Abstract
Background
Symptomatic epilepsy is a common symptom of glioblastoma, which may occur in different stages of disease. There are discrepant reports on association between early seizures and glioblastoma survival, even less is known about the background of these seizures. We aimed at analyzing the risk factors and clinical impact of perioperative seizures in glioblastoma.
Methods
All consecutive cases with de-novo glioblastoma treated at our institution between 01/2006 and 12/2018 were eligible for this study. Perioperative seizures were stratified into seizures at onset (SAO) and early postoperative seizures (EPS, ≤21days after surgery). Associations between patients characteristics and overall survival (OS) with SAO and EPS were addressed.
Results
In the final cohort (n = 867), SAO and EPS occurred in 236 (27.2%) and 67 (7.7%) patients, respectively. SAO were independently predicted by younger age (P = .009), higher KPS score (P = .002), tumor location (parietal lobe, P = .001), GFAP expression (≥35%, P = .045), and serum chloride at admission (>102 mmol/L, P = .004). In turn, EPS were independently associated with tumor location (frontal or temporal lobe, P = .013) and pathologic laboratory values at admission (hemoglobin < 12 g/dL, [P = .044], CRP > 1.0 mg/dL [P = 0.036], and GGT > 55 U/L [P = 0.025]). Finally, SAO were associated with gross-total resection (P = .006) and longer OS (P = .030), whereas EPS were related to incomplete resection (P = .005) and poorer OS (P = .009).
Conclusions
In glioblastoma patients, SAO and EPS seem to have quite different triggers and contrary impact on treatment success and OS. The clinical characteristics of SAO and EPS patients might contribute to the observed survival differences.
Keywords: epilepsy, glioblastoma, predictor, survival
Key Points.
Perioperative epilepsy independently predicts glioblastoma survival.
Seizures at onset are associated with longer survival after glioblastoma surgery.
In contrast, early postoperative seizures are related to poor outcome.
Importance of the Study.
To date, there is a large discrepancy regarding the rate, risk factors, and predictive value of perioperative epileptic seizures in patients with glioblastoma. In this large consecutive glioblastoma cohort, we evaluated over 100 patient/tumor-specific variables with regard to the association with seizures at onset (SAO) and early postoperative seizures (EPS). SAO were independently associated with younger age, better preoperative clinical performance, higher GFAP expression, and higher serum chloride levels. In addition, SAO were related to more radical extent of resection and longer overall survival. In contrast, EPS were strongly associated with presence of systemic disorders like anemia, infection, and liver dysfunction as well as incomplete tumor resection and poorer overall survival. Our results encourage further analysis of the effect of perioperative seizures on glioblastoma survival.
Symptomatic seizures are common in primary and secondary brain tumors.1 In case of glioblastoma, 1 of 4 individuals shows seizures at onset (SAO) of disease as first clinical symptom.2 In addition, glioblastoma patients frequently develop secondary epilepsy due to rapid tumor progression.3
Several previous reports have addressed the association between SAO occurrence and glioblastoma survival, both with positive2,4–8 and negative9–17 results. The majority of recent studies has specifically focused on the role of antiepileptic drugs (AED) on the survival effect of SAO.6,12,18–24 However, a recent large pooled analysis of prospective clinical trials25 has failed to show any survival benefit from the use of AED in glioblastoma.
In this context, the knowledge of SAO predictors might be essential for a better understanding of these early glioblastoma-associated seizures and possible links with patients survival. Unfortunately, evidence on SAO predictors in glioblastoma is sparse, since the majority of SAO-related studies is based on either heterogenous (high and low grade) glioma cohorts or small glioblastoma populations.6,14,26,27 Even less is known on the rate, risk factors and clinical impact of early postoperative seizures (EPS), which are not related to tumor progression and therefore have unclear pathophysiologic background.
Accordingly, this study analyzed the risk factors for SAO and the relationship of SAO with overall survival (OS) in a large consecutive glioblastoma cohort. Moreover, we addressed the predictors and the clinical impact of EPS after glioblastoma surgery, as well as the similarities and differences between SAO and EPS.
Materials and Methods
Patient Population and Clinical Management
All consecutive cases with newly diagnosed glioblastoma treated between January 2006 and December 2018 in our institution were eligible for this study. The exclusion criteria were the following: (a) pediatric cases (<18 years old), (b) previous history of epilepsy, and (c) extracranial location. The retrospective study was conducted in accordance with the STROBE guidelines and was approved by the Institutional Ethics Committee, University of Duisburg-Essen (15-6504-BO).
All cases were histologically confirmed via stereotactic biopsy or tumor resection. Early postoperative MRI within 72 h after surgery was performed after tumor resection. Standard chemoradiation with concomitant and adjuvant temozolomide28 was initiated after surgery. Patients with poor perioperative neurological condition and/or without willingness for further treatment, were referred to best supportive care.
According to the current guidelines,29 AED treatment was usually initiated after the first seizure event. Prophylactic use of AED was not routinely performed in the cohort, except for selected cases.
Data Management
The following patients characteristics were collected from the electronic medical records: demographic (age and sex) and anthropometric parameters (body height, weight, and body mass index), medical history (arterial hypertension, diabetes mellitus, history of cancer, and hypothyroidism), KPS score at admission, tumor location, extent of resection (EOR), immunohistochemical and molecular-genetic parameters (expression of glial fibrillary acidic protein [GFAP], p53 and Ki-67 proliferation index, the isocitrate dehydrogenase 1 gene [IDH1] mutation, and O6-methylguanine DNA methyltransferase [MGMT] promoter methylation status). In addition, 27 routine laboratory measurements at admission and 3 surrogate markers were assessed (see the Supplementary Table S1 with the full list of over 100 variables correlated with the study endpoints). Finally, overall survival or date of the last outpatient follow-up was assessed.
