Abstract
Linac‐based STereotactic Arrhythmia Radioablation (STAR) is a safety and effective approach for selected patients with ventricular arrhythmias.
Keywords: electrical storm ‐ Italy, Stereotactic arrhythmia radioablation
Linac‐based STereotactic Arrhythmia Radioablation (STAR) is a safety and effective approach for selected patients with ventricular arrhythmias.
1. INTRODUCTION
We reported the first patient treated with STereotactic Arrhythmia Radioablation (STAR) in Italy. A 67‐year‐old man with electrical storm and left ventricular thrombosis. He underwent STAR with a dose of 25 Gy in one fraction (treatment plan delivered in 6 minutes). ICD interventions stopped after four months from treatment.
Cardiovascular diseases are still the first cause of mortality, despite the efficacy of new treatment and therapeutic advances for improving its prognosis. Cardiac arrhythmias are a major health burden, 1 , 2 and especially ventricular arrhythmias are associated with poor prognosis and reduced quality of life. Therapeutic options include moderately effective medication, antitachycardia pacing or shock by an implantable cardioverted defibrillator (ICD) when needed and later catheter‐based ablation of arrhythmogenic substrates in the heart.
Catheter ablation aims to eliminate the electrical cells that cause cardiac arrhythmias. To date, ablative techniques use either radiofrequency (RF) energy or cryoablation. In both cases, there is tissue necrosis and consequently the inactivation of the substrate underlying arrhythmia. 3
Several patients, due to their cardiac history and surgical interventions or both mitral and aortic mechanical valves or clots formations in left ventricle, could not be underwent to catheter ablation. Therefore, noninvasive therapeutic alternatives are warranted. STereotactic Arrhythmia Radioablation (STAR) with precise high dose of radiation to a well‐defined targets potentially guided by previous cardiac diagnostic tools was used for treated these patients. 1
STAR is an advanced form of radiation therapy that delivers noninvasive, image‐guided, precise high dose of radiation to targets reducing dose exposure to adjacent normal tissue and minimizing the treatment toxicity. Unlike catheter‐based ablation, which utilizes either RF energy to heat tissue or freezing (cryoablation) to injure tissues, radiotherapy utilizes high dose of radiation to ablate the target. From a radiobiological point of view, the higher dose of radiation (STAR) may theoretically produce greater biological cell kill respect conventional radiotherapy. Its action mechanism is partly unknown. The tissue damage is probably a multifactorial result, due in part to the double‐strand breaks in the DNA, which it leads to apoptosis, but also to vascular damage and consequent ischemic cell death. 4
To date, there are still few studies conducted on human population. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 Considering a so restricted number of treated patients, the validation of the efficacy and safety of this treatment approach still needs confirmation on a large scale, before it can be considered a standard therapeutic option in patients with cardiac arrhythmias. To date, in the STAR treatment experiences, patients were treated with a dose of 25Gy delivered in a single fraction.
Recent technological advances, especially in Linac‐based stereotactic radiotherapy including Volumetric arc therapy (VMAT) and Flattening Filter Free (FFF), 13 , 14 , 15 , 16 may enable faster and more conformal STAR treatment. Here, the first patient treated in Italy with STAR was reported.
2. MATERIALS AND METHODS
A 67‐year‐old man with ischemic cardiomyopathy and low ejection fraction (25%) developed an electrical storm (44 ICD interventions in 2 months) and was evaluated by our Cardiologist Team on September 2019. More than 20 000 premature ventricular contractions (PVC)/day were recorded during therapy with amiodarone 200mg once a day and metoprolol 100mg twice a day. Catheter ablation was hampered due to left ventricular apical aneurism with thrombosis. The cardiac surgeon considered the intervention not feasible due to excessive risk. Thus, patient was enrolled for a STAR. Premature ventricular contraction morphology on 12‐lead ECG was analyzed to localize the area of interest into the left ventricle myocardium (Figure 1). Twelve‐lead ECG of ventricular tachycardia (VT) morphology was not available.
