Abstract
Objectives
Healthy aging is associated with impairments in motor functioning. Such functioning is not limited to the physical execution of actions, but also involves cognitive processes that allow for goal-directed behavior. The present study examined whether aging affects 2 of such cognitive components that control motor functioning, namely action planning and action adaptation, and whether age effects are associated across components.
Method
A group of 103 participants aged 18–82 years performed 2 tasks that have previously been linked to action planning and adaptation, respectively.
Results
Despite observations that aging was associated with slower and less accurate responses, Bayesian models showed evidence indicating that older age was not associated with poorer action planning and conflict adaptation.
Discussion
These findings challenge the view that healthy aging is associated with a general deficit in motor functioning and suggest that some cognitive aspects of motor control may be relatively spared.
Keywords: Adaptation, Motor functioning, Planning
Human movement control is not limited to the physical execution of actions, but also involves cognitive components that are essential for humans to engage in goal-directed behavior (Abrahamse et al., 2013; McDougle et al., 2016; Prinz et al., 2009; Ruitenberg et al., 2015). These include, but are not limited to, action planning and selection, action initiation, online adaptation during actual execution, and inhibition of an ongoing action. We collectively refer to these components as action control. In this study, we focus on action planning and adaptation and investigate the effect of healthy aging on each of these components. In addition, we evaluate whether age-related differences in action control are independent or associated across components.
Most of our actions are preceded by an intention to act in order to reach a desired effect in the environment. For example, pressing the volume button on a TV remote is primarily based on the individuals’ intention to change the intensity of the sound. One prominent perspective on how we achieve intentional action planning and selection is that people represent actions in terms of their anticipated perceptual effects (i.e., action-effects), which through repeated experience become bound with the movement they accompany (Elsner and Hommel, 2001; Hommel et al., 2001). Specifically, perceptual and action features of an event are thought to become integrated into a mental representation called an “event file.” The binding of perceptual and action features (i.e., visuomotor binding) into such a unitary representation is a basic process that allows for the efficient control of behavior (Hommel, 2004). The planning and selection of an action are enabled via the retrieval of a stored event file: by anticipating the desired effects of an action on the environment and the associated visuomotor features, that action is automatically activated. Performance is typically worse when the stimulus feature but not the action within the retrieved event file matches the required action or vice versa (i.e., partial mismatch) as compared to when the stimulus and action both match or differ, because the partial activation of required events results in suboptimal planning. The performance cost due to a partial mismatch is called the partial repetition cost (PRC; Hommel, 2004). Indirect indications for effects of age on the formation of such action-effects come from a study showing that binding between perceptual and motor features was more flexible in subjects with higher fluid intelligence (Colzato et al., 2006). As aging has been linked to reductions in fluid intelligence (e.g., Yuan et al., 2018), it could be predicted that older age is associated with stronger, less flexible binding. Indeed, prior work has demonstrated that the retrieval and updating of visuomotor bindings are less flexible in older compared to younger adults (Hommel et al., 2011).
Another core component of action control is the ability to adapt an ongoing movement to changing situational demands in order to maintain goal-directed behavior (Botvinick et al., 2001; Notebaert & Verguts, 2008; Verguts & Notebaert, 2009). For instance, imagine you are carrying a birthday cake, when suddenly it slips out of your hand—most likely you will try to catch and save the cake. However, would you have been carrying a tray with cups of hot tea and coffee instead, you probably would not attempt to catch these as you could hurt yourself. In the lab, such adaptation is typically studied via conflict paradigms (e.g., Stroop, Eriksen flanker, Simon task) that include a contextual manipulation aimed at modulating the standard congruency effect where people perform worse on incongruent compared to congruent trials. For example, the congruency effect is typically reduced in contexts with high proportions of incongruent trials (proportion congruency effect) and directly after incongruent as compared to congruent trials (congruency sequence effect [CSE]). Finally, the congruency effect is typically reduced for items that are presented mostly in an incongruent manner as compared to those that are presented mostly in a congruent manner; this is referred to as the item-specific proportion congruency (ISPC) effect (Jacoby et al., 2003; for a review on different manipulations, see Braem et al., 2019). While some previous studies on the relationship between aging and adaptation observed that aging is related to reduced adaptability (Aisenberg et al., 2014, 2018), others found that aging is related to increased adaptability (Aschenbrenner & Balota, 2015, 2017). Moreover, there are also studies that observed that age did not significantly affect adaptation (Bugg, 2014; Larson et al., 2016). These mixed findings thus prompt further investigation of the relationship between aging and adaptation.
The aforementioned studies did not unequivocally demonstrate the effect of healthy aging on action planning/selection and motor adaptation, and additionally did not examine to what extent age effects on these action control processes may be associated. In the present study, we therefore recruited a large sample of healthy participants spanning a wide age range (18–82 years old) and had them perform both a planning and an adaptation task. For action planning, we hypothesized that older age would be associated with stronger, less flexible visuomotor binding (i.e., larger PRCs). Given the mixed indications for age effects on adaptation, we tentatively explored whether age affected this process (i.e., whether there was an age-related difference in the size of the ISPC effect). The novelty of this work is that we sampled across a wide age range, allowing us to evaluate the life span trajectory of changes in action control. That is, the current design allowed us to examine not only if age affects action control but also to evaluate when in the life span these prospective effects begin to emerge. Moreover, whereas prior work typically only assessed one component, we evaluated both planning and adaptation in a within-subject manner, allowing us to examine intraindividual differences in action control and to test whether the hypothesized age differences for the various components occur in parallel or are independent.
Method
Participants
We used the pwr package (http://cran.r-project.org/web/packages/pwr/) in R to estimate the sample size required to detect an age association of small-to-medium effect size with an alpha level of 0.05 and statistical power of 0.80; results indicated that we required a minimum of 92 participants. A total of 105 healthy adults volunteered to participate in the current study. The data from two participants were excluded due to technical issues with the presentation/registration software. As such, the final sample comprised 103 participants (39 males) ranging in age from 18 to 82 years (M = 46.5 ± 19.2 years). During data collection, we monitored inclusion to ensure there would be participants in each age decade; the age distribution of our final sample is shown in Supplementary Figure 1. All participants reported to have normal or corrected to normal vision (i.e., no colorblindness; glasses or lenses were allowed). According to scores on Annett’s Handedness Inventory (Annett, 1970), 94 participants were right-handed and 9 were left-handed. Scores on the Dutch Reading Test for Adults (the Dutch version of the National Adult Reading Test; Schmand et al., 1992) indicated that IQ estimates were within the normal range (M = 117.8 ± 6.7). On the Montreal Cognitive Assessment (MoCA; Nasreddine et al., 2005), none of the participants scored below the cutoff of 23 (cf. Carson et al., 2018) indicative for cognitive impairment (M = 27.7 ± 1.9, range 23–30). All participants provided written informed consent prior to the study. The study adhered to the general ethical protocol of the ethics committee of the Faculty of Psychology and Educational Sciences of Ghent University.
