Abstract
Objectives
We examined the number of years to be lived with and without cognitive impairment and with high self-assessed quality of life (i.e., happiness) among a nationally representative sample of Americans aged 65 years and older. Two key questions are addressed: Can people have a high quality of life despite being cognitively impaired? Which is longer: happy life expectancy or cognitively intact life expectancy?
Method
Data from nine waves of the Health and Retirement Study (1998–2014) were used to estimate transition probabilities into and out of cognitively intact/impaired-un/happy states, as well as to death. Recently extended Bayesian multistate life table methods were used to estimate age-specific cognitively intact and happy life expectancy net of sex, race/ethnicity, education, and birth cohort.
Results
Happiness and cognitive impairment were shown to coexist in both the gross cross-tabulated data and in the life tables. Happy life expectancy is approximately 25% longer than cognitively intact life expectancy at age 65 years, and by age 85, happy life expectancy is roughly double cognitively intact life expectancy, on average.
Discussion
Lack of cognitive impairment is not a necessary condition for happiness. In other words, people can have a high quality of life despite being cognitively impaired.
Keywords: Cognitive impairment, Health and Retirement Study, Mortality, Quality of life, Subjective well-being
The well-being of adults aged 65 years and older is a major concern in the United States, as evidenced by extant policies put in place over 50 years ago to promote at least a basic quality of life (QoL) among the aged population (e.g., Social Security and Medicare). QoL, by many measures, has improved for older Americans. For example, longevity has increased and physical disability has largely been postponed to the last years of life, at least among non-Hispanic whites and blacks (Freedman & Spillman, 2016)—whether the same is true for Hispanics and/or other racial/ethnic minority groups remains unclear (Cantu, Hayward, Hummer, & Chiu, 2013). Somewhat contrastingly, cognitive health has recently emerged as a major concern for QoL among older adults (Plassman et al., 2008). In fact, estimates suggest that approximately a quarter of Americans aged 71 years or older have a cognitive impairment even without dementia (Plassman et al., 2008), thus presenting new challenges to the well-being of older adults.
Although there have been numerous advances in treating chronic diseases such as heart disease and some cancers, there have been far fewer advances in treating diseases that produce cognitive impairment (Daviglus et al., 2010). Emerging evidence shows that there has been a compression of cognitive morbidity, similar to trends in physical morbidity (Crimmins, Saito, & Kim, 2016). However, in light of population aging and the sheer size of the Baby Boom cohort, the overall incidence and prevalence of cognitive impairment is likely to increase. Given that cognitive vitality is a major component of the successful aging paradigm (e.g., Rowe & Kahn, 1997; see also Baltes & Baltes, 1990) and is commonly considered essential for QoL in old age (Gerstorf et al., 2015), research is needed to understand the interrelationship between cognitive impairment and self-assessed QoL (e.g., happiness).
Quality Versus Quantity of Life in an Aging Society
Research since the 1980s has attempted to determine whether our well-known gains in years of life have been accompanied by good quality of those additional years (Robine, Jagger, Mathers, Crimins, & Suzman, 2003). This notion of quality versus quantity is well-reflected in the World Health Organization’s (1997) statement that “increased longevity without quality of life is an empty prize.” In other words, the concern is whether we are simply adding years to life versus adding life to those years.
One common strategy for answering this question is to estimate the number of years an average person in a given population can expect to live in good health (e.g., healthy life expectancy). Specifically, total remaining life expectancy is commonly separated into healthy versus unhealthy years, and if healthy years exceed unhealthy years by a considerable margin, QoL is argued to be relatively good (Robine & Ritchie, 1991). If that proportion of life remaining to be healthy is increasing across time, it offers some support for the compression of morbidity hypothesis.
A well-established literature has shown that older adults can generally expect to live many more years in good health than in poor health, and this result is often taken to imply that QoL is relatively good (see Stiefel, Perla, & Zell, 2010). However, this understanding is almost exclusively based on measures of physical health (e.g., self-rated health and functional limitations). Relatively little is known about the extent to which additional years of life that have been gained over the last several decades are spent cognitively impaired (e.g., cognitively impaired life expectancy; a search for “healthy life expectancy” yields approximately 17,000 results on Google Scholar, whereas “cognitive life expectancy” yields only 12 results [date of search: November 21, 2018]), but an emerging literature is tackling this question.
Cognitive functioning is generally measured using instruments such as the Mini-Mental State Examination (MMSE), and cognitive life expectancy is computed by separating remaining life expectancy into the number of years an individual lives with and without cognitive impairment. This emerging research has shown that at age 65 years, average cognitively intact life expectancy is 14.1 years for females and 12.5 years for males, and average cognitively impaired life expectancy is 6.2 years and 5.2 years, respectively (Crimmins et al., 2016). In other words, at age 65 years females can expect 69.5%, and males can expect 70.6%, of their remaining life to be lived cognitively intact. Although there are small gender differences in cognitive life expectancy, there is some evidence to suggest that cognitive life expectancy differs substantially by race/ethnicity. For example, blacks can expect approximately 20% fewer remaining years of life to be cognitively intact versus impaired compared to whites (Garcia et al., 2017). Despite such disparities, cognitively intact years appear to outnumber cognitively impaired years across sex and race/ethnic divides, at least until the latest years of life. Thus, although racial/ethnic disparities are evident, QoL, as objectively assessed by cognitive functioning, appears to be relatively high across the older population.
Although cognitive health and physical health are important quality-of-life components, it is increasingly recognized that they are not the only, or even the most important, components. Research over the last decade has focused increasingly on happiness (and life satisfaction) as one of the key markers of QoL (George, 2010). In short, the argument is if we want to know how good someone’s QoL is, why not simply ask her/him whether she/he is happy (see Kahneman, Diener, & Schwarz, 1999)? An individual’s perceived level of happiness (or life satisfaction) is arguably a better measure of her/his QoL than a researcher’s imposed view of quality based on health measures (Layard, 2010).
