Abstract
Purpose of review
Vasculitides can affect small, medium and/or large vessels, leading to end-organ damage, decreased quality of life and death. Glucocorticoids remain the backbone of treatment for systemic vasculitis but are associated with numerous toxicities. In recent years, the efficacy of glucocorticoid-sparing biologic and novel small molecule therapies has been demonstrated.
Recent findings
In giant cell arteritis, tocilizumab was superior to glucocorticoid monotherapy in maintenance remission and cumulative glucocorticoid exposure and is now approved for the treatment of giant cell arteritis. In addition to the previously demonstrated efficacy of rituximab for remission induction in antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis, recent trials have also demonstrated its superiority for remission maintenance compared to alternative approaches. Mepolizumab is superior to standard of care alone with regard to remission rates and glucocorticoid-sparing effect in refractory eosinophilic granulomatosis with polyangiitis. Avacopan has shown significant promise in ANCA-associated vasculitis as part of a glucocorticoid-free induction regimen in a recently completed phase 3 trial. Use of biologics in rarer vasculitides remains guided by reports from small case series.
Summary
Biologics and other novel therapies have an increasingly important role in the management of systemic vasculitis. Additional studies are needed to define their optimal use and to guide their use in more rare forms of vasculitis.
Keywords: biologic, glucocorticoid, vasculitis
INTRODUCTION
Vasculitis refers to inflammation involving the vessel wall and is often categorized according to the size of the affected vessels (i.e. small, medium or large). These conditions may be idiopathic in cause (i.e. primary) or may develop in the context of another underlying disease (e.g. infection and malignancy) or exposure to medication (e.g. hydralazine) or environmental toxin (e.g. levamisole). Glucocorticoids are a cornerstone of therapy for many of the primary vasculitides. However, glucocorticoids are associated with numerous toxicities such that while taking them, 90% of patients experience at least one adverse event, such as hypertension, diabetes, cataracts, glaucoma, osteoporosis and serious infections, among others (Fig. 1) [1,2]. The association between adverse events and glucocorticoid exposure is dose dependent; for each 1000 mg increase in cumulative glucocorticoid exposure, the risk of an adverse event increases by up to 5% [3]. Thus, for chronic conditions like many types