Apparatus and Procedure
At the start of the experiment, participants signed the informed consent form and provided demographical information. They then completed the handedness inventory, as well as the Dutch version of the National Adult Reading Test and the MoCA to assess their verbal intelligence and global cognitive abilities, respectively. Finally, each subject performed the experimental tasks described below. Stimulus presentation and data recording were controlled by E-prime © 2.0 software, running on a Dell Latitude E5550 laptop computer. Responses were given on a standard qwerty computer keyboard.
Experimental Tasks
Action planning and selection
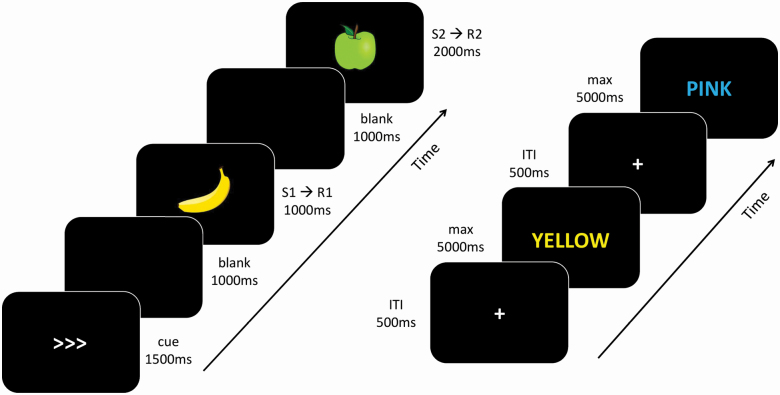
To assess this action control component, we used the event-file task (e.g., Colzato et al., 2012; Hommel, 1998; Hommel et al., 2011). In this task, illustrated in Figure 1, participants were instructed to manually respond to two successive stimuli (S1 and S2) that varied in shape and color. Stimuli consisted of pictures of yellow or green apples or bananas, presented against a black background. The first response (R1) was cued before S1 presentation, by either a left or a right arrow. Upon presentation of S1, participants had to press the arrow key that corresponded to the cue as quickly and accurately as possible. Thus, independent of which stimulus was presented, participants had to respond in line with the direction of the arrow. The response deadline was set at 1,000 ms. For the second response (R2), participants had to follow a predefined decision rule based on the shape of S2: in case of a banana, they had to press the left arrow key and in case of an apple they had to press the right arrow key (counterbalanced across subjects). The color of S2 was always an irrelevant feature since responses were based solely on the shape of the stimulus. For this second response, the deadline was set at 2,000 ms. Responses were made with the index and middle fingers of the dominant hand of the participant, pressing the left or right arrow key. Participants could familiarize themselves with the task during 10 practice trials, after which they performed five experimental blocks of 32 trials each.
Figure 1.
An overview of the stimulus presentation in the event-file task (left) and Stroop task (right). Note that for the Stroop task all words were actually displayed in Dutch; the figure shows examples of both a congruent (YELLOW in yellow ink) and an incongruent trial (PINK in blue ink) (color figure online).
Conflict adaptation
To assess conflict adaptation, we used a Stroop task in which we manipulated the item-specific proportion of (in)congruent trials (80% vs 20% congruent). This allowed us to evaluate the so-called ISPC effect, a hallmark indicator of conflict adaptation (Jacoby et al., 2003; for a review, see Braem et al., 2019). The ISPC effect refers to the phenomenon that the Stroop effect is typically reduced for items that are presented mostly in an incongruent manner as compared to those that are presented mostly in a congruent manner. Stimuli consisted of eight color words (red, yellow, green, blue, violet, orange, pink, and brown) that were used as words as well as ink colors (the Dutch words were “rood,” “geel,” “groen,” “blauw,” “paars,” “oranje,” “roze,” and “bruin”). Two different subsets of four colors each were used (set 1: red–yellow–violet–brown, set 2: green–blue–pink–orange; cf. Geukes et al., 2015). This was done because some of the older participants served as control subjects in a study on action control in Parkinson’s disease where patients completed the task twice (each time with a different subset to avoid learning effects; these data will be presented elsewhere). Within each color set, colors were paired (red and yellow, violet and brown, green and orange, blue and pink) so that unique congruent and incongruent combinations for each item were presented equally often throughout the task and contingency problems were avoided (e.g., Braem et al., 2019; Duthoo et al., 2014; Schmidt & Besner, 2008). For one of the pairs of a color set, 80% of the trials were congruent, while for the other pair of that color set, only 20% of the trials were congruent. For example, for color set 1, the words red and yellow were shown in their congruent ink color in 80% of the trials, while the words violet and brown were only shown in their congruent ink color in 20% of the trials. This proportion was counterbalanced across participants and allowed us to obtain an index for the ISPC effect by subtracting the difference between incongruent trials and congruent trials in the 20% congruent condition from the difference between incongruent and congruent trials in the 80% congruent condition.
Participants were instructed to respond to the ink color of the stimulus by pressing one of four keys on the keyboard (c, v, b, or n) with the fingers of their preferred hand. Each key corresponded to a particular color. As Figure 1 illustrates, each trial started with a black screen on which a white fixation cross was shown in the center of the display. After 500 ms, the fixation cross disappeared and was replaced by a Stroop stimulus (printed in Arial font with a height of 1.5 cm). A trial ended after participants pressed one of the four keys or 5,000 ms had passed. After an incorrect response, a red X was shown for 2,000 ms in the center of the screen. Participants could familiarize themselves with the task and stimulus-response mapping during a practice block of 16 randomly ordered congruent and incongruent trials (50% congruent). During practice, they were presented a sheet of paper showing the four response options and their corresponding colors as a memory aid. They then performed four experimental blocks of 160 randomly ordered trials each, with the restriction that a trial could not require the same response (and thus have the same ink color) as the previous one. Participants could take self-paced breaks between the blocks.