In contrast to physical and cognitive health, happiness generally increases across the life course, at least until one’s mid-to-late 60s when it begins to slowly decline (Bardo, 2017; Bardo, Lynch, & Land, 2017). This age pattern suggests that good physical health and cognitive functioning may not be necessary components for happiness. In fact, research on happiness has found that gains in happy life expectancy (the number of years an average person can expect to live with high levels of perceived happiness) have outpaced gains in disability-free life expectancy (Yang, 2008), and these findings have been taken to suggest that “individuals with chronic illness and disability can sustain a decent sense of well-being” (Yang, 2008, p. 1248). In addition, recent research focused on cognitive functioning and perceived quality-of-life trajectories suggests that the development of a cognitive impairment is only weakly associated with small decreases in subjective well-being (SWB; (Martyr et al., 2018), which may in part be due to increased experience in dealing with age-related losses (see Braun, Schmukle, & Kunzmann, 2017; see also Lynch & George, 2002). Building on this emerging evidence, we ask two basic questions: Can people have a high QoL despite being cognitively impaired? Which is longer: happy life expectancy or cognitively intact life expectancy? We address these questions in this article.
Methods
Data
Data for this study are from the Health and Retirement Study (HRS), a panel study of persons older than 50 years (and their spouses) that has been administered biannually since 1992 (Health and Retirement Study, 2014). In the mid-1990s, the HRS was merged with the Asset and Health Dynamics among the Oldest Old (AHEAD) study, a study of persons older than 70 years. Measurement inconsistencies between the two studies, and within the studies over time, make it difficult to use health data prior to 1998. Thus, we use study data from 1998 forward (to 2014). RAND has developed a consolidated data file (RAND Center for the Study of Aging, 2018); we use this file to enhance replicability of our results. The original sample consists of 37,495 respondents. However, we restrict the analyses to persons who survive beyond 1998, who are observed alive on at least one occasion, who are a member of an HRS birth cohort, and to one person per household. Our initial sample size is 20,651.
For the analyses, we constructed a person-spell data set, where a spell is defined as the time span between waves (2 years). For each spell, we must know a respondent’s level of cognitive impairment and happiness (and survival status) at both the beginning and end of the spell, with the status at the end of the spell for time t also representing the status at the beginning of the spell for time t + 2. We eliminated person-spell records in which the respondent was younger than 65 years, because the mechanisms that underlie the relationship between happiness and cognitive impairment are arguably distinct from those who are younger. Specifically, older adults are more resilient to the impacts of cognitive impairment, and/or cognitive impairment is more normative in old age and easier to adjust to compared to such losses in earlier years of life (see Braun et al., 2017).
Overall, there are 12,704 persons who were observed for at least one spell in which they were at least 65 years of age. Of these, after listwise deletion of those for whom we could not reasonably impute cognitive impairment status or happiness, our analytic sample consisted of 11,964 respondents (5.8% missing compared to 12,704) who ultimately contribute a total of 53,120 person spells.
Measures
Estimates of cognitively intact and happy life expectancy require information on age, cognitive functioning, happiness, and mortality. Cognitive functioning is assessed in the HRS with the use of a version of the MMSE that includes six tasks that measure memory, working memory, speed of mental processing, knowledge and language, and orientation. Recent research has used scores from a subset of this instrument (i.e., immediate word recall of 10 words, delayed word recall of the same 10 words, 5 trials of Serial 7s, and backward counting) to assess cognitive functioning (e.g., Crimmins et al., 2016). Scores from each of these four tasks were combined to form a scale that ranges from 0 to 27. On the basis of the concordance of this scale with diagnoses of cognitive impairment in a subsample of the HRS (see Langa et al., 2005), respondents with scores from 12 to 27 are classified as being cognitively intact. Thus, respondents with scores from 0 to 11 are considered to be cognitively impaired. These questions were not asked of proxies, many of whom acted as proxies in part because of cognitive impairment of primary respondents. Previous research has used a count of instrumental activities of daily living (IADL) limitations, direct assessments of memory, and an interviewer assessment of difficulty completing the interview because of cognitive limitations, to supplement missing cognitive scores (see Crimmins et al., 2016). However, only the IADL information is available in the RAND version of the HRS. Thus, we use a count of two or more IADL limitations as indicative of cognitive impairment when the cognitive functioning scores were missing (our cognitively intact life expectancy results are comparable to Crimmins and colleagues [2016]).
To further assess the robustness of our imputation approach for missing cognitive functioning information, we regressed cognitive impairment on IADL limitations, which yielded an intercept of 14.56 and a slope of −1.85. At 2 IADLs, the expected cognitive score is just under our cutoff of 11 (10.87). Further, a crosstab of binary IADL classification with binary impairment among person-spell records in which both were observed indicated that 73.44% of records would be appropriately classified into impaired/unimpaired states based on the 2+ IADL criterion. Specifically, of those with fewer than 2 IADLs (92.1% of the records), 74.3% were unimpaired (and would be classified as such), whereas 25.7% were impaired (but would have been classified as unimpaired). Of those with 2+ IADLs (7.9% of the records), 36.6% were unimpaired (but would have been classified as impaired), whereas 63.4% were impaired (and would have been classified as such). These results suggest that respondents who are missing cognitive functioning information but have 2+ IADL limitations are more likely to be cognitively impaired than unimpaired (roughly twice as likely). Dropping such cases using listwise deletion would therefore underestimate cognitive impairment, whereas our approach may overestimate it. The “true” results, then should fall somewhere between these two approaches. Thus, we also estimated life tables using listwise deletion and obtained similar results, suggesting that the missingness and our handling of it probably has little impact on substantive conclusions (results obtained under listwise deletion available upon request). Overall, it seems the 2+ IADL criterion used for imputation of missing cognitive functioning information is reasonable, although not perfect.