of vasculitis, glucocorticoid-sparing therapies are critical for improving patient outcomes. Although conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) are often used, there is a rapidly expanding role for biologic medications and other oral, small molecule, targeted therapies in a variety of systemic vasculitides. For the purpose of this review, we define a biologic medication as one that is produced from or contains components of a living organism. In this review, we will focus on recent updates in the management of primary vasculitides, with a focus on recent advances in biologic and small molecule targeted therapy.
FIGURE 1.
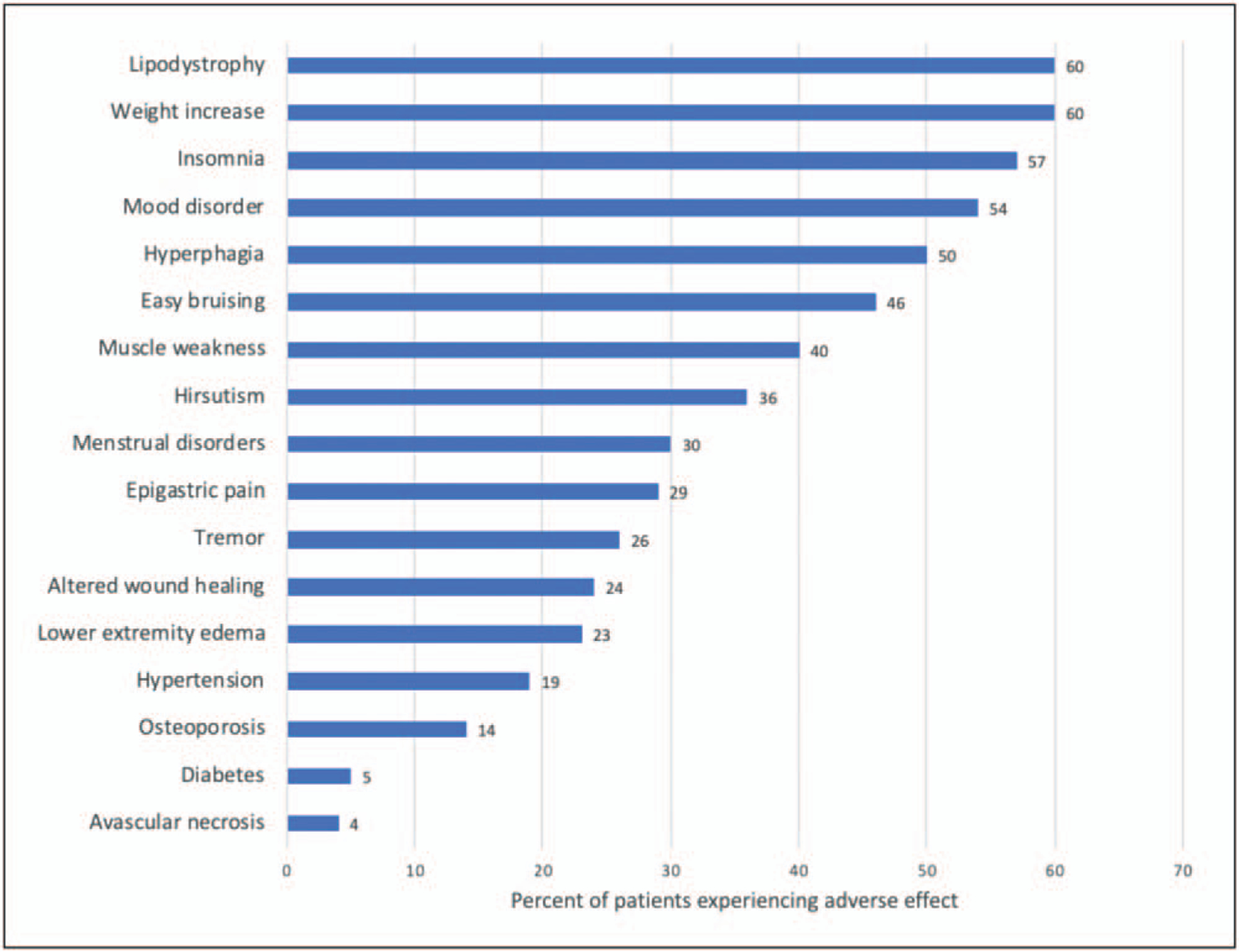
Percentage of patients experiencing various glucocorticoid-related adverse effects after 6 months of glucocorticoid treatment by self-reported questionnaire. Data source: [2].
GIANT CELL ARTERITIS
Giant cell arteritis (GCA) is one of the large-vessel vasculitides and is the most common form of primary vasculitis. It has an annual incidence of 17 per 100,000 people over 50 years old in North America [4] and most often affects women of northern European descent who are over 50 years of age. Until recently, glucocorticoid monotherapy (at a dose of 1 mg/kg/day and tapered gradually over at least 1 year) was the standard of care, though some studies suggested a potential glucocorticoid-sparing effect of methotrexate [5].
The management of GCA shifted with the recent regulatory approval of tocilizumab, an interleukin (IL)-6 receptor antagonist, as a glucocorticoid-sparing treatment for GCA (Table 1). An initial small (N = 20) randomized controlled trial assessed the efficacy of tocilizumab and found higher relapse-free survival rates in the tocilizumab group than the placebo group, which received glucocorticoid monotherapy (85 vs. 20% in the placebo group; P = 0.001) [6]. This was followed by the Giant Cell Arteritis Actemra (GiACTA) trial (N = 251) in which patients were randomized to one of four arms; the proportion achieving sustained glucocorticoid-free remission (primary outcome) at 52 weeks was 56% in the weekly tocilizumab group, 53% in the every other week tocilizumab group, 18% in the 52-week prednisone only group and 14% in the 25-week prednisone only group [7]. Serious adverse events were reported more often in the prednisone groups (22–25% in the prednisone groups vs. 14–15% in the tocilizumab groups). The prednisone groups also had greater cumulative glucocorticoid doses over 52 weeks (3296–3818 mg in the prednisone groups vs. 1862 mg in each of the tocilizumab groups) [7].
Table 1.