Data Processing and Analysis
For the action planning and selection task, we filtered out incorrect responses to the second stimulus (R2), as well as responses <200 and >2,000 ms (cf. Colzato et al., 2012). The main outcome measure for this task is the PRC (see Hommel, 2004; Hommel et al., 2011). For each subject, the PRC for the task-relevant visuomotor binding between shape and response is determined by calculating the difference between the mean reaction time (RT) for partial repetitions (shape repeated and response alternated, or vice versa) and the mean RT for complete repetitions and alternations (shape and response both repeated, or both alternated). Larger PRC values represent stronger, less flexible binding. For the sake of completeness, we used similar calculations to determine PRCs for the task-irrelevant binding between color and response (visuomotor), and between shape and color (visuoperceptual).
For the conflict adaptation task, we excluded the first trial of each block, error, and post-error trials, as well as trials of which the RT exceeded the mean RT by more than 3 SDs (cf. Ruitenberg et al., 2019). This latter procedure was done per participant and separately for each proportion and congruency condition, and resulted in removal of 1.78% of the trials. The main outcome measure for this task is the ISPC effect for each participant, which we calculated by subtracting the difference in performance between incongruent and congruent trials on 20% congruent items from the difference on 80% congruent items. Larger ISPC values represent more adaptation.
We calculated our main outcome measures using both raw RTs and subject-based z-scores to control for known age-related changes in processing speed that affect RT performance (cf. Aschenbrenner & Balota, 2017, 2019). To evaluate whether PRC and IPSC effects were present in our entire sample, we ran repeated-measures ANOVAs for each task. To evaluate whether age was associated with individual differences in magnitude of the PRCs and ISPC effect, we ran linear regression analyses. For these latter analyses, we used both the more traditional null-hypothesis significance testing (NHST) approach in SPSS (IBM Corp, 2019) as well as a Bayesian approach in JASP (version 0.11.1; JASP Team, 2019). We included the Bayesian approach as this allowed us to determine whether our data provided evidence in favor of the null or alternative hypothesis, which is especially meaningful in case our NHST results would show nonsignificant associations between age and action control indices (cf. Bugg, 2014; Larson et al., 2016). A BF10 >3 is regarded as moderate evidence for the alternative hypothesis (with BF10 >10 indicating strong evidence), and a BF10 between 1 and 3 as merely anecdotal evidence. In contrast, a BF10 <0.33 is regarded as moderate evidence for the null hypothesis (with BF10 <0.1 indicating strong evidence), and a BF10 between 1 and 0.33 as anecdotal evidence (Dienes, 2011; Nuzzo, 2017). Results pertaining to the ANOVA interaction effects and regression analyses are presented in Table 1.
Table 1.
Overview of the Interaction Effects of the ANOVAs and Linear Regressions With Age for the Raw RT and z-Score Analyses per Experimental Task
| Effect across the entire sample | Association with age | |||
|---|---|---|---|---|
| Task | Raw RTs | z-scores | Raw RTs | z-scores |
| Action planning and selection | ||||
| PRC shape–response | F = 4.97, p = .031, η p2 = .045 | F = 4.20, p = .043, η p2 = .040 | β = .081, t < 1, p = .41 (R2 = .007), BF10 = 0.28 | β = .078, t < 1, p = .43 (R2 = .006), BF10 = 0.27 |
| PRC color–response | F < 1, p = .96, η p2 < .01 | F < 1, p = .90, η p2 < .01 | β = −.11, t = −1.11, p = .26 (R2 = .012), BF10 = 0.36 | β = −.16, t = −1.65, p = .10, (R2 = .026), BF10 = 0.69 |
| PRC shape–color | F = 18.76, p < .001, η p2 = .15 | F = 16.44, p < .001, η p2 = .14 | β = .23, t = 2.40, p = .018 (R2 = .054), BF10 = 2.62 | β = .11, t = 1.15, p = .25, (R2 = .013), BF10 = 0.37 |
| Conflict adaptation | ||||
| ISPC effect | F = 149.17, p < .001, η p2 = .59 | F = 192.87, p < .001, η p2 = .65 | β = .26, t = 2.73, p = .007 (R2 = .069), BF10 = 5.29 | β = .089, t < 1, p = .37 (R2 = .008), BF10 = 0.29 |
Note: ANOVA = analysis of variance; ISPC = item-specific proportion congruency; PRC = partial repetition cost.
Results
Action Planning and Selection
To evaluate general processing speed, we evaluated the average performance in response to the first stimulus (R1). Across our sample, mean RT was 409 ± 95 ms and mean accuracy was 90% ± 14%. Results of linear regression analyses showed that both RT and accuracy were predicted by age, with older age being associated with slower responses, β = .586, t = 7.26, p < .001 (R2 = .343), as well as less accurate responses, β = −.562, t = −6.83, p < .001 (R2 = .316). A similar pattern of results was observed for responses to the second stimulus (R2), with older age again being associated with slower, β = .530, t = 6.28, p < .001 (R2 = .281), and less accurate responses, β = −.432, t = −4.82, p < .001 (R2 = .187).