Our happiness measure is based on a question that asks whether a respondent was happy (1) all/most of the time or (0) some/none of the time in the past week. This measure is similar to other happiness measures in nationally representative social survey data (e.g., the happiness measure in the General Social Survey). Arguably, this measure reflects one’s overall perception of her/his QoL (see Schwarz & Strack, 1999), which is relatively stable across time in contrast to other SWB dimensions (e.g., positive and negative affect; see Arthaud-Day, Rode, Mooney, & Near, 2005; see also Freedman, Carr, Cornman, & Lucas, 2017). The happiness question was asked only of self-respondents. Thus, information for this question is missing when the survey was completed by a proxy (n = 5570 person spells; 9.8%). Missing cases were replaced with responses from the most temporally proximate, prior wave (last observation carried forward). To evaluate the robustness of our findings, we conducted additional analyses in which missing values on the happiness measure were coded/treated as “unhappy.” Those analyses yielded comparable results, as did analyses based on complete case information (results available upon request).
Our predictors of cognitive impairment and happiness include age, sex, race/ethnicity, birth cohort, and education. Age is measured in years, including fractions of years, based on month of birth and month of interview. Sex is measured with a dummy variable (male = 1). Race is measured with two dummy variables (black = 1, other = 1, white = reference). Ethnicity is measured with an indicator for Hispanic ethnicity. Birth cohort is measured as an integer, with 1900 as the reference year (i.e., cohort = birth year − 1900). Finally, education is measured in years of schooling, with a range from 0 to 17+.
Analytic Approach
We estimate years to be lived in cognitively intact/impaired and un/happy states using an extension of multistate life table methods developed initially by Lynch and Brown (2005). Multistate life table methods compute years to be lived in different states by applying age-specific transition probability matrices to a population at a given age and totaling up the time to be lived in each state as the population ages and flows across states based on the transition probabilities. These methods have a long history in demography, but traditional approaches have been limited by the need to disaggregate data in order to “control” on covariates—which leads to cell sizes that are often too small for stable estimation of transition probabilities—and the inability to construct standard errors of state expectancy estimates, which are necessary for the construction of interval estimates and for evaluating statistical hypotheses regarding differences in state expectancies across subpopulations. Various methods have been developed in recent decades that compensate for these problems, facilitating the construction of multistate life tables from panel data. Typically, regression methods are used to estimate transition probabilities as a function of covariates, including age. Bootstrapping of data (Hayward, Rendall, & Crimmins, 1999), sampling of parameters from their assumed asymptotic distributions (Lee & Rendall, 2001), applying the delta method using normality assumptions (Lièvre, Brouard, & Heathcote, 2003), and Bayesian Gibbs sampling (Lynch & Brown, 2005) have each been used to enable construction of interval estimates of state expectancies. Here, we use the Gibbs sampling approach of Lynch and Brown (2005), which has recently been extended to handle state spaces with more than two living states (self-identifying reference omitted). We take a Bayesian approach in part because—unlike the other approaches except for bootstrapping—it does not rely on distributional assumptions to justify construction of interval estimates from point estimates of the parameter and its standard error, and such distributional assumptions become less tenable as state spaces and hence regression model dimensionality, become larger. Bootstrapping can also be problematic as state spaces increase in size, because it is unclear how to handle appropriately bootstrap samples in which transition data are lacking for some transitions.
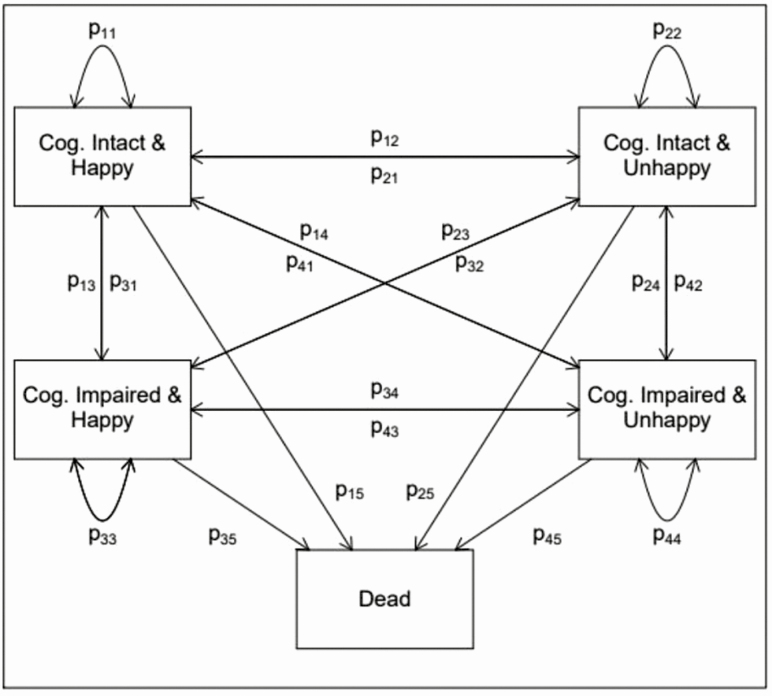
The state space we are interested in is shown in Figure 1. As the figure shows, there are four living states, including (1) cognitively intact and happy (CH), (2) cognitively impaired and happy (IH), (3) cognitively intact and unhappy (CU), and (4) cognitively impaired and unhappy (IU). Individuals may transition between any of these living states across time, they may remain in a state, or they may transition from any state to death, as represented by the arrows and transition probabilities shown in the figure. Thus, at each age, the state space can be represented by a 5 × 5 matrix of transition probabilities, where each row in the matrix represents the state a respondent is in at time t, and each column represents the state a respondent is in at time t + 2 (the time between HRS interview waves is 2 years). Because death is an absorbing state, an individual may experience one of the 20 possible transitions over a spell.
Figure 1.
State space used in multistate life table modeling. Transition probabilities, p, shown between (1) happy and cognitively intact, (2) happy and cognitively impaired, (3) unhappy and intact, (4) unhappy and impaired, and (5) dead states. The general state space is the same across all ages, although the transition probabilities are modeled as a function of age and covariates.