Biologic and small molecule, targeted treatments for rheumatic diseases
| Rheumatic disease | Treatments currently in use | Under evaluation in clinical trials |
|---|---|---|
| Giant cell arteritis | Tocilizumaba | Abatacept Anakinra Upadacitinib |
| ANCA-associated vasculitis | Rituximaba (for remission induction and maintenance) | Avacopan Belimumab |
| Takayasu arteritis | TNF inhibitors Tocilizumaba |
|
| Cryoglobulinemic vasculitis | Rituximab | |
| Primary angiitis of the central nervous system | Rituximab | |
| Behcet’s disease | TNF inhibitors (especially for neuro-Behcet’s) | Ustekinumab Anakinra |
| Polyarteritis nodosa | TNF inhibitors Rituximab Tocilizumab |
TNF, tumor necrosis factor.
Supported by the data from randomized controlled trials; otherwise, data limited to cohort studies, case series or case reports.
Other biologics have been studied in GCA in smaller studies and yielded less dramatic results. Abatacept, a cytotoxic T-lymphocyte-associated protein 4 immunoglobulin, was studied in a randomized controlled withdrawal trial in which all patients received abatacept up-front with glucocorticoids; subsequently, those achieving remission (N = 41) were randomized to continue or discontinue abatacept (both received a total of 28 weeks of glucocorticoids). Relapse-free remission was observed in 48% in the abatacept continuation group compared with 31% in the glucocorticoid monotherapy group (P = 0.049). There was no difference in the frequency or severity of adverse events between the treatment arms. Although promising, additional studies are needed to further evaluate the efficacy of abatacept for GCA [8]. Ustekinumab, an IL-12/23 inhibitor, was evaluated for GCA in an open-label single-arm study (N = 25) that suggested that it may lead to less glucocorticoid exposure and reduce the risk of relapse [9]. However, a subsequent single-arm, open-label study (N = 13) evaluating ustekinumab in combination with a 6-month prednisone taper was terminated early because of 70% of the initially enrolled patients experiencing disease flares [10■].
The precise role of tocilizumab in the approach to GCA management remains controversial and undefined [11]. Additional studies (e.g. trials, cohort studies and cost-effectiveness studies) are needed to evaluate the optimal use of tocilizumab for the treatment of GCA (e.g. timing of initiation and duration of treatment) and its long-term ability to prevent large vessel and other complications. Current guidelines for the management of GCA reflect this uncertainty, recommending initial treatment with high-dose glucocorticoids and the use of tocilizumab in the setting of refractory or relapsing disease or for patients at increased risk of glucocorticoid-related complications [12■■].
The success of the GiACTA trial has prompted tremendous interest in programs evaluating novel approaches to GCA management. Ongoing clinical trials are studying tocilizumab in combination with a short 2-month prednisone taper (ClinicalTrials.gov; NCT03726749), an IL-6 receptor inhibitor (sarilumab) (ClinicalTrials.gov; NCT03600805), and novel targets, such as Janus kinase with upadacitinib (ClinicalTrials.gov; NCT03725202), and IL-1with anakinra (ClinicalTrials.gov; NCT02902731).
TAKAYASU ARTERITIS
Takayasu arteritis is a form of large-vessel vasculitis involving the aorta and its primary branches that tends to affect women under the age of 50 years. Its incidence varies across the globe, with an estimated incidence of 2.6 cases per million in the United States and up to 60 cases per million in Japan [13]. Glucocorticoids in combination with DMARDs, especially conventional synthetic DMARDs (e.g. methotrexate and leflunomide), have traditionally been the standard of care for treatment because of difficulty tapering glucocorticoids to reasonably low doses [12■■]. Recently, biologic DMARDs, such as tumor necrosis factor (TNF) inhibitors and tocilizumab, have been studied and increasingly been used as first-line therapy, though there is a paucity of data to guide these practices.