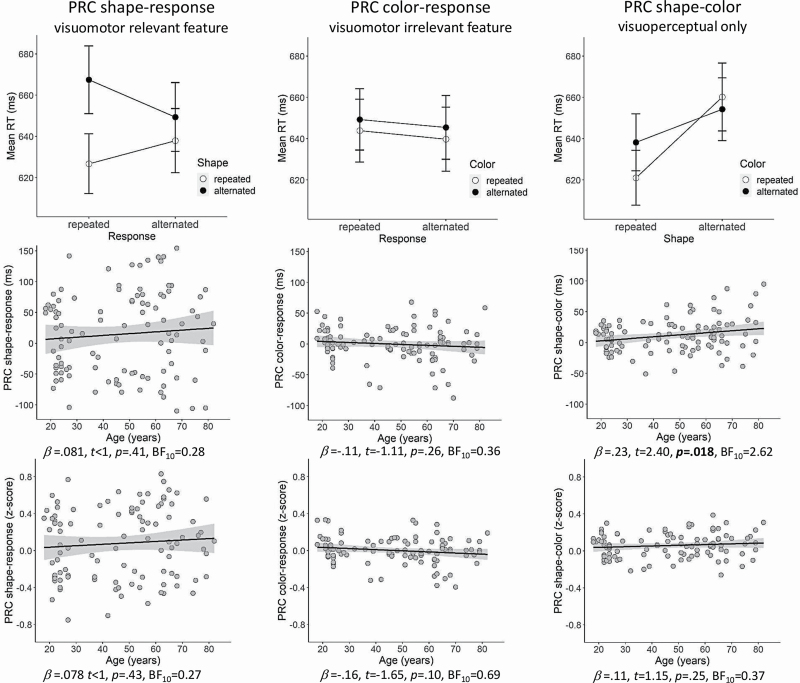
We ran a repeated-measures ANOVA on RTs with the repetition versus alternation of Response from R1 to R2 (2; response repeated vs alternated) and stimulus Shape from S1 to S2 (2; shape repeated vs alternated) as within-subject factors. Results showed a significant interaction between shape and response, F(1,102) = 4.97, p = .031, η p2 = .045, suggesting that repeating both shape and response or alternating both shape and response produced better performance than repeating one and alternating the other. Results of a similar repeated-measures ANOVA including Response and stimulus Color showed no significant interaction, F(1,102)<1, p = .96, which was expected as color was an irrelevant feature in the current task. Finally, results of a repeated-measures ANOVA stimulus Shape and Color (i.e., not including the response component) showed a significant interaction between shape and color, F(1,102) = 18.76, p < .001, η p2 = .15, suggesting that repeating or alternating both visual features produced better performance than repeating one feature and alternating the other. Figure 2 (top panels) shows the interaction effects.
Figure 2.
Top panels: Mean RT (ms) for the three combinations of response, shape, and color repetitions and alternations. Center and bottom panels: Scatterplots illustrating the relationship between age and the various partial repetition costs (PRCs) as reflected in raw RTs (center panels) and z-transformed RTs (bottom panels).
To analyze whether age affected binding, we ran a linear regression analysis on individual PRCs (Figure 2, center panels). Results showed that the PRC for the interaction between shape and response was not significantly predicted by age, β = .081, t < 1, p = .414 (R2 = .007), BF10 = 0.28. We also calculated the PRC for the interaction between color and response, but again results showed that this was not significantly predicted by age, β = −.110, t = −1.113, p = .268 (R2 = .012), BF10 = 0.36. However, results showed that the PRC for the interaction between shape and color was significantly predicted by age, β = .23, t = 2.40, p = .018 (R2 = .054), BF10 = 2.62.
Results of the aforementioned repeated-measures ANOVAs on subject-based z-score transformed RTs showed a significant interaction between shape and response, F(1,102) = 4.20, p = .043, ηp2 = .040, and shape and color, F(1,102) = 16.44, p < .001, ηp2 = .14, but not between color and response, F(1,102) < 1, p = .90, η p2 < .01. Linear regression analysis showed that individual differences in the magnitude of the PRC as reflected in z-scores were not significantly predicted by age for shape–response, p = .43, BF10 = 0.27, color–response, p = .10, BF10 = 0.69, or shape–color, p = .25, BF10 = 0.37 (Figure 2, lower panels).
Conflict Adaptation
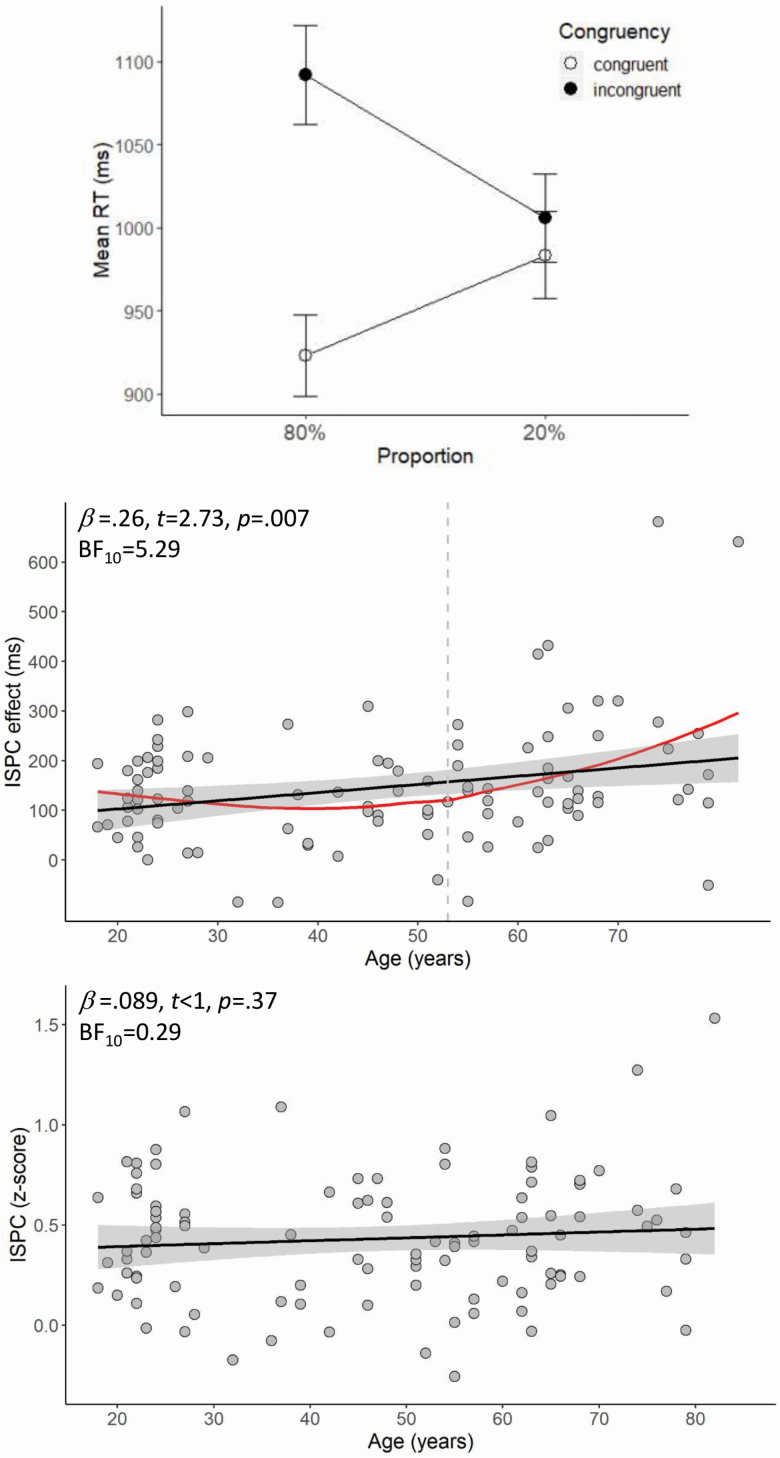
To evaluate whether the ISPC effect was present in the entire sample, we ran a repeated-measures ANOVA on RTs with Proportion (2; 80% vs 20% congruent) and Congruency (2; congruent vs. incongruent) as within-subject variables. Results showed that participants responded slower in the incongruent than congruent condition (1,049 vs 953 ms), F(102) = 197.68, p < .001, ηp2 = .66. Moreover, results showed a significant Proportion × Congruency interaction, F(1,102) = 149.17, p < .001, ηp2 = .59. Individual post hoc ANOVAs for each Proportion condition showed that the RT difference between incongruent and congruent trials was significant both for 80% congruent items (1,092 vs 923 ms), F(1,102) = 221.43, p < .001, η p2 = .68, and for 20% congruent items (1,006 vs. 984 ms), F(1,102) = 13.91, p < .001, η p2 = .12. As Figure 3 (top panel) shows, the congruency effect was larger for the 80% compared to the 20% congruent condition, thus demonstrating the ISPC effect. Next, we evaluated the association between age and the size of the ISPC effect. Results of a linear regression analysis showed that the ISPC effect was predicted by age, β = .26, t = 2.73, p = .007 (R2 = .069), BF10 = 5.29. Specifically, older age was associated with a larger ISPC effect (see Figure 3, center panel).