We model transitions between waves using a multinomial logit model with 19 outcomes reflecting all possible transitions, with remaining in the happy and cognitively intact state as the reference state, and with age and other covariates as predictors of transitions. We use Markov chain Monte Carlo (MCMC) methods to generate 1,000 samples from the posterior distribution of the model parameters. Unlike the usual Maximum Likelihood approach to estimation of this model, which yields a single point estimate for the effects of covariates and a point estimate of their standard errors, MCMC methods yield an entire distribution of model parameters, much like bootstrapping the data and estimating the model repeatedly yields an empirical sample from the sampling distribution of the parameters. Details of MCMC methods in general can be found elsewhere (e.g., Lynch, 2007).
Given a single collection of model parameter estimates/sampled values, transition probability matrices can be constructed by combining the parameter values with a set of covariate values (a covariate profile) to produce a set of predicted values, which can then be converted to probabilities via the usual approach of inverting the multinomial logit link function. Age-specific transition probability matrices are obtained by repeatedly computing the predicted scores, incrementing age across the age range in each computation.
With a complete set of age-specific transition probabilities, life table calculations can proceed via a typical method (e.g., see Palloni (2000) for general details on multistate life table computations; see Lynch and Brown (2005) for more details of the Bayesian approach; technical details for this adaptation of the Lynch/Brown method can be obtained from the authors). First, a “radix” population is established representing the proportion of the population in each state age 0 (here, age 65 years). Our radix, l0, is a 5 × 1 vector with the fifth element set to 0, indicating that no one starts deceased at age 65. The radix population is multiplied by the 5 × 5 transition probability matrix for the age 0 to age 2 spell, and a new vector, l2, is obtained, representing the number of individuals in each state at age 2. This process is repeated across the age range, up to age 100 to provide a collection of vectors representing the number of persons in each state at all ages. Years lived in each state are computed using the linear assumption (reflecting that all transitions between states occur uniformly across each age interval), and state expectancies at a given age are obtained by dividing the total number of years remaining to be lived in each state by the number of survivors to that age.
Repeating the generation of transition probability matrices and the multistate calculations for each of the 1,000 samples of model parameters obtained via MCMC sampling yields a distribution of state expectancies that can be sorted to produce empirical interval estimates by taking the 2.5th and 97.5th percentile values. This entire process can be repeated for different covariate profiles so that specific social and demographic groups may be statistically compared in terms of their relative expectancies.
Results
Descriptive Statistics
Table 1 shows descriptive statistics for the covariates, as well as for cognitive impairment prevalence, happiness prevalence, and mortality incidence by age. The mean age for the analytic person-spell sample was 75.90 years. The majority of person-spells were contributed by females (59%), white-non-Hispanics (74%), and persons with approximately 12 years of education. Approximately 32% of the total person-spells were contributed by respondents who had a cognitive impairment. However, cognitive impairment prevalence is shown to increase across later adulthood. For example, 18% of the person-spells observed among 65- to 69-year-olds, and 71% of the person-spells observed among those aged 90 years and older, were cognitively impaired spells. Approximately 88% of the total person-spells were reported to be lived with high levels of happiness, and this is true across age.
Table 1.
Descriptive Statistics, and Age-Specific Cognitive Impairment Prevalence, Happiness Prevalence, and Mortality Incidence: Health and Retirement Study 1998–2014 (Total Person Spells = 53,120)
| Variable | Mean | SD | Min | Max | |||
|---|---|---|---|---|---|---|---|
| Age | 75.90 | 7.73 | 65 | 110 | |||
| Cohort | 28.98 | 8.83 | −8 | 48 | |||
| Male | 0.41 | 0.49 | 0 | 1 | |||
| Black | 0.15 | 0.35 | 0 | 1 | |||
| Hispanic | 0.08 | 0.27 | 0 | 1 | |||
| Other | 0.03 | 0.18 | 0 | 1 | |||
| Education | 11.95 | 3.41 | 0 | 17 | |||
| Variable | N | 65–69 | 70–74 | 75–79 | 80–84 | 85–89 | 90 years plus |
| Impaired | 16,963 (31.93%) | 2604 (17.94%) | 3064 (23.45%) | 3268 (31.75%) | 3144 (42.78%) | 2786 (56.49%) | 2097 (70.77%) |
| Happy | 46,816 (88.13%) | 12,810 (88.23%) | 11,596 (88.76%) | 9107 (88.49%) | 6469 (88.01%) | 4288 (86.94%) | 2546 (85.93%) |
| Mortality | 5560 (46.47%) | 651 (9.21%) | 779 (11.04%) | 876 (14.22%) | 1063 (23.25%) | 1107 (36.30%) | 1084 (63.17%) |
Observed Transitions
Table 2 shows the observed transitions for moving in and out of cognitively intact/impaired and un/happy states. Three important features of this table are worth noting. First, all cells of the transition space are populated. Thus, there is indeed communication between cognitively intact/impaired and un/happy states: individuals return to the cognitively intact state from the cognitively impaired state, and individuals transition between happy and unhappy states. Second, a substantial number of deaths occur over the study period, with a total of 5,560 deaths (46.47% of the sample). Third, the most common experience is to remain cognitively intact and happy.
Table 2.
Observed Transitions Between Waves in Complete Health and Retirement Study 1998–2014 Person-Spell Data Set (Total Person Spells = 53,120)
| Time t + 2 → Time t ↓ |
CH | CU | IH | IU | D |
|---|---|---|---|---|---|
| CH | 23,981 (45.14%) |
1811 (3.41%) |
4551 (8.57%) |
496 (0.93%) |
1897 (3.57%) |
| CU | 1671 (3.15%) |
1180 (2.22%) |
400 (0.75) |
383 (0.72%) |
390 (0.73%) |
| IH | 2722 (5.12%) |
272 (0.51%) |
7358 (13.85%) |
711 (1.34%) |
2687 (5.06%) |
| IU | 263 (0.50%) |
220 (0.41%) |
695 (1.31%) |
846 (1.59%) |
586 (1.10%) |
| D | 0 | 0 | 0 | 0 | ALL |
Note: CH = cognitively intact-happy; CU = cognitively intact-unhappy; IH = impaired-happy; IU = impaired-unhappy; D = dead. Rows represent state at time t; columns represent state at time t + 2. t and t+2 represent the start and end points of each person spell, regardless of age (i.e., marginalized over age).
