ABSTRACT
Background
Trimethylamine N-oxide (TMAO) is a diet- and microbiome-derived metabolite and a proposed biomarker of adverse cardiometabolic outcomes. TMAO studies have mainly been conducted in individuals with cardiometabolic disease, and studies in population-derived samples are limited.
Objective
We aimed to investigate the associations between plasma TMAO concentrations and its precursors [carnitine, choline, betaine, and dimethylglycine (DMG)] with metabolic syndrome (MetS) scores, preclinical cardiovascular phenotypes, and inflammatory biomarkers (i.e. high-sensitivity C-reactive protein and serum glycoprotein acetyls) in a population-derived cohort of children and their parents.
Methods
The concentrations of TMAO and its precursors were quantified using UHPLC coupled with tandem MS (UHPLC/MS-MS) in 1166 children (mean age 11 y ± 0.5 y, 51% female) and 1324 adults (44 y ± 5.1 y, 87% female) participating in The Growing Up in Australia's Child Health CheckPoint Study. We developed multivariable fractional polynomial models to analyze associations between TMAO, its precursors, MetS (adjusted for sex and age), and cardiovascular phenotypes (adjusted for sex, age, BMI, household income, and the urinary albumin to creatinine ratio). Pearson's correlations were computed to identify associations between TMAO, its precursors, and inflammatory biomarkers.
Results
The concentrations of TMAO precursors, but not TMAO itself, were associated with MetS, cardiovascular phenotypes, and inflammatory biomarkers in children and adults.
Conclusions
TMAO precursors, but not TMAO itself, were associated with adverse cardiometabolic and inflammatory phenotypes in children and adults. TMAO precursor concentrations may better reflect cardiovascular health and inflammatory status within the wider population. Replication in other population settings and mechanistic studies are warranted.
Keywords: TMAO, children, adults, Growing Up in Australia, epidemiology, cardiovascular preclinical phenotypes
TMAO precursor concentrations may better reflect cardiometabolic health and inflammatory status within the wider population.
Introduction
Trimethylamine N-oxide (TMAO) is a diet- and microbiome-derived metabolite suggested to be a predictive biomarker and functional mediator in cardiovascular disease (CVD) (1). Several mechanisms have been proposed to explain the TMAO-CVD relation including decreased reverse cholesterol transport, increased foam cell formation, heightened platelet aggregation, and an upregulation of proinflammatory pathways, all of which are known risk factors for the development and progression of atherosclerosis by favoring vascular plaque build-up and inflammatory responses (1–4). Several animal intervention studies and human prospective and cross-sectional studies highlight that TMAO concentrations are associated with unfavorable metabolic and cardiovascular phenotypes, with some inconsistencies identified across studies (2, 5–14). Nevertheless, associations between TMAO concentrations and adverse cardiovascular outcomes are stronger in individuals with CVD (11), compared with healthy populations (15), and are concentration dependent (3, 16).
TMAO studies are typically conducted in individuals with metabolic disease or CVD, with a paucity in healthy populations or children (13, 15, 17, 18). Furthermore, cohort studies rarely include adults and children from the same family, limiting the ability to identify intergenerational relations between TMAO concentrations and cardiometabolic outcomes. Identifying TMAO-cardiometabolic outcome associations across generations is important given that CVDs originate in early life (19). Therefore, characterizing the concentrations of TMAO and that of its precursors [e.g. choline, carnitine, dimethylglycine (DMG), and betaine] in a population-based sample of children and adults may identify pathways for intervention and prevention of metabolic diseases across 2 different generations.
Metabolic syndrome (MetS) is an aggregate of anthropometric and metabolic factors that increase the risk of CVD (20), 1 of the leading causes of death worldwide (21). Therefore, identifying early cardiovascular risk phenotypes, including MetS, is important for the preventative treatment and monitoring of individuals at increased risk. Preclinical cardiovascular phenotypes are predictive of CVD in adults. Carotid intima-media thickness (cIMT) and distensibility, measures of arterial stiffness and blood pressure, and retinal microvascular structure are increasingly used preclinical cardiovascular phenotypes (22). Identifying novel associations between blood metabolites, risk factors, and preclinical cardiovascular phenotypes in a population-derived cohort, beyond those that are already established [e.g. glucose, blood lipids, and ω-3 index (23, 24)], may further allow early risk stratification and inform preventative and mechanistic investigations.
We previously characterized parent-child concordance (N = 1324 parents and N = 1166 children), age, and sex-effects on plasma concentrations of TMAO and its precursors (i.e. carnitine, choline, betaine, and DMG) (25). We also evaluated associations between concentrations of TMAO and precursors and self-reported dietary intakes of animal protein (i.e. red meat, meat products, chicken, fish, milk products, and cheese) and fast-food meals (25). We reported strong family effects (i.e. 13–37% of variability explained), a positive association with age, higher concentrations in males, and positive associations with reported fish and red meat intakes (25). Building on these findings, in this population-derived cohort of parents and children (aged 11–12 y), we aimed to investigate how concentrations of TMAO and its precursors are associated with: 1) MetS scores; 2) cardiovascular preclinical phenotypes; and 3) circulating inflammatory biomarkers.
Methods
Ethical approval, consent, and sample collection
The Royal Children's Hospital (Melbourne, Australia) Human Research Ethics Committee (33225D) and the Australian Institute of Family Studies Ethics Committee approved The Child Health CheckPoint study (CheckPoint). The population sample included in the current analysis consists of 1874 parent-child dyads who participated in the Growing Up in Australia's Child Health CheckPoint study. The Checkpoint study was a biomedical assessment nested between 2 waves (5 and 6 ) of the Longitudinal Study of Australian Children (LSAC) (26, 27) (Supplemental Figure 1). Parents or caregivers provided consent for themselves and their child to participate in CheckPoint and for the collection and research use of their blood samples (22).
Procedures
All cardiovascular and covariate measures were collected from study participants during the CheckPoint biomedical assessment. Glycoprotein acetyls (GlycA) were previously measured by Checkpoint team collaborators, and MetS was previously computed and published by members of the Checkpoint team (28). The present study included the UHPLC coupled with tandem MS (UHPLC/MS-MS) profiling of TMAO and its precursors, the measure of high-sensitivity C-reactive protein (hsCRP), as well as the statistical analysis of associations between TMAO and cardiometabolic outcomes.
Following an average 4-h fast, venous blood was collected from adults and children and split into components including 6 plasma aliquots and 6 serum aliquots processed within ∼1 h, as previously described (22). For the current study, a total of 2490 plasma samples (1166 children and 1324 adults) were shipped, on dry ice in thermally monitored boxes, to the Liggins Institute. Sample tubes were randomized onto 96-well FluidX plates, keeping parent-child pairs (1123 pairs) on the same plate, then stored at −80°C prior to each assay, as previously described (25).
Quantitation of TMAO and its precursors in plasma
Semifasted EDTA plasma TMAO, carnitine, choline, betaine, and DMG concentrations were measured using 2 previously described analytical assays (29). Briefly, sample preparation (i.e. protein precipitation, mixing, and filtration) was conducted on an EpMotion 5075vt robot fitted with a thermal mixer and vacuum manifold (Eppendorf). This was followed by UHPLC/MS-MS, performed on a Vanquish UHPLC+ system, coupled with a TSQ Quantiva triple quadrupole mass spectrometer (Thermo Scientific).
MetS scores
MetS scores were previously derived (28) from concentrations of serum HDL cholesterol, serum triglycerides, serum glucose, and systolic blood pressure (SBP), standardized by sex in the children subgroup or by sex and age (in decades) in the adults (30, 31). Principal component analysis (PCA) of the standardized components was used to extract a factor loading for each variable. Each component was multiplied by its loading factor and all were summed to derive the continuous MetS score.
Cardiovascular phenotype measures
All measures were collected from participants during the CheckPoint biomedical assessment. cIMT and carotid distensibility measures were derived from carotid artery ultrasound measurements conducted at end-diastole using a portable ultrasound (GE Healthcare Vivid i BT06 with 10 MHz linear array probe), as previously described (32). Pulse wave velocity (PWV) was derived from measures of the time taken for the arterial waveform to propagate from the carotid to the femoral artery using a neck tonometer and blood pressure thigh cuff [SphygmoCor XCEL (AtCor Medical)], as well as the carotid-femoral distance travelled measured using a tape measure, as previously detailed (33). Systolic blood pressure and diastolic blood pressure (SBP and DBP) of participants were measured using a SphygmoCor XCEL (AtCor Medical), in supine position after several minutes of rest, as previously described (34). Retinal photographs were taken in a dark room using a retinal camera (Canon CR-DGi) fitted with a digital single-lens reflex (SLR) camera (Canon EOS 60D), capturing the macula and optic disc (34, 35). Central retinal artery equivalent (CRAE) and central retinal vein equivalent (CRVE) were calculated using the Big 6 revised Knudston–Parr formula, as previously described (34, 35). Mean concentrations of triplicate measurements of SBP, DBP, and PWV excluding missing values were computed for each individual.