The evaluation of histological specimens was based on the original reports of 2 clinical neuropathologists, as described previously.30 All histological and molecular findings were reviewed in accordance with the 2016 Classification of the Central Nervous System Tumors of the World Health Organization.31 Tumor location was assessed upon the review of the preoperative MRI imaging. In addition, postoperative MRI scans were analyzed with regard to EOR. The cases without contrast-enhancing residual after tumor resection were considered gross-total resection (GTR), the remaining cases were regarded as tumor debulking. All available preoperative and postoperative MRI scans were reviewed by the first author (Y.A.) blinded at this time for any clinical information.
The diagnosis of epilepsy was based on the occurrence of clinical symptoms suspicious for seizures (involuntary movements, abnormal sensory signs, or an altered mental status). Additionally, patients underwent an electroencephalogram in case of questionable/nonconclusive clinical symptoms. All patients with symptomatic epilepsy were consulted by in-house epileptologists, for diagnosis confirmation, assessment of seizure semiology, and medical treatment. Epileptic seizures leading to the radiographic diagnosis of glioblastoma were regarded as SAO. Postoperative epileptic seizures occurring up to 3 weeks after surgery and prior to the begin of chemoradiation were regarded as EPS. When available, semiology (secondary generalized, simple or complex focal, or status epilepticus) and timing (in relation to the surgery day) of the seizures were documented. The hospital records were also reviewed for AED treatment.
Study Endpoints and Statistical Analysis
The following primary endpoints were addressed in this study: (a) independent predictors of SAO and (b) EPS and (c) association between the SAO/EPS and OS. The secondary endpoint of the study was the evaluation of the patients’ characteristics related to the seizure semiology.
Statistical analyses were performed with the help of PRISM (version 5.0, GraphPad Software Inc.) and SPSS (version 25, SPSS Inc., IBM). Patients’ baseline characteristics were expressed as mean ± standard deviation (SD) or percentage of patients, as appropriate. For OS data, median values with interquartile range were reported. Differences with a P ≤ .05 were regarded as statistically significant.
First, all associations between the potential risk factors and the study endpoints were tested using univariate analysis. For univariate correlations, differences between continuous variables were analyzed using the Student’ t-test for normally distributed data and the Mann–Whitney U test for non-normal distributed data; associations between categorical variables were analyzed using the chi-square or Fisher’s exact tests, as appropriate.
Laboratory measurements were evaluated as continuous variables and in dichotomized manner, according to the common reference values for upper and lower ranges. For laboratory tests showing significant associations with the endpoints only as continuous variables, an additional dichotomization was performed upon the cutoffs defined on the receiver operating characteristic (ROC) curve. For immunohistochemical tumor characteristics, the dichotomization was applied using the cutoffs reported previously in the literature and/or the results from the ROC curves.
The significant correlations from univariate analyses were then evaluated in a multivariate analysis. Binary logistic regression analysis was used for the identification of independent predictors of SAO/EPS, as well as for their relation to EOR. Laboratory markers were first tested in a separate multivariate assessment prior to the inclusion in the final regression analysis. For correlation between SAO/EPS and OS, a Cox proportional-hazards model was applied by adding the common outcome confounders (age, KPS, EOR, molecular markers, and adjuvant treatment). Missing data were replaced using multiple imputation.
Data Availability Statement
Any data not published within the article will be shared in anonymized manner by request from any qualified investigator.
Results
Patient Population
After the exclusion of noneligible cases (age < 18 years, n = 7; history of epilepsy, n = 6; and spinal glioblastoma, n = 1), 867 individuals were included in the final analysis. The baseline characteristics of the cohort are presented in Table 1.
Table 1.
Baseline Characteristics of 867 Glioblastoma Patients Included in the Final Analysis
| Parameter | N (%) or Mean (±SD) |
|---|---|
| Age, years | 63.83 (±11.51) |
| Sex, male | 501 (57.8%) |
| Medical history | |
| Arterial hypertension | 450 (51.9%) |
| Diabetes mellitus | 150 (17.3%) |
| Hypothyroidism | 104 (12.0%) |
| History of cancer | 127 (14.6%) |
| KPS at admissiona | |
| Good (90–100%) | 338 (39.7%) |
| Reduced (70–80%) | 414 (48.6%) |
| Poor (≤60%) | 100 (11.7%) |
| EOR, biopsy | 247 (28.5%) |
| Tumor location | |
| Frontal lobe | 305 (35.2%) |
| Temporal lobe | 239 (27.6%) |
| Parietal lobe | 103 (11.9%) |
| Occipital lobe | 129 (14.9%) |
| Midline/Infratentorial/Bi-hemispheric | 91 (10.5%) |
| Molecular status | |
| MGMT methylation | 310 (41.6%b) |
| IDH1 mutation | 17 (3.1%c) |
EOR, extent of resection; IDH1-Isocitrate dehydrogenase 1 mutation; MGMT, O[6]-methylguanine-DNA methyltransferase promoter methylation; N, number of cases; SD, standard deviation.
a15 cases (1.7%) with missing KPS score.
b121 cases (14%) with missing MGMT promoter methylation status.
c311 cases (35.9%) with missing IDH1 mutation status.
Perioperative Seizures: Occurrence and Management
The rates of SAO and EPS in the cohort were 27.2% (n = 236) and 7.7% (n = 67), respectively. Due to initially minor radiographic findings, a watch-and-wait strategy was applied in 14 SAO patients, resulting in late surgery between 2 and 11 months after seizure onset (mean interval: 5.71 months). In the remaining cases, all patients (with or without SAO) were operated within 2 weeks after the radiographic diagnosis. EPS were documented on the mean postoperative day 4.6 (±5.0). Twenty-three glioblastoma patients developed >1 separate seizure event during the perioperative course. Of them, 10 patients showed SAO and EPS.