Data from both retrospective and prospective open-label series suggest that TNF inhibitors can reduce disease activity and glucocorticoid exposure in Takayasu arteritis, though results should be interpreted with caution as there have not been randomized controlled trials evaluating the efficacy of these medications [12■■]. A recent small (N = 36) randomized controlled trial evaluated the efficacy of tocilizumab in Takayasu arteritis to prevent relapse after remission was achieved with glucocorticoids. In that trial, tocilizumab decreased the time to relapse of disease in the per-protocol analysis [hazard ratio 0.3, 95% confidence interval (CI): 0.11–1.00, P = 0.03], though this difference was not significant in the intention to treat analysis (hazard ratio 0.4, 95% CI: 0.15–1.10; P = 0.06) [13]. Additional trials with large cohorts are need to further assess the efficacy of tocilizumab and TNF inhibitors for the treatment of Takayasu arteritis. The decision to use either a TNF inhibitor or tocilizumab for Takayasu arteritis should be based on the patient’s comorbidities and the presence of any relevant contraindications to either medication [12■■].
ANTINEUTROPHIL CYTOPLASMIC ANTIBODY-ASSOCIATED VASCULITIS
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a category of diseases that includes granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA) and eosinophilic granulomatosis with polyangiitis (eGPA), all of which tend to affect small vessels and are often characterized by the presence of antibodies to either myeloperoxidase or proteinase 3. For moderate to severe disease, remission induction therapy typically involved the combination of cyclophosphamide and glucocorticoids until the RAVE trial (N = 197) demonstrated the noninferiority of rituximab (anti-CD20 monoclonal antibody) when compared to cyclophosphamide for remission induction (64 vs. 53%, respectively; P < 0.001 for noninferiority) [14]. Today, providers have a number of treatment options for the management of AAV, including rituximab, cyclophosphamide, mepolizumab, azathioprine, methotrexate and mycophenolate mofetil, guided by disease type and severity, treatment phase, patient preference and comorbidities [15]. Recently, a number of additional trials have further evaluated rituximab, as well as mepolizumab, in AAV.
MAINRITSAN1 was a randomized controlled trial (N = 115) that demonstrated the superiority of rituximab over azathioprine for remission maintenance (major relapse rates of 5 vs. 29%; P = 0.002) and comparable safety profiles of both drugs in patients with GPA or MPA [16]. These results have been confirmed in those with relapsing AAV in the recently completed, but not yet published, RITAZAREM randomized controlled trial [17■]. Notably, rituximab was associated with improved survival in long-term follow-up of MAINRITSAN1 participants [18].
Following demonstration of rituximab’s efficacy for remission maintenance, additional trials were conducted to better define its use in AAV. The MAINRITSAN2 trial (N = 162) randomized patients with GPA or MPA to fixed retreatment (every 6 months) or a tailored-approach to retreatment (when CD19+ B cells or ANCA reappeared) for remission maintenance over 18 months, with the primary endpoints of number of relapses or worsening disease activity measured at month 28. Those randomized to tailored dosing required fewer rituximab infusions (3 vs. 5) but did not experience a significantly higher risk of relapse than those randomized to fixed dosing (10 vs. 17%; P = 0.2) [19]. Although the results from MAINRITSAN 2 are compelling, there is a concern that there was a numerical difference in the proportion flaring with tailored therapy that may have been statistically significant if the trial cohort has been larger.
Most recently, the MAINRITSAN3 trial randomized 97 patients with GPA or MPA who had completed MAINRITSAN2 to an additional 18 months of rituximab or placebo. In this trial, those randomized to continue rituximab had superior relapse-free survival compared to those randomized to discontinue rituximab (96 vs. 74%, respectively; P = 0.008) [20■■]. Rates of hypogammaglobulinemia, a concern with prolonged rituximab exposure, were similar in the rituximab and placebo-treated patients. The rates of serious infection were numerically greater in the group randomized to continued rituximab compared to placebo (12 vs. 9%) [20■■]. Additional studies, including cost-effectiveness studies, are needed to define the optimal role for different maintenance strategies in AAV, especially those using varying approaches to frequency and duration of rituximab use.
Although belimumab, a monoclonal antibody targeting B-lymphocyte stimulator (BLyS), also targets B cells, it was not found to have benefit with regard to risk of relapse when added to azathioprine for remission maintenance in patients with GPA or MPA [21■]. Although there is not a clear role for belimumab in AAV management at this time, it was observed in the trial that none of the patients who received rituximab for induction and were subsequently treated with belimumab had a relapse, raising the question of whether belimumab may have a role in patients who receive rituximab for remission induction [21■].