Inflammatory markers
Plasma C-reactive protein was measured by particle-enhanced immunological agglutination using the Roche Diagnostics hsCRP assay on a Hitachi Cobas c311 analyzer (Hitachi High Technologies Corporation). Serum GlycA were measured using the Nightingale NMR metabolomics panel (Nightingale Health), as previously described (36). GlycA was chosen given its increasingly promising utility as a novel biomarker of systemic inflammation in pediatric and adult populations (37, 38).
Covariate measures
Household income was previously calculated by adding self-reported weekly incomes of both parents as well as those of other adults in the household, as part of the economic information collected in the LSAC (wave 6).
BMI in kg/m2 was calculated from weight and height measurements. Weight was measured once, wearing light clothing without shoes or socks on 4-limb segmental (InBody230, Biospace) or 2-limb (Tanita BC-351) body composition scales. Standing height was measured without shoes or socks (2–3 times if the first 2 measures differed by >0.5 cm) on a portable rigid stadiometer (Invicta, IP0955), as previously described (22). BMI z-scores were derived from BMI measures for children only using reference datasets (CDC and UK90), as previously described (39).
The urinary albumin to creatinine ratio (UACR) was measured from urine samples collected in a polypropylene sterile pot, stored at 4°C until processing (∼3–13-h processing time), then aliquoted and stored at −80°C prior to UACR analysis. A Cobas Integra 400 plus analyzer was used to measure UACR, albumin was measured using an immunoturbimetric assay, and creatinine using the enzymatic colorimetric method, as previously described (40).
Statistical analysis
Statistical analyses were conducted in R programming environment version 3.6.1 (41). All metabolites were adjusted for plate effects before normality checking and statistical modeling using the random effect function (RANEF) in lme4 R package (42). Plate effects were removed for each compound separately to eliminate any technical batch effects. To ensure that the interaction of other random factors that may be different across plates does not get erased by removing plate effects (e.g. more females on 1 plate compared to another by random chance), we developed a mixed model that included generation × sex as an interaction of fixed effects, as well as family (i.e. parent-child dyad) and plate as 2 random effects, and removed the random effect of the “plate” factor from each model. The plate-adjusted variable was subsequently used in statistical analyses. TMAO and DMG concentrations were positively skewed in the population, and therefore were log-transformed. Carnitine, choline, and betaine concentrations were normally distributed.
Associations between TMAO and its precursors with MetS scores and cardiovascular phenotypes were identified using multivariable fractional polynomial models in the mfp package in R (43, 44). Each generation (adults versus children) was modeled separately. The multivariate model with MetS scores was adjusted for age and sex in each generation. To test associations with cardiovascular phenotypes (i.e. cIMT and carotid distensibility, measures of arterial stiffness and blood pressure, and retinal microvascular structure), 2 models were computed: 1) adjusted for age, sex, and household income (Model 1); and 2) adjusted for age, sex, the UACR ratio as a measure of kidney function, household income, as well as BMI (Model 2). Models containing all metabolites (multimetabolite models) were initially computed followed by a stepwise removal of metabolites that were not associated with the outcome of interest within the model (P > 0.05). Cook's distance plots were computed for each model separately to identify and remove potential outliers that may be driving the model using a stringent Cook's distance cut-off of >0.5. Effect sizes were calculated as SDs of the metabolite multiplied by the estimate of the metabolite in the reduced version of the fully adjusted model (Model 2). The reported R2in all statistical models were the adjusted R2 accounting for the number of predictors in the model.
When several metabolites were associated with the outcome of interest, a correlation matrix was generated to identify whether these metabolites were behaving as a profile of metabolites or as separate entities. Pearson's correlations and associated tests were generated using the corr.test function in R, corrected for multiple testing using the default Holm method in R.
Results
Sample characteristics
The cohort (Table 1) consisted of 2490 individuals (1121 parent-child pairs), 1166 children (mean age 11 y ± 0.5 y, 51% female) and 1324 parents (mean age 44 y ± 5.1 y, 87% female). Median (lower; upper quartile) BMI in adults was 26.5 (23.4; 31.0) and mean (SD) BMI z-score (0.3 ± 0.9) in children. Over 78% of our sample scored in the middle to least disadvantaged Socio-Economic Indexes for Areas (SEIFA) score (quintiles 3–5).
TABLE 1.
Sample characteristics
| Children | Adults | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | All | Male | Female | All | Male | Female | |
| N (%) | 1166 | 565 (49%) | 601 (51%) | 1324 | 174 (13%) | 1150 (87%) | |
| Age in years, mean ± SD | 11.4 ± 0.5 | 11.4 ± 0.5 | 11.5 ± 0.5 | 43.9 ± 5.1 | 46.2 ± 6.4 | 43.6 ± 4.8 | |
| BMI rounded, kg/m2 | Median (LQ; UQ) | 18.4 (16.8; 20.6) | 18.1 (16.7; 20.2) | 18.8 (17.0; 21.1) | 26.5 (23.4; 31.0) | 27.4 (25.2; 31.1) | 26.3 (23.1; 31.0) |
| CV (%) | 10.2 | 9.5 | 10.8 | 14.0 | 10.5 | 14.6 | |
| BMI z-scores | Mean ± SD | 0.3 ± 0.9 | 0.3 ± 0.9 | 0.3 ± 0.9 | — | — | — |
| Biological parent of child, N | — | — | — | 1313 | 172 | 1141 | |
| Metabolic syndrome scores without BMI | Median (LQ; UQ) | −0.2 (−1; 0.8) | −0.2 (−1; 0.8) | −0.2 (−1.0; 0.8) | −0.203 (−1.2; 0.9) | −0.1 (−1.2; 0.9) | −0.2 (−1.2; 0.9) |
| Far wall average IMT overall mean of valid frames, mm | Mean ± SD | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 |
| CV, % | 20 | 20 | 20 | 16.7 | 16.7 | 16.7 | |
| Carotid distensibility, % | Mean ± SD | 17.3 ± 3.2 | 17.1 ± 3.1 | 17.5 ± 3.2 | 8.8 ± 2.0 | 8.5 ± 2.1 | 8.8 ± 2.0 |
| CV, % | 18.5 | 18.1 | 18.3 | 22.7 | 24.7 | 22.7 | |
| CRAE, µm | Mean ± SD | 159.3 ± 11.8 | 156.0 ± 11.8 | 162.0 ± 11.1 | 151.2 ± 13.7 | 148.0 ± 13.8 | 152.0 ± 13.7 |
| CV, % | 7.4 | 7.6 | 6.8 | 9.1 | 9.3 | 9.0 | |
| CRVE, µm | Mean ± SD | 230.8 ± 16.3 | 228.0 ± 15.7 | 234.0 ± 16.4 | 219.0 ± 18.5 | 215.0 ± 19.2 | 220.0 ± 18.3 |
| CV, % | 7.1 | 6.9 | 7.0 | 8.4 | 8.9 | 8.3 | |
| Pulse wave velocity, m/sec | Mean ± SD | 4.4 ± 0.5 | 4.5 ± 0.6 | 4.4 ± 0.5 | 7.0 ± 1.1 | 7.6 ± 1.2 | 6.9 ± 1.1 |
| CV, % | 11.4 | 13.3 | 11.4 | 15.7 | 15.8 | 15.9 | |
| Systolic blood pressure, mmHg | Mean ± SD | 120.6 ± 12.9 | 121.0 ± 13.2 | 120.0 ± 12.6 | 120.7 ± 12.7 | 128.0 ± 11.8 | 119 ± 12.5 |
| CV, % | 10.7 | 10.9 | 10.5 | 10.5 | 9.2 | 10.5 | |
| Diastolic blood pressure, mmHg | Mean ± SD | 63.1 ± 5.7 | 62.7 ± 6.0 | 63.4 ± 5.5 | 73.7 ± 8.6 | 77.8 ± 8.7 | 73.0 ± 8.4 |
| CV (%) | 9.0 | 9.6 | 8.7 | 11.7 | 11.2 | 11.5 | |
| hs-CRP, mg/L | Median (LQ; UQ) | 0.1 (0.02; 0.5) | 0.1(0.01; 0.5) | 0.15 (0.03; 0.5) | 1.0 (0.4; 2.7) | 1.0 (0.4; 2.0) | 1.0 (0.4; 2.8) |
| CV (%) | 92.3 | 96.1 | 88.7 | 74.2 | 66.7 | 75 | |
| Glyc-A, mmol/L | Median (LQ; UQ) | 1.0 (0.9; 1.04) | 1.0 (0.9; 1.04) | 1.0 (0.9; 1.04) | 1.0 (0.9; 1.1) | 1.1 (1.0; 1.2) | 1.0 (0.9; 1.1) |
| CV (%) | 7.2 | 7.2 | 7.2 | 10 | 9.1 | 10 | |
| Australian state of current residence: State (N) | New South Wales (359); Victoria (261); Queensland (221);South Australia (92); West Australia (139); Tasmania (40);Northern Territory (17); Australian Capital Territory (38) | New South Wales (391); Victoria (311); Queensland (240);South Australia (108); West Australia (164); Tasmania(46); Northern Territory (18); Australian Capital Territory(47) | |||||
| Socio-Economic Indexes for Areas (SEIFA) disadvantageQuintile (N) | Most Disadvantaged (83); Second Most (171); Middle(199); Second Least (272); Least Disadvantaged (442) | Most Disadvantaged (94); Second Most (193); Middle(233); Second Least (304); Least Disadvantaged (501) | |||||
Skewed variables were reported as medians and lower/upper quartiles, and normally distributed variables as means and SDs.