AED treatment was initiated after the occurrence of first seizure(s) in all cases except for 13 individuals with SAO. In addition, 13 patients received prophylactic AED treatment without preceding seizures. There was a wide range of AED used in the cohort, with levetiracetam and valproic acid as the most common drugs.
Predictors of Perioperative Seizures
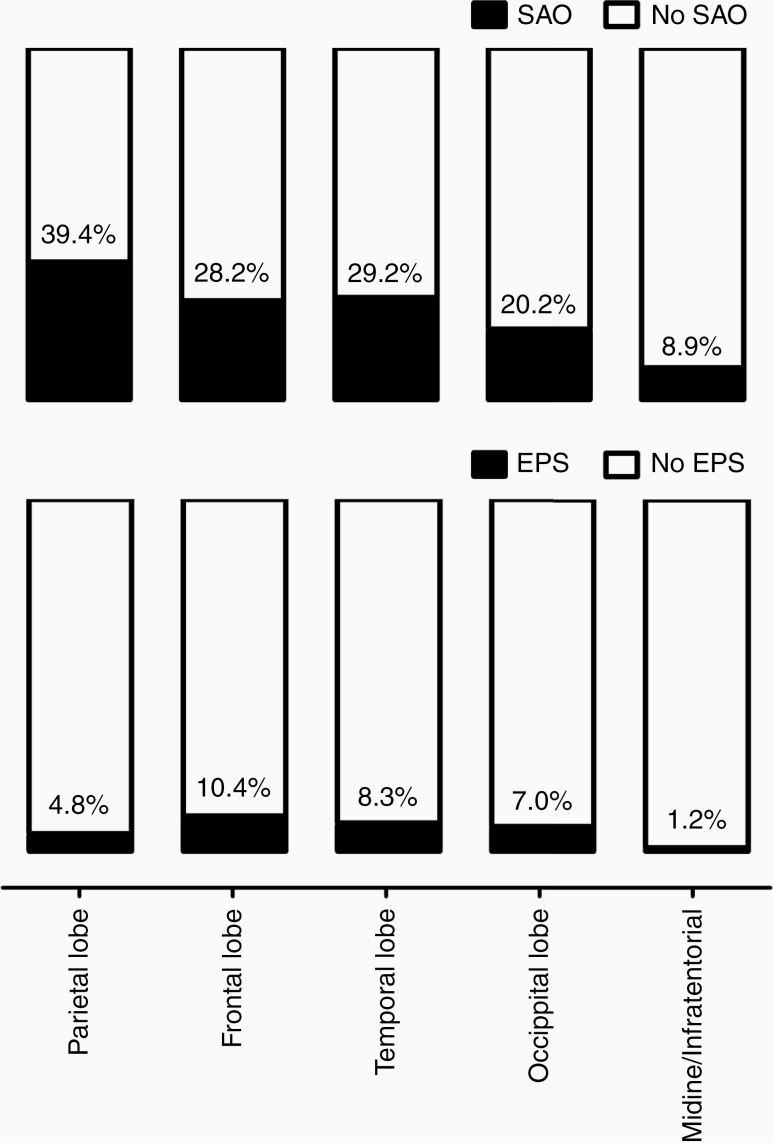
SAO and EPS showed partially contrary correlations with the baseline characteristics (see Table 2 for the univariate analyses of the results reaching the significance level for at least one study endpoint, for the full list of all associations see the Supplementary Table S1). In particular, SAO were more common in individuals with younger age (P = .001) and higher preoperative KPS score (P < .0001). The tumors located in the parietal lobe (odds ratio [OR]: 1.96, P = .003, see Figure 1 for the distribution of SAO/EPS rates in different brain areas), those with higher GFAP expression (≥35%, OR: 1.88, P = .05) and more radical EOR (tumor resection vs biopsy [OR: 1.87, P = .001] and GTR vs debulking [OR: 1.64, P = .006]) were also associated with SAO. In turn, EPS were related to comorbidities (arterial hypertension [OR: 1.74, P = .040] and history of cancer [OR: 1.87, P = .049]) and tumors located in the frontal or temporal lobe (OR: 2.07, P = .017). Moreover, there was an inverse association between the EOR and occurrence of EPS in the individuals undergoing tumor resection (GTR vs debulking, OR: 0.42, P = .005). Finally, certain admission laboratory variables were also associated with the SAO and EPS. Of note, pathologic laboratory values were more characteristic for EPS than for SAO.
Table 2.