Mepolizumab, a monoclonal antibody against IL-5, prevents interaction between IL-5 and the surface of eosinophils and was recently evaluated in a randomized clinical trial (N = 136) for the treatment of eGPA, a condition characterized by eosinophilic infiltration in affected tissue. The addition of mepolizumab to standard immunosuppressive therapy in patients with relapsing or refractory eGPA unable to discontinue glucocorticoids led to higher rates of remission (accrued remission of at least 24 weeks in 28 vs. 3%; P < 0.001) and less glucocorticoid exposure over 1 year (mean prednisone dose of 9.2 vs. 13.5 mg) when compared with placebo, prompting regulatory approval [22]. Mepolizumab may be particularly helpful for eGPA cases with asthma and or sino-nasal disease requiring chronic glucocorticoids. Notably, this trial excluded patients with severe eGPA (e.g. glomerulonephritis and cardiomyopathy).
In addition to recent developments using B-cell and eosinophil-targeted therapy, other biologics have been studied for AAV in small trials. In an open-label trial (N = 20) of patients with nonsevere GPA, 90% of abatacept-treated patients had disease improvement, 80% achieved remission and 73% discontinued prednisone. These compelling findings require additional study in a randomized controlled trial [23].
Following the results of two promising phase 2 clinical trials [24,25], a steroid-free regimen with avacopan, a nonbiologic oral antagonist of the human C5a receptor, was recently found to be noninferior to standard glucocorticoid regimens for remission induction in GPA or MPA when combined with rituximab or cyclophosphamide in a large randomized controlled trial (N = 331). Preliminary results indicate that avacopan led to higher rates of sustained remission (65.7 vs. 54.9%, respectively; P = 0.007 for superiority of avacopan), reduced glucocorticoid toxicity and higher quality of life scores when compared to standard glucocorticoid-containing regimens [26].
Ongoing clinical trials in AAV are evaluating a low-dose glucocorticoid regimen beginning at 0.5 mg/kg/day (ClinicalTrials.gov; NCT02198248), benralizumab versus mepolizumab for eGPA (ClinicalTrials.gov; NCT04157348) and the combination of rituximab and belimumab (ClinicalTrials.gov; NCT03967925). There are also other trials evaluating other complement inhibitors, like IFX-1, which binds to C5a (ClinicalTrials.gov; NCT03712345).
CRYOGLOBULINEMIC VASCULITIS
Cryoglobulinemic vasculitis is a small-vessel vasculitis caused by cryoglobulinemic immune complex deposition. The most common form of cryoglobulinemic vasculitis is due to hepatitis C infection. The treatment approach is multipronged and includes treatment of the underlying cause, which is especially important in cases due to viral illnesses or malignancy, targeting circulating B cells with rituximab to decrease the production of cryoglobulins in severe disease, and the addition of plasmapheresis in severe cases (e.g. glomerulonephritis, diffuse alveolar hemorrhage and vasculitic neuropathy) [27]. Rituximab has been found to be efficacious and steroid sparing in both viral and nonviral-associated cryoglobulinemic vasculitis with estimated efficacy of 67% for peripheral neuropathy, 77% for weakness, 79% for arthralgia and 85% for cutaneous ulcers [28].
PRIMARY ANGIITIS OF THE CENTRAL NERVOUS SYSTEM
Primary angiitis of the central nervous system (PACNS), or CNS vasculitis, has traditionally been treated with glucocorticoids with or without cyclophosphamide. The relapsing nature of this disease and toxicity of prolonged cyclophosphamide exposure has led to interest in trying biologics for the treatment of PACNS. Rituximab, in particular, has been reported to have potential efficacy in PACNS in several case reports or series [29,30]. Indeed, the inflammatory infiltrate in PACNS is often lymphocytic, with a frequent predominance of B lymphocytes, supporting the use of a B-cell depleting agent [29,30].
BEHCET’S DISEASE
Behcet’s disease is characterized by recurrent oral and/or genital ulcers and features of a variable vessel vasculitis (small, medium and large vessels may be affected). Although medications, such as colchicine, azathioprine and apremilast, are effective for mucocutaneous ulcers, the vascular manifestations of Behcet’s disease often require additional therapy [31■,32]. TNF inhibitors are recommended in refractory cases of Behcet’s disease and as first-line therapy in cases with neurologic involvement. Infliximab has been the most studied TNF inhibitor for the treatment of Behcet’s, followed by adalimumab [33]. In a case series of patients (N = 27) with Behcet’s disease manifesting as refractory vasculitis, 80% experienced complete clinical remission within 3 months of initiating a TNF inhibitor [34]. There have also been case series reporting the successful use of ustekinumab and anakinra for refractory Behcet’s disease, though additional studies are needed [33,35].
