred to a center with experienced obstetricians and an NICU where full adequate support can be given to the pregnant mothers and infants of less than 32 weeks.
2.2. Supplemental oxygen
2.2.1. Background
The immature retina of a preterm infant is susceptible to insults that disrupt neuro‐vascular growth, leading to ROP. Suppression of growth factors due to hyperoxia results in an arrest of retinal vascularization (phase 1). Subsequently, the increasing metabolically active, yet poorly vascularized retina becomes hypoxic, stimulating growth factor‐induced vasoproliferation (phase 2), which eventually can cause retinal detachment. 9
Giving supplemental oxygen that results in high oxygen tension and saturation is a significant risk factor for the development of ROP. Studies have convincingly shown that restricting the use of oxygen in the first weeks after birth reduces the incidence of ROP. 10 Both the concentration of oxygen is given, and the resulting oxygen saturation must be controlled. Oxygen blenders control the percentage of oxygen given, and transcutaneous oxygen saturation monitors are the most widely used systems to control the oxygen saturation in the blood.
2.2.2. Clinical management
Many studies have evaluated the most optimal setting of oxygen saturation limits. 10 Setting the low limits of the saturation monitor at 84% is related to an increased death rate. Setting the upper limit of the monitor above 95% increases the risk of developing ROP. Based on several studies, 11 , 12 it is advisable to aim for a saturation between 91% and 95%. To achieve this, the oxygen saturation monitor's alarm limits should be set between 90% and 96%. 13
The supplemental oxygen should be controlled in each newborn by close monitoring the oxygen concentration administered and the resulting oxygen saturation in the infant. Infants should not be treated in a facility where these systems are not available, and they must be transferred to a facility where these systems are available. In all hospitals where infants are born, oxygen blenders must be available, both in the delivery room and in the neonatal ward. In case no oxygen blender is available, oxygen must be diluted with room air because 100% oxygen is extremely hazardous for the newborn infant. The desired oxygen concentration can be calculated using the following formula {(n1x21) + (n2x100)}: (n1 + n2)} (n1 is the flowrate of air, n2 the flowrate of oxygen). A 60% oxygen concentration will be obtained from diluting 1 L/min of oxygen with 1 L/min of air. When 2 L/min of room air is given together with 1 L/min of oxygen, the resulting oxygen concentration is 47%. When 3 L/min of room air is given together with 1 L/min of oxygen, the resulting oxygen concentration is 37%. A scheme “rules of eight” how to dilute oxygen and the resulting oxygen concentration is given in Table 1. The oxygen concentration in the oxygen/air mixture can be calculated using the following formula
TABLE 1.
Oxygen concentration resulting from mixing air and oxygen
| Pure oxygen (100%) L/min | Pressurized air (oxygen 21%)L/min | Resulting oxygen concentration (%) |
|---|---|---|
| 0 | 8 (0.5‐10) | 21 |
| 0.25 | 7.75 (7,5‐7.75) | 23 |
| 0.5 | 7.5 (7‐10) | 26 (25‐26) |
| 1 | 7 (7‐9) | 31 (29‐31) |
| 2 | 6 | 40 |
| 3 | 5 | 51 |
| 4 | 4 | 61 |
| 5 | 3 | 70 |
| 6 | 2 | 80 |
| 7 | 1 | 90 |
| 8 (0.5‐10) | 0 | 100 |
Oxygen concentration should be titrated according to the values found with the oxygen saturation monitor. In case no oxygen saturation monitor is available, the concentration of supplemental oxygen should be reduced to a level where the first signs of cyanosis are present, whereafter, the oxygen concentration is increased with 5% to 10%. 14 Fluctuations in the oxygen saturation must be prevented since fluctuations and periods with hyper‐ and hypoxia have been shown to increase the risk for ROP. 15 There is no need to use medical gas as compressed air. As used for diving, compressed air can safely be used for the newborn infant when flowmeters are available that can deliver amounts of 1 to 10 L/min.
3. OTHER FACTORS INCREASING THE RISK TO DEVELOP ROP
3.1. Maternal factors
3.1.1. Background
Hypertension in pregnant mothers is suggested to increase the risk of ROP by influencing vascular growth factors. A meta‐analysis found, however, no relation between hypertension in the mother and ROP. 16 Smoking is a risk factor, most likely to cause growth restriction due to smoking. 17 Chorioamnionitis might increase the risk of ROP by both preterm birth and inflammatory mediators, as shown in a recent review on risk factors for ROP. 18 Giving corticosteroids to mothers who might deliver before 32 weeks reduces the risk for BPD. This medication is related to less severe lung problems and more general aging effects of corticosteroids. 19 , 20 A lower incidence of BPD will result in less oxygen supplementation to the infant and thereby a lower risk of developing ROP.
3.1.2. Clinical management
Optimal antenatal care is essential to reduce the risk of preterm labor and improve the fetus's condition. Transferring a pregnant mother to a center is mandatory in case of complications in pregnancy below 32 weeks. Mothers with severe complications at higher gestational ages also must be referred. A course of corticosteroids, 12 mg betamethasone for 2 days, should be given to all mothers who might deliver before 34 weeks.