Figure 3.
Top panel: Mean RT (ms) for congruent and incongruent trials as a function of proportion congruency. Center and bottom panels: The black line in the scatterplots illustrates the relationship between age and the item-specific proportion congruency (ISPC) effect as reflected in RTs (center) and z-scores (bottom). The red line shows the LOESS smoothed age trajectory; the dashed line indicates the age at which changes started (color figure online).
To analyze the age of onset for the changes in conflict adaptation, we used locally weighted polynomial regression (LOESS) smoothing to describe the age trajectory (see Chan et al., 2014; Westlye et al., 2010). This approach is similar to spline smoothing and has been shown to be superior for describing age effects compared to models including a quadratic or cubic term, as well as being more robust to variations in the age range (Fjell et al., 2010). Subsequently, we determined the age of onset by identifying the inflection point for the trajectory. This approach revealed that the onset of age differences was at 53 years (see Figure 3, center panel).
Results of a repeated-measures ANOVA on z-score transformed RTs showed that participants responded slower in the incongruent than congruent condition (.213 vs −.078), F(102) = 277.05, p < .001, η p2 = .73. Moreover, results showed a significant Proportion × Congruency interaction, F(1,102) = 192.87, p < .001, η p2 = .65. Individual post hoc ANOVAs for each Proportion condition showed that the RT difference between incongruent and congruent trials was significant both for 80% congruent items (.339 vs −.167), F(1,102) = 336.82, p < .001, η p2 = .77, and for 20% congruent items (.087 vs .012), F(1,102) = 17.10, p < .001, η p2 = .14. However, results of a linear regression analysis showed that individual differences in the magnitude of the ISPC effect as reflected in z-scores were not significantly predicted by age, β = .089, t < 1, p = .37 (R2 = .008), BF10 = 0.29 (Figure 3; bottom panel).
Associations Between Action Planning and Conflict Adaptation
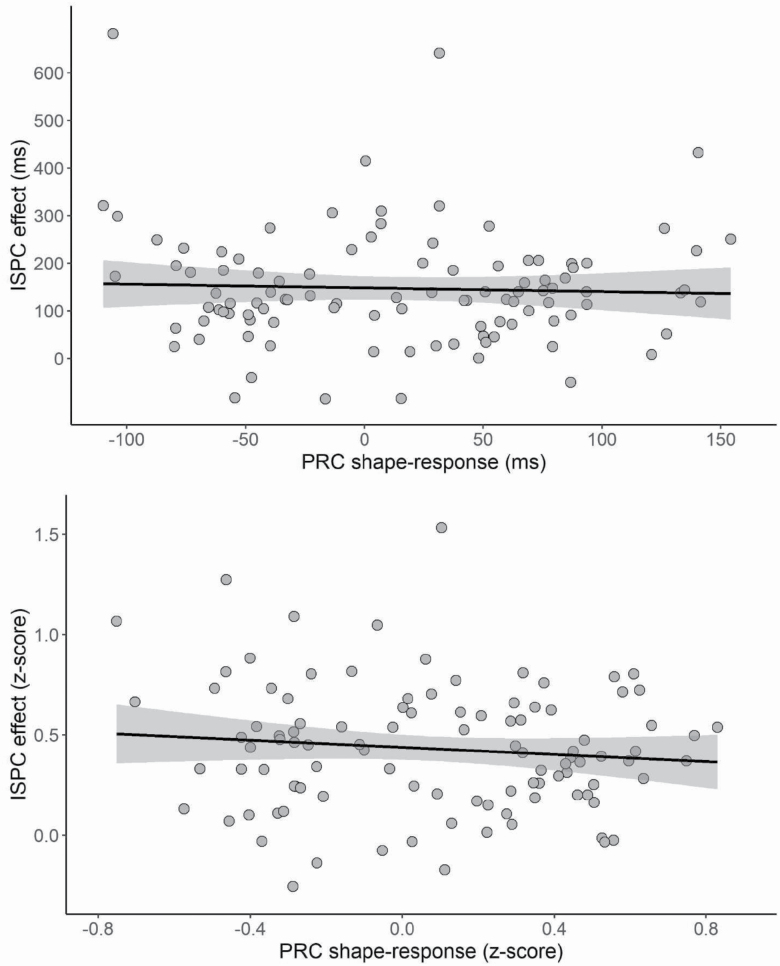
Finally, we performed a correlation analysis between the shape–response PRC in the action planning task and ISPC effect in the Stroop task to evaluate whether individual differences in the two tasks were associated. Results showed no significant correlation for raw RTs, r(103) = −.043, p = .66, BF10 = 0.13, or z-scores, r(103) = −.108, p = .27, BF10 = 0.22, suggesting that performance in the two tasks is relatively independent (Figure 4).
