Abstract
Objective
It has often been reported that dual-task (DT) performance declines with age. Physical exercise can help improve cognition, but these improvements could depend on cognitive functions and age groups. Moreover, the mechanisms supporting this enhancement are not fully elucidated. This study investigated the impacts of physical exercise on single- and dual-task performance in younger-old (<70) and older-old (70+) adults. The study also assessed whether the training effect on cognition was mediated by improvement in cardiorespiratory fitness.
Methods
One hundred forty-three participants (65–89 years) took part in a physical exercise intervention for 3 months or were assigned to a control group. All participants completed a DT paradigm and an estimated measure of cardiorespiratory fitness. Regression models were used to test the training effect on these outcomes, and mediation analyses were used to determine whether the training-related cognitive changes were mediated by changes in cardiorespiratory fitness.
Results
In 70+, training predicted improved processing speed (βc = −.33) and cardiorespiratory fitness (βa = .26) and the effect of training on processing speed was fully mediated by change in cardiorespiratory fitness (βab = −.12). In <70, training predicted improvement in task-set cost (βc = −.26) and change in cardiorespiratory fitness (βa = .30) but improvement in task-set cost was not entirely mediated by change in cardiorespiratory fitness.
Discussion
Results are discussed in terms of the mechanisms supporting DT performance improvement following physical exercise training in older adults.
Keywords: Divided attention, Executive functions, Physical activity
The decline in cognitive functioning with aging is well-documented (Verhaeghen & Salthouse, 1997). It has also been frequently reported that attentional control is affected early in the course of aging leading to impaired ability to perform concurrent multiple tasks (McDowd & Shaw, 2000). Deficit in managing two or more tasks simultaneously has an important impact on everyday functioning and activities of daily living. Dual-task (DT) paradigms are often used in cognitive aging research as they reflect real-world scenarios (e.g., conversing while driving, navigating while walking, writing while listening) that involve managing multiple tasks concurrently. Individual differences in DT performances are multidetermined and likely rely on the integrity of multiple executive control mechanisms (Fraser & Bherer, 2013) that are also involved in other executive function tasks such as the Stroop task or the TrailB. However, many recent studies suggest that performances in DT paradigms have demonstrated predictive validity for everyday hazardous situations that are common among older adults such as automobile accidents and fall rates (Ball, Owsley, Sloane, Roenker, & Bruni, 1993; Verghese et al., 2002). Moreover, walking and simultaneously performing a cognitive task has been shown to impair gait performances, and lower abilities in performing this motor-cognitive DT would be responsible for recurrent falls (Beauchet et al., 2008).
The reduced ability of older adults to manage several tasks simultaneously can be partly explained by a decrease in processing speed, a reduced ability to maintain and prepare response alternatives in working memory, and a decline in the ability to execute/manage simultaneous responses (Fraser & Bherer, 2013). It has also been suggested that age does not affect DT abilities in a linear way but instead that age induces a shift in DT strategy as supported by brain imaging studies (Braver, Gray, & Burgess, 2007). In a context of increased attentional demands like those imposed in DT situations, younger adults tend to use a more proactive strategy, which implies that they better keep active maintenance of context information in working memory (Braver et al., 2007). In contrast, older adults tend to rely on reactive processing which is invoked only as needed on a just-in-time basis (Braver et al., 2007). Others have argued that aging is not simply a matter of strategy differences between younger and older adults but also a matter of the ability to interleave and simulantaneoulsy perform multiple tasks (Kramer & Kray, 2006; Kramer & Madden, 2008). Be that as it may, evidence suggests that DT strategies could differ according to the age of the participants and therefore inclusion of participants with broad age range could limit our understanding of age-related difficulty in dual tasking (Kelly et al., 2014). The present study explored age-related performance separately in younger-old (<70) and older-old (70+) adults. The choice of the 70 years threshold was data-driven as it corresponds to the median of the participants included in the present study. Moreover, in a review, Baltes and Smith mentioned that most people maintain their level of everyday intelligence or mental achievement until around age 70 and that today’s 70-year-olds are comparable to 65-year-olds who lived 30 years ago (Baltes & Smith, 2003), confirming the interest of testing such a threshold.
Increasing evidence supports the notion that the age-related cognitive decline can be reduced by lifestyle factors such as physical activities (Bherer, 2015; Kramer & Colcombe, 2018). Physical exercise training, especially if involving an aerobic training component, has a positive effect on cognition that goes beyond the mere effects of expectations or placebo effect, and could undeniably improve various cognitive functions (Stothart, Simons, Boot, & Kramer, 2014). In a seminal meta-analysis, Colcombe and Kramer (2003) reported that physical exercise training was associated with improvement in several cognitive functions with a more pronounced improvement in executive control processes in adults between 55 and 80 (Colcombe & Kramer, 2003). Physical training, in particular when including aerobic components, ranging from 8 to 72 weeks, has been shown to be able to improve cognitive functions, including memory, attention, processing speed, and executive function (Smith et al., 2010). Reviews suggest that aerobic exercise enhances a wide range of cognitive functions and that even other types of exercise training, such as resistance training, may also benefit cognition (Bherer, Erickson, & Liu-Ambrose, 2013). Specifically, a recent meta-analysis has found that adults above 50 years of age who exercise regularly significantly improve across a spectrum of cognitive tests of memory (short-term and working), attention (sustained alertness), and executive functioning (Northey, Cherbuin, Pumpa, Smee, & Rattray, 2018). Importantly, the positive effects of physical training on cognition has been shown to be moderated by the age of the participants, the proportion of men and women in the studies and the length of training sessions (Kramer et al., 2003). However, some meta-analyses and studies that have included wide age ranges, both men and women, and diverse training types have reported nonsignificant results of exercise intervention on cognition. It thus seems that both, age-related decline in DT ability, and the physical training effect on cognition could be dependent on the age of participants. Very few studies have systematically explored whether physical exercise training would benefit cognition the same in younger-old adults and older-old adults.