3.2. Place of delivery
3.2.1. Background
The risk of developing ROP is higher in infants who have severe illness in the post‐natal period. The risk of developing severe ROP is higher for an infant born in a hospital not equipped with adequate facilities or a hospital without well‐trained personnel. This risk is also higher when a child needs a post‐natal transfer to the NICU, as found both in the CRYO‐ROP and ET‐ROP studies. 21 , 22
3.2.2. Clinical management
An important method to reduce the incidence of ROP, therefore, is to transfer a mother (before delivery) to a center with a neonatal intensive care unit where next to controlling oxygen delivery and oxygen saturation, full support for a very preterm infant is possible. All mothers who might deliver before 32 weeks should be transferred to the perinatal center, while those who might deliver between 32 and 36 weeks should be transferred to district hospitals or alternative hospitals with a newborn intensive care center to anticipate for possible intensive care that might be needed by the infant after birth.
3.3. Infant factors
3.3.1. Resuscitation
Background
In almost all cases, it is unnecessary to administer supplemental oxygen when resuscitating term or preterm infants. The outcome of resuscitation is not better when 100% oxygen is given, compared to room air. Resuscitation with 100% oxygen will induce oxygen radicals, which are incredibly toxic for newborn infants. Lower levels of oxygen scavengers, compounds that reduce oxygen toxicity, are seen up to 4 weeks after resuscitation with 100% oxygen. The oxygen radicals might induce the development of ROP later on. 23 Therefore, international resuscitation counsels advise that they use room air to resuscitate term infants and infants of 32 weeks and higher. There is a slow increase in oxygen saturation in all healthy, term and preterm infants after birth 13 (Figure 1). This saturation indicates no need to give supplemental oxygen when the oxygen saturation is still 75% to 80% 5 minutes after birth.
FIGURE 1.
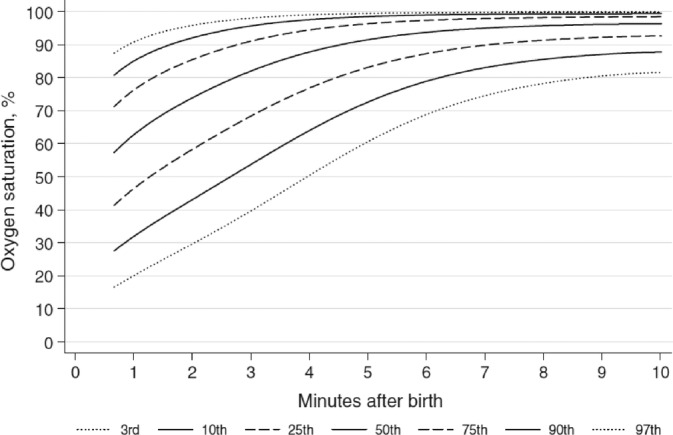
Oxygen saturation in the first 10 minutes after birth (3rd, 10th, 25th, 50th, 75th, 90th, and 97th SpO2 percentiles for all preterm and term infants with no medical intervention after birth) 13
In infants born after 28 to 32 weeks, it is advised to resuscitate with 21% to 30% oxygen, after less than 28 weeks with 30% oxygen. A meta‐analysis by Saugstad et al showed that preterm babies ≤32 weeks' gestation resuscitated with lower (0.21‐0.3) vs higher (0.6‐1.0) initial FiO2 showed no differences in morbidity but exhibited an almost significant trend toward reduced mortality. 24 A systematic review with meta‐analysis published in 2019 by Welsford et al concluded that preterm newborns' ideal initial FiO2 is still unknown. However, the majority of newborns' ≤32 weeks' gestation will require some oxygen supplementation. 25 Suggestions regarding the amount FiO2 that should be given to newborn infants in the delivery room and the corresponding SpO2 targets are shown in Table 2. 23 , 26
TABLE 2.
Suggestions regarding the amount of oxygen supplementation in the delivery room
| Gestational age | Initial FiO2 | Target SpO2 at 5 minutes |
|---|---|---|
| <37 weeks | 0.21 | 85%‐90% |
| 33+0 to 36+6 weeks | 0.21 | 85% |
| 29+0 to 32+6 weeks | 0.21‐0.30 | 80%‐85% |
| ≤28 weeks | 0.3 | 80% |
Practical clinical management
Resuscitation in term infants and infants born after at least 32 weeks must be started using room air. At a gestational age of 28 to 32 weeks, the advice is to resuscitate with 21% to 30% oxygen, below 28 weeks with 30% oxygen. 13 Higher levels of oxygen are indicated when the goals for oxygen saturation, as described above, are not reached. These guidelines conform to the recommendations of the National Resuscitation Foundation. 27 , 28
In cases where supplemental oxygen is given, the resulting oxygen saturation must be monitored. The amount of oxygen given must be controlled by using an air/oxygen blender. In cases where the blender is not available, resuscitation with a mixture of 3.5 L/min room air and 0.5 L/min oxygen, resulting in a mixture with 30% oxygen, is sufficient in almost all cases.