Figure 1.
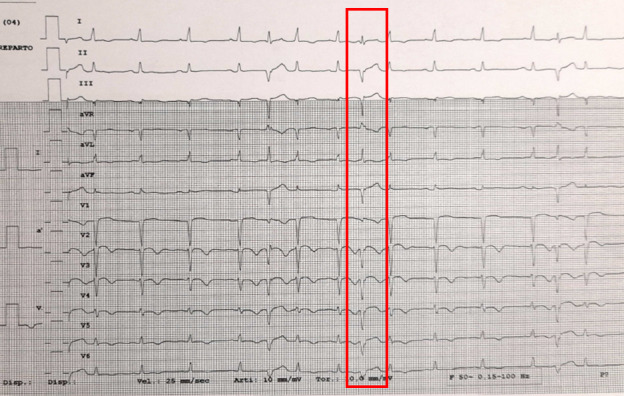
12‐lead ECG showing ventricular ectopy
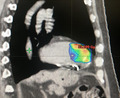
In order to determine the target myocardial tissue and treatment results, a merge study including PVC morphology, cardiac‐gated cardiac tomography (CT), myocardial 18F‐Fluorodeoxyglucose positron emission tomography (18F‐FDG PET‐CT) and 99mTc‐Sestamibi Gated single‐photon emission computed tomography (SPET‐CT) was performed before the treatment. PVC morphology on 12‐lead ECG was compatible with an origin from the interventricular septum (Figure 1) and, furthermore, 18F‐FDG PET‐CT and 99mTc‐Sestamibi Gated SPET‐CT were used to identify vital myocardium in that area. After these analyses, the final target area was defined on the interventricular septum (Figure 2).
Figure 2.
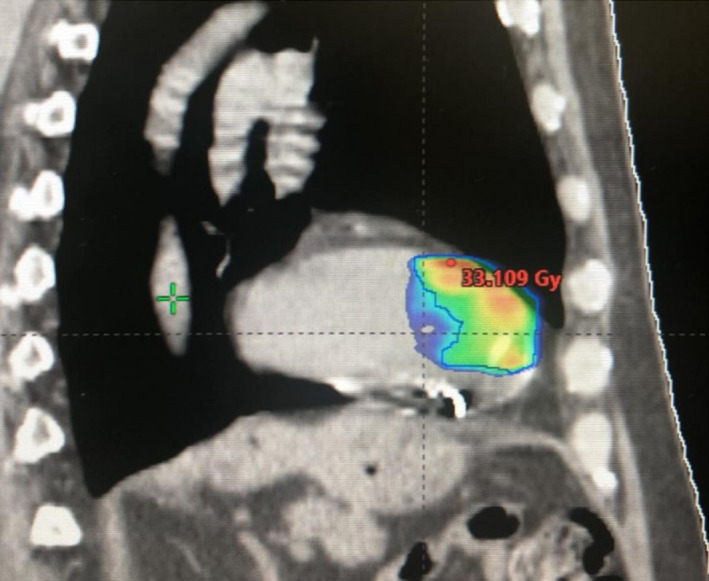
Treatment planning
For radiotherapy (RT) planning purposes, a 4‐dimensional computed tomography (4‐DCT) scan (2 mm slice thickness) was obtained with patients in the supine position using a personalized immobilization device. 4‐DCT scan was used to the compensation of cardiac and respiratory target motion.
The diagnostic images (Cardio‐CT, 18F‐FDG PET‐CT and 99mTc‐Sestamibi Gated SPET‐CT) were exported to the Eclipse® treatment planning system (Varian Medical System), and fused with the treatment planning CT. The final target volume was defined by the radiation oncologists in collaboration with electrophysiologists and radiologists. Organs at risk, including the lung, heart, left coronary, esophagus, spine, and ICD, were also delineated.