With regard to dual-tasking ability, to the best of our knowledge, only two intervention studies have investigated the specific effect of exercise on DT performances, demonstrating contradictory findings (Hawkins, Kramer, & Capaldi, 1992; Madden, Blumenthal, Allen, & Emery, 1989). In Hawkins and colleagues (1992), participants (aged 63–82) randomly assigned to a 10-week aquatic aerobic fitness program showed significant improvements in DT but not single task performances (Hawkins et al., 1992). In contrast, Madden and colleagues (1989), did not find any beneficial effects on DT performances in older adults between 60 and 83 after a 12-week aerobic intervention (Madden et al., 1989). Moreover, these two studies did not investigate training effect on specific component of DT performances, such as speed of processing, the ability to prepare, and maintain response alternatives in working memory (Fraser & Bherer, 2013), although Kramer and colleagues (1999), did report improved task-switching following 6 months of aerobic exercise training in older adults (Kramer et al., 1999).
The discrepancy in results of previous studies could lie in the difference in training-related aerobic fitness improvement as further investigation found that higher cardiorespiratory fitness (CRF) could predict better DT performances (Wong et al., 2015) or at least better response preparation in tasks that involve reaction time performances (Renaud, Bherer & Maquestiaux, 2010). Indeed, higher CRF has been shown to be related to brain structural modification, to changes in functional connectivity and to higher cerebral blood volume that could enhance cognitive functioning (Kramer & Colcombe, 2018; Voss, Vivar, Kramer, & van Praag, 2013). However, most of the studies showing links between CRF and cognition are cross-sectional and cannot conclude on causal relationships. These studies highlight the need for longitudinal intervention study to test the mediating aspect of CRF on the effects of aerobic training on DT performances. Although there are an increasing number of randomized controlled trials that support the relationship between fitness training and aspects of cognition, causal relationship between increased CRF and increased cognitive performances remains uncertain (Kramer & Colcombe, 2018). The primary aim of the present study was to investigate if physical exercise training would improve DT performances in younger-old and older-old adults. The secondary aim was to determine whether improvement in DT would be mediated by CRF improvement in these two groups.
Method
Recruitment
Data for this study were collected from three intervention research protocols performed in the research center of a geriatric institution. Each study involved older adult participants randomly assigned to a physical training intervention that aimed to improve CRF or to a passive control group. Participants were independent leaving community dwellers recruited from public advertisements (flyers and newspapers) and from the research center’s participant pool. A telephone-based screening interview was used to assess the eligibility of each candidate, that is that they did not engage in structured physical activity more than twice a week. All participants underwent a complete geriatric assessment to ensure that they could perform a physical exercise program at low risk. Participants were excluded if they showed signs of dementia [MMSE cutoff score of 26/30 (Folstein, Folstein, & McHugh, 1975)], or depression [>10 at the Geriatric depression scale (Yesavage et al., 1982)]. Participants were also excluded if they reported a history of neurological disease, a major surgery in the year preceding the study or uncorrected auditory or visual impairments.
Physical exercise interventions had a duration of 12 weeks, the training programs took place in a research-dedicated gymnasium located in a geriatric hospital institution and were supervised by a certified kinesiologist. Basic principles and guidelines for exercise programming were followed, including adequate warm-up and cool-down periods, progressive and gradual increments in exercise duration, and energy expenditure (Nelson et al., 2007). The exercise sessions were slightly different depending on the protocols. Each session was conducted by kinesiologists and included 10–40 min of aerobic workout (using treadmills, fast walking, recumbent bikes, and elliptical), and 10–15 min of strength exercises (using resistance cables) or stretching exercises. The intensity and duration of the aerobic exercises were increased individually, according to the participants progression. The 3-month training usually started with shorter aerobic workouts and finished with aerobic workout reaching around 40 min. Borg Rating of Perceived Exertion scale (0–10) was used at each training to adapt training and determine the target intensity and duration in order to make sure the participant’s training reached moderate to hard intensity. Training protocols are more extensively described elsewhere (Desjardins-Crepeau et al., 2016; Langlois et al., 2013; Renaud, Maquestiaux, Joncas, Kergoat & Bherer, 2010). Trained participants came three times a week at the laboratory to complete three exercise training sessions in two protocols (Langlois et al., 2013; Renaud, Maquestiaux, Joncas, Kergoat & Bherer, 2010) or two exercise sessions and a web-lesson in one protocol (Desjardins-Crepeau et al., 2016). Note that difference in protocol is taken into account in the results section.
Measures
DT paradigm
DT performance was assessed within a 2-week delay before and after intervention. The DT paradigm involved performing two concurrent multiple-choice discrimination tasks. Each task required the discrimination between visual or auditory stimuli (Bherer et al., 2006). Responses were provided on computer keyboards, and reaction time was recorded in ms. Consistent with previous studies using this paradigm (Bherer et al., 2005, 2006, 2008a; Lussier, Gagnon, & Bherer, 2012), response accuracy was generally very high (usually >90%) and did not show significant changes with fitness. Therefore, further analysis in the present study were based on reaction time only. First, participants completed blocks of trials composed of only one of the two tasks (pure single-task trials [SP]), then participants responded to only one task in the DT condition (single-task trials mixed with DT trials [SM]), and finally participants executed two motor responses to stimuli from two different tasks (DT trials [DM]). These three different types of trials can provide valuable information on the mechanisms involved in DT performance. First, the pure single-task trials reflect processing speed abilities. Second, dividing performances in the SM by the performances in SP reflects the ability to maintain different response alternatives in memory and to prepare to answer to multiple tasks while controlling for speed of execution of one task. Heretofore, we will refer to this performance cost as a task-set cost (TSC). And finally, the ability to manage and execute two simultaneous tasks can be assessed by the dual-task cost (DTC), which is calculated by dividing DM by SM. In this study, training effects were tested on the three outcomes SP, TSC, and DTC, which are thought to reflect three important processes involved in dual-tasking (Fraser & Bherer, 2013), respectively, speed of processing, the ability to prepare, and maintain response alternatives in working memory or the ability to coordinate multiple responses execution. Raw scores for the DT outcomes are presented in Supplementary Material.
