Abstract
Background
There is a clear need for a better assessment of independent risk factors for in-hospital mortality, intensive care unit admission, and bacteremia in patients presenting with suspected sepsis at the emergency department.
Methods
A prospective observational cohort study including 1690 patients was performed. Two multivariable logistic regression models were used to identify independent risk factors.
Results
Sequential organ failure assessment (SOFA) score of ≥2 and serum lactate of ≥2mmol/L were associated with all outcomes. Other independent risk factors were individual SOFA variables and systemic inflammatory response syndrome variables but varied per outcome. Mean arterial pressure <70 mmHg negatively impacted all outcomes.
Conclusions
These readily available measurements can help with early risk stratification and prediction of prognosis.
Keywords: bacteremia, emergency department, ICU admission, risk factors, sepsis
Sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection. Despite international efforts, mortality remains high [1]. A rapid identification of severely ill patients is necessary, and several risk-scoring systems have been proposed. The Third International Consensus Conference introduced sequential organ failure assessment (SOFA) as a risk-assessment tool for mortality. Sepsis, defined as SOFA ≥2, is associated with 10% mortality risk [2]. Sequential organ failure assessment has been evaluated in the emergency department (ED) [3]. In addition, the quick SOFA (qSOFA) has been proposed for risk stratification in the ED but showed a low sensitivity for predicting unfavorable outcomes [4]. Furthermore, the previous sepsis guidelines-based systemic inflammatory response syndrome (SIRS) criteria were shown to have better performances than qSOFA [4]. Other scoring models include the modified early warning score, which performed better than qSOFA for risk stratification [5]. On the other hand, prediction models for bacteremia have not been well implemented in clinical practice [6]. Fast and easily measurable risk factors in the ED could help in early triage and close monitoring of severely ill patients. The objective of this study was to identify potential risk factors associated with in-hospital mortality, intensive care unit (ICU) admission, and bacteremia at the start of a new episode of suspected sepsis.
METHODS
A prospective observational cohort study was performed at the ED of a 981-bed teaching hospital in Hasselt, Belgium between February 2019 and March 2020 as part of the Fast Assay for Pathogen Identification and Characterization (FAPIC) project (ClinicalTrials.gov Identifier NCT03841162). All adult patients presenting with suspected sepsis (all patients for whom blood cultures were drawn) were asked to participate. The study was approved by the medical ethics committees of Jessa hospital and Hasselt University, and written informed consent was obtained from all participants. Patients were included after collection of the first set of blood cultures at each new suspected sepsis episode (minimal interval of 7 days between positive blood cultures with the same pathogen and at least 24 hours between positive cultures with different organisms). Blood cultures were performed using the BACTEC FX (Becton Dickinson) system, bacterial identification by MALDI-TOF Biotyper (Bruker), and susceptibility testing by the Phoenix system TM 100 (Becton Dickinson). Other microbiological tests (including cultures of urine, lower respiratory tract, and samples of specific foci, urinary antigen tests for Streptococcus pneumoniae and Legionella pneumophila and nasopharyngeal swabs for virologic polymerase chain reaction) were performed if deemed relevant by the treating physician.
Clinical and laboratory parameters were collected at the start of each new episode. Emergency department physicians ordered clinical, biochemical, and microbiological tests guided by a suspected sepsis protocol in place at the ED. Clinical parameters included body temperature, heart rate, mean arterial pressure (MAP), oxygen saturation (SaO2) and partial oxygen pressure (PaO2), Glasgow Coma scale (GCS), the presence of central lines at admission, vasopressor use, and oxygen requirements. Laboratory testing included white blood cell count (WBC), platelet count, hemoglobin, red blood cell distribution width (RDW), C-reactive protein (CRP), creatinine, urea, lactate dehydrogenase (LDH), bilirubin, alanine aminotransferase (ALT), and aspartate aminotransferase (AST). Additional biochemical tests ordered as part of the FAPIC study were serum lactate and ferritin, based on recent insights regarding their association with sepsis mortality [2, 7]. Recorded patient outcomes were in-hospital mortality, ICU admission (at any time during hospital admission), and the presence of bacteremia. All final infection diagnoses recorded by the treating physicians were validated by an experienced infectious diseases physician (I.C.G.) not involved in the care of study patients, according to infectious diseases definitions [8–10]. Definitions are provided in Supplementary Table 1. Descriptive statistics were used to present patient’s characteristics. Continuous data are shown as median (interquartile range) and categorical data are shown as frequency (percentages). Two multivariable logistic regression models were built for each outcome. Model 1 included SOFA score ≥2, and model 2 included individual SOFA score variables to evaluate the association of these variables separately. All parameters were inserted in the starting model. A backwards selection based on significance level P < .05 was used. Odds ratios (ORs) were calculated to define independent risk factors. Sensitivity, specificity, and positive and negative predictive values were calculated for all models. All analyses were done using SPSS version 25 (IBM, Chicago, IL).