3.3.2. Optimal nutrition and growth
Background
Infants with a low weight gain after birth have a higher risk of developing ROP as compared to those who gain weight following the intrauterine growth curve. 7 Low weight gain can be caused by low nutrient intake. However, it can also be an indicator of severe illness. Studies have shown that a deficiency of specific nutrients, particularly n‐3 poly‐unsaturated essential fatty acids is related to a higher risk of ROP development. 29 Many TPN solutions contain only small amounts of these fatty acids. In cases of severe illnesses, there might be a low weight gain and corresponding low IGF‐1 levels. IGF‐1 is an anabolic hormone promoting anabolism of many tissues, including retinal vessels. Low IGF‐1 levels are associated with increased risks for ROP. 30 Hyperglycemia is related to ROP's higher incidences, while hyperglycemia is mainly seen in extremely sick and preterm infants. 9 Severe illness increases the risk of developing ROP, most likely through the presence of pro‐inflammatory cytokines. 31 Human milk is recommended to all preterm infants after birth and effectively prevents necrotizing enterocolitis and late‐onset sepsis. 32 , 33 Human milk has also been shown to reduce the incidence of ROP. 34 , 35 , 36 This might be due to a number of antioxidant components that could be potentially protective against ROP or the presence of long chain polyunsaturated fatty acids in human milk. 9
Clinical management
Starting nutrition on the first day after birth is essential. Enteral feeding can be started in almost all infants on the first day with preferably human milk at a dose of 10 to 20 mL/kg BW/day and increased with 20 mL/kg/day. Starting parenteral feeding with amino acids at a dose of 2 to 3 g/kg BW/day and lipids in a dose of 1 g/kg/day directly after birth are essential to prevent muscle breakdown, promote muscle gain, and optimize the immune response.
3.3.3. Respiratory problems
Background
Respiratory problems and the use of various techniques to treat these problems, such as mechanical ventilation, high‐frequency oscillation, and continuous positive airway pressure (CPAP), are associated with a higher incidence of ROP. 37 , 38 Treating respiratory problems is related to the incidence of ROP, most likely due to supplemental oxygen. The more oxygen is used, and the longer the duration of respiratory support, the higher the risk of developing ROP. Therefore, preventing respiratory problems and shortening respiratory support by lung‐protective ventilation strategies are most important in preventing ROP.
Clinical management
Corticosteroids should be given to mothers threatening to deliver <34 weeks. Start with CPAP in all preterm infants <32 weeks. Give surfactant when oxygen needs >40%, especially when a CPAP level of 8 to 12 cm H2O is needed to reach a sufficient oxygen saturation. 39 , 40 Recent studies 1 , 2 showed a lower incidence of ROP in infants treated with minimally invasive surfactant administration compared to endotracheal intubation. Use lung‐protective ventilation strategies by using only synchronized ventilation modes with volume guarantee, limit tidal volumes to 5 to 7 mL/kg BW, and use at least 5 cm PEEP. Be aggressive in weaning from the ventilator and extubate as soon as possible.
3.3.4. Prevention of late onset sepsis
Background
Infections, particularly late‐onset sepsis (LOS), increase the risk of developing ROP. 41 , 42 Pro‐inflammatory compounds such as prostaglandins and cytokines influence the development of the vasculature, thereby developing ROP. 43
Clinical management
The risk of newborn infants to develop LOS is highly dependent on the quality of care in the NICU. Proper hygiene policies such as strict hand washing protocols, clean materials, and aseptic procedures decrease the risk. The risk to develop LOS is lower in infants who are gaining weight well, and who have not been treated with broad‐spectrum antibiotics.
3.3.5. Blood and exchange transfusions
Background
Many studies showed that blood transfusions increase the risk of ROP. 44 , 45 , 46 A study in Indonesia showed that the risk was also increased when an exchange transfusion was done. 47 The increased risk might be due to either the administration of adult hemoglobin or the iron overload resulting from the transfusions. Iron overload increases the risks of oxygen radicals. Adult hemoglobin causes a higher delivery of oxygen to tissues compared to fetal hemoglobin. Some studies found that anemia was related to an increased risk of ROP, as well as the administration of EPO. 48 Delayed umbilical cord clamping—DCC—(not earlier than 1 minute after birth) is recommended for improved maternal and infant health and nutrition outcomes. 49 In preterm infants, delayed umbilical cord clamping is associated with significant neonatal benefits, including the better establishment of red blood cell volume and decreased need for blood transfusion. 50 This intervention is appropriate not only in sick but also in stable preterm who do not require positive pressure ventilation. DCC intervention should be considered because iron deficiency anemia is one of the four significant malnutrition problems in vulnerable age groups, including pregnant mothers in Indonesia, next to protein‐energy deficiency, vitamin A deficiency, and iodine deficiency. Preventing anemia by delayed cord clamping, therefore, is of special advantage in Indonesia. Hyperbilirubinemia is an increased serum bilirubin level that can be prevented by early determination of the bilirubin level. Early identification of increased bilirubin levels will reduce the need for exchange transfusions. 51
Clinical management
The most frequent reason for blood transfusion is blood taking. Micro methods for laboratory determinations are needed. Regarding the implementation of DCC for the prevention of anemia in premature infants in Indonesia, we tried to implement this routine in our hospital. Unfortunately, there is no detailed data regarding the use of DCC in our country. National guidelines are needed so that this relatively easy‐to‐perform procedure can be widely implemented. Hyperbilirubinemia must be prevented by early determination of increased serum bilirubin, together with early use of phototherapy.
4. STOP: R1O2P3
We created the mnemonic “STOP ‐ R1O2P3” to make it easier to remember the steps to be taken to prevent ROP.
4.1. S (Start)
To stop ROP, it starts with preventing ROP antenatally by preventing preterm birth by referring to the mother. She might deliver before 32 weeks to a perinatal center and give corticosteroids to the mother in case of threatening preterm labor <34 weeks.