CV are calculated as: SDs/means × 100 for normally distributed variables, and as coefficients of quartile variation for skewed variables: CQV = [(Q3 − Q1)/Q3 + Q1] × 100.
CQV, coefficients of quartile variation; CRAE, central retinal artery equivalent; CRVE, central retinal vein equivalent; IMT, intima-media thickness; LQ, lower quartile; UQ, upper quartile.
Associations of TMAO and its precursors’ concentrations with MetS scores
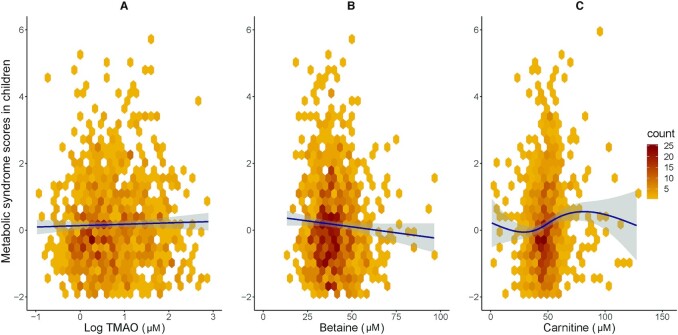
TMAO concentrations were not associated with MetS scores in either adults or children (Tables 2 and 3, and Figures 1 and 2).
TABLE 2.
Multivariate fractional polynomial and linear regression model results with MetS scores in adults and children
| Generation | Metabolite or flexible nonlinear functions from multivariate model1 | Estimate full model | P value full model | Estimate reduced model | P value reduced model | Adjusted R2linear model | F statistic linear model | P value linear model | |
|---|---|---|---|---|---|---|---|---|---|
| Adults | TMAO2 | −0.03 | 0.57 | — | — | 0.05 | 14.35 | <0.0001 | |
| Carnitine | −0.002 | 0.39 | — | — | |||||
| Choline | 0.11 | <0.0001 | 0.10 | <0.0001 | |||||
| Betaine | −0.03 | <0.0001 | −0.03 | <0.0001 | |||||
| DMG2 | 0.43 | 0.005 | 0.44 | 0.004 | |||||
| Children | TMAO2 | −0.05 | 0.40 | — | — | 0.03 | 7.02 | <0.0001 | |
| Carnitine | ([Carnitine + 1.9]/100)2 | 1.53 | <0.0001 | 1.50 | <0.0001 | ||||
| ([Carnitine + 1.9]/100)2 × log([Carnitine + 1.9]/100) | −2.23 | <0.0001 | −2.21 | <0.0001 | |||||
| Betaine | −0.01 | 0.003 | −0.01 | 0.004 | |||||
| DMG2 | 0.17 | 0.29 | — | — | |||||
| Choline | −0.006 | 0.78 | — | — |
Flexible nonlinear functions are computed for variables that do not exhibit a monotonic relation with the outcome of interest.
Log transformed variables.
DMG, dimethylglycine; TMAO, trimethylamine N-oxide.
TABLE 3.
Effect sizes of TMAO/precursor concentrations on MetS scores in adults and children
| Generation | Metabolite or flexible nonlinear functions from multivariate model1 | SD of metabolite | Estimate in model | Effect size (SD × 1-unit change) |
|---|---|---|---|---|
| Adults | Betaine | 12.10 | −0.03 | −0.36 |
| Choline | 2.65 | 0.11 | 0.29 | |
| DMG2 | 0.35 | 0.44 | 0.15 | |
| Children | Betaine | 10.97 | −0.01 | −0.13 |
Flexible nonlinear functions are computed for variables that do not exhibit a monotonic relation with the outcome of interest.
Log transformed variables.
MetS, metabolic syndrome; TMAO, trimethylamine N-oxide.
FIGURE 1.
Hexagonal plots of TMAO (A) and precursors choline (B), betaine (C), and DMG (D) with metabolic syndrome scores in adults. Plasma concentrations of choline, betaine, and DMG, but not those of TMAO, are strongly associated with increased metabolic syndrome scores in adults. Trendline of association in navy blue, 95% CI in gray. DMG, dimethylglycine; TMAO, trimethylamine N-oxide.
FIGURE 2.
Hexagonal plots of TMAO (A) and precursors betaine (B) and carnitine (C) with metabolic syndrome scores in children. Plasma concentrations of betaine and carnitine, but not those of TMAO, are associated with increased metabolic syndrome scores in children. Trendline/curve of association in navy blue, 95% CI in gray. TMAO, trimethylamine N-oxide.
Adult plasma choline and DMG concentrations were positively associated with increasing MetS scores (Table 2), with effect sizes of +0.29 and +0.15 (calculated as the SD of metabolite × metabolite estimate in the model), respectively (P < 0.0001) (Table 3). By contrast, betaine was negatively associated with increasing MetS scores (effect size: −0.36; P < 0.0001).
Children's betaine concentrations were negatively associated with MetS (effect size: −0.13; P = 0.004) (Figure 2). The association for carnitine with MetS in children was nonmonotonic (Supplemental Figure 2), so the effect size for carnitine cannot be quantified in a single number as it depends on the starting point on the curve of association. Therefore, the fractional polynomial fitted uses a function of 2 different transformations of carnitine to model this (Table 2). However, the adjusted R2 of the model (containing betaine, child age, and child sex) with (R2 = 0.03; P < 0.0001) and without the flexible nonlinear function of carnitine (R2 = 0.005; P = 0.04) highlights that the inclusion of carnitine improves the fit of the model with MetS in children. Children's choline and DMG concentrations were not associated with MetS.
When assessing correlations between compounds that were associated with MetS, the concentrations of betaine, choline, and DMG were positively correlated with each other in adults (correlation coefficient: R >0.3; P < 0.0001) (Supplemental Table 1). However, the concentrations of betaine and carnitine function scores were not correlated in children (R = 0.04, and P = 0.26; Supplemental Table 1).
Associations of TMAO and its precursors’ concentrations with cIMT
In children, TMAO concentrations were negatively associated with carotid far-wall cIMT after adjusting for age, sex, and household income (Model 1) (Tables 4 and 5). However, there was no evidence of an association in the fully adjusted Model 2. Conversely, betaine concentrations were positively associated with cIMT (effect size: +0.01; P = 0.001) and DMG concentrations negatively associated with cIMT (effect size: −0.01; P = 0.003), even after full covariate adjustment in children. In adults, there was no evidence for an association for TMAO or any of its precursors with cIMT in either model (Tables 4 and 5).
TABLE 4.