Multistate Life Tables
Table 3 shows results for total life expectancy (TLE) and expected years of life in each of the four intact/impaired-un/happy states at ages 65 and 85 years, respectively. In order to show results for an average person, covariates were set at mean values when generating the life tables. Note that total remaining years of life reflect the sum of remaining years expected in each of the four states (i.e., TLE = CH + CU + IH + IU). At age 65, one can expect to live an additional 17.82 (95% CI: 17.54, 18.10) years of life (CI denotes “Credible Interval,” which is the Bayesian version of the classical “Confidence Interval.” The interpretation differs in the Bayesian paradigm: the interpretation is that the parameter of interest falls within the interval with probability .95.), and TLE decreases to 2.52 years (95% CI: 2.33, 2.71) at age 85 years, on average.
Table 3.
Life Expectancy: Total and in Each of the Four States, With 95% CIs: Results of Multistate Life Tables Computed From Health and Retirement Study 1998–2014 Data. (Total Person Spells = 53,120)
| At age 65 years | At age 85 years | |
|---|---|---|
| Total life expectancy | 17.82 (17.54, 18.10) | 2.52 (2.33, 2.71) |
| Cognitively intact-happy | 11.30 (11.11, 11.49) | 0.89 (0.80, 0.96) |
| Cognitively intact-unhappy | 1.38 (1.31, 1.44) | 0.13 (0.11, 0.15) |
| Impaired-happy | 4.35 (4.19, 4.52) | 1.27 (1.15, 1.41) |
| Impaired-unhappy | 0.79 (0.73, 0.86) | 0.23 (0.19, 0.28) |
Note: Results from model with covariates set at mean values: cohort = 1930, education 12 years, male = 0.41, black = 0.15, Hispanic = 0.08, other = 0.03. Sum of life expectancy in states may not add to total expectation because of rounding.
Expected remaining total years of life were separated into intact/impaired-un/happy years. At age 65 years, approximately 11.30 (95% CI: 11.11, 11.49) remaining years of life are expected to be spent both cognitively intact and happy, 1.38 (95% CI: 11.11, 11.49) years are expected to be cognitively intact and unhappy, 0.79 (95% CI: 0.73, 0.86) years are expected to be cognitively impaired and unhappy, and 4.35 (95% CI: 4.19, 4.52) years are expected to be lived cognitively impaired but happy, on average. Further, by age 85 years, a substantial number of remaining years are expected to be spent cognitively impaired but happy (see Table 3). In fact, very few remaining years of cognitively impaired life are unhappy years, on average.
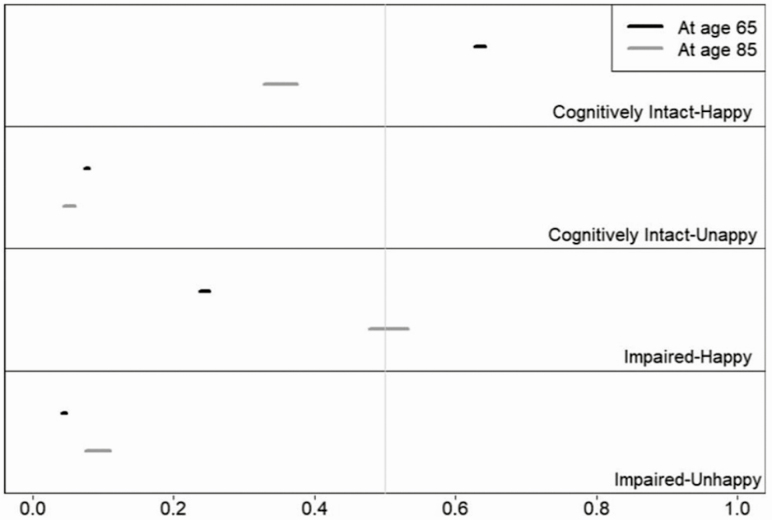
The proportion of remaining life individuals can expect to live in different states is another important summary (see Figure 2). At age 65 years, the greatest proportion of remaining life can be expected to be spent cognitively intact and happy (63% [95% CI: 0.63, 0.64]), followed by cognitively impaired but happy (24% [95% CI: 0.24, 0.25]), cognitively intact and unhappy (8% [95% CI: 0.07, 0.08]), and cognitively impaired and unhappy (4% [95% CI: 0.04, 0.05]). By age 85 years, individuals can expect to spend the largest remaining proportion of life cognitively impaired but happy (51% [95% CI: 0.48, 0.53]), followed by cognitively intact and happy (35% [95% CI: 0.33, 0.38]), cognitively impaired and unhappy (9% [95% CI: 0.07, 0.11]), and cognitively intact and unhappy (5% [95% CI: 0.04, 0.06]).
Figure 2.
Proportion of life expectancy in each of the four states, with 95% CIs: Results of multistate life tables computed from Health and Retirement Study 1998–2014 data (total person spells = 53,120). Results from model with covariates set at mean values: cohort = 1930, education 12 years, male = 0.41, black = 0.15, Hispanic = 0.08, other = 0.03. Sum of proportions in states may not add to 1 because of rounding. Vertical gray reference lines denote 0.50.