Figure 4.
Scatterplots illustrating the relationship between individual differences in the shape–response PRC and item-specific proportion congruency (ISPC) effect as reflected in raw RTs (top panel) and z-transformed RTs (bottom panel).
Exploratory Analysis
Following up on our finding that general processing speed appears to be age-sensitive, we ran a Brinley-type analysis on our data to examine whether this could potentially explain the observed individual differences in PRC and ISPC effects. To this end, we first performed a median-split on age and determined mean performance per condition in each task for the younger adult (18–48 years) and older adult (51–82 years) groups. We then plotted performance in the two age groups against each other (see Supplementary Figure 2) and calculated the slope of a fitted linear function on the data via a regression analysis. Results showed that the slope was 1.53, t = 23.97, p < .001, suggesting that older adults showed general cognitive slowing regardless of the experimental task at hand.
Discussion
The present study examined whether healthy aging impacts cognitive control processes underlying motor functioning, and whether age effects are associated between processes. Using an individual differences approach, we evaluated whether action planning as reflected in visuomotor PRCs and conflict adaptation as reflected in the ISPC effect were affected by aging. We found that these main indices of action control could be observed across the entire sample. However, results of our NHST analyses in combination with Bayesian evidence generally showed moderate support for the null-hypothesis, indicating that despite reaction time slowing and decrements in accuracy, aging is not associated with changes in action planning or adaptability and that individual differences in these action control abilities are unrelated. Our findings challenge the view that healthy aging is associated with a general deficit in motor functioning, and instead suggest that at least some cognitive aspects of motor control may be relatively spared.
We were able to replicate binding across perceptual and motor features across our entire sample, but in contrast to our hypothesis and previous findings by Hommel et al. (2011), we found no evidence that aging affected such binding. Unexpectedly, we did find indications that aging was associated with stronger—that is, less flexible—visuoperceptual feature binding (although this effect was not observed when the data were transformed to subject-based z-scores). Based on the results from our sample, aging seems to affect binding in visual perception rather than binding across perception and action. An explanation for the discrepancy in findings between the present study and Hommel et al. (2011) may pertain to the types of stimulus-features used in the experimental task. Specifically, the stimuli in our task varied in shape and color (i.e., both visuoperceptual features), whereas the stimuli in Hommel et al.’s task varied in shape and location (i.e., a visuoperceptual and a spatial feature; note that shape was the task-relevant feature in both studies). Hommel et al. (2011) found that visuomotor binding was less flexible in older compared to younger adults, yet they found no evidence for age differences in binding involving spatial features. Combining these prior findings with our current results, it could be proposed that age affects visuomotor binding only for stimuli that have a single, task-relevant visual feature (e.g., shape) in addition to a task-irrelevant spatial feature (location), but not for stimuli that have both task-relevant and task-irrelevant visual features (e.g., shape and color). This may further be related to the fact that visual features such as shape and color are both processed in the occipital lobe (e.g., Bartels & Zeki, 2008; Kourtzi & Kanwisher, 2001) and may therefore be harder to disentangle in a visuomotor representation, while location is processed in the parietal lobe (e.g., Pisella et al., 2004) and can more easily be separated from the visuomotor representation.
For conflict adaptation, our raw RT results showed evidence in favor of the alternative hypothesis that older age is associated with increased adaptation, but the fact that this relationship disappeared with z-scores suggests that this result can likely be attributed to age effects on processing speed rather than adaptability. This is further corroborated by the result of our exploratory analysis, although it should be noted that the Brinley-type analysis cannot conclusively confirm this causal interpretation. Recent work suggests that directionality of age effects on conflict adaptation may further be specific to the experimental task that is being used to study this ability. Specifically, Aschenbrenner and Balota (2017) had a group of younger adults and a group of older adults perform a Stroop task, Simon task, and Eriksen flanker task. They observed that compared to younger adults, older adults showed more adaptation in the Stroop task, but less adaptation in the Simon and flanker tasks. They used the CSE (the observation that the congruency effect is typically reduced after incongruent as compared to congruent trials) as an index for adaptation. As both the CSE and ISPC effect are thought to rely on reactive, transient (as compared to proactive, sustained) adaptations in attentional settings (Bugg, 2014; Funes et al., 2010; Torres-Quesada et al., 2013), it could be argued that our present observation of absent age effects on adaptation may be conflict type-specific. At the same time, there are recent indications that these conflict adaptation indices may arise from independent mechanisms (Aschenbrenner & Balota, 2019), opening up the possibility that aging may be associated with changes in some adaptation mechanisms but not others. Future studies should systematically manipulate conflict type and behavioral index using within-subject approaches to investigate the respective role of each factor in the relationship between aging and action control.
A strength of the present study is that we assessed two action control components within the same participants. In addition, we included participants across a wide age range. Whereas previous studies have typically only examined one action control component and compared extreme age groups (e.g., Aschenbrenner & Balota, 2017; Bugg, 2014; Hommel et al., 2011; Larson et al., 2016), the current design allowed us to not only examine age differences in action control, but also to evaluate when in the life span any observed effects emerged and whether age differences would be related across action control components. A further strength is that we used Bayesian models that allowed us to interpret nonsignificant NHST results. A limitation of the present work is that behavioral measures may not directly reflect brain mechanisms and as such the absence of effect of healthy aging on behavioral outcomes does not refute the possibility of effects at the neural level. For example, there may be age-related changes in brain recruitment during the task that allow older adults to maintain intact performance (i.e., compensatory mechanism; Reuter-Lorenz & Cappell, 2008), which could obscure age differences to be reflected in behavior. Future studies should combine behavioral paradigms with neuroimaging methods to further our understanding of the effects of healthy aging on action control.
Overall, results of the present study showed evidence that older age is not associated with poorer action planning and conflict adaptation, indicating that some cognitive aspects of motor control are relatively spared in healthy aging. Future studies should use systematic within-subject, multimethod approaches to elucidate the role of experimental design factors in the relationship between healthy aging and action control at both the behavioral and neural level.
Supplementary Material
Supplementary data are available at The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences online.
Supplementary Figure 1. Age-density histogram with overlaid density plot showing the age distribution of participants in the study.