Cardiorespiratory fitness protocols and evaluations
CRF was assessed by estimating VO2max from two validated submaximal evaluations of aerobic capacity: the Rockport 1-mile fitness walking test (R1MWT) and the 6-minute walk test (6MWT). The Rockport 1-mile test was used in two protocols. The time taken by the participant to walk 1 mile (1.6 kilometers) as quickly as possible was recorded. The CRF was estimated using the following standard equations: VO2max (mL/kg/min) = 132.853 − 0.0769 [weight (lb)] − 0.3877 [age (year)] + 6.315 [gender (males = 1, females = 0)] − 3.2649 [walked time (min)] − 0.1565 [postexercise heart rate (beats per minute)] (Kline et al., 1987). In the other included protocol, the 6-min walking test was used. The distance covered by the participants over a time of 6 min was recorded. The CRF was estimated using the following standard: VO2max (mL/kg/min) = 70.161 + 0.023 × [walked distance (m)] − 0.276 × [weight (kg)] − 6.79 × [sex (males = 0, females = 1)] − 0.193 × [resting HR (beats per minute)] − 0.191 × [age (year)] (Burr, Bredin, Faktor, & Warburton, 2011).
Data Analyses
Z score changes were calculated from each study across all measures: SP, TSC, DTC, and CRF, which allows consistent comparison of effect sizes across studies between training conditions (training vs control). Z-scores were calculated by subtracting individual scores from the protocol’s grand mean, divided by the protocol’s standard deviation (pre- and postcombined). Z score change was calculated by taking the difference between the pre- and post-z scores. For SP, TSC, and DTC negative z score change represented a reduction in reaction time and in cost, so a better performance. For CRF, a positive z score change represented a higher estimated VO2.
The sample was partitioned into younger–old adults (YOA) and older–old adults (OOA) which included participants <70 and ≥70, respectively. Participants characteristics were compared between study condition (training vs control) and age groups (OOA and YOA) using F tests.
Mediation Analyses
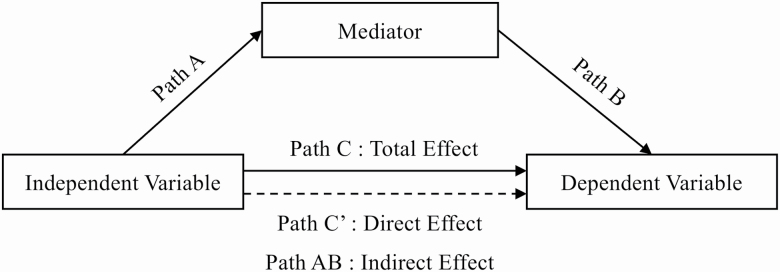
Mediation models involved a three-output analyses process (Paths A–C′ as illustrated in Figure 1) (MacKinnon, 2008), which has been applied in previous studies (Kaushal, Rhodes, Meldrum, & Spence, 2018). They were conducted in YOA and OOA separately. To allow comparable interpretation of the models, the interaction between age group and CRF z score change was also tested.
Figure 1.
Path analysis model of mediation involving a three-output analyses process.
Training condition effect on DT outcomes (Path C)
The first step was to test if the training condition (training vs control) predicted the dependent variables (SP, TSC, and DTC z score changes) (Path C) using ordinary least squares regression. The physical training effect on cognition have been found to be moderated by sex and training protocols (Kramer et al., 2003). Therefore, we controlled for biological sex and the protocol of origin of the participants. When a training condition effect was detected on z score changes (Path C), a mediation analysis was conducted to determine whether the detected effect was mediated by the CRF z score change.
Training condition effect on CRF (Path A) and CRF effect on DT outcomes (Path B)
In presence of significant Path C, the second step analyzed univariate regression coefficients of CRF z score changes between the two training conditions (Path A). This test identifies if training conditions differed between CRF z score changes. This was followed by Path B analysis to determine whether CRF z score changes predicted z score changes in DT outcomes.
Direct (Path C′) and indirect effect (Path AB) of training condition on DT outcomes
The final step estimated the direct (Path C′) of training condition on the change in outcome variables after controlling for CRF and the indirect effect (Path AB). Direct and indirect effects (Path AB) were computed with bias-corrected bootstrap 95% confidence intervals based on 5,000 bootstrap samples (Hayes, 2013). The significance of mediation was indicated if the confidence intervals in Path AB did not cross through zero. Partial mediation was denoted if confidence intervals of Path C′ crossed through zero and full mediation was denoted if confidence intervals of Path C′ did not cross through zero. All analyses were performed using IBM SPSS Statistics for Windows, Version 24.0 with the PROCESS macro for mediation analyses (Hayes, 2013).
Results
Participant Characteristics
Participant characteristics for YOA and OOA can be found in Table 1. No significant differences (p > .05) were found between intervention condition (training vs control) in sex, age, MMSE, CRF, and level of education at baseline. Z score changes of SP, TSC, and DTC are presented in Table 2.
Table 1.
Participants Characteristics
| Younger–old adults | Older–old adults | |||||
|---|---|---|---|---|---|---|
| Characteristics | Control group | Intervention group | p Value | Control group | Intervention group | p Value |
| Sample | 19 | 49 | 33 | 42 | ||
| Sex (% of men) | 11% | 18% | .438 | 21% | 33% | .253 |
| Age (years) | 64.32 (3.4) | 65.27 (2.81) | .243 | 75.03 (3.65) | 76.10 (4.63) | .283 |
| MMSE (/30) | 29.06 (1.08) | 28.69 (0.99) | .201 | 28.70 (1.04) | 28.61 (1.32) | .758 |
| CRF(mL/kg/min) | 23.40 (6.45) | 24.95 (7.39) | .466 | 23.96 (8.01) | 19.67 (12.17) | .148 |
| Education (years) | 12.93 (2.74) | 14.84 (3.50) | .061 | 13.08(.627) | 13.60 (.556) | .537 |
Note. MMSE = Mini-mental State Examination; CRF = cardiorespiratory fitness.