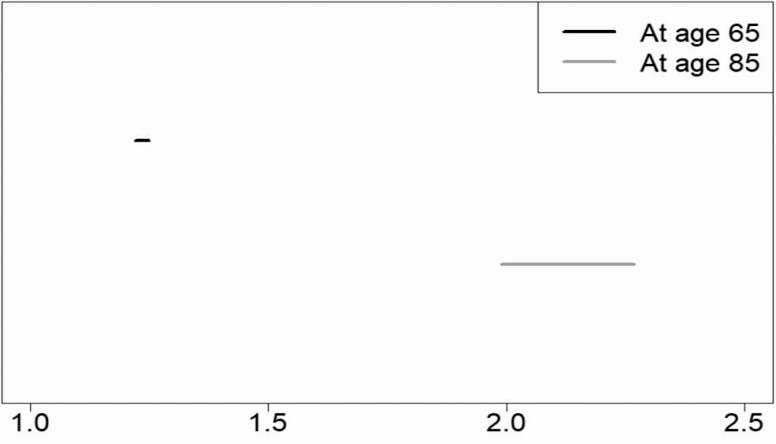
In order to see which is longer, happy life expectancy or cognitively intact life expectancy, we examined the ratio of happy years to cognitively intact years. Figure 3 shows that at age 65 years, happy life expectancy is 23% (95% CI: 1.22, 1.25) greater than cognitively intact life expectancy. Further, a clear age pattern is apparent: expected happy years increasingly outnumber cognitively intact years across later adulthood. For example, at age 85 years, happy life expectancy is 113% (95% CI: 1.99, 2.27) greater than cognitively intact life expectancy. In sum, cognitive impairment does not equate to unhappiness, and this is especially true in the latest years of life.
Figure 3.
Length of happy life expectancy versus cognitively intact life expectancy: 95% CIs for ratio of happy life expectancy to cognitively intact life expectancy: Results of multistate life tables computed from Health and Retirement Study 1998–2014 data (total person spells = 53,120). Results from model with covariates set at mean values: cohort = 1930, education 12 years, male = 0.41, black = 0.15, Hispanic = 0.08, other = 0.03. Sum of proportions in states may not add to 1 because of rounding.
Discussion
We set out to answer two basic questions regarding the intersection of cognitive impairment and subjectively assessed QoL among adults aged 65 years and older in the United States. First, cognitive vitality is a major component of the successful aging paradigm (Rowe & Kahn, 1997) and is commonly considered essential for QoL in old age (Gerstorf et al., 2015). Yet, it remained unknown whether lack of cognitive impairment is a necessary condition for happiness. Thus, we asked whether people can have a high QoL despite having a cognitive impairment. Our results suggest that the answer to this question is “yes.” Happiness and cognitive impairment were shown to coexist not only in the gross cross-tabulated data but also in the life tables. In fact, approximately a quarter of remaining life at age 65 years, and one-half at age 85, can be expected to be spent cognitively impaired but still happy.
Traditionally, research has examined QoL in terms of objective life circumstances or as a sum of ratings in different aspects of life (e.g., Quality of Life in Alzheimer’s Disease [QOL-AD] assessment tool) based on either self or proxy reports (see Stoner, Stansfeld, Orrell, & Spector, 2017). According to a recent meta-analysis, either no or only small associations were found with factors related to QoL (see Martyr et al., 2018). Nonetheless, supportive relationships, social engagement, everyday functioning, physical and mental health, and high-quality care were identified as the most critical aspects of life for those with a cognitive impairment (Martyr et al., 2018).
Although continued efforts to identify and improve critical aspects of life for those with a cognitive impairment are undoubtedly needed in view of population aging, this line of inquiry is conceptually distinct from that which focuses on self-assessments of overall QoL (i.e., SWB; happiness). Specifically, research from the subfield of positive psychology has clearly shown that traditional QoL measures and self-assessed happiness reflect unique constructs (Kahneman et al., 1999; Seligman & Csikszentmihalyi, 2000). Gerontological researchers have long recognized this conceptual distinction, and have played a central role in further highlighting the importance of examining happiness as a key marker of QoL (see George, 2010). Despite major contributions in happiness studies from scholars across a wide array of disciplines who are collectively concerned with aging-related social and psychological phenomena, relatively little was known about length of subjectively assessed QoL in terms of cognitive impairment.
Second, limited evidence has shown that declines in cognitive functioning have only a small or no effect on SWB (Allerhand, Gale, & Deary, 2014; Braun et al., 2017). These findings are congruent with an alternative orientation to the successful aging paradigm, which suggests that older adults become better equipped to deal with age-related losses in functioning as they age (Baltes & Baltes, 1990; see also Martin et al, 2015). In the same vein, successful aging also involves “optimizing life expectancy while at the same time minimizing physical, psychological, and social morbidity” (Fries, 1990). To these regards, extant research has shown that happy life expectancy exceeds active life expectancy, and this has been taken to suggest that QoL is good among the older population (even among those with a physical impairment; Yang, 2008).
However, length of QoL in terms of cognitive impairment was unknown. Thus, to address whether increased longevity has been accompanied by better quality of additional years of life we asked which is longer, happy life expectancy or cognitively intact life expectancy? We found that happy life expectancy is approximately 22%–25% longer than cognitively intact life expectancy at age 65 years, and by age 85, happy life expectancy is approximately twice than that of cognitively intact life expectancy. In sum, these findings align more closely with the selective optimization with compensation model of successful aging (Baltes & Baltes, 1990) relative to predominate models typically based on objective definitions of health and well-being (e.g., Rowe & Kahn, 1997).
This study has a few important limitations. First, cognitive functioning for proxies in this study was based on IADL information, whereas previous studies have used additional information (e.g., direct assessments of memory, and an interviewer assessment of difficulty completing the interview because of cognitive limitations) to supplement missing cognitive scores (see Crimmins et al., 2016). Although our cognitively intact life expectancy results are comparable to Crimmins and colleagues (2016), and our robustness analyses support the current imputation approach, these differences in how cognitive functioning for proxies was assessed should be considered when comparing the present results to previous studies.