Shortened List of Univariate Assessments Between the Patients’ Characteristics and the Occurrence of SAO and EPS (for the full list with over 100 tested variables—see the Supplementary Table S1)
| Parameter | SAO | EPS | ||
|---|---|---|---|---|
| OR (95% CI)/mean (±SD) | P-value | OR (95% CI)/mean (±SD) | P-value | |
| Age | 61.24 (±12.16) vs 64.21 (±12.42) | .001 | 63.62 (±13.49) vs 63.39 (±12.32) | .677 |
| Arterial hypertension | 0.94 (0.69–1.27) | .695 | 1.74 (1.02–2.96) | .040 |
| History of cancer | 0.72 (0.46–1.14) | .193 | 1.87 (1.03–3.40) | .049 |
| Location: parietal lobe | 1.96 (1.28–3.01) | .003 | 0.57 (0.22–1.44) | .327 |
| Location: frontal/temporal lobe | 1.28 (0.93–1.76) | .149 | 2.07 (1.15–3.75) | .017 |
| EOR: Surgery vs Biopsy | 1.87 (1.30–2.68) | .001 | 1.72 (0.92–3.21) | .092 |
| EOR: GTR vs Debulking | 1.64 (1.15–2.35) | .006 | 0.42 (0.23–0.76) | .005 |
| GFAP staining. ≥ 35% | 1.88 (1.02–3.47) | .053 | 0.91 (0.37–2.28) | .812 |
| KPS % continuous | 83.16 (±12.19) vs 78.67 (±13.18) | <.0001 | 78.09 (±13.74) vs 80.09 (±12.99) | .304 |
| Erythrocyte < 4.0/pL | 2.12 (0.99–4.55) | .079 | 4.28 (1.74–10.48) | .004 |
| WBC > 11/nL | 0.40 (0.28–0.55) | <.0001 | 1.09 (0.66–1.82) | .796 |
| Hemoglobin < 12 g/dL | 1.43 (0.75–2.72) | .285 | 3.34 (1.53–7.29) | .005 |
| Hematocrit < 35% | 1.44 (0.70–2.94) | .332 | 3.16 (1.33–7.55) | .015 |
| PLT > 400/nL | 0.32 (0.11–0.90) | .022 | 0.32 (0.04–2.34) | .352 |
| Sodium < 140 mmol/L | 0.67 (0.49–0.94) | .019 | 1.17 (0.70–1.97) | .591 |
| Chloride > 102 mmol/L | 1.72 (1.22–2.42) | .002 | 0.84 (0.50–1.43) | .581 |
| Calcium > 2.35 mmol/L | 1.75 (1.24–2.46) | .002 | 0.49 (0.26–0.95) | .039 |
| Urea > 20 mg/dL | 0.53 (0.39–0.73) | <.0001 | 1.62 (0.97–2.71) | .068 |
| GGT > 55 U/L | 0.99 (0.67–1.47) | 1.000 | 2.19 (1.26–3.81) | .008 |
| Total protein <7.0 g/dL | 0.67 (0.48–0.92) | .014 | 1.83 (1.05–3.18) | .033 |
| CRP >=1.0 mg/dL | 0.96 (0.58–1.59) | .898 | 3.00 (1.39–6.51) | .006 |
| PLT/WBC > 25 | 1.64 (1.20–2.24) | .002 | 0.82 (0.50–1.38) | .519 |
| WBC/MPV < 0.9 | 2.69 (1.87–3.88) | <.0001 | 1.12 (0.63–2.00) | .768 |
CRP, C-reactive protein; EOR, extent of resection; GFAP, glial fibrillary acidic protein immunohistochemistry staining percentage; GGT, gamma-glutamyl-transferase; GTR, gross total resection; MPV, mean platelet volume; OR, odds ratio; PLT, blood platelets; SD, standard deviation; WBC, white blood cells. Differences with a P ≤ .05 were regarded as statistically significant.
Figure 1.
Incidence of SAO and EPS depending on tumor location
In the multivariate analysis (see Table 3 and Supplementary Table S2), SAO were independently associated with younger age (P = .009), higher KPS (P = .002), tumor location (parietal lobe, P = .001), higher GFAP expression (≥35%, P = .045), and serum chloride levels (>102 mmol/L, P = .004). In turn, EPS were independently associated with tumor location (frontal or temporal lobe, P = .013) and the following admission laboratory parameters: increased c-reactive protein (>1.0 mg/dL, P = .036) and Gamma-Glutamyl-Transferase levels (>55 U/L, P = .025), and presence of anemia (hemoglobin < 12 g/dL, P = .044/red blood cells count < 4/nL, P = .041, see also Supplementary Table S3 for the appropriate multivariate analysis).
Table 3.
Multivariate Analysis of Independent Predictors of SAO and EPS
| Parameters | aOR (95% CI) | P-value |
|---|---|---|
| Predictors of SAO | ||
| Location: parietal lobe | 2.13 (1.37–3.33) | .001 |
| Age, years | 0.98 (0.97–0.99) | .009 |
| KPS score, % | 1.02 (1.01–1.04) | .002 |
| IHC: GFAP ≥35% | 2.07 (1.02–4.20) | .045 |
| Blood test: Chloride >102 U/L | 1.68 (1.18–2.39) | .004 |
| Blood test: Calcium >2.35 mmol/L | 1.29 (0.87–1.93) | .206 |
| Predictors of EPS | ||
| Location: frontal/temporal lobe | 2.34 (1.20–4.56) | .013 |
| Comorbidity: Arterial hypertension | 1.52 (0.86–2.68) | .154 |
| Comorbidity: Second malignancy | 1.62 (0.85–3.07) | .144 |
| Blood test: Hemoglobin <12 g/dL | 2.32 (1.02–5.24) | .044 |
| Blood test: GGT >55 U/L | 1.94 (1.09–3.47) | .025 |
| Blood test: CRP >1.0 mg/dL | 2.45 (1.07–5.58) | .036 |
aOR, adjusted odds ratio; CRP, C-reactive protein; IHC, immunohistochemistry; GFAP, glial fibrillary acidic protein staining percentage; GGT, gamma-glutamyl-transferase. Differences with a P ≤ .05 were regarded as statistically significant.
Impact of Perioperative Seizures on OS
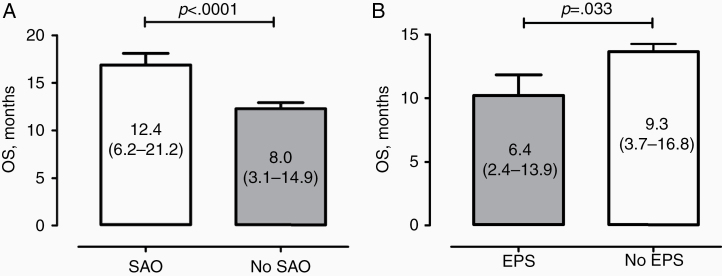
Correlation between perioperative seizures and patients’ survival showed contrary effects of SAO and EPS. In particular, OS was significantly longer in individuals with SAO than without (12.4 vs 8.0 months, P < .0001, Figure 2A). In contrast, patients with EPS had poorer outcome, as compared to the counterparts without EPS (6.4 vs 9.3 months, P = .033, Figure 2B). Of note, glioblastoma individuals with SAO without surgery delay showed a trend to longer OS than the above-mentioned 14 cases with delayed surgery after SAO (13.0 vs 7.7 months, P = .1073).