Table 2.
Z Score Changes in DT Scores and Cardiorespiratory Fitness
| Younger–old adults | Older–old adults | |||
|---|---|---|---|---|
| Z score changes | Control group | Intervention group | Control group | Intervention group |
| SP Z score change | −.285 (.642) | .045 (.788) | .114 (.472) | −.208 (.754) |
| TSC Z score change | −.006 (.897) | −.470 (1.04) | −.483 (1.341) | −.053 (1.06) |
| DTC Z score change | .224 (.903) | .195 (1.12) | −.109 (1.256) | .041 (1.14) |
| CRF Z score change | −.001 (.364) | .264 (.294) | .056 (.338) | .234 (.622) |
Note. SP = simple pure trial; TSC = task-set cost; DTC = dual-task cost; CRF = cardiorespiratory fitness.
Mediation Analyses
Training condition effect on DT outcomes (Path C)
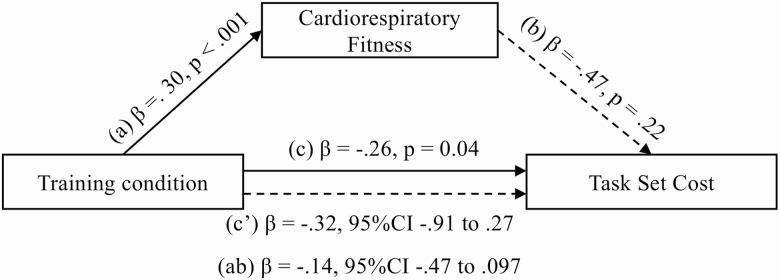
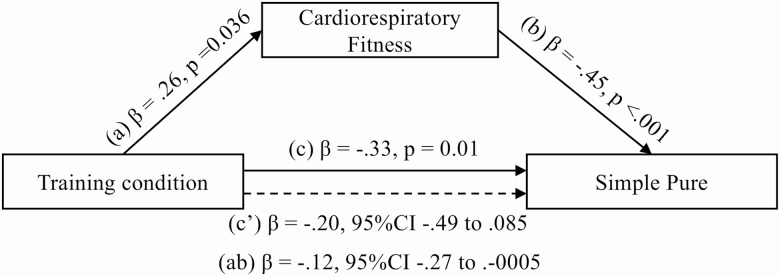
Ordinary least regression square tests in the YOA found training condition to predict TSC z score change, β = −.26, SE = .13, p = .040 (Figure 2) but not SP and DTC (p > .05). The regression test in the OOA participants found training condition to predict SP z score change, β = −.33, SE = .12, p = .011 (Figure 3) but not TSC and DTC (p > .05). These findings support the relationship between training condition and the respective DT outcomes (Path C) and support the hypotheses to test the two mediation models for each age group.
Figure 2.
Mediation of training condition effect on task-set cost by cardiorespiratory fitness in younger–old adults.
Figure 3.
Mediation of training condition effect on pure single-task trials by cardiorespiratory fitness in older–old adults.
Training condition effect on CRF (Path A) and CRF effect on DT outcomes (Path B)
Path A found training condition to significantly predict the change in CRF in both OOA, β = .26, SE = .12, p = .036 and YOA, β = . 31, SE = .09, p < .001 models. No interaction effect between age group and CRF z score change was found. Similar effects of training on CRF were therefore found in OOA and YOA. Path B in the OOA model revealed CRF to predict SP, β = −.45, SE = .13, p < .001. However, CRF did not predict TSC, β = −.47, SE = .39, p = .227 in the YOA model.
Direct (Path C′) and indirect effect (Path AB) of training condition on DT outcomes
The YOA model found the confidence intervals of both the direct (Path C′) (β = −.32, SE = .30, 95% CI = −.91 to .27), and indirect pathways (Path AB) (β = −.14, SE = .14, 95% CI = −.47 to .10) to cross through zero, thus giving inconclusive results for the full mediation of the physical exercise training effect on TSC by CRF.
The OOA model found training condition to no longer directly predict SP after accounting for the effects from CRF (β = −.20, SE = .15, 95% CI = −.49 to .09, p = .165) (Path C′). In addition, the confidence interval for the indirect pathway did not cross through zero (β = −.12, SE = .07, 95% CI = −.27 to −.0005) (Path AB), thus, demonstrating full mediation of the physical exercise training effect on SP by CRF.
Discussion
The present study investigated if physical exercise training would improve DT performances in younger–old and older–old adults and determine if this improvement would be mediated by an estimated measure of CRF. Results showed improvements in two (SP and TSC) of the three subcomponents of DT paradigm, where performance in SP significantly improved in OOA and TSC decreased among YOA. However, no significant improvements were found in DTC. The findings add some support to previous studies that showed that regularly active seniors (age 61–81) demonstrated faster processing speed and better overall DT performances (Marmeleira, Ferreira, Melo, & Godinho, 2012) but previous interventions findings on exercise as a predictor of DT are limited and mixed (Hawkins et al., 1992; Madden et al., 1989).
Results reported here are partly in line with previous studies in addition to providing novel findings. Madden and colleagues (1989), reported that physical exercise training while improving CRF would not improve DT outcomes. Hawkins and colleagues (1992) also noted improvement in CRF and in reaction time in a DT condition, but reported no effect on speed of processing (i.e., single task conditions). Hawkins and colleagues (1992) suggested that methodological or task differences among studies can account for results discrepancies. In fact, Madden and colleagues could have used a DT paradigm that was not demanding enough, and therefore potentially not inducing a large TSC, to detect training related changes. This hypothesis could not be validated due to the absence of a comparable assessment of TSC within the DT paradigm in Madden’s and Hawkins’ studies. The present study is the first to directly investigate physical exercise training effect on both TSC and DTC, with the rationale that both task costs reflect different processes involved in DT performance. Although the DT task used in the present study was quite simple and involved a low working memory load, results showed improvement in TSC and not in DTC after the exercise intervention. This is in line with Hawkins and colleagues’ suggestion that physical exercise training impacts TSC, which emphasizes the importance of investigating different aspects of the DT performance to better account for the exercise training effects. However, the results reported here suggest that training effects can also be observed in relatively easy tasks that do not induce large TSC or that do not involve a high working memory load.