A dose of 25 Gy in one fraction, optimized to have a prescription isodose line of 75%, was prescribed to the planning target volume (PTV). 17 Treatment plan was generated on Varian TrueBeamTM v. 2.7, and the treatment time was only of 6 minutes (Figure 2).
3. RESULTS
Patient was treated on 6 September 2019. IGRT (image‐guided radiotherapy) with Cone Beam CT and SGRT (surface‐guided radiotherapy) with Align‐RT (Vision RT) were used to reduce set‐up error and to monitoring patients during fraction. The treatment plan was delivered in 6 minutes.
After 3 months from radioablation, no acute side effects were registered. The patients stopped amiodarone assumption at 3 months after procedure.
At 1 month, the intracardiac defibrillator did not record VTs. The VEs decreased from 24000/24h to 123/24h. The patient had 2 sustained VT at second month from procedure, successfully interrupted by DC shock. At third month from procedure, a VT was interrupted by overdrive pacing. All these postprocedure VTs had a different cycle length when compared with the preprocedure VT.
After fourth months, the patient did not experience other ICD interventions (Figure 3). At nuclear medicine imaging, performed after six months from procedure, 18F‐FDG PET‐CT showed a small area of myocardial necrosis region in the site of STAR treatment and 99mTc‐Sestamibi Gated SPET‐CT, performed with same parameters before and after treatment, an objective improvement of kinetics and left ventricle ejection fraction (40%). After 6 months from STAR, no late side effects were registered.
Figure 3.
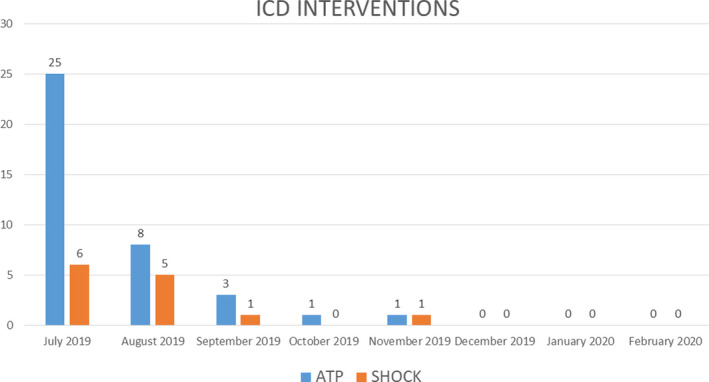
ICD interventions
4. DISCUSSION
For cardiac arrhythmias, which are associated with poor prognosis and quality of life, therapeutic options include catheter‐based ablation of arrhythmogenic substrates. Due to several patients’ issues, catheter‐based ablation approach could not be proposed. Therefore, noninvasive therapeutic alternatives, including STAR, are warranted. 18
Preclinical and early clinical data suggest that STAR with a single dose of 25Gy could be an efficacy and safe treatment for these patients. Due to the technological improvements in Linac‐based stereotactic treatments, including VMAT, IGRT, and FFF, 19 , 20 the possibility of Linac‐based STAR will increase in the course of the next future.
Based on the latter considerations, we reported the first patient treated with STAR in Italy. Linac‐based STAR treatment plan was clinically acceptable and efficient, showing a better target coverage, sparing critical structure with a delivered time of 6 minutes. For more precise therapy, a CBCT was performed before treatment to reduce set‐up errors, while to reduce intrafraction errors SGRT was used.
To date, there are still few studies conducted on human population. 5 , 6 , 7 , 21 In particular, these studies reported that this new strategy was associated to a low procedure risk, a reduced procedure time and a significant reduction of ventricular arrhythmias during follow‐up. In several cases, cyber knife technology was used to delivered the STAR, but the here presented case with Linac‐based technology showed the reduction of treatment time (6 minus vs until 70 minutes with CK); safety and efficacy of cardiac ablation for VT. In fact, in terms of safety, patient received the high dose without acute side effect registered during the first 30 days from STAR and late effects after 6 months from treatment.