Multivariate and linear models of TMAO/precursor concentrations with cardiovascular preclinical phenotypes
| Metabolite or flexible nonlinear functions from multivariate model1 | Model 1 adjusted for age, sex, and | Model 2 adjusted for age, sex, household income, UACR, and BMI | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cardiovascular outcome | household income reduced model | Estimate full | P full | Estimate reduced | P reduced | Adjusted R2of reduced | |||||
| Generation | Estimate | P | model | model | model | model | model | F | P | ||
| Far wall intima-media thickness | Children | TMAO2 | −4e−3 | 0.05 | −3e−3 | 0.17 | — | — | 0.03 | 4.61 | <0.0001 |
| Betaine | 5e−4 | 0.001 | 6e−4 | 0.001 | 6e−4 | 0.001 | |||||
| DMG2 | −1e−2 | 0.01 | −2e−2 | 0.01 | −2e−2 | 0.003 | |||||
| Carnitine | — | — | 7e−5 | 0.59 | — | — | |||||
| Choline | — | — | −4e−4 | 0.64 | — | — | |||||
| Adults | TMAO2 | — | — | 5e−3 | 0.06 | — | — | — | — | — | |
| Betaine | — | — | 2e−4 | 0.31 | — | — | |||||
| Carnitine | — | — | 2e−4 | 0.14 | — | — | |||||
| DMG2 | — | — | −1e−2 | 0.11 | — | — | |||||
| Choline | — | — | −6e−4 | 0.53 | — | — | |||||
| Carotid distensibility | Children | TMAO2 | — | — | −8e−2 | 0.54 | — | — | 0.02 | 3.77 | 0.001 |
| Betaine | 3e−2 | 0.003 | 3e−2 | 0.02 | 3e−2 | 0.003 | |||||
| Choline | — | — | 4e−2 | 0.49 | — | — | |||||
| DMG2 | — | — | 8e−3 | 0.98 | — | — | |||||
| Carnitine | — | — | 4e−3 | 0.56 | — | — | |||||
| Adults | TMAO | — | — | −8e−2 | 0.31 | — | — | 0.17 | 35.23 | <0.0001 | |
| DMG2 | 0.41 | 0.01 | 0.44 | 0.03 | 0.44 | 0.01 | |||||
| Choline | — | — | −1e−2 | 0.61 | — | — | |||||
| Carnitine | — | — | −1e−3 | 0.78 | — | — | |||||
| Betaine | — | — | 4e−3 | 0.48 | — | — | |||||
| Systolic blood pressure | Children | TMAO2 | — | — | −0.71 | 0.18 | — | — | — | — | — |
| Betaine | — | — | −0.04 | 0.28 | — | — | |||||
| Carnitine | — | — | 9e−3 | 0.77 | — | — | |||||
| DMG2 | — | — | 0.06 | 0.96 | — | — | |||||
| Choline | — | — | −0.04 | 0.84 | — | — | |||||
| Adults | TMAO | — | — | −0.10 | 0.80 | — | — | 0.31 | 74.21 | <0.0001 | |
| Choline | 0.80 | <0.0001 | 0.37 | 0.01 | 0.32 | 0.01 | |||||
| DMG | — | — | −1.14 | 0.29 | — | — | |||||
| Betaine | −0.19 | <0.0001 | −0.10 | 0.001 | −0.12 | <0.0001 | |||||
| Carnitine | — | — | −0.003 | 0.90 | — | — | |||||
| Diastolic blood pressure | Children | TMAO2 | — | — | −0.32 | 0.15 | — | — | 0.05 | 8.89 | <0.0001 |
| Betaine | –0.07 | <0.0001 | −0.07 | 0.0001 | –0.07 | <0.0001 | |||||
| Carnitine | 0.03 | 0.02 | 0.02 | 0.09 | 0.03 | 0.02 | |||||
| DMG2 | — | — | 0.03 | 0.96 | — | — | |||||
| Choline | — | — | 0.06 | 0.50 | — | — | |||||
| Adults | TMAO2 | — | — | −0.09 | 0.76 | — | — | 0.17 | 30.50 | <0.0001 | |
| Choline | 0.41 | <0.0001 | 0.24 | 0.02 | 0.23 | 0.02 | |||||
| Betaine | −0.11 | <0.0001 | −0.07 | 0.003 | −0.07 | 0.0003 | |||||
| DMG2 | — | — | −1.72 | 0.03 | −1.72 | 0.03 | |||||
| Carnitine | — | — | −0.003 | 0.85 | — | — | |||||
| Pulse wave velocity | Children | TMAO2 | — | — | 0.001 | 0.95 | — | — | 0.13 | 25.76 | <0.0001 |
| Betaine | −0.01 | <0.0001 | −0.01 | 0.0001 | −0.01 | <0.0001 | |||||
| Choline | — | — | 0.001 | 0.88 | — | — | |||||
| DMG2 | — | — | −0.01 | 0.81 | — | — | |||||
| Carnitine | — | — | −0.0003 | 0.80 | — | — | |||||
| Adults | TMAO2 | — | — | −0.07 | 0.06 | — | — | 0.23 | 54.92 | <0.0001 | |
| Choline | 0.05 | <0.0001 | 0.02 | 0.09 | — | — | |||||
| Betaine | −0.02 | <0.0001 | −9e−3 | 0.004 | −0.01 | 0.0003 | |||||
| DMG2 | — | — | −0.15 | 0.16 | — | — | |||||
| Carnitine | — | — | 2e−3 | 0.42 | — | — | |||||
| Central retinal artery equivalent (CRAE) | Children | TMAO2 | — | — | 0.32 | 0.52 | — | — | 0.07 | 11.44 | <0.0001 |
| Carnitine | — | — | 0.06 | 0.02 | 0.05 | 0.04 | |||||
| Choline | — | — | −0.15 | 0.43 | — | — | |||||
| Betaine | — | — | −0.03 | 0.46 | — | — | |||||
| DMG2 | — | — | −0.44 | 0.75 | — | — | |||||
| Adults | TMAO2 | — | — | 0.09 | 0.87 | — | — | 0.07 | 11.63 | <0.0001 | |
| Model 1 and 2 reduced model: ([choline]/10)−1 Model 2 nonreduced: ([choline]/10)−2 | 6.73 | <0.0001 | 1.45 | 0.001 | 5.50 | 0.0002 | |||||
| Betaine | 0.11 | 0.002 | 0.09 | 0.03 | 0.10 | 0.01 | |||||
| Carnitine | — | — | −0.04 | 0.13 | — | — | |||||
| DMG2 | — | — | 0.12 | 0.93 | — | — | |||||
| Central retinal vein equivalent (CRVE) | Children | TMAO2 | — | — | −0.18 | 0.79 | — | — | — | — | — |
| Betaine | — | — | −5e−3 | 0.92 | — | — | |||||
| DMG2 | — | — | −0.69 | 0.72 | — | — | |||||
| Carnitine | — | — | 0.06 | 0.16 | — | — | |||||
| Choline | — | — | 0.01 | 0.96 | — | — | |||||
| Adults | TMAO2 | — | — | −0.53 | 0.48 | — | — | — | — | — | |
| Carnitine | — | — | −0.05 | 0.20 | — | — | |||||
| Betaine | — | — | −0.007 | 0.89 | — | — | |||||
| DMG2 | — | — | −0.38 | 0.84 | — | — | |||||
| Choline | — | — | −0.11 | 0.67 | — | — | |||||
Flexible nonlinear functions are computed for variables that do not exhibit a monotonic relation with the outcome of interest.
Log transformed variables.
DMG, dimethylglycine; TMAO, trimethylamine N-oxide; UACR, urinary albumin to creatinine ratio.
TABLE 5.
Effect sizes of TMAO/precursor concentrations on cardiovascular preclinical phenotypes in adults and children
| Cardiovascular outcome | Generation | Metabolite or flexible nonlinear functions from reduced Model 21 | Metabolite SD2 | Estimate reduced model 2 | Effect size (SD × 1-unit change) |
|---|---|---|---|---|---|
| Carotid intima-media thickness | Children | Betaine | 10.97 | 6e−4 | 0.01 |
| DMG3 | 0.32 | −2e−2 | −0.01 | ||
| Carotid distensibility | Children | Betaine | 10.97 | 3e−2 | 0.32 |
| Adults | DMG3 | 0.35 | 0.44 | 0.15 | |
| Systolic blood pressure | Adults | Betaine | 12.04 | −0.12 | −1.44 |
| Choline | 2.62 | 0.32 | 0.84 | ||
| Diastolic blood pressure | Children | Betaine | 10.97 | −0.07 | −0.73 |
| Carnitine | 15.54 | 0.03 | 0.45 | ||
| Adults | Betaine | 12.10 | −0.07 | −0.84 | |
| Choline | 2.65 | 0.23 | 0.61 | ||
| DMG3 | 0.35 | −1.72 | −0.60 | ||
| Pulse wave velocity (PWV) | Children | Betaine | 10.97 | −0.01 | −0.11 |
| Adults | Betaine | 12.04 | −0.01 | −1.32 | |
| Central retinal artery equivalents (CRAE) | Children | Carnitine | 15.55 | 0.05 | 0.78 |
| Adults | Choline1 | 0.28 | 5.50 | 1.54 | |
| Betaine | 12.04 | 0.10 | 1.23 |
Flexible nonlinear functions are computed for variables that do not exhibit a monotonic relation with the outcome of interest: e.g. choline = 1/choline.