4.2. T (Tiered referral system)
Improvement of neonatal care facilities must start in the regional general hospital and continue in the referral hospital. A tiered standard referral system is needed to make it possible for patients to get services that meet their needs. Stakeholders must realize the establishment of regional perinatal/neonatal intensive care centers with complete facilities. Mothers with complications in pregnancy and tiny VLBW infants at high risk must be sent to the hospital with a tiered referral system.
Complications in pregnancy <32 weeks—send the mother to the hospital with NICU
Complications in pregnancy 32 to 36 weeks—send the mother to the hospital with NICU or district hospital
An infant born <32 weeks or birth weight < 1250 g—send to hospital with NICU
An infant born 32 to 34 weeks or birth weight 1250 to 1500 g—send to a district hospital
4.3. O (ophthalmology screening and treatment)
4.3.1. Screening infants for ROP
Background
Most cases of ROP resolve naturally. However, there will be progression to severe disease in some infants that might lead to blindness. When the progression of ROP to severe stages is detected in time, treatment can prevent blindness progression. Therefore, all infants with a risk to develop ROP must be screened. Screening protocols are published, mainly for HIC countries. 52 , 53 , 54 , 55 They include a screening of all infants born before 32 weeks. The screening usually starts at 4 to 6 weeks postnatally (but at least 31 weeks postmenstrual age) and continues until the retina's complete vascularization. However, these guidelines need adaptation for Indonesia, as ROP is also seen in infants with gestational ages above 32 weeks. We developed the National ROP Screening and Treatment Guideline for preterm babies in Indonesia at National Neonatologist‐Ophthalmologist Workshops, which should be used in all NICUs in Indonesia. 56 These guidelines advise the following indications for ROP screening 56 , 57 :
Screen all infants born before ≤34 weeks and/or BW ≤1500 g.
Infants with greater BW or older GA should be screened for ROP in case the infants received supplemental oxygen, either at birth or in the first weeks of life. This screening can be requested by a neonatologist or pediatrician and depends on the severity of neonatal risk factors.
Time of first examination
If the GA is more than 30 weeks, baby is examined 2 to 4 weeks after birth
If the GA is less than or equal to 30 weeks, check 4 weeks after birth
Infants with 37 weeks or more gestational age do not need to do ROP screening
At least one examination before discharge from the hospital
Further screening depends on the findings of the first examination
Repeat screening every week or 2 weeks until complete vascularization or ROP is confirmed
Continue screening until there is complete regression.
If ROP is progressing to type 1 ROP, urgent treatment is needed.
It is necessary to monitor the infants after discharge to ensure that their disease does not develop into stages requiring treatment.
Infants with ROP have an increased risk for other pathologies such as high‐level myopia, squint, and cortical brain damage. Therefore, they require a long‐term follow‐up so that their problems can be appropriately managed.
At each ROP screening session, an ophthalmologist needs to know how to classify the severity of ROP in each eye examined. This classification will provide information about the prognosis and give guidelines for making decisions about screening and treatment. The International Committee for the Classification of ROP has classified it using the following criteria (Figure 2): (a) The severity of the ROP, (b) The zone in the retina where ROP is found, (c) The extent of the ROP, (d) Whether the retinal blood vessels are dilated and/or tortuous (pre‐plus or plus disease), (e) Whether aggressive posterior ROP is present. Furthermore, the severity of ROP is divided into five categories, namely stages 1 to 5. 58
FIGURE 2.
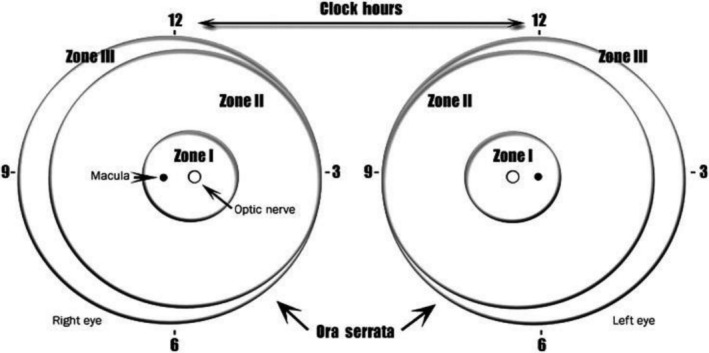
Scheme of the retina of both eyes showing three‐zone borders and clock hours used to describe the location and extent of retinopathy of prematurity. 58
4.3.2. Treatment options for ROP
Background
In ROP type 1, there is a clear risk of developing blindness, and treatment is indicated. In the case, ROP type 2, a “wait and watch” approach is indicated. The definition of type 1 and 2 ROP is as follows:
Type I ROP (high‐risk ROP)
Zone I, any stage ROP with a plus disease or
Zone I, stage 3, with or without plus disease or
Zone II, stage 2 or 3 ROP, with plus disease
Type II ROP (low risk)
Zone I, stage 1 or 2 with no plus disease or
Zone II, stage 3 with no plus disease
ROP treatment
The first‐line treatment for ROP is laser retinal photocoagulation. This procedure is quite safe if applied properly and can prevent retinal detachment. 56 , 57 , 59 , 60 In all HIC guidelines, laser treatment is the first treatment; second is intra‐vitreal injection with anti‐vascular endothelial growth factor (VEGF). This intervention might cause more myopia later, and there is doubt about its safety for disturbed angiogenesis in other (growing) organs. Indonesia's ROP guidelines also place laser treatment as first‐line therapy. If the laser is technically not possible to perform (no pupillary response after mydriatics administration), then anti‐VEGF is recommended. The absence of a laser device should not cause delay or cessation of therapy. In this case, the patient should be referred to as soon as the patient is stable enough to be transported to an institution with the facilities and competence for ROP therapy. Laser treatment requires a trained and highly skilled ophthalmologist. 56 , 57
4.4. P (program, implementation, and supporting system)
A comprehensive program to reduce the incidence of the devastating disease ROP is needed. Collaborative efforts involving obstetricians, neonatologists, ophthalmologists, nursing staff, and epidemiologists are needed to reduce the incidence of ROP in LMIC caused by the increased survival of VLBW infants, made possible by the increasing number of neonatal intensive care centers.