In terms of clinical results, the first patients treated with STAR reported a better clinical outcome in terms of ICD interventions, ejection fraction, and drug administration. Moreover, this is the first report about use of 18F‐FDG PET‐CT data to plan and evaluate STAR treatment for ventricular arrhythmias.
Respect to catheter ablation, in which the reduction/absence of ventricular ectopies and VT was reported after few days from procedure, the consideration of time of the clinical results should be crucial for patients enrolled for STAR.
On the other hand, the absence of acute side effects and risk procedure was very interesting for selected patients who could be treated directly with STAR.
5. CONCLUSIONS
Linac‐based STAR is a safety and effective approach for selected patients with VT. Moreover, considering a so restricted number of treated patients, the validation of this treatment approach still needs confirmation on a large scale, before it can be considered a standard therapeutic option in patients with cardiac arrhythmias.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
Fiorentino Alba, Di Monaco Antonio, Surgo Alessia, and Grimaldi Massimo: conceptualized and designed the study, and analyzed and interpreted the data. Vitulano Nicola, Gregucci Fabiana, Carbonara Roberta, Quadrini Federico, and Bonaparte Ilaria: acquired the data. Ludovico Elena: acquired the data, analyzed and interpreted the data. Rubini Giuseppe: analyzed and interpreted the data. All the authors were involved in drafting the manuscript or revising it critically for important intellectual content; gave final approval of the version to be published; participated sufficiently in the work to take public responsibility for appropriate portions of the content and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
ETHICAL CONSIDERATIONS
The patient signed the informed consent before the study.
ACKNOWLEDGMENTS
Published with written consent of the patient.
Fiorentino A, Di Monaco A, Surgo A, et al. Linac‐based STereotactic Arrhythmia Radioablation (STAR) of ventricular tachycardia: Case report and literature review. Clin Case Rep.2021;9:362–366. 10.1002/ccr3.3530
Alba Fiorentino and Antonio Di Monaco equally contributed to this work.
DATA AVAILABILITY STATEMENT
All main data are reported in the manuscript.
REFERENCES
- 1. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129‐2200. [DOI] [PubMed] [Google Scholar]
- 2. Zecchin M, Severgnini M, Fiorentino A, et al. Management of patients with cardiac implantable electronic devices (CIED) undergoing radiotherapy: A consensus document from Associazione Italiana Aritmologia e Cardiostimolazione (AIAC), Associazione Italiana Radioterapia Oncologica (AIRO), Associazione Italiana Fisica Medica (AIFM). Int J Cardiol. 2018;255:175‐183. [DOI] [PubMed] [Google Scholar]
- 3. Ernst S. Catheter ablation: general principles and advances. Card Electrophysiol Clin. 2017;9:311‐317. [DOI] [PubMed] [Google Scholar]
- 4. Song CW, Cho LC, Yuan J, Dusenbery KE, Griffin RJ, Levitt SH. Radiobiology of stereotactic body radiation therapy/stereotactic radiosurgery and the linear‐quadratic model. Int J Radiat Oncol Biol Phys. 2013;87:18‐19. [DOI] [PubMed] [Google Scholar]
- 5. Cuculich PS, Schill MR, Kashani R, et al. Noninvasive Cardiac Radiation for Ablation of Ventricular Tachycardia. N Engl J Med. 2017;377:2325‐2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Loo BW Jr, Soltys SG, Wang L, et al. Stereotactic ablative radiotherapy for the treatment of refractory cardiac ventricular arrhythmia. Circ Arrhythm Electrophysiol. 2015;8:748‐750. [DOI] [PubMed] [Google Scholar]