Our results also highlight the importance of taking into account age in the study of the relationship between physical exercise training effect on DT performance. This could also help explain discrepancy between past studies and the present one. Indeed, previous studies did not investigate separately YOA and OOA while results reported here suggest the exercise effects differ according to the age of the participants. Showing evidence that training effects might be more sensitive in older seniors suggests that not taking age into account could have masked training effects in previous studies.
Altogether results of the present study suggest that physical exercise training can improve performance differently in a DT paradigm in YOA and OOA. OOA showed improvement in speed of processing (i.e., in the single task condition), which is of importance given that processing speed declines early in the course of aging (Penke et al., 2010). In YOA, improved TSC suggests an enhanced ability to maintain and manipulate information in working memory, a process that is also highly age-sensitive (Kray & Lindenberger, 2000).
Older–Old Adults Mediation Model
The present study is the first to test the mediating effect of CRF changes on DT performances in older adults. The results showed that improvement in estimated CRF entirely mediated the relationship between exercise intervention group and cognitive outcomes (SP). For instance, the association between the estimated CRF and processing speed reflected by reaction time in SP trials (Path B of the mediation models) parallels previous findings showing that higher level of CRF might have a protective effect against age-associated decline in the execution of speeded motor responses among 70–79 year older adults (Renaud, Bherer et al., 2010). The present findings also aligned with a meta-analysis by Angevaren, Aufdemkampe, Verhaar, Aleman, and Vanhees (2008) which demonstrated an impact of aerobic exercise on CRF (Path A) that coincided with the improvement of cognitive speed in older adults (Angevaren et al., 2008). Another meta-analysis from Smith and colleagues (2010) confirmed (Path C) where older individuals assigned to an aerobic exercise training showed improvements in processing speed with less convincing effects on working memory (Smith et al., 2010). Colcombe and colleagues (2004) offers the most probable mechanical explanation of the present results by suggesting that higher fitness is associated with a better functioning of key aspects of the attentional network (Colcombe et al., 2004) specifically the prefrontal and parietal cortices, while DTC has been reported to be specifically associated with inferior frontal gyri independently of fitness level (Herath, Klingberg, Young, Amunts, & Roland, 2001).
Mediation Model of Younger–Old Adults
The YOA model found older individuals below 70 years who engaged in physical training could improve the ability to maintain different response alternatives in working memory and enhanced response preparation, reflected here by a reduction in TSC. This finding is in accordance with Hötting and colleagues (2012) who suggested that physical exercise training in adults aged between 40 and 56 has specific beneficial effects on memory functions rather than a broader range of cognitive functions (Hötting et al., 2012). In addition, Renaud, Bherer et al., and colleagues (2010) suggested that CRF is associated with more efficient response preparation in seniors between 60 and 79 years. However, Renaud, Bherer et al., and colleagues’ study was cross-sectional and could not conclude on causal effects of CRF on cognition highlighting the need for evaluating longitudinal intervention effects. The present study demonstrated that in adults below 70, the change in estimated CRF related to physical training does not predict TSC suggesting that other mechanisms were also likely implicated in the positive effects of physical exercise training. This is in accordance with a study by Smiley-Oyen, Lowry, Francois, Kohut, and Ekkekakis (2008) who reported that aerobic training-related improvement of higher cognitive function was not related to actual CRF changes (Smiley-Oyen et al., 2008). A study by Wong and colleagues (2015) reported that greater activation of several brain regions was a mediator of the relationship between cardiorespiratory fitness and DT processing. However, in Wong’s study, the association between cardiorespiratory fitness and DT performance was not significant supporting the notion that other mechanisms beyond CRF could be at work. Hence, in addition to other additional mediators that could explain covariance between the exercise training and TSC, there could be another construct (structural brain changes) that could mediate this relationship. Some mechanisms which could have contributed to the improvement of TSC include an increased level of neurotransmitters (e.g., norepinephrine) (Etnier et al., 1997), structural and functional changes in the brain (Bherer et al., 2013) and higher vascularization (Etnier et al., 1997). Possible weight loss related to the training in the present study, could also play a significant positive effect on cognition through its impact on the reduction of insulin resistance and oxidative stress (Veronese et al., 2017). Further research is needed to test these hypotheses and account for additional explanatory mechanisms of the improvement in TSC.
Differences Between Both Models
Because no different training effect on CRF change between age groups was detected, the difference in results between YOA and OOA could be explained by the use of the age-related difference in DT mechanisms. Braver and colleagues (2007) proposed an age-related shift between two strategies for the management of task demands and this was supported by neuroimaging correlates. Younger adults would use a more proactive strategy with active maintenance of context information in working memory, while older adults would rather rely on a more reactive processing based on cognitive speed (Braver et al., 2007). This proposed age-related difference in strategy could help account for findings of the present study. Reduction in TSC in YOA suggests greater working memory involvement after the intervention, in line with a proactive strategy. In the OOA group, improvement in performance was explained by increased processing and/or psychomotor speed, which would enable them to be more efficient in a reactive manner. It thus seems to be the case that exercise training did not lead to a change in response strategy among YOA and OOA but rather reinforced the commonly used processing strategy. Moreover, brains retain some degree of plasticity well into older adulthood that allows training-induced structural changes (Erickson et al., 2007), and while the cognitive plasticity still allows improvement of attention (Bherer et al., 2008b), it has been suggested that in memory domains cognitive plasticity decreases with age and make positive training effects on memory performances more difficult (Baltes & Kliegl, 1992). This could also explain why only younger seniors improved TSC in the present study.