A network of regional neonatal intensive care centers with complete facilities is needed. A plan for these centers must be made in consultation with and supported by all stakeholders, including the Government and The Indonesian National Health Insurance System. The program to stop ROP includes prevention, early detection, and screening followed by appropriate therapy to optimize the quality of life of affected newborns.
All level 3 NICUs should have the adequate materials and knowledge to work according to the STOP‐ROP protocol and have access to an ophthalmologist who has the knowledge and materials to screen for ROP—and treatment—if indicated.
Based on the studies on ROP conducted in Indonesia and other literature, 3 , 4 , 5 , 61 , 62 we propose the following guideline to prevent and screen preterm infants for ROP. The abbreviation “R1O2P3” is used to facilitate the procedures that must be standard practice in all perinatal/neonatal units (Figure 3).
(R1) After birth, Resuscitation is started with room air in a term and late preterm infants. The recommendation for preterm infants of 28 to 32 weeks is 21% to 30% oxygen, below 28 weeks 30%. 24 Supplemental oxygen is guided by targeted oxygen saturation levels as advised by international resuscitation counsels (2 minutes 60%, 3 minutes 70%, 4 minutes 80%, 5 minutes 85%). 27 , 28
(O1). When Oxygen is given, use a blender. When oxygen is given, use a transcutaneous saturation monitor. The saturation target should be at 90 to 92%.
(O2) Give Optimal nutrition and maintain normal glucose levels. Start enteral, preferably mothers' own milk and parenteral nutrition on day one, and give at least 2 g/kg/day amino acids or protein on day one and 3 to 4 g/kg BW/day after that.
(P1) Prevent pulmonary injury by giving early CPAP immediately after birth to infants <32 weeks and surfactant when the infants using CPAP FiO2 > 40% despite 10 to 12 cm H2O.
(P2) Prevent infection by proper hand hygiene and clean materials and procedures
(P3) Last but not least, prevent blood‐ and exchange transfusion by restricting blood sampling using micro‐lab techniques, prevent anemia, and hyperbilirubinemia.
FIGURE 3.
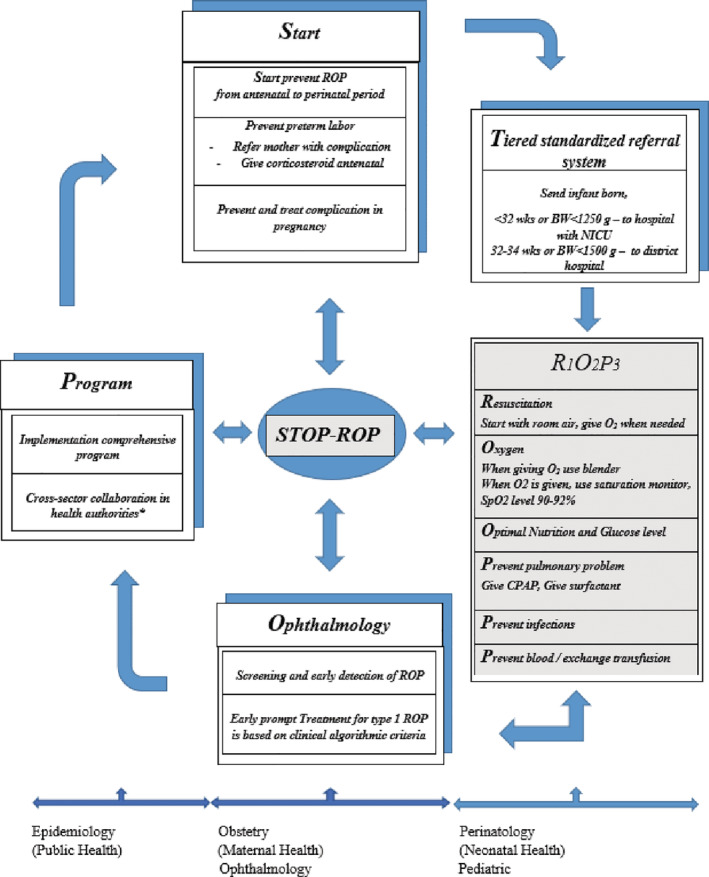
Diagram/flowchart “How to prevent ROP with STOP−ROP?”