Figure 2.
OS (median values including the interquartile range in parentheses) of the patients in the cohort depending on the occurrence of SAO (A) or EPS (B) in the perioperative course.
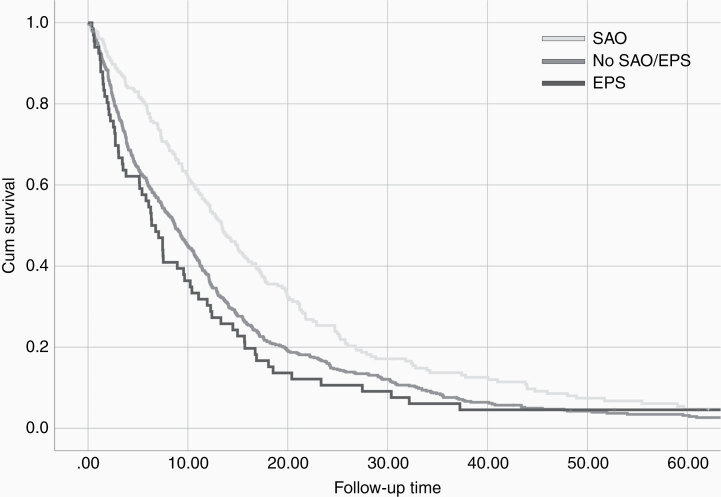
The multivariate analysis for independent OS predictors (adjusted for patients’ age, KPS, EOR, MGMT/IDH1 status, and postoperative chemoradiation, see Supplementary Table S4) confirmed the positive impact of SAO (adjusted hazard ratio [aHR]: 0.82, 95% confidence interval [CI]: 0.69–0.98, P = .03) and negative impact of EPS (aHR: 1.41, 95% CI: 1.09–1.83, P = .009) on the OS. Kaplan–Meier Survival plot (Figure 3) shows different survival curves in the cohort depending on the presence or absence of SAO and EPS.
Figure 3.
Kaplan–Meier survival plot showing the survival differences between the patients with SAO, EPS, and no perioperative seizures (no SAO and no EPS).
Seizure Semiology
Of the cases with documented seizure type (n = 262/293), patients developed more frequently secondary generalized seizures (n = 134, 51.1%) than simple (n = 114, 43.5%) or complex focal (n = 13, 5%) convulsions. One patient (0.4%) presented with epileptic status at disease onset. Individuals with secondary generalized seizures were significantly younger, than the patients with other seizure types (59.57 [±11.55] vs 63.74 [±11.38] years, P = .003). Remaining patients’ characteristics showed no significant associations with the seizure semiology. Finally, there was a trend toward a longer OS in case of secondary generalized seizures (12.3 vs 10.5 months, P = .074).
Discussion
The risk factors for perioperative seizures in glioblastoma remain poorly understood. Moreover, the clinical impact of SAO and EPS is still a matter of debate. In this large consecutive series of glioblastoma patients, we have identified different risk patterns for the occurrence of SAO and EPS. In addition, there was a contrary effect of SAO and EPS on OS.
Epileptogenesis of SAO
The studies on the predictors of SAO are mostly based on heterogenous glioma cohorts and presume higher risk of SAO (and of symptomatic seizures in general) in low-grade glioma, partially due to the survival differences compared with high-grade glioma.1,32,33 Recent reports pointed to the crucial role of IDH1 mutation in tumor epileptogenesis.14,26,34,35 Other molecular-genetic and immunohistochemical tumor markers like 1p19q-codeletion, MGMT promoter methylation, BDNF, ADK, BRAF V600E mutations, MMP-9, expressions of miR-128, nuclear protein Ki-67, p53, RINT1, and VLGR, EGFR amplification, and PI3K-mTOR pathway were also addressed, and partially linked with the risk of symptomatic epilepsy in glioma patients.1,3,32,36,37
As to the studies based on glioblastoma cohorts, younger age,14,34 certain tumor locations,13,14 expression of p5334 and glutamine synthetase,10,15 higher preoperative KPS score,14 smaller volume of tumor, intratumoral necrosis and peri-tumoral edema,10,11 and statin medication (inversely)11 were reported to be associated with SAO. At the same time, other studies did not identify any relationship between the patients’ age,13 tumor location,10 and size13 with the occurrence of preoperative seizures. The major limitation of all these studies refers to mostly smaller cohort size and missing evaluation of the independent predictive value of each of the reported risk factors.
Our large glioblastoma-based cohort confirmed the essential role of such predictors like younger age, higher KPS score and tumor location in the genesis of SAO. In addition, we identified 2 other independent risk factors for SAO—higher expression of GFAP in tumor tissue and high-normal to increased levels of serum chloride (>102 mmol/L). In glial cells, GFAP is involved in cytoskeleton architecture, maintaining the mechanical strength, the associations with surrounding neurons and the blood–brain barrier.30 The relationship between GFAP expression and seizure activity in astrocytes has been addressed in experimental epilepsy studies beyond oncological research,38,39 and has been described in selected cases of glioma patients.40 Whether higher GFAP expression leads to increased epileptogenicity or vice versa remains uncertain, since increase of GFAP expression secondary to epileptic seizures was demonstrated experimentally.41 Notably, higher GFAP expression in low-grade glioma was previously reported.30,42 This circumstance is in line with potential epileptogenic role of GFAP in glial tumors, since higher prevalence of epilepsy in low- versus high-grade glioma has already been mentioned before. In virtue of all these findings, further research of the role of GFAP in seizure activity of glial tumors is mandatory.