POLYARTERITIS NODOSA
Polyarteritis nodosa (PAN) is a form of medium-vessel vasculitis, most often affecting the skin, nerves, gastrointestinal tract and kidneys. Although it was historically associated with hepatitis B viral (HBV) infections, the frequency of PAN cases associated with hepatitis B has decreased significantly over time, largely in part because of the widespread hepatitis B vaccination [36]. Although treatment of HBV-associated PAN requires antiviral treatment, glucocorticoids have been the cornerstone of therapy for idiopathic PAN, with cyclophosphamide reserved for more severe disease. TNF inhibitors, rituximab and tocilizumab have been reported to potentially have efficacy in PAN, but these data are limited to case series [37–39].
CONCLUSION
Biologics and novel targeted synthetic drugs are playing an increasingly important role as effective glucocorticoid-sparing medications for vasculitis. In particular, the management of GCA and AAV has evolved substantially in recent years with the completion of pivotal clinical trials. Tocilizumab represents the first effective glucocorticoid-sparing agent for GCA and avacopan may lead to a glucocorticoid-free remission induction regimen for AAV. However, additional studies in these and other vasculitides are needed to define the optimal role of biologics and other novel glucocorticoid-sparing therapies.
KEY POINTS.
Because of the chronicity of many primary vasculitides and the risk of long-term glucocorticoid toxicity, glucocorticoid-sparing medications play a key role in the management.
Several biologics, including tocilizumab for GCA, rituximab for ANCA-associated vasculitis and mepolizumab for eosinophilic GPA, have received regulatory approval for treatment based on randomized controlled trials, though data supporting the use of these and other biologics in other vasculitides remain limited to case series and other small studies.
Ongoing clinical trials evaluating the efficacy and glucocorticoid-sparing effects of biologics and novel small molecules are expected to further inform the management of systemic vasculitis moving forward.
Financial support and sponsorship
NSB is supported by the National Institutes of Health Ruth L. Kirschstein Institutional National Research Service Award (T32-AR-007258). ZSW is funded by NIH/NIAMS (K23AR073334 and L30 AR070520). The National Institute of Health had no role in the design or authorship of this publication.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.Broder MS, Sarsour K, Chang E, et al. Corticosteroid-related adverse events in patients with giant cell arteritis: a claims-based analysis. Semin Arthritis Rheum 2016; 46:246–252. [DOI] [PubMed] [Google Scholar]
- 2.Morin C, Fardet L. Systemic glucocorticoid therapy: risk factors for reported adverse events and beliefs about the drug. A cross-sectional online survey of 820 patients. Clin Rheumatol 2015; 34:2119–2126. [DOI] [PubMed] [Google Scholar]
- 3.Gale S, Wilson JC, Chia J, et al. Risk associated with cumulative oral glucocorticoid use in patients with giant cell arteritis in real-world databases from the USA and UK. Rheumatol Ther 2018; 5:327–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzalez-Gay MA, Vazquez-Rodriguez TR, Lopez-Diaz MJ, et al. Epidemiology of giant cell arteritis and polymyalgia rheumatica. Arthritis Rheum 2009; 61:1454–1461. [DOI] [PubMed] [Google Scholar]
- 5.Mahr AD, Jover JA, Spiera RF, et al. Adjunctive methotrexate for treatment of giant cell arteritis: an individual patient data meta-analysis. Arthritis Rheum 2007; 56:2789–2797. [DOI] [PubMed] [Google Scholar]
- 6.Villiger PM, Adler S, Kuchen S, et al. Tocilizumab for induction and maintenance of remission in giant cell arteritis: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 2016; 387:1921–1927. [DOI] [PubMed] [Google Scholar]
- 7.Stone JH, Tuckwell K, Dimonaco S, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med 2017; 377:317–328. [DOI] [PubMed] [Google Scholar]
- 8.Langford CA, Cuthbertson D, Ytterberg SR, et al. A Randomized, double-blind trial of abatacept (CTLA-4Ig) for the treatment of giant cell arteritis. Arthritis Rheumatol 2017; 69:837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conway R, O’Neill L, Gallagher P, et al. Ustekinumab for refractory giant cell arteritis: a prospective 52-week trial. Semin Arthritis Rheum 2018; 48:523–528. [DOI] [PubMed] [Google Scholar]
- 10. ■.Matza MA, Fernandes AD, Stone JH, Unizony SH. Ustekinumab for the treatment of giant cell arteritis. Arthritis Care Res 2020; doi: 10.1002/acr.24200. [Online ahead of print] [DOI] [PubMed] [Google Scholar]; This open-label study of ustekinumab in giant cell arteritis was terminated early because of 77% of patients failing to achieve the primary endpoint of prednisone-free remission.