Calculated following Cook's plot outlier (Cook's distance >0.5) removal from each model.
Log transformed variables.
DMG, dimethylglycine; TMAO, trimethylamine N-oxide.
Associations of TMAO and its precursors’ concentrations with carotid distensibility
Child betaine concentrations were positively associated with carotid distensibility (effect size +0.32; P = 0.003) following covariate adjustment (Tables 4 and 5). By contrast, only DMG concentrations were positively associated (effect size: +0.15; P = 0.01) with carotid distensibility after covariate adjustment in adults.
Associations of TMAO and its precursors with blood pressure and PWV
Plasma TMAO was not associated with blood pressure or PWV in either generation (Tables 4 and 5). Betaine concentrations (effect size: −1.44; P = 0.002) were negatively and choline concentrations positively (effect size: +0.84; P = 0.03) associated with SBP in adults but not in children. Consistent with this, the concentrations of betaine (effect size −0.84; P = 0.0003), and DMG (effect size: −0.60; P = 0.03) were negatively, whereas choline was positively (effect size: +0.61; P = 0.02) associated with DBP in adults even after covariate adjustment.
In children, none of the metabolites were markedly associated with SBP. However, the concentrations of carnitine (effect size +0.45; P = 0.02) were positively and those of betaine (effect size: −0.73; P < 0.0001) negatively associated with DBP in children.
In both children and adults, only betaine concentrations were negatively associated with PWV (children: effect size: −0.11; P < 0.0001; adults: effect size: −1.32; P = 0.0003).
Associations of TMAO and precursors with microvascular parameters
In children, carnitine concentrations were positively associated with CRAE (effect size: +0.78; P = 0.04) (Tables 4 and 5). Plasma concentrations of choline were negatively (effect size: +1.54 for 1/choline; P = 0.0002), and betaine positively associated with CRAE in adults (effect size: +1.23; P = 0.01). There were no identifiable associations between any of the metabolites and CRVE in either generation (P > 0.05 for all).
Plasma concentrations of metabolites associated with cIMT, carotid distensibility, blood pressure, and CRAE were correlated with each other (−0.26 <R <0.48; P < 0.01) (Supplemental Table 2).
Associations of TMAO and its precursors’ concentrations with inflammatory markers in adults
Plasma TMAO concentrations were not associated with hsCRP or GlycA concentrations in either children or adults (Supplemental Table 3). By contrast, although weak, plasma betaine concentrations were negatively, and choline concentrations were positively associated with hsCRP (R = −0.13; P < 0.0001 for betaine; R = 0.1; P < 0.001 for choline) and GlycA concentrations (R = −0.09; P = 0.01 for betaine; R = 0.2; P < 0.0001 for choline) in adults.
Discussion
In this population-derived cohort of Australian children and adults, concentrations of TMAO precursors (i.e. betaine, choline, carnitine, and DMG), but not TMAO itself, were associated with MetS scores, cardiovascular phenotypes, and inflammatory markers in both children and adults.
We did not identify associations between TMAO plasma concentrations and cIMT, carotid distensibility, PWV, SBP, DBP, microvascular structure, or inflammatory biomarkers. Our findings are at odds with previous data reporting positive associations between TMAO concentrations and adverse cardiovascular outcomes (11, 13, 45–47), although previous findings have been inconsistent (15, 48, 49). However, it is possible that TMAO concentrations are exclusively associated with cardiovascular markers at: 1) later stages of CVD and/or in metabolically unhealthy individuals (5, 45, 50); 2) at higher baseline TMAO concentrations (3, 51); or 3) with other cardiovascular and inflammatory markers (4, 47, 52). The majority of studies analyzing the TMAO-CVD relation target individuals with CVD (7, 10, 11) and largely have not identified strong associations between TMAO concentrations and adverse cardiovascular outcomes in population settings (15). Therefore, prospective studies are required to characterize TMAO concentrations in healthy individuals and determine the relation between TMAO concentrations and cardiometabolic risk.
It is possible that TMAO concentrations reflect, rather than cause, increased cardiovascular risk (53). For example, TMAO concentrations are dependent upon rates of excretion by the kidneys and kidney function (50, 54), and renal dysfunction increases cardiovascular risk (55). Inclusion of UACR, a proxy of kidney function, as a covariate in our models decreased the strength of association of TMAO with cIMT in children. Therefore, associations between TMAO and CVD outcomes may reflect unmeasured confounding (50). It is thus important to consider the contributory roles of covariates (e.g. kidney function) when analyzing and interpreting associations between TMAO concentrations and cardiovascular outcomes.
Betaine, choline, DMG, and carnitine concentrations were all associated with ≥2 of the cardiometabolic outcome measures in our study. Consistent with our findings, several studies report associations between TMAO precursors and adverse cardiometabolic outcomes (5, 13, 56–58). For example, choline has positive, and betaine negative, associations with MetS risk factors (i.e. blood lipids, BMI, body fat percentage, waist circumference, glucose, and blood pressure) in middle-aged and elderly women and men (56). Carnitine, choline, and betaine concentrations strongly predict increased major cardiovascular events in patients with pre-existing cardiovascular risk (13). Plasma DMG concentrations have been robustly associated with acute myocardial infarction; improved prediction of acute myocardial infarction in models that included common cardiovascular risk factors; and were associated with mortality from acute myocardial infarction (57, 58). Positive associations have also been reported for carnitine concentrations with MetS, although no significant link was found with blood pressure (5).
TMAO precursor concentrations were strongly associated with metabolic outcomes in our study, despite no evidence of association with TMAO per se. We suggest that TMAO precursors may exhibit some of their effects via metabolic pathways that do not involve TMAO and that precursor concentrations may be better markers of cardiovascular health within the wider population. Choline, betaine, DMG, and carnitine are central to metabolic pathways involved in vital physiological functions, such as lipid metabolism, that do not involve TMAO, for example 1-C metabolism and fatty acid β-oxidation (57, 59, 60). In our study, choline, betaine, and DMG were strongly correlated with each other, suggesting that they function as a profile of metabolites. Strong associations between these precursors and cardiometabolic outcomes may reflect alterations in the 1-C metabolic pathway, as previously proposed (56). Choline, betaine, and DMG can directly and indirectly form phosphatidylcholine, the main constituent of lipoproteins (e.g. VLDL and LDL), which are key shuttles for lipid exportation from the liver (57, 60). Moreover, carnitine is a rate-limiting step in long-chain fatty acid β-oxidation (59). Therefore, associations of these metabolites with health outcomes may potentially be mediated by pathways involved in lipid metabolism independently of TMAO concentrations.
Previous studies have demonstrated that the association of TMAO precursors with cardiovascular outcomes is weaker when TMAO concentrations are low (2, 9). Moreover, increased metabolic risk is observed in situations where concentrations of precursors and TMAO were concomitantly elevated (61). The observation that TMAO concentrations strengthen the association between precursors and cardiovascular outcomes indicates a possible synergistic effect between TMAO and its precursors. This could be explained by a potential interplay between TMAO synthesis and the 1-C metabolic pathway. Previous studies have highlighted marked correlations between plasma TMAO and homocysteine concentrations (62). TMAO concentrations were also reported to be negatively correlated with the S-adenosylmethionine (SAM)/S-adenosylhomocysteine (SAH) ratio (61). This is consistent with the possibility that a downregulation of 1-C metabolism, frees up common precursors that are subsequently converted into TMAO (61). SAM concentrations were also profiled in our study (data not shown). TMAO and SAM concentrations were weakly correlated [R = 0.07; CI (0.03; 0.11); P < 0.001], recognizing the limitation that these correlations are based on single semifasted plasma measurements. Additional supporting evidence has also been reported in an intervention study where combined vitamin B and D supplementation lowered both plasma homocysteine and TMAO concentrations (63). As such, it is likely that an individual's baseline health status determines the “balance” in precursor utilization between 1-C metabolism and TMAO synthesis.