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
AUTHOR CONTRIBUTIONS
Conceptualization: Johanes Edy Siswanto, Pieter J. J. Sauer
Data Curation: Johanes Edy Siswanto, Peter Dijk, Rita S. Sitorus, Pieter J. J. Sauer
Investigation: Johanes Edy Siswanto, Peter Dijk, Rita S. Sitorus, Pieter J. J. Sauer
Methodology: Johanes Edy Siswanto, Peter Dijk, Arend F. Bos, Pieter J. J. Sauer
Project Administration: Johanes Edy Siswanto, Arend F. Bos
Supervision: Arend F. Bos
Validation: Arend F. Bos, Rita S. Sitorus, Sudarto Ronoatmodjo, Pieter J. J. Sauer
Visualization: Johanes Edy Siswanto, Peter Dijk, Pieter J. J. Sauer
Writing ‐ Original Draft Preparation: Johanes Edy Siswanto, Pieter J. J. Sauer
Writing ‐ Review & Editing: Johanes Edy Siswanto, Peter Dijk, Arend F. Bos, Rita S. Sitorus, Asri C. Adisasmita, Sudarto Ronoatmodjo, Pieter J. J. Sauer
All authors have read and approved the final version of the manuscript.
Johanes Edy Siswanto had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.
TRANSPARENCY STATEMENT
Johanes Edy Siswanto affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
ACKNOWLEDGMENTS
This work was supported by Harapan Kita Women and Children Hospital, National Centre of Women and Children's Health, Jakarta, Indonesia and Department of Pediatrics, Beatrix Children's Hospital, University Medical Center Groningen, Groningen, the Netherlands.
Siswanto JE, Dijk PH, Bos AF, et al. How to prevent ROP in preterm infants in Indonesia? Health Sci Rep. 2021;4:e219 10.1002/hsr2.219
Funding information The authors have not declared a specific grant for this research from any funding agency in public, commercial, or not‐for‐profit sectors.
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
REFERENCES
- 1. Härtel C, Paul P, Hanke K, et al. Less invasive surfactant administration and complications of preterm birth. Sci Rep. 2018;8(1):8333 10.1038/s41598-018-26437-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Han T, Liu H, Zhang H, et al. Minimally invasive surfactant administration for the treatment of neonatal respiratory distress syndrome: a multicenter randomized study in China. Front Pediatr. 2020;8:1‐12. 10.3389/fped.2020.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siswanto JE, Sauer PJ. Retinopathy of prematurity in Indonesia: incidence and risk factors. J Neonatal Perinatal Med. 2017;10:85‐90. [DOI] [PubMed] [Google Scholar]
- 4. Siswanto JE, Widodo NH, Sauer PJJ. Eleven years of retinopathy of prematurity in one neonatal intensive care unit in Jakarta, Indonesia. Arch Dis Child. 2018;103:619‐621. [DOI] [PubMed] [Google Scholar]
- 5. Blencowe H, Lawn JE, Vazquez T, Fielder A, Gilbert C. Preterm‐associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr Res. 2013;74:35‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stoll BJ, Hansen NI, Bell EF, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993‐2012. JAMA. 2015;314:1039‐1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu C, Löfqvist C, Smith LEH, VanderVeen D, Hellström A, WINROP Consortium., and the WINROP Consortium . Importance of early postnatal weight gain for Normal retinal angiogenesis in very preterm infants. Arch Ophthalmol. 2012;130:992‐999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hirabayashi H, Honda S, Morioka I, et al. Inhibitory effects of maternal smoking on the development of severe retinopathy of prematurity. Eye. 2010;24:1024‐1027. [DOI] [PubMed] [Google Scholar]
- 9. Hellström A, Smith LEH, Dammann O. Retinopathy of prematurity. Lancet. 2013;382:1445‐1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Askie LM, Henderson‐Smart DJ, Ko H. Restricted versus liberal oxygen exposure for preventing morbidity and mortality in preterm or low birth weight infants. Cochrane Database Syst Rev. 2009;1:CD001077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sola A, Golombek SG, Montes Bueno MT, et al. Safe oxygen saturation targeting and monitoring in preterm infants: can we avoid hypoxia and hyperoxia? Acta Paediatr. 2014;103:1009‐1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McClure C, Jang SY, Fairchild K. Alarms, oxygen saturations, and SpO2 averaging time in the NICU. J Neonatal Perinatal Med. 2016;9:357‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dawson JA, Kamlin COF, Vento M, et al. Defining the reference range for oxygen saturation for infants after birth. Pediatrics. 2010;125:e1340‐e1347. [DOI] [PubMed] [Google Scholar]
- 14. Sola A, Chow L, Rogido M. Retinopathy of prematurity and oxygen therapy: a changing relationship. An Pediatr (Barc). 2005;1:48‐61. [DOI] [PubMed] [Google Scholar]
- 15. York JR, Landers S, Kirby RS, Arbogast PG, Penn JS. Arterial oxygen fluctuation and retinopathyof prematurity in very‐low‐birth‐weight infants. J Perinatol. 2004;24:82‐87. [DOI] [PubMed] [Google Scholar]