Supplementary Figure 2. Brinley plot showing the mean RT per condition in each experimental task for the two age groups (based on a median-split).
Acknowledgments
We thank Lorenza Colzato and Nelleke van Wouwe for sharing the code of their event-file task, and Sarah de Pue and Laura Pollentier for their assistance with data collection. Data were collected while M. F. L. Ruitenberg was affiliated with the Department of Experimental Psychology at Ghent University, Belgium. The data that support the findings of this study are available on request from the corresponding author. This study was not preregistered.
Funding
This work was supported in part by a postdoctoral fellowship from the Ghent University Special Research Fund (BOF 15/PDO/135) awarded to M. F. L. Ruitenberg.
Conflict of Interest
None declared.
References
- Abrahamse E. L., Ruitenberg M. F., de Kleine E., & Verwey W. B (2013). Control of automated behavior: Insights from the discrete sequence production task. Frontiers in Human Neuroscience, 7, 82. doi: 10.3389/fnhum.2013.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aisenberg D., Sapir A., d’Avossa G., & Henik A (2014). Long trial durations normalise the interference effect and sequential updating during healthy aging. Acta Psychologica, 153, 169–178. doi: 10.1016/j.actpsy.2014.10.005 [DOI] [PubMed] [Google Scholar]
- Aisenberg D., Sapir A., Close A., Henik A., & d’Avossa G (2018). Right anterior cerebellum BOLD responses reflect age related changes in Simon task sequential effects. Neuropsychologia, 109, 155–164. doi: 10.1016/j.neuropsychologia.2017.12.012 [DOI] [PubMed] [Google Scholar]
- Annett M. (1970). A classification of hand preference by association analysis. British Journal of Psychology (London, England: 1953), 61, 303–321. doi: 10.1111/j.2044-8295.1970.tb01248.x [DOI] [PubMed] [Google Scholar]
- Aschenbrenner A. J., & Balota D. A (2015). Interactive effects of working memory and trial history on Stroop interference in cognitively healthy aging. Psychology and Aging, 30, 1–8. doi: 10.1037/pag0000012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschenbrenner A. J., & Balota D. A (2017). Dynamic adjustments of attentional control in healthy aging. Psychology and Aging, 32, 1–15. doi: 10.1037/pag0000148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschenbrenner A. J., & Balota D. A (2019). Additive effects of item-specific and congruency sequence effects in the vocal Stroop task. Frontiers in Psychology, 10, 860. doi: 10.3389/fpsyg.2019.00860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels A., & Zeki S (2008). The architecture of the colour centre in the human visual brain: New results and a review. The European Journal of Neuroscience, 12, 172–193. doi: 10.1046/j.1460-9568.2000.00905.x [DOI] [PubMed] [Google Scholar]
- Botvinick M. M., Braver T. S., Barch D. M., Carter C. S., & Cohen J. D (2001). Conflict monitoring and cognitive control. Psychological Review, 108, 624–652. doi: 10.1037/0033-295x.108.3.624 [DOI] [PubMed] [Google Scholar]
- Braem S., Bugg J. M., Schmidt J. R., Crump M. J. C., Weissman D. H., Notebaert W., & Egner T (2019). Measuring adaptive control in conflict tasks. Trends in Cognitive Sciences, 23, 769–783. doi: 10.1016/j.tics.2019.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugg J. M. (2014). Evidence for the sparing of reactive cognitive control with age. Psychology and Aging, 29, 115–127. doi: 10.1037/a0035270 [DOI] [PubMed] [Google Scholar]
- Carson N., Leach L., & Murphy K. J (2018). A re-examination of Montreal Cognitive Assessment (MoCA) cutoff scores. International Journal of Geriatric Psychiatry, 33, 379–388. doi: 10.1002/gps.4756 [DOI] [PubMed] [Google Scholar]
- Chan M. Y., Park D. C., Savalia N. K., Petersen S. E., & Wig G. S (2014). Decreased segregation of brain systems across the healthy adult lifespan. Proceedings of the National Academy of Sciences of the United States of America, 111, E4997–E5006. doi: 10.1073/pnas.1415122111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colzato L. S., van Wouwe N. C., Hommel B., Zmigrod S., Ridderinkhof K. R., & Wylie S. A (2012). Dopaminergic modulation of the updating of stimulus-response episodes in Parkinson’s disease. Behavioural Brain Research, 228(1), 82–86. doi: 10.1016/j.bbr.2011.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colzato L. S., van Wouwe N. C., Lavender T. J., & Hommel B (2006). Intelligence and cognitive flexibility: Fluid intelligence correlates with feature “unbinding” across perception and action. Psychonomic Bulletin & Review, 13, 1043–1048. doi: 10.3758/bf03213923 [DOI] [PubMed] [Google Scholar]
- Dienes Z. (2011). Bayesian versus orthodox statistics: Which side are you on? Perspectives on Psychological Science, 6(3), 274–290. doi: 10.1177/1745691611406920 [DOI] [PubMed] [Google Scholar]
- Duthoo W., Abrahamse E. L., Braem S., Boehler C. N., & Notebaert W (2014). The heterogeneous world of congruency sequence effects: An update. Frontiers in Psychology, 5, 1001. doi: 10.3389/fpsyg.2014.01001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsner B., & Hommel B (2001). Effect anticipation and action control. Journal of Experimental Psychology. Human Perception and Performance, 27, 229–240. doi: 10.1037//0096-1523.27.1.229 [DOI] [PubMed] [Google Scholar]
- Fjell A. M., Walhovd K. B., Westlye L. T., Østby Y., Tamnes C. K., Jernigan T. L., Gamst A., & Dale A. M (2010). When does brain aging accelerate? Dangers of quadratic fits in cross-sectional studies. Neuroimage, 50, 1376–1383. doi: 10.1016/j.neuroimage.2010.01.061 [DOI] [PubMed] [Google Scholar]
- Funes M. J., Lupiáñez J., & Humphreys G (2010). Sustained vs. transient cognitive control: Evidence of a behavioral dissociation. Cognition, 114, 338–347. doi: 10.1016/j.cognition.2009.10.007 [DOI] [PubMed] [Google Scholar]