Strengths, Limitations, Future Directions
Although our study makes several advancements in the literature, there are some limitations worth noting. An indirect measure of CRF was used. Specifically, CRF was estimated using validated methods through estimation from Rockport and 6-min walking tests which both are convenient to use in older adults and show high correlation with direct VO2 measures (Burr et al., 2011; Kline et al., 1987). However, the estimated CRF was calculated based on the weight of the participants. Weight loss could possibly improve memory, attention, and executive functions through pathways that could overlap with cardiorespiratory fitness training (Veronese et al., 2017). Further studies should therefore confirm the present findings with gold standard measurements of VO2 measures. Moreover, while the sample size of the present study did not allow more complex analyses, future research with larger sample sizes should try to determine finer age cutoffs through moderated mediation analysis. This would also allow to test and understand which mediation paths are affected by aging and determine if age moderates the direct relationship between training and cognition, or if it moderates the relationship between training and CRF or between CRF and cognition.
Overall, the study contributes to the literature. To the best of our knowledge, this is the first study to test estimated aerobic fitness as a mediator between exercise training and DT outcomes. As mentioned by a meta-analysis by Kramer and Colcome (2003), aerobic training’s effects on cognition are indeed moderated by age but also sex and training protocols. This was considered as participants were partitioned into separate age groups, and we controlled for the sex and the protocols in the analysis. Therefore, the present results seem valid for both men and women, and for physical exercise training ranging from two to three times a week for 12 weeks. Mediation analyses are particularly crucial for empirical data as although an intervention can identify which constructs have changed, mediation identifies and explains why the dependent variable changed. This approach also addresses a recent call to use advanced model analyses for exercise cognition literature (Boisgontier & Cheval, 2016).
Conclusion
In summary, findings of the present study suggest that exercise interventions can help improve aspects of DT performance in older adults. While this is true for both younger–old and older–old adults, the improvement was observed on different outcomes of the task (speed vs working memory component) and differently mediated by estimated CRF among age groups. Further studies using brain imaging (e.g., like Wong et al., 2015) would help further explain the brain adaptation mechanisms that support exercise-related improvement in DT performances in older adults.
Funding
This study was supported by a Canadian Institutes of Health Research (CIHR) grant (#187596). L. Desjardins-Crépeau and F. Langlois were supported by a doctoral fellowship from the CIHR, A. Langeard was supported by a Fonds de Recherce Québec (FRQS-INSERM) salary grant, N. Kaushal and T. Vrinceanu by a FRQS fellowship and L. Bherer was supported by the Canada Research Chair Program.
Conflict of Interest
None reported.
Supplementary Material
References
- Angevaren M., Aufdemkampe G., Verhaar H. J., Aleman A., & Vanhees L (2008). Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Systematic Reviews, 3(3), CD005381. doi:10.1002/14651858.CD005381.pub3 [DOI] [PubMed] [Google Scholar]
- Ball K., Owsley C., Sloane M. E., Roenker D. L., & Bruni J. R (1993). Visual attention problems as a predictor of vehicle crashes in older drivers. Investigative Ophthalmology & Visual Science, 34, 3110–3123. [PubMed] [Google Scholar]
- Baltes P. B., & Kliegl R (1992). Further testing of limits of cognitive plasticity: Negative age differences in a mnemonic skill are robust. Developmental Psychology, 28(1), 121–125. doi:10.1037/0012-1649.28.1.121 [Google Scholar]
- Baltes P. B., & Smith J (2003). New frontiers in the future of aging: From successful aging of the young old to the dilemmas of the fourth age. Gerontology, 49, 123–135. doi:10.1159/000067946 [DOI] [PubMed] [Google Scholar]
- Beauchet O., Annweiler C., Allali G., Berrut G., Herrmann F. R., & Dubost V (2008). Recurrent falls and dual task-related decrease in walking speed: Is there a relationship? Journal of the American Geriatrics Society, 56, 1265–1269. doi:10.1111/j.1532-5415.2008.01766.x [DOI] [PubMed] [Google Scholar]
- Bherer L. (2015). Cognitive plasticity in older adults: Effects of cognitive training and physical exercise. Annals of the New York Academy of Sciences, 1337, 1–6. doi:10.1111/nyas.12682 [DOI] [PubMed] [Google Scholar]
- Bherer L., Erickson K. I., & Liu-Ambrose T (2013). A review of the effects of physical activity and exercise on cognitive and brain functions in older adults. Journal of Aging Research, 2013, 657508. doi:10.1155/2013/657508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bherer L., Kramer A. F., Peterson M. S., Colcombe S., Erickson K., & Becic E (2005). Training effects on dual-task performance: Are there age-related differences in plasticity of attentional control? Psychology and Aging, 20, 695–709. doi:10.1037/0882-7974.20.4.695 [DOI] [PubMed] [Google Scholar]
- Bherer L., Kramer A. F., Peterson M. S., Colcombe S., Erickson K., & Becic E (2006). Testing the limits of cognitive plasticity in older adults: Application to attentional control. Acta Psychologica, 123, 261–278. doi:10.1016/j.actpsy.2006.01.005 [DOI] [PubMed] [Google Scholar]