Limitations
This study was conducted in a relatively large Australian cohort of children and adults (total N = 2490), included TMAO as well as its main precursors, and adjusted for common cardiovascular covariates (i.e. age, sex, BMI, household income, and renal function). Despite these strengths, TMAO concentrations exhibit intraindividual variability, therefore 1 measurement in a cross-sectional study is insufficient to draw conclusive results of associations with cardiometabolic outcomes (64). Moreover, low semifasting plasma TMAO concentrations do not necessarily equate to a low-TMAO production capacity in individuals, which may be a more robust indicator of TMAO-health associations (65). Additionally, trimethylamine (TMA), the reduced form of TMAO was not profiled in our study although proposed as the potentially overlooked culprit of adverse cardiometabolic outcomes (66, 67). TMA was not quantitated as it typically requires an ethyl bromoacetate derivatization prior to LC/MS analysis, which would have interfered with the detection of other compounds profiled in our analytical panel (e.g. amino acids) (68). The adult subgroup consisted mainly of females with an unbalanced 1:10 male to female ratio compared with a 1:1 ratio in children. The CheckPoint cohort was predominantly European Caucasian and is relatively more advantaged than the wider Australian population (over 78% of our population scored in the middle-least disadvantaged SEIFA score compared with around 62% in the general Australian population) (69, 70). Finally, this study did not include analysis of the stool microbiome (71).
Conclusion
In our study of Australian children and adults, we did not find evidence of associations between TMAO concentrations and adverse cardiometabolic phenotypes nor inflammatory markers. However, choline, betaine, DMG, and carnitine concentrations were all strongly associated with several cardiometabolic outcome measures as well as inflammatory markers in both generations. We propose that the impact of TMAO concentrations on metabolic outcomes is likely to depend on baseline health status. Interventions aiming to target TMAO concentrations should therefore assess the effects of changes in TMAO concentrations on several factors that may affect different “layers” of an individual's health. We also speculate that the balance between the involvement of precursors in 1-C metabolism and TMAO formation may become dysregulated in disease states as a compensatory mechanism in response to metabolic damage. Given the disparity in results between healthy and unhealthy populations, and given how understudied the profiles of TMAO/precursors are in healthy population settings and across different generations, researchers should refrain from extrapolating associations between TMAO and CVD in individuals with CVD to the general population (65).
Supplementary Material
ACKNOWLEDGEMENTS
This manuscript uses unit record data from Growing Up in Australia, the Longitudinal Study of Australian Children. The study is conducted in partnership between the Department of Social Services (DSS), the Australian Institute of Family Studies (AIFS), and the Australian Bureau of Statistics (ABS). The findings and views reported in this manuscript are those of the authors and should not be attributed to DSS, AIFS, or the ABS.
The authors’ responsibilities were as follows—MW, RS, and DPB: designed this study; MW, RS, DPB, and JMO: designed the substudy of micronutrients; SA: conducted the laboratory work, performed all statistical analyses, and wrote the manuscript; KL and SAC: provided data support; BJ: provided statistical support; EBT: assisted with laboratory analyses; KL, SAC, BJ, EBT, JAK, KL, MW, RS, DPB, and JMO: discussed and edited the manuscript; MW, RS, DPB, and JMO: supervised SA; and all authors: read and approved the final manuscript.
Author Biographical
This study was funded by a Ministry of Business, Innovation and Employment (MBIE) Catalyst grant [The New Zealand-Australia Life Course Collaboration on Genes, Environment, Nutrition and Obesity (GENO); UOAX1611 to JMO]. SA is the recipient of a New Zealand International Doctoral Research Scholarship 2017. DB is supported by a National Health and Medical Research Council (Australia) Senior Research Fellowship (1064629) and Investigator Grant (1175744). Research at the Murdoch Children's Research Institute is supported by the Victorian Government's Operational Infrastructure Program. The funding bodies did not play any role in the study.
Author disclosures: The authors report no conflicts of interest.
Supplemental Figures 1 and 2 and Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
DPB and JMO'S are joint corresponding authors.
Abbreviations used: cIMT, carotid intima-media thickness; CRAE, central retinal artery equivalent; CRVE, central retinal vein equivalent; CVD, cardiovascular disease; DBP, diastolic blood pressure; DMG, dimethylglycine; GlycA, glycoprotein acetyls; hsCRP, high-sensitivity C-reactive protein; LSAC, Longitudinal Study of Australian Children; MetS, metabolic syndrome; PWV, pulse wave velocity; SAM, S-adenosylmethionine; SBP, systolic blood pressure; SEIFA, Socio-Economic Indexes for Areas; TMAO, trimethylamine N-oxide; UACR, urinary albumin to creatinine ratio.
Contributor Information
Stephanie Andraos, Liggins Institute, The University of Auckland, Auckland, New Zealand.
Beatrix Jones, Department of Statistics, Faculty of Science, The University of Auckland, Auckland, New Zealand.
Katherine Lange, The Murdoch Children's Research Institute, Parkville, Victoria, Australia; Department of Paediatrics, University of Melbourne, Parkville, Victoria, Australia.
Susan A Clifford, The Murdoch Children's Research Institute, Parkville, Victoria, Australia; Department of Paediatrics, University of Melbourne, Parkville, Victoria, Australia.
Eric B Thorstensen, Liggins Institute, The University of Auckland, Auckland, New Zealand.
Jessica A Kerr, The Murdoch Children's Research Institute, Parkville, Victoria, Australia; Department of Paediatrics, University of Melbourne, Parkville, Victoria, Australia.
Melissa Wake, The Murdoch Children's Research Institute, Parkville, Victoria, Australia; Department of Paediatrics, University of Melbourne, Parkville, Victoria, Australia.
Richard Saffery, The Murdoch Children's Research Institute, Parkville, Victoria, Australia; Department of Paediatrics, University of Melbourne, Parkville, Victoria, Australia.
David P Burgner, The Murdoch Children's Research Institute, Parkville, Victoria, Australia; Department of Paediatrics, University of Melbourne, Parkville, Victoria, Australia; Department of Paediatrics, Monash University, Clayton, Victoria, Australia.
Justin M O'Sullivan, Email: justin.osullivan@auckland.ac.nz, Liggins Institute, The University of Auckland, Auckland, New Zealand; MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, United Kingdom.