- Geukes S., Gaskell M. G., & Zwitserlood P (2015). Stroop effects from newly learned color words: Effects of memory consolidation and episodic context. Frontiers in Psychology, 6, 278. doi: 10.3389/fpsyg.2015.00278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommel B. (1998). Event files: Evidence for automatic integration of stimulus-response episodes. Visual Cognition, 5, 183–216. [Google Scholar]
- Hommel B. (2004). Event files: Feature binding in and across perception and action. Trends in Cognitive Sciences, 8, 494–500. doi: 10.1016/j.tics.2004.08.007 [DOI] [PubMed] [Google Scholar]
- Hommel B., Kray J. & Lindenberger U (2011). Feature integration across the lifespan: Stickier stimulus–response bindings in children and older adults. Frontiers in Psychology, 2, 268. doi: 10.3389/fpsyg.2011.00268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommel B., Müsseler J., Aschersleben G., & Prinz W (2001). The Theory of Event Coding (TEC): A framework for perception and action planning. The Behavioral and Brain Sciences, 24(5), 849–878; discussion 878. doi: 10.1017/s0140525x01000103 [DOI] [PubMed] [Google Scholar]
- IBM Corp (2019). IBM SPSS Statistics for Windows (Version 26.0) [Computer software]. [Google Scholar]
- Jacoby L. L., Lindsay D. S., & Hessels S (2003). Item-specific control of automatic processes: Stroop process dissociations. Psychonomic Bulletin & Review, 10(3), 638–644. doi: 10.3758/bf03196526 [DOI] [PubMed] [Google Scholar]
- JASP Team (2019). JASP (Version 0.11.1) [Computer software]. [Google Scholar]
- Kourtzi Z., & Kanwisher N (2001). Representation of perceived object shape by the human lateral occipital complex. Science (New York, N.Y.), 293(5534), 1506–1509. doi: 10.1126/science.1061133 [DOI] [PubMed] [Google Scholar]
- Larson M. J., Clayson P. E., Keith C. M., Hunt I. J., Hedges D. W., Nielsen B. L., & Call V. R (2016). Cognitive control adjustments in healthy older and younger adults: Conflict adaptation, the error-related negativity (ERN), and evidence of generalized decline with age. Biological Psychology, 115, 50–63. doi: 10.1016/j.biopsycho.2016.01.008 [DOI] [PubMed] [Google Scholar]
- McDougle S. D., Ivry R. B., & Taylor J. A (2016). Taking aim at the cognitive side of learning in sensorimotor adaptation tasks. Trends in Cognitive Sciences, 20, 535–544. doi: 10.1016/j.tics.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine Z. S., Phillips N. A., Bédirian V., Charbonneau S., Whitehead V., Collin I., Cummings J. L., & Chertkow H (2005). The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53, 695–699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- Notebaert W., & Verguts T (2008). Cognitive control acts locally. Cognition, 106, 1071–1080. doi: 10.1016/j.cognition.2007.04.011 [DOI] [PubMed] [Google Scholar]
- Nuzzo R. L. (2017). An introduction to Bayesian data analysis for correlations. PM & R: The Journal of Injury, Function, and Rehabilitation, 9, 1278–1282. doi: 10.1016/j.pmrj.2017.11.003 [DOI] [PubMed] [Google Scholar]
- Pisella L., Berberovic N., & Mattingley J. B (2004). Impaired working memory for location but not for colour or shape in visual neglect: A comparison of parietal and non-parietal lesions. Cortex, 40, 379–390. doi: 10.1016/s0010-9452(08)70132-1 [DOI] [PubMed] [Google Scholar]
- Prinz W., Aschersleben G., & Koch I (2009). Cognition and action. In Morsella E., Bargh J. A., & Gollwitzer P. M. (Eds.), Oxford handbook of human action (pp. 35–71). Oxford University Press Inc. [Google Scholar]
- Reuter-Lorenz P. A., & Cappell K. A (2008). Neurocognitive aging and the compensation hypothesis. Current Directions in Psychological Science, 17, 177–182. doi: 10.1111/j.1467-8721.2008.00570.x [DOI] [Google Scholar]
- Ruitenberg M. F., Duthoo W., Santens P., Notebaert W., & Abrahamse E. L (2015). Sequential movement skill in Parkinson’s disease: A state-of-the-art. Cortex, 65, 102–112. doi: 10.1016/j.cortex.2015.01.005 [DOI] [PubMed] [Google Scholar]
- Ruitenberg M. F. L., Abrahamse E. L., Santens P., & Notebaert W (2019). The effect of dopaminergic medication on conflict adaptation in Parkinson’s disease. Journal of Neuropsychology, 13, 121–135. [DOI] [PubMed] [Google Scholar]
- Schmand B., Lindeboom J., & van Harskamp F (1992). Nederlandse Leestest voor Volwassenen (Dutch Adult Reading Test). Swets & Zeitlinger. [Google Scholar]
- Schmidt J. R., & Besner D (2008). The Stroop effect: Why proportion congruent has nothing to do with congruency and everything to do with contingency. Journal of Experimental Psychology, 34, 514–523. doi: 10.1037/0278-7393.34.3.514 [DOI] [PubMed] [Google Scholar]
- Torres-Quesada M., Funes M. J., & Lupianez J (2013). Dissociating proportion congruent and conflict adaptation effects in a Simon-Stroop procedure. Acta Psychologica, 14, 203–210. doi: 10.1016/j.actpsy.2012.11.015 [DOI] [PubMed] [Google Scholar]
- Verguts T., & Notebaert W (2009). Adaptation by binding: A learning account of cognitive control. Trends in Cognitive Sciences, 13, 252–257. doi: 10.1016/j.tics.2009.02.007 [DOI] [PubMed] [Google Scholar]
- Westlye L. T., Walhovd K. B., Dale A. M., Bjørnerud A., Due-Tønnessen P., Engvig A., Grydeland H., Tamnes C. K., Ostby Y., & Fjell A. M (2010). Life-span changes of the human brain white matter: Diffusion tensor imaging (DTI) and volumetry. Cerebral Cortex, 20, 2055–2068. doi: 10.1093/cercor/bhp280 [DOI] [PubMed] [Google Scholar]
- Yuan P., Voelkle M. C., & Raz N (2018). Fluid intelligence and gross structural properties of the cerebral cortex in middle-aged and older adults: A multi-occasion longitudinal study. Neuroimage, 172, 21–30. doi: 10.1016/j.neuroimage.2018.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.