Moreover, we analyzed a wide range of admission laboratory values and found independent associations between higher serum chloride levels and the occurrence of SAO in glioblastoma. The interpretation of admission blood tests with regard to SAO epileptogenesis is generally problematic. The altered laboratory values might rather be secondary to seizure event(s), than provide the insights into the systemic processes, which promote the seizures. This would particularly explain higher levels of calcium and sodium in serum of individuals with SAO, because seizures can result in hypercalcemia and hypernatremia.43 As to higher chloride levels in SAO patients, this finding might have certain causal implications. The impact of chloride ions on seizure activity in neuronal cells is widely acknowledged and related to gamma-Aminobutyric acid (GABA) receptor activity.44,45 Experimental research with glial tumor cells has demonstrated that elevated intracellular chloride concentrations cause hyperpolarization of GABAergic neurons and lead to reduced network.1,46 In consequence, cumulative reduction of the inhibitory postsynaptic potential due to reduced GABAergic synaptic density is supposed to foster.1,46,47 Therefore, our findings strengthen the current hypotheses on the epileptogenesis of gliomas.
EPS: A Strong Negative Predictor
In contrast to delayed seizures due to postoperative tumor progression, less is known about the incidence and the genesis of EPS. This is the first study addressing the risk factors for and clinical consequences of EPS after glioblastoma surgery. Unlike SAO, EPS were more related to systemic dysfunctions present at the time of admission like anemia, systemic infection, and altered liver homeostasis. In addition, previous medical history (arterial hypertension and history of cancer) was also associated with EPS risk, however only in univariate analysis. It has been reported that various systemic diseases like endocrine, electrolyte and autoimmune disorders, organ dysfunction and failure, cancer, and paraneoplastic disorders facilitate seizure activity.48 Therefore, the risk of EPS after glioblastoma surgery might already be estimated preoperatively allowing timely selection of the patients requiring special postoperative care.
Not less interesting is the association of EPS with EOR and OS. The link between incomplete tumor resection and postoperative seizure risk has already been reported for glial13 and metastatic brain49 tumors. In summary, poorer outcome of glioblastoma individuals with EPS might be related to incomplete tumor resection and presence of above-mentioned systemic disorders.
Symptomatic Seizures in Glioblastoma: Is There Any Link With Outcome?
A large number of studies has addressed the association between early symptomatic seizures and glioblastoma survival reporting on longer OS in patients with SAO.2,4–8 Noteworthy, there are also several publications not confirming the predictive impact of SAO on patients survival.9–17 Moreover, many recent studies focused not on the SAO event as outcome confounder, but on the potential effect of AED on glioblastoma survival. Antitumoral activity of different AED was analyzed in numerous clinical and experimental studies.1,12,23,33,50 Unfortunately, the results are strongly conflicting, both with18–21 and without6,12,22–25 any association between AED use and survival. A recent large pooled analysis of prospective clinical trials25 has failed to show any survival benefit from the use of AED in newly diagnosed glioblastoma.
Our results might shed some light on the backgrounds of discrepant results of the previous studies on the role of AED. Occurrence of EPS also necessitates early initiation of AED treatment. In turn, EPS are associated with poorer survival after glioblastoma surgery, as we demonstrate in the present study. Therefore, future studies on AED effect on glioblastoma survival should also take the timing and causal background of perioperative seizures into account.
We could show independent association between SAO and OS. The vast majority of the studies, which failed to show a benefit of SAO on glioblastoma survival were based on relatively small cohorts and their results were therefore statistically underpowered. A recent meta-analysis2 also showed extended OS in individuals with SAO. In virtue of the findings of the present and previous studies, the following major conclusions regarding the SAO and OS can be made: (a) occurrence of SAO is associated with more favorable outcome of glioblastoma; (b) this effect might be related to earlier diagnosis of glioblastoma, since these patients present with higher KPS score and smaller tumor burden, and the SAO individuals with surgery delay seem to “lose” the survival benefit; (c) clinical characteristics of SAO patients like younger age and more radical EOR might also contribute to better treatment results.
Study Limitations
Retrospective design presents the major limitation of this study. Therefore, there is certain portion of missing data due to incomplete documentation in the hospital records. Regarding the molecular features of glioblastoma, missing data is mainly related to later implementation of molecular markers into the diagnostic set-up of glioblastoma, for example, IDH1 mutation status. These variables are insofar important because molecular tumor characteristics might present the causal link explaining the association between the seizure risk and survival in glioblastoma patients. Therefore, further research on the role of already established and novel molecular tumor markers on epileptogenesis in glioblastoma are mandatory. Nevertheless, we present a study based on a large consecutive series and adjust the study results for relevant endpoint confounders and information bias using multivariate analysis and multiple imputation.
Conclusions
In glioblastoma patients, SAO are independently associated with younger age, better preoperative clinical performance, certain tumor characteristics (location in the parietal lobe and higher GFAP expression), and serum ion alterations (higher chloride levels). In addition, SAO are related to more radical EOR and favorable OS. In contrast, EPS are strongly associated with presence of systemic disorders (anemia, infection, and liver dysfunction), incomplete tumor resection and poorer OS. Our results encourage further analysis of the effect of perioperative seizures on glioblastoma survival.
Supplementary Material
Funding
We acknowledge the support by the Open Access Publication Fund of the University of Duisburg-Essen.
Conflict of interest statement. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Authorship Statement. All authors designed the study, interpreted the data, critically revised and provided final approval for the manuscript, and are accountable for the accuracy of its contents. Study conception and design: R.J. and Y.A. Acquisition of data: R.J., Y.A., L.R., A.S., and L.L. Analysis and interpretation of data: R.J., Y.A., M.D., D.P., P.D., K.W., A.J., and B.S. Drafting of manuscript: R.J. and Y.A. Critical revision: C.Q., M.G., and M.S. Project administration: R.J. and U.S.