References
- 1. Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, DuGar B, Feldstein AE, Britt EB, Fu X, Chung Y-Met al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li Let al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhu W, Wang Z, Tang WHW, Hazen SL. Gut microbe-generated trimethylamine N-oxide from dietary choline is prothrombotic in subjects. Circulation. 2017;135:1671–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Seldin MM, Meng Y, Qi H, Zhu W, Wang Z, Hazen SL, Lusis AJ, Shih DM. Trimethylamine N‐oxide promotes vascular inflammation through signaling of mitogen‐activated protein kinase and nuclear factor‐κB. JAHA. 2016;5:e002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gao X, Tian Y, Randell E, Zhou H, Sun G. Unfavorable associations between serum trimethylamine N-oxide and l-carnitine levels with components of metabolic syndrome in the newfoundland population. Front Endocrinol (Lausanne). 2019;10:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cheng F, Li W, Liu G, Tang Y. In silico ADMET prediction: recent advances, current challenges and future trends. Curr Top Med Chem. 2013;13(11):1273–89. [DOI] [PubMed] [Google Scholar]
- 7. Yu D, Shu X-O, Rivera ES, Zhang X, Cai Q, Calcutt MW, Xiang Y-B, Li H, Gao Y-T, Wang TJet al. Urinary levels of trimethylamine-N-oxide and incident coronary heart disease: a prospective investigation among urban Chinese adults. J Am Heart Assoc. 2019;8:e010606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lever M, George PM, Slow S, Bellamy D, Young JM, Ho M, McEntyre CJ, Elmslie JL, Atkinson W, Molyneux SLet al. Betaine and trimethylamine-N-oxide as predictors of cardiovascular outcomes show different patterns in diabetes mellitus: an observational study. PLoS One. 2014;9:e114969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang Z, Tang WHW, Buffa JA, Fu X, Britt EB, Koeth RA, Levison BS, Fan Y, Wu Y, Hazen SL. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur Heart J. 2014;35:904–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dong Z, Liang Z, Guo M, Hu S, Shen Z, Hai X. The association between plasma levels of trimethylamine N-oxide and the risk of coronary heart disease in Chinese patients with or without type 2 diabetes mellitus. Dis Markers. 2018;2018:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li XS, Obeid S, Wang Z, Hazen BJ, Li L, Wu Y, Hurd AG, Gu X, Pratt A, Levison BSet al. Trimethyllysine, a trimethylamine N-oxide precursor, provides near- and long-term prognostic value in patients presenting with acute coronary syndromes. Eur Heart J. 2019;40:2700–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Qi J, You T, Li J, Pan T, Xiang L, Han Y, Zhu L. Circulating trimethylamine N-oxide and the risk of cardiovascular diseases: a systematic review and meta-analysis of 11 prospective cohort studies. J Cell Mol Med. 2018;22(1):185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heianza Y, Ma W, Manson JE, Rexrode KM, Qi L. Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: a systematic review and meta‐analysis of prospective studies. J Am Heart Assoc. 2017;6(7):e004947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaysen GA, Johansen KL, Chertow GM, Dalrymple LS, Kornak J, Grimes B, Dwyer T, Chassy AW, Fiehn O. Associations of trimethylamine N-oxide with nutritional and inflammatory biomarkers and cardiovascular outcomes in patients new to dialysis. J Ren Nutr. 2015;25:351–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meyer KA, Benton TZ, Bennett BJ, Jacobs DR, Lloyd‐Jones DM, Gross MD, Carr JJ, Gordon‐Larsen P, Zeisel SH. Microbiota‐dependent metabolite trimethylamine N‐oxide and coronary artery calcium in the Coronary Artery Risk Development in Young Adults Study (CARDIA). J Am Heart Assoc. 2016;5(10)::e003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reiner MF, Müller D, Gobbato S, Stalder O, Limacher A, Bonetti NR, Pasterk L, Méan M, Rodondi N, Aujesky Det al. Gut microbiota-dependent trimethylamine-N-oxide (TMAO) shows a U-shaped association with mortality but not with recurrent venous thromboembolism. Thromb Res. 2019;174:40–7. [DOI] [PubMed] [Google Scholar]
- 17. Leal‐Witt MJ, Llobet M, Samino S, Castellano P, Cuadras D, Jimenez‐Chillaron JC, Yanes O, Ramon‐Krauel M, Lerin C. Lifestyle intervention decreases urine trimethylamine N‐oxide levels in prepubertal children with obesity. Obesity. 2018;26:1603–10. [DOI] [PubMed] [Google Scholar]
- 18. Hsu C-N, Lu P-C, Lo M-H, Lin I-C, Chang-Chien G-P, Lin S, Tain Y-L. Gut microbiota-dependent trimethylamine N-oxide pathway associated with cardiovascular risk in children with early-stage chronic kidney disease. Int J Mol Sci. 2018;19(12):3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Skilton MR, Celermajer DS, Cosmi E, Crispi F, Gidding SS, Raitakari OT, Urbina EM. Natural history of atherosclerosis and abdominal aortic intima-media thickness: rationale, evidence, and best practice for detection of atherosclerosis in the young. JCM. 2019;8:1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. International Diabetes Federation . The IDF consensus worldwide definition of the Metabolic Syndrome. [Internet]. 2006. Available from: https://www.idf.org/e-library/consensus-statements/60-idfconsensus-worldwide-definitionof-the-metabolic-syndrome.html. [Google Scholar]
- 21. Dagenais GR, Leong DP, Rangarajan S, Lanas F, Lopez-Jaramillo P, Gupta R, Diaz R, Avezum A, Oliveira GBF, Wielgosz Aet al. Variations in common diseases, hospital admissions, and deaths in middle-aged adults in 21 countries from five continents (PURE): a prospective cohort study. Lancet. 2019;395(10226):785–94. [DOI] [PubMed] [Google Scholar]
- 22. Clifford SA, Davies S, Wake M, Team CHCP. Child Health CheckPoint: cohort summary and methodology of a physical health and biospecimen module for the Longitudinal Study of Australian Children. BMJ Open. 2019;9:3–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harris WS. The omega-3 index as a risk factor for coronary heart disease. Am J Clin Nutr. 2008;87:1997S. [DOI] [PubMed] [Google Scholar]
- 24. Natto ZS, Yaghmoor W, Alshaeri HK, Van Dyke TE. Omega-3 fatty acids effects on inflammatory biomarkers and lipid profiles among diabetic and cardiovascular disease patients: a systematic review and meta-analysis. Sci Rep. 2019;9:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Andraos S, Lange K, Clifford SA, Jones B, Thorstensen EB, Kerr JA, Wake M, Saffery R, Burgner DP, O'Sullivan JM. Plasma trimethylamine N-oxide (TMAO) and its precursors: population epidemiology, parent-child concordance, and associations with reported dietary intake in 11–12-year-old children and their parents. Curr Dev Nutr. 2020;4(7):nzaa103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Edwards B. Growing up in Australia: The Longitudinal Study of Australian children: entering adolescence and becoming a young adult. Family Matters. 2014;95::5–14. [Google Scholar]
- 27. Sanson A, Johnstone R. The LSAC Research Consortium & FaCS LSAC Project Team. Growing Up in Australia takes its first steps. Family Matters. 2004;46–53. [Google Scholar]
- 28. Lycett K, Juonala M, Magnussen CG, Norrish D, Mensah FK, Liu R, Clifford SA, Carlin JB, Olds T, Saffery Ret al. Body mass index from early to late childhood and cardiometabolic measurements at 11 to 12 years. Pediatrics. 2020; 146(2):e20193666. [DOI] [PubMed] [Google Scholar]
- 29. Andraos S, Goy M, Albert BB, Kussmann M, Thorstensen EB, O'Sullivan JM. Robotic automation of a UHPLC/MS-MS method profiling one-carbon metabolites, amino acids, and precursors in plasma. Anal Biochem. 2020;592:113558. [DOI] [PubMed] [Google Scholar]
- 30. Wijndaele K, Beunen G, Duvigneaud N, Matton L, Duquet W, Thomis M, Lefevre J, Philippaerts RM. A continuous metabolic syndrome risk score: utility for epidemiological analyses. Diabetes Care. 2006;29:2329–9. [DOI] [PubMed] [Google Scholar]
- 31. Gurka MJ, Ice CL, Sun SS, DeBoer MD. A confirmatory factor analysis of the metabolic syndrome in adolescents: an examination of sex and racial/ethnic differences. Cardiovasc Diabetol. 2012;11:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu RS, Dunn S, Grobler AC, Lange K, Becker D, Goldsmith G, Carlin JB, Juonala M, Wake M, Burgner DP. Carotid artery intima-media thickness, distensibility and elasticity: population epidemiology and concordance in Australian children aged 11–12 years old and their parents. BMJ Open. 2019;9:23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kahn FK, Wake M, Lycett K, Clifford S, Burgner DP, Goldsmith G, Grobler AC, Lange K, Cheung M. Vascular function and stiffness: population epidemiology and concordance in Australian children aged 11–12 years and their parents. BMJ Open. 2019;9:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dascalu J, Liu M, Lycett K, Grobler AC, He M, Burgner DP, Wong TY, Wake M. Retinal microvasculature: population epidemiology and concordance in Australian children aged 11–12 years and their parents. BMJ Open. 2019;9:44–52..9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Knudtson MD, Lee KE, Hubbard LD, Wong TY, Klein R, Klein BEK. Revised formulas for summarizing retinal vessel diameters. Curr Eye Res. 2003;27:143–9. [DOI] [PubMed] [Google Scholar]
- 36. Ellul S, Wake M, Clifford SA, Lange K, Würtz P, Juonala M, Dwyer T, Carlin JB, Burgner DP, Saffery R. Metabolomics: population epidemiology and concordance in Australian children aged 11–12 years and their parents. BMJ Open. 2019;9:106–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Collier F, Ellul S, Juonala M, Ponsonby A-L, Vuillermin P, Saffery R, Burgner D. Glycoprotein acetyls (GlycA) at 12 months are associated with high-sensitivity C-reactive protein and early life inflammatory immune measures. Pediatr Res. 2019;85:584–5. [DOI] [PubMed] [Google Scholar]
- 38. Connelly MA, Otvos JD, Shalaurova I, Playford MP, Mehta NN. GlycA, a novel biomarker of systemic inflammation and cardiovascular disease risk. J Transl Med. 2017;15:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Clifford SA, Gillespie AN, Olds T, Grobler AC, Wake M. Body composition: population epidemiology and concordance in Australian children aged 11–12 years and their parents. BMJ Open. 2019;9:95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Larkins NG, Kim S, Carlin JB, Grobler AC, Burgner DP, Lange K, Craig JC, Wake M. Albuminuria: population epidemiology and concordance in Australian children aged 11–12 years and their parents. BMJ Open. 2019;9:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. R Core Team . R: The R Project for Statistical Computing. R Foundation for Statistical Computing, Vienna (Austria). [Internet]. [cited 2019 Aug 8]. Available from: https://www.r-project.org/. [Google Scholar]
- 42. Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Soft. 2015;67:1–48. [Google Scholar]
- 43. Ambler G, Benner A. mfp: Multivariable Fractional Polynomials. [Internet]. [Accessed 2019 Aug 8]. Available from: https://cran.r-project.org/package=mfp.