- 7. Jumeau R, Ozsahin M, Schwitter J, et al. Rescue procedure for an electrical storm using robotic non‐invasive cardiac radio‐ablation. Radiother Oncol. 2018;128:189‐191. [DOI] [PubMed] [Google Scholar]
- 8. Stöhr EJ, Shave RE, Baggish AL, Weiner RB. Left ventricular twist mechanics in the context of normal physiology and cardiovascular disease: a review of studies using speckle tracking echocardiography. Am J Physiol Heart Circ Physiol. 2016;311:H633‐644. [DOI] [PubMed] [Google Scholar]
- 9. Robinson CG, Samson PP, Moore KMS, et al. Phase I/II Trial of Electrophysiology‐Guided Noninvasive Cardiac Radioablation for Ventricular Tachycardia. Circulation. 2019;139:313‐321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cvek J, Neuwirth R, Knybel L, et al. Cardiac radiosurgery for malignant ventricular tachycardia. Cureus. 2014;6:e190. [Google Scholar]
- 11. Gianni C, Rivera D, Burkhardt JD, et al. Stereotactic Arrhythmia Radioablation for Refractory Scar‐Related Ventricular Tachycardia. Heart Rhythm. 2020;17(8):1241‐1248. [DOI] [PubMed] [Google Scholar]
- 12. Lloyd MS, Wight J, Schneider F, et al. Clinical experience of stereotactic body radiation for refractory ventricular tachycardia in advanced heart failure patients. Heart Rhythm. 2020;17:415‐422. [DOI] [PubMed] [Google Scholar]
- 13. Mazzola R, Fersino S, Alongi P, et al. Stereotactic body radiation therapy for liver oligometastases: predictive factors of local response by 18F‐FDG‐PET/CT. Br J Radiol. 2018;91:20180058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mazzola R, Fersino S, Aiello D, et al. Linac‐based stereotactic body radiation therapy for unresectable locally advanced pancreatic cancer: risk‐adapted dose prescription and image‐guided delivery. Strahlenther Onkol. 2018;194:835‐842. [DOI] [PubMed] [Google Scholar]
- 15. Alongi F, Mazzola R, Fiorentino A, et al. Phase II study of accelerated Linac‐based SBRT in five consecutive fractions for localized prostate cancer. Strahlenther Onkol. 2019;195:113‐120. [DOI] [PubMed] [Google Scholar]
- 16. Franco P, De Bari B, Ciammella P, et al. The role of stereotactic ablative radiotherapy in oncological and non‐oncological clinical settings: highlights from the 7th Meeting of AIRO–Young Members Working Group (AIRO Giovani). Tumori. 2014;100:e214‐219. [DOI] [PubMed] [Google Scholar]
- 17. Bonaparte I, Gregucci F, Surgo A, Carbonara R, Fiorentino A. A treatment planning study for linac based Stereotactic arrhythmia radio ablation (STAR) of ventricular tachycardia. Abstract: Cureus in press; 2020. [DOI] [PubMed] [Google Scholar]
- 18. Fiorentino A, Gregucci F, Bonaparte I, et al. “Stereotactic ablative radiation therapy (STAR) for cardiac arrythmia: a new therapeutic option?” Radiol Med. 2020;May 13. [DOI] [PubMed] [Google Scholar]
- 19. Fiorentino A, Giaj‐Levra N, Tebano U, et al. Stereotactic ablative radiation therapy for brain metastases with volumetric modulated arc therapy and flattening filter free delivery: feasibility and early clinical results. Radiol Med. 2017;122:676‐682. [DOI] [PubMed] [Google Scholar]
- 20. Alongi F, Fiorentino A, Mancosu P, et al. Stereotactic radiosurgery for intracranial metastases: linac‐based and gamma‐dedicated unit approach. Expert Rev Anticancer Ther. 2016;16:731‐740. [DOI] [PubMed] [Google Scholar]
- 21. Sharma A, Wong D, Weidlich G, et al. Noninvasive stereotactic radiosurgery (CyberHeart) for creation of ablation lesions in the atrium. Heart Rhythm. 2010;7:802‐810. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All main data are reported in the manuscript.