References
- 1. Goldstein ED, Feyissa AM. Brain tumor related-epilepsy. Neurol Neurochir Pol. 2018;52(4):436–447. [DOI] [PubMed] [Google Scholar]
- 2. Lu VM, Jue TR, Phan K, McDonald KL. Quantifying the prognostic significance in glioblastoma of seizure history at initial presentation: a systematic review and meta-analysis. Clin Neurol Neurosurg. 2018;164:75–80. [DOI] [PubMed] [Google Scholar]
- 3. Vecht CJ, Kerkhof M, Duran-Pena A. Seizure prognosis in brain tumors: new insights and evidence-based management. Oncologist. 2014;19(7):751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Flanigan PM, Jahangiri A, Kuang R, et al. Improved survival with decreased wait time to surgery in glioblastoma patients presenting with seizure. Neurosurgery. 2017;81(5):824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lorimer CF, Hanna C, Saran F, Chalmers A, Brock J. Challenges to treating older glioblastoma patients: the influence of clinical and tumour characteristics on survival outcomes. Clin Oncol (R Coll Radiol). 2017;29(11):739–747. [DOI] [PubMed] [Google Scholar]
- 6. Toledo M, Sarria-Estrada S, Quintana M, et al. Prognostic implications of epilepsy in glioblastomas. Clin Neurol Neurosurg. 2015;139:166–171. [DOI] [PubMed] [Google Scholar]
- 7. Yuile P, Dent O, Cook R, Biggs M, Little N. Survival of glioblastoma patients related to presenting symptoms, brain site and treatment variables. J Clin Neurosci. 2006;13(7):747–751. [DOI] [PubMed] [Google Scholar]
- 8. Berendsen S, Varkila M, Kroonen J, et al. Prognostic relevance of epilepsy at presentation in glioblastoma patients. Neuro Oncol. 2016;18(5):700–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dobran M, Nasi D, Chiriatti S, et al. Prognostic factors in glioblastoma: is there a role for epilepsy? Neurol Med Chir (Tokyo). 2018;58(3):110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dührsen L, Sauvigny T, Ricklefs FL, et al. Seizures as presenting symptom in patients with glioblastoma. Epilepsia. 2019;60(1):149–154. [DOI] [PubMed] [Google Scholar]
- 11. Henker C, Kriesen T, Scherer M, et al. Association between tumor compartment volumes, the incidence of pretreatment seizures, and statin-mediated protective effects in glioblastoma. Neurosurgery. 2019;85(4):E722–E729. [DOI] [PubMed] [Google Scholar]
- 12. Jaeckle KA, Ballman K, Furth A, Buckner JC. Correlation of enzyme-inducing anticonvulsant use with outcome of patients with glioblastoma. Neurology. 2009;73(15):1207–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liang S, Zhang J, Zhang S, Fu X. Epilepsy in adults with supratentorial glioblastoma: incidence and influence factors and prophylaxis in 184 patients. PLoS One. 2016;11(7):e0158206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mineo JF, Bordron A, Baroncini M, et al. Prognosis factors of survival time in patients with glioblastoma multiforme: a multivariate analysis of 340 patients. Acta Neurochir (Wien). 2007;149(3):245–252; discussion 252-243. [DOI] [PubMed] [Google Scholar]
- 15. Rosati A, Poliani PL, Todeschini A, et al. Glutamine synthetase expression as a valuable marker of epilepsy and longer survival in newly diagnosed glioblastoma multiforme. Neuro Oncol. 2013;15(5):618–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shin JY, Kizilbash SH, Robinson S, Uhm JH, Jatoi A. Incidence, characteristics, and implications of seizures in patients with Glioblastoma. Am J Hosp Palliat Care. 2017;34(7):650–653. [DOI] [PubMed] [Google Scholar]
- 17. Salvati M, Bruzzaniti P, Relucenti M, et al. Retrospective and randomized analysis of influence and correlation of clinical and molecular prognostic factors in a mono-operative series of 122 patients with glioblastoma treated with STR or GTR. Brain Sci. 2020;10(2):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barker CA, Bishop AJ, Chang M, Beal K, Chan TA. Valproic acid use during radiation therapy for glioblastoma associated with improved survival. Int J Radiat Oncol Biol Phys. 2013;86(3):504–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kerkhof M, Dielemans JC, van Breemen MS, et al. Effect of valproic acid on seizure control and on survival in patients with glioblastoma multiforme. Neuro Oncol. 2013;15(7):961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weller M, Gorlia T, Cairncross JG, et al. Prolonged survival with valproic acid use in the EORTC/NCIC temozolomide trial for glioblastoma. Neurology. 2011;77(12):1156–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim YH, Kim T, Joo JD, et al. Survival benefit of levetiracetam in patients treated with concomitant chemoradiotherapy and adjuvant chemotherapy with temozolomide for glioblastoma multiforme. Cancer. 2015;121(17):2926–2932. [DOI] [PubMed] [Google Scholar]
- 22. Knudsen-Baas KM, Engeland A, Gilhus NE, Storstein AM, Owe JF. Does the choice of antiepileptic drug affect survival in glioblastoma patients? J Neurooncol. 2016;129(3):461–469. [DOI] [PubMed] [Google Scholar]
- 23. Rigamonti A, Imbesi F, Silvani A, et al. Antiepileptic treatment and survival in newly diagnosed glioblastoma patients: retrospective multicentre study in 285 Italian patients. J Neurol Sci. 2018;390:14–19. [DOI] [PubMed] [Google Scholar]