- 16. Zhu T, Zhang L, Zhao F, Qu Y, Mu D. Association of maternal hypertensive disorders with retinopathy of prematurity: a systematic review and meta‐analysis. PLOS One. 2017;12(4):e0175374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Spiegler J, Jensen R, Segerer H, et al. Influence of smoking and alcohol during pregnancy on outcome of VLBW infants. Z Geburtshilfe Neonatol. 2013;217:215‐219. [DOI] [PubMed] [Google Scholar]
- 18. Kim SJ, Port AD, Swan R, et al. Retinopathy of prematurity: a review of risk factors and their clinical significance. Surv Ophthalmol. 2018;5:618‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Crowther CA, Middleton PF, Voysey M, et al. Effects of repeat prenatal corticosteroids given to women at risk of preterm birth: an individual participant data meta‐analysis. PLoS Med. 2019;4:e1002771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Trembath A, Laughon MM. Predictors of bronchopulmonary dysplasia. Clin Perinatol. 2012;39(3):585‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schaffer DB, Palmer EA, Plotsky DF, et al. Prognostic factors in the natural course of retinopathy of prematurity. The cryotherapy for retinopathy of prematurity cooperative group. Ophthalmology. 1993;2:230‐237. [DOI] [PubMed] [Google Scholar]
- 22. Good WV, Hardy RJ, Dobson V, et al. The incidence and course of retinopathy of prematurity: findings from the early treatment for retinopathy of prematurity study. Pediatrics. 2005;1:15‐23. [DOI] [PubMed] [Google Scholar]
- 23. Saugstad OD, Oei J, Lakshminrusimha S, et al. Oxygen therapy of the newborn from molecular understanding to clinical practice. Pediatr Res. 2019;85:20‐29. [DOI] [PubMed] [Google Scholar]
- 24. Saugstad OD, Aune D, Aguar M, Kapadia V, Finer N, Vento M. Systematic review and meta‐analysis of optimal initial fraction of oxygen levels in the delivery room at 32 weeks. Acta Paediatr. 2014;103:744‐751. [DOI] [PubMed] [Google Scholar]
- 25. Welsford M, Nishiyama C, Shortt C, et al. Initial oxygen use for preterm newborn resuscitation: a systematic review with meta‐analysis. Pediatrics. 2019;1; pii:e20181828. [DOI] [PubMed] [Google Scholar]
- 26. Lara‐Cantón I, Solaz A, Parra‐Llorca A, et al. Optimal inspired fraction of oxygen in the delivery room for preterm infants. Children (Basel). 2019;6:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wyckoff MH, Aziz K, Escobedo MB, et al. Part 13: neonatal resuscitation: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(suppl 2):S543‐S560. [DOI] [PubMed] [Google Scholar]
- 28. Escobedo MB, Aziz K, Kapadia VS, et al. 2019 American Heart Association focused update on neonatal resuscitation. An update to the American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2019;140:e922‐e930. [DOI] [PubMed] [Google Scholar]
- 29. Beken S, Dilli D, Fettah ND, Kabataş EU, Zenciroğlu A, Okumuş N. The influence of fish‐oil lipid emulsions on retinopathy of prematurity in very low birth weight infants: a randomized controlled trial. Early Hum Dev. 2014;90(1):27‐31. [DOI] [PubMed] [Google Scholar]
- 30. Hellstrom A, Engstrom E, Hard AL, et al. Postnatal serum insulin‐like growth factor I deficiency is associated with retinopathy of prematurity and other complications of premature birth. Pediatrics. 2003;112:1016‐1020. [DOI] [PubMed] [Google Scholar]
- 31. Lee J, Dammann O. Perinatal infection, inflammation, and retinopathy of prematurity. Semin Fetal Neonatal Med. 2012;17:26‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Herrmann K, Carroll K. An exclusively human milk diet reduces necrotizing enterocolitis. Breastfeed Med. 2014;4:184‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. 2011;3:255‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bharwani SK, Green BF, Pezzullo JC, Bharwani SS, Bharwani SS, Dhanireddy R. Systematic review and meta‐analysis of human milk intake and retinopathy of prematurity: a significant update. J Perinatol. 2016;36:913‐920. [DOI] [PubMed] [Google Scholar]
- 35. Zhou J, Shukla VV, John D, Chen C. Human milk feeding as a protective factor for retinopathy of prematurity: a meta‐analysis. Pediatrics. 2015;136(6):e1576‐e1586. [DOI] [PubMed] [Google Scholar]
- 36. Manzoni P, Stolfi I, Pedicino R, et al. Human milk feeding prevents retinopathy of prematurity (ROP) in preterm VLBW neonates; Italian task force for the study and prevention of neonatal fungal infections. Italian Society of Neonatology Early Hum Dev. 2013;89(suppl 1):S64‐S68. [DOI] [PubMed] [Google Scholar]
- 37. Ying GS, Quinn GE, Wade KC, et al. Predictors for the development of referral‐warranted retinopathy of prematurity in the telemedicine approaches to evaluating acute‐phase retinopathy of prematurity (e‐ROP) study. JAMA Ophthalmol. 2015;133(3):304‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Holmstrom G, Broberger U, Thomassen P. Neonatal risk factors for retinopathy of prematurity—a population‐based study. Acta Ophthalmol Scand. 1998;76:204‐207. [DOI] [PubMed] [Google Scholar]
- 39. Te Pas AB, Spaans VM, Rijken M, et al. Early nasal continuous positive airway pressure and low threshold for intubation in very preterm infants. Acta Paediatr. 2008;97:1049‐1054. [DOI] [PubMed] [Google Scholar]