- 11.Hellmann DB. Giant-cell arteritis: more ecstasy, less agony. N Engl J Med 2017; 377:385–386. [DOI] [PubMed] [Google Scholar]
- 12. ■■.Hellmich B, Agueda A, Monti S, et al. 2018 Update of the EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis 2020; 79:19–30. [DOI] [PubMed] [Google Scholar]; The updated EULAR guidelines for large-vessel vasculitis recommend tocilizumab in patients with GCA with refractory or relapsing disease or an increased risk of glucocorticoid toxicity. They recommend TNF inhibitors or tocilizumab in relapsing or refractory Takayasu arteritis.
- 13.Nakaoka Y, Isobe M, Takei S, et al. Efficacy and safety of tocilizumab in patients with refractory Takayasu arteritis: results from a randomised, double-blind, placebo-controlled, phase 3 trial in Japan (the TAKT study). Ann Rheum Dis 2018; 77:348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010; 363:221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallace ZS, Miloslavsky EM. Management of ANCA associated vasculitis. BMJ 2020; 368:m421. [DOI] [PubMed] [Google Scholar]
- 16.Guillevin L, Pagnoux C, Karras A, et al. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med 2014; 371:1771–1780. [DOI] [PubMed] [Google Scholar]
- 17. ■.Smith R, Jayne D, Merkel PA. A randomized, controlled trial of rituximab versus azathioprine after induction of remission with rituximab for patients with ANCA-associated vasculitis and relapsing disease [abstract 806]. Arthritis Rheumatol 2019; 71: [Google Scholar]; The RITAZAREM trial randomized patients with relapsing ANCA-associated vasculitis to either rituximab or azathioprine as maintenance therapy and found that rituximab was superior to azathioprine in preventing further relapse (HR 0.36; 95% CI 0.23–0.57).
- 18.Terrier B, Pagnoux C, Perrodeau E, et al. Long-term efficacy of remission-maintenance regimens for ANCA-associated vasculitides. Ann Rheum Dis 2018; 77:1150–1156. [DOI] [PubMed] [Google Scholar]
- 19.Charles P, Terrier B, Perrodeau E, et al. Comparison of individually tailored versus fixed-schedule rituximab regimen to maintain ANCA-associated vasculitis remission: results of a multicentre, randomised controlled, phase III trial (MAINRITSAN2). Ann Rheum Dis 2018; 77:1143–1149. [DOI] [PubMed] [Google Scholar]
- 20. ■■.Charles P, Perrodeau E, Samson M, et al. Long-term rituximab use to maintain remission of antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med 2020; 173:179–187. [DOI] [PubMed] [Google Scholar]; The MAINRITSAN3 trial randomized patients from MAINRITSAN2 to receive an additional 18 months of rituximab or placebo; they found improved relapse-free survival at 28 months in the rituximab group (98 vs. 74%; P = 0.008).
- 21. ■.Jayne D, Blockmans D, Luqmani R, et al. Efficacy and safety of belimumab and azathioprine for maintenance of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized controlled study. Arthritis Rheumatol 2019; 71:952–963. [DOI] [PMC free article] [PubMed] [Google Scholar]; Following induction therapy, this trial randomized patients with ANCA-associated vasculitis to belimumab or placebo in addition to azathioprine and glucocorticoids and found no significant difference in the maintenance of remission between the belimumab and placebo groups.