- 24. Tsai HC, Wei KC, Tsai CN, et al. Effect of valproic acid on the outcome of glioblastoma multiforme. Br J Neurosurg. 2012;26(3):347–354. [DOI] [PubMed] [Google Scholar]
- 25. Happold C, Gorlia T, Chinot O, et al. Does valproic acid or levetiracetam improve survival in glioblastoma? a pooled analysis of prospective clinical trials in newly diagnosed Glioblastoma. J Clin Oncol. 2016;34(7):731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Feyissa AM, Worrell GA, Tatum WO, et al. Potential influence of IDH1 mutation and MGMT gene promoter methylation on glioma-related preoperative seizures and postoperative seizure control. Seizure. 2019;69:283–289. [DOI] [PubMed] [Google Scholar]
- 27. Lote K, Stenwig AE, Skullerud K, Hirschberg H. Prevalence and prognostic significance of epilepsy in patients with gliomas. Eur J Cancer. 1998;34(1):98–102. [DOI] [PubMed] [Google Scholar]
- 28. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 29. Sirven JI, Wingerchuk DM, Drazkowski JF, Lyons MK, Zimmerman RS. Seizure prophylaxis in patients with brain tumors: a meta-analysis. Mayo Clin Proc. 2004;79(12):1489–1494. [DOI] [PubMed] [Google Scholar]
- 30. Ahmadipour Y, Gembruch O, Pierscianek D, Sure U, Jabbarli R. Does the expression of glial fibrillary acid protein (GFAP) stain in glioblastoma tissue have a prognostic impact on survival? Neurochirurgie. 2020;66(3):150–154. [DOI] [PubMed] [Google Scholar]
- 31. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 32. Chen DY, Chen CC, Crawford JR, Wang SG. Tumor-related epilepsy: epidemiology, pathogenesis and management. J Neurooncol. 2018;139(1):13–21. [DOI] [PubMed] [Google Scholar]
- 33. Maschio M. Brain tumor-related epilepsy. Curr Neuropharmacol. 2012;10(2):124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Toledo M, Sarria-Estrada S, Quintana M, et al. Epileptic features and survival in glioblastomas presenting with seizures. Epilepsy Res. 2017;130:1–6. [DOI] [PubMed] [Google Scholar]
- 35. Woo PY, Chan DT, Chan KY, et al. Risk factors for seizures and antiepileptic drug-associated adverse effects in high-grade glioma patients: A multicentre, retrospective study in Hong Kong. Surg Pract. 2015;19(1):2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ertürk Çetin Ö, İşler C, Uzan M, Özkara Ç. Epilepsy-related brain tumors. Seizure. 2017;44:93–97. [DOI] [PubMed] [Google Scholar]
- 37. Kerkhof M, Vecht CJ. Seizure characteristics and prognostic factors of gliomas. Epilepsia. 2013;54(Suppl 9):12–17. [DOI] [PubMed] [Google Scholar]
- 38. Li D, Li P, He Z, et al. Human intravenous immunoglobulins suppress seizure activities and inhibit the activation of GFAP-positive astrocytes in the hippocampus of picrotoxin-kindled rats. Int J Neurosci. 2012;122(4):200–208. [DOI] [PubMed] [Google Scholar]
- 39. Çavdar S, Kuvvet Y, Sur-Erdem I, Özgür M, Onat F. Relationships between astrocytes and absence epilepsy in rat: an experimental study. Neurosci Lett. 2019;712:134518. [DOI] [PubMed] [Google Scholar]
- 40. You G, Sha Z, Jiang T. The pathogenesis of tumor-related epilepsy and its implications for clinical treatment. Seizure. 2012;21(3):153–159. [DOI] [PubMed] [Google Scholar]
- 41. Steward O, Torre ER, Tomasulo R, Lothman E. Seizures and the regulation of astroglial gene expression. Epilepsy Res Suppl. 1992;7:197–209. [PubMed] [Google Scholar]
- 42. Rutka JT, Murakami M, Dirks PB, et al. Role of glial filaments in cells and tumors of glial origin: a review. J Neurosurg. 1997;87(3):420–430. [DOI] [PubMed] [Google Scholar]
- 43. Castilla-Guerra L, del Carmen Fernández-Moreno M, López-Chozas JM, Fernández-Bolaños R. Electrolytes disturbances and seizures. Epilepsia. 2006;47(12):1990–1998. [DOI] [PubMed] [Google Scholar]
- 44. Nardone R, Brigo F, Trinka E. Acute symptomatic seizures caused by electrolyte disturbances. J Clin Neurol. 2016;12(1):21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Raimondo JV, Burman RJ, Katz AA, Akerman CJ. Ion dynamics during seizures. Front Cell Neurosci. 2015;9:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. MacKenzie G, O’Toole KK, Moss SJ, Maguire J. Compromised GABAergic inhibition contributes to tumor-associated epilepsy. Epilepsy Res. 2016;126:185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pallud J, Le Van Quyen M, Bielle F, et al. Cortical GABAergic excitation contributes to epileptic activities around human glioma. Science Translational Medicine. 2014;6(244):244ra289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hoerth MT, Sirven JI. Seizures due to systemic disease. In: Lewis SL, ed. Neurological Disorders Due to Systemic Disease. Chapter 6; Hoboken, New Jersey, USA: Wiley-Blackwell; 2013:107–126. [Google Scholar]
- 49. Wu A, Weingart JD, Gallia GL, et al. Risk factors for preoperative seizures and loss of seizure control in patients undergoing surgery for metastatic brain tumors. World Neurosurg. 2017;104:120–128. [DOI] [PubMed] [Google Scholar]
- 50. Lange F, Weßlau K, Porath K, et al. AMPA receptor antagonist perampanel affects glioblastoma cell growth and glutamate release in vitro. PLoS One. 2019;14(2):e0211644. Cupica vidius? Quon nes et; nos aus poraedest? Hem nestiam auceperes poerium iam ac fursupi [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Any data not published within the article will be shared in anonymized manner by request from any qualified investigator.