- 40. Dargaville P, Aiyappan A, De Paoli AG, et al. Continuous positive airway pressure failure in preterm infants: incidence. Predict Conseq Neonatol. 2013;1:8‐14. [DOI] [PubMed] [Google Scholar]
- 41. Tolsma KW, Allred EN, Chen ML, et al. Neonatal bacteremia and retinopathy of prematurity: the ELGAN study. Arch Ophthalmol Chic Ill 1960. 2011;12:1555‐1563. [DOI] [PubMed] [Google Scholar]
- 42. Chiang MF, Arons RR, Flynn JT, et al. Incidence of retinopathy of prematurity from 1996 to 2000: analysis of a comprehensive New York state patient database. Ophthalmology. 2004;7:1317‐1325. [DOI] [PubMed] [Google Scholar]
- 43. de Souza Rugolo LM, Bentlin MR, Mussi‐Pinhata M, et al. Late‐onset sepsis in very low birth weight infants: a Brazilian neonatal research network study. J Trop Pediatr. 2014;60:415‐421. [DOI] [PubMed] [Google Scholar]
- 44. Hesse L, Eberl W, Schlaud M, Poets CF. Blood transfusion. Iron load and retinopathy of prematurity. Eur J Pediatr. 1997;156:465‐470. [DOI] [PubMed] [Google Scholar]
- 45. Dani C, Reali MF, Bertini G, Martelli E, Pezzati M, Rubaltelli FF. The role of blood transfusions and iron intake on retinopathy of prematurity. Early Hum Dev. 2001;62:57‐63. [DOI] [PubMed] [Google Scholar]
- 46. Howarth C, Banerjee J, Aladangady N. Red blood cell transfusion in preterm infants: current evidence and controversies. Neonatology. 2018;114:7‐16. [DOI] [PubMed] [Google Scholar]
- 47. Siswanto JE, Ronoatmodjo S, Adisasmita A, Soemantri A, Sitorus RS, Sauer PJJ. Risk factors for the development and progression of retinopathy of prematurity in preterm infants in Indonesia. J Neonatal Perinatal Med. 2019;13:253‐260. 10.3219/NPM-190219. [DOI] [PubMed] [Google Scholar]
- 48. Rivera JC, Holm M, Austeng D, et al. Retinopathy of prematurity: inflammation, choroidal degeneration, and novel promising therapeutic strategies. J Neuroinflammation. 2017;14:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. WHO . Optimal timing of cord clamping for the prevention of iron deficiency anemia in infants. e‐Library of Evidence for Nutrition Actions (eLENA). https://www.who.int/elena/titles/full_recommendations/cord_clamping/en/
- 50. ACOG Committee Opinion , Number 684, American College of Obstetricians and Gynecologists. Delayed umbilical cord clamping after birth. Obstet Gynecol 2020;136;e100‐6. [DOI] [PubMed] [Google Scholar]
- 51. Smitherman H, Stark AR, Bhutan VK. Early recognition of neonatal hyperbilirubinemia and its emergent management. Semin Fetal Neonatal Med. 2006;11:214‐224. [DOI] [PubMed] [Google Scholar]
- 52. Jefferies AL, Canadian Paediatric Society, Fetus and Newborn Committee . Retinopathy of prematurity: recommendations for screening. Paediatr Child Health. 2010;10:667‐670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Section on Ophthalmology, American Academy of Pediatrics, American Academy of Ophthalmology, American Association for Pediatric Ophthalmology and Strabismus . Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2006;117:572‐576. [DOI] [PubMed] [Google Scholar]
- 54. Fierson WM, American Academy of Pediatrics Section on Ophthalmology; American Academy of Ophthalmology; American Association for Pediatric Ophthalmology and Strabismus; American Association of Certified Orthoptists . Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2018;6:e20183061. [DOI] [PubMed] [Google Scholar]
- 55. The RCPCH in collaboration with the Royal College of Ophthalmologists, British Association of Perinatal Medicine and BLISS . Screening and Treatment of Retinopathy of Prematurity ‐ clinical guideline. 2008.
- 56. Sitorus RS, and POKJA Nasional ROP . Pedoman nasional skrining dan terapi ROP pada bayi prematur di Indonesia. Jakarta. FKUI, PERDAMI, IDAI. 2011.
- 57. Quinn G, Gilbert C. Treating ROP: how and when. Commun Eye Health J. 2017;30:59. [PMC free article] [PubMed] [Google Scholar]
- 58. International Committee for the Classification of Retinopathy of Prematurity . The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. 2005;123:991‐999. [DOI] [PubMed] [Google Scholar]
- 59. William V, Good MD, Early Treatment for Retinopathy of Prematurity Cooperative Group . Final results of the early treatment for retinopathy of prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc. 2004;102:219‐250. [PMC free article] [PubMed] [Google Scholar]
- 60. Liegl R, Hellström A, Smith LEH. Retinopathy of prematurity: the need for prevention. Eye Brain. 2016;8:91‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gilbert C, Blencowe H. Retinopathy of prematurity: it is time to take action. Commun Eye Health J. 2017;30:45‐48. [PMC free article] [PubMed] [Google Scholar]
- 62. Hariharan L, Gilbert CE, Quinn GE, et al. Reducing blindness from retinopathy of prematurity (ROP) in Argentina through collaboration, advocacy and policy implementation. Health Policy Plan. 2018;0:1‐12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.