- 22.Wechsler ME, Akuthota P, Jayne D, et al. Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N Engl J Med 2017; 376:1921–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langford CA, Monach PA, Specks U, et al. An open-label trial of abatacept (CTLA4-IG) in nonsevere relapsing granulomatosis with polyangiitis (Wegener’s). Ann Rheum Dis 2014; 73:1376–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jayne DRW, Bruchfeld AN, Harper L, et al. Randomized trial of C5a receptor inhibitor avacopan in ANCA-associated vasculitis. J Am Soc Nephrol 2017; 28:2756–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merkel PA, Niles JL, Jimenez R et al. A randomized clinical trial of CCX168, an orally administered C5aR inhibitor for treatment of patients with ANCA-associated vasculitis. Abstract 2016 ACR/ARHP Annual Meeting. 2016. [Google Scholar]
- 26.Merkel PA, Jayne D, Yue H, et al. , on behalf of the ADVOCATE Study Group. A randomized, double-blind, active-controlled study of avacopan in antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis. Ann Rheum Dis 2020; 79:8. [Google Scholar]
- 27.Goglin S, Chung SA. Current treatment of cryoglobulinemic vasculitis. Curr Treat Options Rheumatol 2016; 2:213–224. [Google Scholar]
- 28.Roccatello D, Saadoun D, Ramos-Casals M, et al. Cryoglobulinaemia. Nat Rev Dis Primers 2018; 4:11. [DOI] [PubMed] [Google Scholar]
- 29.de Boysson H, Arquizan C, Guillevin L, Pagnoux C. Rituximab for primary angiitis of the central nervous system. J Rheumatol 2013; 40:2102–2103. [DOI] [PubMed] [Google Scholar]
- 30.Salvarani C, Brown RD Jr, Huston J III, et al. Treatment of primary CNS vasculitis with rituximab: case report. Neurology 2014; 82:1287–1288. [DOI] [PubMed] [Google Scholar]
- 31. ■.Hatemi G, Mahr A, Ishigatsubo Y, et al. Trial of apremilast for oral ulcers in Behcet’s syndrome. N Engl J Med 2019; 381:1918–1928. [DOI] [PubMed] [Google Scholar]; This phase 3 trial demonstrated a greater reduction in the number of oral ulcers with apremilast compared to placebo in patients with Behcet’s syndrome.
- 32.Hatemi G, Silman A, Bang D, et al. Management of Behcet disease: a systematic literature review for the European League Against Rheumatism evidence-based recommendations for the management of Behcet disease. Ann Rheum Dis 2009; 68:1528–1534. [DOI] [PubMed] [Google Scholar]
- 33.Muratore F, Pazzola G, Soriano A, et al. Unmet needs in the pathogenesis and treatment of vasculitides. Clin Rev Allergy Immunol 2018; 54:244–260. [DOI] [PubMed] [Google Scholar]
- 34.Aksoy A, Yazici A, Omma A, et al. Efficacy of TNFalpha inhibitors for refractory vascular Behcet’s disease: a multicenter observational study of 27 patients and a review of the literature. Int J Rheum Dis 2020; 23:256–261. [DOI] [PubMed] [Google Scholar]
- 35.Mirouse A, Barete S, Monfort JB, et al. Ustekinumab for Behcet’s disease. J Autoimmun 2017; 82:41–46. [DOI] [PubMed] [Google Scholar]
- 36.Cui F, Shen L, Li L, et al. Prevention of chronic hepatitis B after 3 decades of escalating vaccination policy, China. Emerg Infect Dis 2017; 23: 765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ginsberg S, Rosner I, Slobodin G, et al. Infliximab for the treatment of refractory polyarteritis nodosa. Clin Rheumatol 2019; 38:2825–2833. [DOI] [PubMed] [Google Scholar]
- 38.Krusche M, Ruffer N, Kotter I. Tocilizumab treatment in refractory polyarteritis nodosa: a case report and review of the literature. Rheumatol Int 2019; 39:337–344. [DOI] [PubMed] [Google Scholar]
- 39.Loricera J, Blanco R, Hernandez JL, et al. Biologic therapy in ANCA-negative vasculitis. Int Immunopharmacol 2015; 27:213–219. [DOI] [PubMed] [Google Scholar]