- 44. Royston P, Altman DG. Regression using fractional polynomials of continuous covariates: parsimonious parametric modelling. Applied Statistics. 1994;43:429. [Google Scholar]
- 45. Tang WHW, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Qi J, You T, Li J, Pan T, Xiang L, Han Y, Zhu L. Circulating trimethylamine N-oxide and the risk of cardiovascular diseases: a systematic review and meta-analysis of 11 prospective cohort studies. J Cell Mol Med. 2018;22:185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Randrianarisoa E, Lehn-Stefan A, Wang X, Hoene M, Peter A, Heinzmann SS, Zhao X, Königsrainer I, Königsrainer A, Balletshofer Bet al. Relationship of serum trimethylamine N-oxide (TMAO) levels with early atherosclerosis in humans. Sci Rep. 2016;6:26745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mente A, Chalcraft K, Ak H, Davis AD, Lonn E, Miller R, Potter MA, Yusuf S, Anand SS, McQueen MJ. The relationship between trimethylamine-N-oxide and prevalent cardiovascular disease in a multiethnic population living in Canada. Can J Cardiol. 2015;31(9):1189–94. [DOI] [PubMed] [Google Scholar]
- 49. Yin J, Liao S-X, He Y, Wang S, Xia G-H, Liu F-T, Zhu J-J, You C, Chen Q, Zhou Let al. Dysbiosis of gut microbiota with reduced trimethylamine-N-oxide level in patients with large-artery atherosclerotic stroke or transient ischemic attack. J Am Heart Assoc. 2015;4(11):e002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mueller DM, Allenspach M, Othman A, Saely CH, Muendlein A, Vonbank A, Drexel H, von Eckardstein A. Plasma levels of trimethylamine-N-oxide are confounded by impaired kidney function and poor metabolic control. Atherosclerosis. 2015;243:638–44. [DOI] [PubMed] [Google Scholar]
- 51. Barrea L, Annunziata G, Muscogiuri G, Di Somma C, Laudisio D, Maisto M, de Alteriis G, Tenore GC, Colao A, Savastano S. Trimethylamine-N-oxide (TMAO) as novel potential biomarker of early predictors of metabolic syndrome. Nutrients. 2018;10(12):1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Manor O, Zubair N, Conomos MP, Xu X, Rohwer JE, Krafft CE, Lovejoy JC, Magis AT. A multi-omic association study of trimethylamine N-oxide. Cell Rep. 2018;24:935–46. [DOI] [PubMed] [Google Scholar]
- 53. DiNicolantonio JJ, McCarty M, OKeefe J. Association of moderately elevated trimethylamine N-oxide with cardiovascular risk: is TMAO serving as a marker for hepatic insulin resistance. Open Heart. 2019;6:e000890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stubbs JR, House JA, Ocque AJ, Zhang S, Johnson C, Kimber C, Schmidt K, Gupta A, Wetmore JB, Nolin TDet al. Serum trimethylamine-N-oxide is elevated in CKD and correlates with coronary atherosclerosis burden. JASN. 2016;27:305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Konstantinova SV, Tell GS, Vollset SE, Nygård O, Ø Bleie, Ueland PM. Divergent associations of plasma choline and betaine with components of metabolic syndrome in middle age and elderly men and women. J Nutr. 2008;138:914–20. [DOI] [PubMed] [Google Scholar]
- 57. Tveitevåg Svingen GF, Ueland PM, Pedersen EKR, Schartum-Hansen H, Seifert R, Ebbing M, Løland KH, Tell GS, Nygård O. Plasma dimethylglycine and risk of incident acute myocardial infarction in patients with stable angina pectoris. Arterioscler Thromb Vasc Biol. 2013;33:2041–8. [DOI] [PubMed] [Google Scholar]
- 58. Svingen GF, Schartum-Hansen H, Ueland PM, Pedersen ER, Seifert R, Ebbing M, Bønaa KH, Mellgren G, Nilsen DW, Nordrehaug JEet al. Elevated plasma dimethylglycine is a risk marker of mortality in patients with coronary heart disease. Eur J Prev Cardiolog. 2015;22:743–52. [DOI] [PubMed] [Google Scholar]
- 59. Longo N, Frigeni M, Pasquali M. Carnitine transport and fatty acid oxidation. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2016;1863:2422–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schwab U, Alfthan G, Aro A, Uusitupa M. Long-term effect of betaine on risk factors associated with the metabolic syndrome in healthy subjects. Eur J Clin Nutr. 2011;65:70–6. [DOI] [PubMed] [Google Scholar]
- 61. Obeid R, Awwad HM, Rabagny Y, Graeber S, Herrmann W, Geisel J. Plasma trimethylamine N-oxide concentration is associated with choline, phospholipids, and methyl metabolism. Am J Clin Nutr. 2016;103(3):703–11. [DOI] [PubMed] [Google Scholar]
- 62. Malinowska AM, Szwengiel A, Chmurzynska A. Dietary, anthropometric, and biochemical factors influencing plasma choline, carnitine, trimethylamine, and trimethylamine-N-oxide concentrations. Int J Food Sci Nutr. 2017;68:488–95. [DOI] [PubMed] [Google Scholar]
- 63. Obeid R, Awwad HM, Kirsch SH, Waldura C, Herrmann W, Graeber S, Geisel J. Plasma trimethylamine-N-oxide following supplementation with vitamin D or D plus B vitamins. Mol Nutr Food. 2017;61:1600358. [DOI] [PubMed] [Google Scholar]
- 64. McEntyre CJ, Lever M, Chambers ST, George PM, Slow S, Elmslie JL, Florkowski CM, Lunt H, Krebs JD. Variation of betaine, N,N- dimethylglycine, choline, glycerophosphorylcholine, taurine and trimethylamine-N-oxide in the plasma and urine of overweight people with type 2 diabetes over a two-year period. Ann Clin Biochem. 2015;52:352–60. [DOI] [PubMed] [Google Scholar]
- 65. Arduini A, Zammit VA, Bonomini M. Identification of trimethylamine N-oxide (TMAO)-producer phenotype is interesting, but is it helpful?. Gut. 2020;69(2):400–1. [DOI] [PubMed] [Google Scholar]
- 66. Ufnal M. Trimethylamine, a toxic precursor of trimethylamine oxide, lost in medical databases. J Nutr. 2020;150(2):419. [DOI] [PubMed] [Google Scholar]
- 67. Jaworska K, Bielinska K, Gawrys-Kopczynska M, Ufnal M. TMA (trimethylamine), but not its oxide TMAO (trimethylamine-oxide), exerts hemodynamic effects—implications for interpretation of cardiovascular actions of gut microbiome. Cardiovasc Res. 2019;115(14):1948–9. [DOI] [PubMed] [Google Scholar]
- 68. Veeravalli S, Karu K, Phillips IR, Shephard EA. A highly sensitive liquid chromatography electrospray ionization mass spectrometry method for quantification of TMA, TMAO and creatinine in mouse urine. MethodsX. 2017;4:310–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Australian Bureau of Statistics. Population. [Internet]. 2019. [Accessed 2019 Aug 8]. Available from: https://www.abs.gov.au/population. [Google Scholar]
- 70. Australian Bureau of Statistics . Census of Population and Housing: Socio-Economic Indexes for Areas (SEIFA), Australia, 2016[Internet]. 2018. [Accessed 2019 Aug 8]. Available from: https://www.abs.gov.au/.
- 71. Cho CE, Taesuwan S, Malysheva OV, Bender E, Tulchinsky NF, Yan J, Sutter JL, Caudill MA. Trimethylamine-N-oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: a randomized controlled trial. Mol Nutr Food Res. 2017;61:1600324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.