- Bherer L., Kramer A. F., Peterson M. S., Colcombe S., Erickson K., & Becic E. (2008a). Transfer effects in task-set cost and dual-task cost after dual-task training in older and younger adults: Further evidence for cognitive plasticity in attentional control in late adulthood. Experimental Aging Research, 34, 188–219. doi:10.1080/03610730802070068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bherer L., Kramer A. F., Peterson M. S., Colcombe S., Erickson K., & Becic E. (2008b). Transfer effects in task-set cost and dual-task cost after dual-task training in older and younger adults: Further evidence for cognitive plasticity in attentional control in late adulthood. Experimental Aging Research, 34, 188–219. doi:10.1080/03610730802070068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisgontier M. P., & Cheval B (2016). The ANOVA to mixed model transition. Neuroscience and Biobehavioral Reviews, 68, 1004–1005. doi:10.1016/j.neubiorev.2016.05.034 [DOI] [PubMed] [Google Scholar]
- Braver T. S., Gray J. R., & Burgess G. C (2007). Explaining the many varieties of working memory variation: Dual mechanisms of cognitive control. Variation in Working Memory, New York, NY, US: Oxford University Press. 76–106. doi:10.1093/acprof:oso/9780195168648.003.0004 [Google Scholar]
- Burr J. F., Bredin S. S., Faktor M. D., & Warburton D. E (2011). The 6-minute walk test as a predictor of objectively measured aerobic fitness in healthy working-aged adults. The Physician and Sportsmedicine, 39, 133–139. doi:10.3810/psm.2011.05.1904 [DOI] [PubMed] [Google Scholar]
- Colcombe S., & Kramer A. F (2003). Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychological Science, 14, 125–130. doi:10.1111/1467-9280.t01-1-01430 [DOI] [PubMed] [Google Scholar]
- Colcombe S. J., Kramer A. F., Erickson K. I., Scalf P., McAuley E., Cohen N. J.,…Elavsky S (2004). Cardiovascular fitness, cortical plasticity, and aging. Proceedings of the National Academy of Sciences of the United States of America, 101, 3316–3321. doi:10.1073/pnas.0400266101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins-Crépeau L., Berryman N., Fraser S. A., Vu T. T., Kergoat M. J., Li K. Z.,…Bherer L (2016). Effects of combined physical and cognitive training on fitness and neuropsychological outcomes in healthy older adults. Clinical Interventions in Aging, 11, 1287–1299. doi:10.2147/CIA.S115711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K. I., Colcombe S. J., Wadhwa R., Bherer L., Peterson M. S., Scalf P. E.,…Kramer A. F (2007). Training-induced plasticity in older adults: Effects of training on hemispheric asymmetry. Neurobiology of Aging, 28, 272–283. doi:10.1016/j.neurobiolaging.2005.12.012 [DOI] [PubMed] [Google Scholar]
- Etnier J. L., Salazar W., Landers D. M., Petruzzello S. J., Han M., & Nowell P (1997). The influence of physical fitness and exercise upon cognitive functioning: A meta-analysis. Journal of Sport and Exercise Psychology, 19(3), 249–277. doi:10.1123/jsep.19.3.249 [Google Scholar]
- Folstein M. F., Folstein S. E., & McHugh P. R (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198. doi:10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Fraser S., & Bherer L (2013). Age-related decline in divided-attention: From theoretical lab research to practical real-life situations. Wiley Interdisciplinary Reviews. Cognitive Science, 4, 623–640. doi:10.1002/wcs.1252 [DOI] [PubMed] [Google Scholar]
- Hawkins H. L., Kramer A. F., & Capaldi D (1992). Aging, exercise, and attention. Psychology and Aging, 7, 643–653. [DOI] [PubMed] [Google Scholar]
- Hayes A. F. (2013). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach New York, NY: Guilford Press. [Google Scholar]
- Herath P., Klingberg T., Young J., Amunts K., & Roland P (2001). Neural correlates of dual task interference can be dissociated from those of divided attention: An fMRI study. Cerebral Cortex (New York, N.Y.: 1991), 11, 796–805. doi:10.1093/cercor/11.9.796 [DOI] [PubMed] [Google Scholar]
- Hötting K., Reich B., Holzschneider K., Kauschke K., Schmidt T., Reer R.,…Röder B (2012). Differential cognitive effects of cycling versus stretching/coordination training in middle-aged adults. Health Psychology, 31, 145–155. doi:10.1037/a0025371 [DOI] [PubMed] [Google Scholar]
- Kaushal N., Rhodes R. E., Meldrum J. T., & Spence J. C (2018). Mediating mechanisms in a physical activity intervention: A test of habit formation. Journal of Sport & Exercise Psychology, 40, 101–110. doi:10.1123/jsep.2017-0307 [DOI] [PubMed] [Google Scholar]
- Kelly M. E., Loughrey D., Lawlor B. A., Robertson I. H., Walsh C., & Brennan S (2014). The impact of exercise on the cognitive functioning of healthy older adults: A systematic review and meta-analysis. Ageing Research Reviews, 16, 12–31. doi:10.1016/j.arr.2014.05.002 [DOI] [PubMed] [Google Scholar]
- Kline G. M., Porcari J. P., Hintermeister R., Freedson P. S., Ward A., McCarron R. F.,…Rippe J. M (1987). Estimation of VO2max from a one-mile track walk, gender, age, and body weight. Medicine and Science in Sports and Exercise, 19, 253–259. [PubMed] [Google Scholar]
- Kramer A. F., & Colcombe S (2018). Fitness effects on the cognitive function of older adults: A meta-analytic study-revisited. Perspectives on Psychological Science, 13, 213–217. doi:10.1177/1745691617707316 [DOI] [PubMed] [Google Scholar]
- Kramer A. F., Colcombe S. J., McAuley E., Eriksen K. I., Scalf P., Jerome G. J.,…Webb A. G (2003). Enhancing brain and cognitive function of older adults through fitness training. Journal of Molecular Neuroscience, 20, 213–221. doi:10.1385/JMN:20:3:213 [DOI] [PubMed] [Google Scholar]
- Kramer A. F., Hahn S., Cohen N. J., Banich M. T., McAuley E., Harrison C. R.,…Colcombe A (1999). Ageing, fitness and neurocognitive function. Nature, 400, 418–419. doi:10.1038/22682 [DOI] [PubMed] [Google Scholar]
- Kramer A. F., & Kray J (2006). Aging and attention. In E. Bialystok, & F. I. M. Craik (Eds.), Lifespan cognition: Mechanisms of change (pp. 57–69). New York, NY: Oxford University Press. doi:10.1093/acprof:oso/9780195169539.003.0005 [Google Scholar]
- Kramer A. F., & Madden D. J (2008). Attention. In Craik F. I. M. & Salthouse T. A. (Eds.), The handbook of aging and cognition (3rd ed., pp. 189–250). New York, NY: Psychology Press. doi:10.1080/01924780903295796 [Google Scholar]