Second, happiness assessments involve a cognitive process that requires one to be able to evaluate the overall quality of her/his life (see Schwarz & Strack, 1999). Thus, concern may be warranted regarding the ability of people with a cognitive impairment to report reliably on their own happiness. Here it is important to reemphasize that happiness information was available only for respondents who were capable of completing the HRS survey, and missing responses (often due to the use of a proxy because of cognitive impairment) were replaced with the proximally nearest response. However, sensitivity analyses (in which respondents with missing happiness information were considered “unhappy”) yielded comparable results. Furthermore, given that respondents with severe cognitive limitations were not asked the happiness question, impairment severity is unable to be further classified (e.g., a distinction between those with mild cognitive impairment versus those with dementia; see Schoeni, Freedman, & Langa, 2018). Still, whether people with a, perhaps even considerable, cognitive impairment can accurately assess their own happiness is unknown. In addition, although there is strong empirical evidence to suggest that the current happiness measure is relatively stable across time (see Arthaud-Day et al., 2005), this remains an open debate (see Lucas, 2007). Finally, QoL is an overarching construct, and happiness is only one (important) element of living well. Further research is needed to better understand how people with cognitive impairment experience other aspects of QoL (e.g., positive and negative affect).
Conclusions
The main goals of this study were to see whether lack of cognitive impairment is a necessary condition for happiness, and to determine whether the number of remaining years of life one can expect to live cognitively impaired outnumber the number of remaining years that she/he can expect to live with high levels of self-assessed happiness, on average. Our findings indicate that happiness and cognitive impairment are not closely linked, and that Americans aged 65 years and older can expect to live substantially more happy years of life than years spent cognitively impaired. Future research should focus on identifying the underlying mechanisms that promote stability in self-assessed happiness despite loss in cognitive functioning.
Funding
This work was supported by a pilot grant through Duke University’s Center for Population Health and Aging (CPHA), which is funded by the National Institutes of Health (NIH; 2P30-AG034424).
Acknowledgments
Thank you to the anonymous reviewers who helped us to improve this manuscript. All data, analytic methods, and study materials will be provided by the corresponding author upon request. This study was not preregistered.
References
- Allerhand M., Gale C. R., & Deary I. J (2014). The dynamic relationship between cognitive function and positive well-being in older people: A prospective study using the English Longitudinal Study of Aging. Psychology and Aging, 29, 306–318. doi:10.1037/a0036551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthaud-Day M. L., Rode J. C., Mooney C. H., & Near J. P (2005). The subjective well-being construct: A test of its convergent, discriminant, and factorial validity. Social Indicators Research, 74, 445–476. doi:10.1007/s11205-004-8209-6 [Google Scholar]
- Baltes P. B., & Baltes M. M (1990). Psychological perspectives on successful aging: The model of selective optimization with compensation. In Baltes P. B. & Baltes M. M. (Eds.), Successful aging: Perspectives from the behavioral sciences (Vol. 1, pp. 1–34). United Kingdom: Cambridge University Press. [Google Scholar]
- Bardo A. R. (2017). A life course model for a domains-of-life approach to happiness: Evidence from the United States. Advances in Life Course Research, 33, 11–22. doi:10.1016/j.alcr.2017.06.002 [Google Scholar]
- Bardo A. R., Lynch S. M., & Land K. C (2017). The importance of the baby boom cohort and the great recession in understanding age, period, and cohort patterns in happiness. Social Psychological and Personality Science, 8, 341–350. doi:10.1177/1948550616673874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun T., Schmukle S. C., & Kunzmann U (2017). Stability and change in subjective well-being: The role of performance-based and self-rated cognition. Psychology and Aging, 32, 105–117. doi:10.1037/pag0000153 [DOI] [PubMed] [Google Scholar]
- Cantu P. A., Hayward M. D., Hummer R. A., & Chiu C. T (2013). New estimates of racial/ethnic differences in life expectancy with chronic morbidity and functional loss: Evidence from the National Health Interview Survey. Journal of Cross-Cultural Gerontology, 28, 283–297. doi:10.1007/s10823-013-9206-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins E. M., Saito Y., & Kim J. K (2016). Change in cognitively healthy and cognitively impaired life expectancy in the United States: 2000-2010. SSM Population Health, 2, 793–797. doi:10.1016/j.ssmph.2016.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daviglus M. L., Bell C. C., Berrenttini W., Bowen P. E., Connolly E. S. Jr, Cox N. J., . . . Trevisan M (2010). National Institutes of Health state-of-the-Science conference statement: Preventing Alzheimer disease and cognitive decline. Annals of Internal Medicine, 153, 176–181. doi:10.7326/0003-4819-153-3-201008030-00260 [DOI] [PubMed] [Google Scholar]
- Freedman V. A., Carr D., Cornman J. C., & Lucas R. E (2017). Impairment severity and evaluative and experienced well-being among older adults: Assessing the role of daily activities. Innovation in Aging, 1, igx010. doi:10.1093/geroni/igx010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman V. A., & Spillman B. C (2016). Active life expectancy in the older US population, 1982-2011: Differences between blacks and whites persisted. Health Affairs (Project Hope), 35, 1351–1358. doi:10.1377/hlthaff.2015.1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries J. F. (1990). Medical perspectives upon successful aging. In Baltes P. B., & Baltes M. M. (Eds.), Successful aging: Perspectives from the behavioral sciences (pp. 35–49). United Kingdom: Cambridge University Press. [Google Scholar]
- Garcia M. A., Downer B., Chiu C.-T., Saenz J. L., Rote S., & Wong R (2017). Racial/ethnic and nativity differences in cognitive life expectancies among older adults in the United States. The Gerontologist, 59, 281–289. doi:10.1093/geront/gnx142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George L. K. (2010). Still happy after all these years: Research frontiers on subjective well-being in later life. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 65B, 331–339. doi:10.1093/geronb/gbq006 [DOI] [PubMed] [Google Scholar]
- Gerstorf D., Hülür G., Drewelies J., Eibich P., Duezel S., Demuth I., … Lindenberger U (2015). Secular changes in late-life cognition and well-being: Towards a long bright future with a short brisk ending? Psychology and Aging, 30, 301–310. doi:10.1037/pag0000016 [DOI] [PubMed] [Google Scholar]
- Hayward M. D., Rendall M., & Crimmins E (1999). Evaluating group differences in healthy life expectancy: The estimation of confidence intervals for multistate life table expectancies. Paper presented at the annual meeting of the Gerontological Society of America, San Francisco, CA.