- Kray J., & Lindenberger U (2000). Adult age differences in task switching. Psychology and Aging, 15, 126–147. doi:10.1037/0882-7974.15.1.126 [DOI] [PubMed] [Google Scholar]
- Langlois F., Vu T. T., Chassé K., Dupuis G., Kergoat M. J., & Bherer L (2013). Benefits of physical exercise training on cognition and quality of life in frail older adults. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 68, 400–404. doi:10.1093/geronb/gbs069 [DOI] [PubMed] [Google Scholar]
- Lussier M., Gagnon C., & Bherer L (2012). An investigation of response and stimulus modality transfer effects after dual-task training in younger and older. Frontiers in Human Neuroscience, 6, 129. doi:10.3389/fnhum.2012.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon D. P. (2008). Introduction to statistical mediation analysis. New York, NY: Lawrence Erlbaum Associates. doi:10.4324/9780203809556 [Google Scholar]
- Madden D. J., Blumenthal J. A., Allen P. A., & Emery C. F (1989). Improving aerobic capacity in healthy older adults does not necessarily lead to improved cognitive performance. Psychology and Aging, 4, 307–320. doi:10.1037/0882-7974.4.3.307 [DOI] [PubMed] [Google Scholar]
- Marmeleira J., Ferreira I., Melo F., & Godinho M (2012). Associations of physical activity with driving-related cognitive abilities in older drivers: An exploratory study. Perceptual and Motor Skills, 115, 521–533. doi:10.2466/10.06.25.PMS.115.5.521-533 [DOI] [PubMed] [Google Scholar]
- McDowd J. M., & Shaw R. L (2000). Attention and aging: A functional perspective. In Craik F. I. M. & Salthouse T. A. (Eds.), The handbook of aging and cognition (2nd ed., pp. 221–292). Mahwah, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Nelson M. E., Rejeski W. J., Blair S. N., Duncan P. W., Judge J. O., & King A. C (2007). Physical activity and public health in older adults: Recommendation from the American College of Sports Medicine and the American Heart Association. Circulation, 116, 1094–1105. doi:10.1161/circulationaha.107.185650 [DOI] [PubMed] [Google Scholar]
- Northey J. M., Cherbuin N., Pumpa K. L., Smee D. J., & Rattray B (2018). Exercise interventions for cognitive function in adults older than 50: A systematic review with meta-analysis. British Journal of Sports Medicine. 52(3), 154–160. doi:10.1136/bjsports-2016-096587 [DOI] [PubMed] [Google Scholar]
- Penke L., Muñoz Maniega S., Murray C., Gow A. J., Hernández M. C., Clayden J. D.,…Deary I. J (2010). A general factor of brain white matter integrity predicts information processing speed in healthy older people. The Journal of Neuroscience, 30, 7569–7574. doi:10.1523/JNEUROSCI.1553-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud M., Bherer L., & Maquestiaux F (2010). A high level of physical fitness is associated with more efficient response preparation in older adults. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 65, 317–322. doi:10.1093/geronb/gbq004 [DOI] [PubMed] [Google Scholar]
- Renaud M., Maquestiaux F., Joncas S., Kergoat M. J., & Bherer L (2010). The effect of three months of aerobic training on response preparation in older adults. Frontiers in Aging Neuroscience, 2, 148. doi:10.3389/fnagi.2010.00148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley-Oyen A. L., Lowry K. A., Francois S. J., Kohut M. L., & Ekkekakis P (2008). Exercise, fitness, and neurocognitive function in older adults: The “selective improvement” and “cardiovascular fitness” hypotheses. Annals of Behavioral Medicine, 36, 280–291. doi:10.1007/s12160-008-9064-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. J., Blumenthal J. A., Hoffman B. M., Cooper H., Strauman T. A., Welsh-Bohmer K.,… Sherwood A (2010). Aerobic exercise and neurocognitive performance: A meta-analytic review of randomized controlled trials. Psychosomatic Medicine, 72, 239–252. doi:10.1097/PSY.0b013e3181d14633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stothart C. R., Simons D. J., Boot W. R., & Kramer A. F (2014). Is the effect of aerobic exercise on cognition a placebo effect? PLoS One, 9, e109557. doi:10.1371/journal.pone.0109557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J., Buschke H., Viola L., Katz M., Hall C., Kuslansky G., & Lipton R (2002). Validity of divided attention tasks in predicting falls in older individuals: A preliminary study. Journal of the American Geriatrics Society, 50, 1572–1576. doi:10.1046/j.1532-5415.2002.50415.x [DOI] [PubMed] [Google Scholar]
- Verhaeghen P., & Salthouse T. A (1997). Meta-analyses of age-cognition relations in adulthood: Estimates of linear and nonlinear age effects and structural models. Psychological Bulletin, 122, 231–249. doi:10.1037/0882-7974.3.2.173 [DOI] [PubMed] [Google Scholar]
- Veronese N., Facchini S., Stubbs B., Luchini C., Solmi M., Manzato E.,…Fontana L (2017). Weight loss is associated with improvements in cognitive function among overweight and obese people: A systematic review and meta-analysis. Neuroscience and Biobehavioral Reviews, 72, 87–94. doi:10.1016/j.neubiorev.2016.11.017 [DOI] [PubMed] [Google Scholar]
- Voss M. W., Vivar C., Kramer A. F., & van Praag H (2013). Bridging animal and human models of exercise-induced brain plasticity. Trends in Cognitive Sciences, 17, 525–544. doi:10.1016/j.tics.2013.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C. N., Chaddock-Heyman L., Voss M. W., Burzynska A. Z., Basak C., Erickson K. I.,…Kramer A. F (2015). Brain activation during dual-task processing is associated with cardiorespiratory fitness and performance in older adults. Frontiers in Aging Neuroscience, 7, 154. doi:10.3389/fnagi.2015.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage J. A., Brink T. L., Rose T. L., Lum O., Huang V., Adey M., & Leirer V. O (1982). Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research, 17, 37–49. doi:10.1016/0022-3956(82)90033-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.