- Health and Retirement Study (2014). RAND HRS longitudinal file 2014 (v2) public use dataset. Ann Arbor, MI:University of Michigan. [Google Scholar]
- Kahneman D., Diener E., & Schwarz N. (Eds.). (1999). Well-being: Foundations of hedonic psychology. New York, NY: Russell Sage Foundation. [Google Scholar]
- Langa K. M., Plassman B. L., Wallace R. B., Herzog A. R., Heeringa S. G., Ofstedal M. B., … Willis R. J (2005). The aging, demographics, and memory study: Study design and methods. Neuroepidemiology, 25, 181–191. doi:10.1159/000087448 [DOI] [PubMed] [Google Scholar]
- Layard R. (2010). Economics. Measuring subjective well-being. Science (New York, N.Y.), 327, 534–535. doi:10.1126/science.1186315 [DOI] [PubMed] [Google Scholar]
- Lee M. A., & Rendall M. S (2001). Self-employment disadvantage in the working lives of blacks and females. Population Research and Policy Review, 20, 291–320. doi: 10.1023/A:101188701 [Google Scholar]
- Lièvre A., Brouard N., & Heathcote C (2003). The estimation of health expectancies from cross-longitudinal surveys. Mathematical Population Studies, 10, 211–248. doi:10.1080/713644739 [Google Scholar]
- Lucas R. (2007). Adaptation and the set-point model of subjective well-being: Does happiness change after major life events? Current Directions in Psychological Science, 16, 75–79. doi:10.1111/j.1467-8721.2007.00479.x [Google Scholar]
- Lynch S. M. (2007). Introduction to applied Bayesian statistics and estimation for social scientists. Springer Science & Business Media. [Google Scholar]
- Lynch S. M., & Brown J. S (2005). A new approach to estimating life tables with covariates and constructing interval estimates of life table quantities. Sociological Methodology, 35, 177–225. doi: 10.1111/j.0081-1750.2006.00168.x [Google Scholar]
- Lynch S. M., & George L. K (2002). Interlocking trajectories of loss-related events and depressive symptoms among elders. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 57, S117–S125. doi:10.1093/geronb/57.2.s117 [DOI] [PubMed] [Google Scholar]
- Martin P., Kelly N., Kahana B., Kahana E., Willcox B. J., Willcox D. C., & Poon L. W (2015). Defining successful aging: A tangible or elusive concept? The Gerontologist, 55, 14–25. doi:10.1093/geront/gnu044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martyr A., Nelis S. M., Quinn C., Wu Y. T., Lamont R. A., Henderson C., … Clare L (2018). Living well with dementia: A systematic review and correlational meta-analysis of factors associated with quality of life, well-being and life satisfaction in people with dementia. Psychological Medicine, 48, 2130–2139. doi:10.1017/S0033291718000405 [DOI] [PubMed] [Google Scholar]
- Palloni A. (2000). Increment-decrement lifetables. In Preston S. H., Heuveline P., & Guillot M. (Eds.), Demography: Measuring and modeling population processes (pp. 256–272). Malden, MA: Blackwell. [Google Scholar]
- Plassman B. L., Langa K. M., Fisher G. G., Heeringa S. G., Weir D. R., Ofstedal M. B., … Wallace R. B (2008). Prevalence of cognitive impairment without dementia in the United States. Annals of Internal Medicine, 148, 427–434. doi:10.7326/0003-4819-148-6-200803180-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAND Center for the Study of Aging (2018). RAND HRS Longitudinal File 2014 (v2). Santa Monica, CA: RAND Corporation. [Google Scholar]
- Robine J.-M., Jagger C., Mathers C. D., Crimins E. M., & Suzman R. M (2003). Determining health expectancies. West Sussex, England: Wiley. [Google Scholar]
- Robine J. M., & Ritchie K (1991). Healthy life expectancy: Evaluation of global indicator of change in population health. BMJ (Clinical research ed.), 302, 457–460. doi:10.1136/bmj.302.6774.457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe J. W. & Kahn R. L (1997). Successful aging. The Gerontologist, 37, 433–440. doi:10.1093/geront/37.4.433 [DOI] [PubMed] [Google Scholar]
- Schoeni R. F., Freedman V. A., & Langa K. M (2018). Introduction to a supplement on population level trends in dementia: Causes, disparities, and projections. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 73, S1–S9. doi:10.1093/geronb/gby007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz N., & Strack F (1999). Reports of subjective well-Being: Judgmental processes and their methodological implications. In Strack F., Argyle M., & Schwarz N. (Eds.), Subjective well-being: An interdisciplinary perspective (pp. 27–47). New York, NY: Oxford Press. [Google Scholar]
- Seligman M. E., & Csikszentmihalyi M (2000). Positive psychology. An introduction. The American Psychologist, 55, 5–14. doi:10.1007/978-94-017-9088-8_18 [DOI] [PubMed] [Google Scholar]
- Stiefel M. C., Perla R. J., & Zell B. L (2010). A healthy bottom line: Healthy life expectancy as an outcome measure for health improvement efforts. The Milbank Quarterly, 88, 30–53. doi:10.1111/j.1468-0009.2010.00588.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoner C. R., Stansfeld J., Orrell M., & Spector A (2017). The development of positive psychology outcome measures and their uses in dementia research: A systematic review. Dementia, 1–22. doi:10.1177/1471301217740288 [DOI] [PubMed] [Google Scholar]
- WHO (1997). The world health report 1997. Conquering suffering: Enriching humanity. Geneva, Switzerland: World Health Organization. [PubMed] [Google Scholar]
- Yang Y. (2008). Long and happy living: Trends and patterns of happy life expectancy in the U.S., 1970-2000. Social Science Research, 37, 1235–1252. doi:10.1016/j.ssresearch.2007.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]