Patient Consent Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Documented approval for this study was obtained from the Ethics committees of Hasselt University and Jessa Hospital (18.106/infect18.03 and 19.51/infect.19.02). Written informed consent was obtained from all participants.
RESULTS
In total, 1690 admissions to the ED of 1545 unique patients were included. Patient characteristics, patient outcomes, and infection diagnoses are shown in Table 1. Median age was 70 years (55–80), and 976 patients (57.8%) were male. Median Charlson Comorbidity Index (CCI) was 1 (0–3). Cardiac comorbidities, renal insufficiency, and chronic pulmonary diseases were most prevalent. Median SOFA score was 2 (0–3), 193 (11.4) patients had central lines, 316 patients (18.9%) required oxygen therapy, and 17 patients (1.0%) required the use of vasopressors. All clinical and laboratory measurements are shown in Supplementary Table 2. Overall, 90 patients (5.3%) died in hospital, and 131 patients (7.8%) were admitted to the ICU. There were 253 (15.0%) admissions with true bacteremia and 82 (4.9%) with contaminated blood cultures. Isolated pathogens and major resistances are shown in Supplementary Table 3.
Table 1.
Patient Characteristics, Disease Severity, Clinical Outcomes, Final Infection Diagnoses, and Proportion of Patients With Bacteremia
| Variable | Total n = 1690 | ||
|---|---|---|---|
| Demographics | |||
| Age (years; median IQR) | 70 (55–80) | ||
| Sex | |||
| Male | 976 (57.8) | ||
| Female | 714 (42.2) | ||
| Charlson Comorbidity Index | 1 (0–3) | ||
| Cardiac comorbidities | 301 (17.8) | ||
| Hypertension | 373 (22.1) | ||
| Chronic pulmonary disease | 263 (15.6) | ||
| Cerebrovascular diseasea | 139 (8.2) | ||
| Renal insufficiency | 255 (15.1) | ||
| Liver disease | 53 (3.1) | ||
| Diabetes | 255 (15.1) | ||
| Solid malignancies | 176 (10.4) | ||
| Solid metastatic malignancies | 177 (10.5) | ||
| Hematological malignancies | 53 (3.2) | ||
| SOFA score at admission (median IQR) | 2 (0–3) | ||
| Central lineb at admission | 193 (11.4) | ||
| Oxygen Therapy | |||
| Noninvasive | 307 (18.2) | ||
| Invasive | 11 (0.7) | ||
| Vasopressor use | 17 (1.0) | ||
| Outcomes | |||
| In-hospital mortality | 90 (5.3) | ||
| ICU admission | 131 (7.8) | ||
| Bacteremia | 253 (15.0) | ||
| LOS (days; median IQR) | 5 (3–10) | ||
| ICU LOS (days; median IQR) | 3 (2–8) | ||
| Clinical Infection Diagnosis | Bacteremia (n = 253) | No Bacteremia (n = 1437) | |
| Pneumonia | 291 (17.2) | 24 (9.5) | 267 (18.6) |
| ABSSSI | 164 (9.7) | 14 (5.5) | 150 (10.4) |
| Intra-abdominal infection | 158 (9.3) | 43 (17.0) | 115 (8.0) |
| Urosepsis | 97 (5.7) | 97 (38.3) | 0 (0.0) |
| Influenza | 97 (5.7) | 0 (0.0) | 97 (6.8) |
| lRTI | 93 (5.5) | 0 (0.0) | 93 (6.5) |
| BSI, CLABSI, and endocarditis | 73 (4.3) | 73 (28.9) | 0 (0.0) |
| uUTI | 60 (3.6) | 0 (0.0) | 60 (4.2) |
| Other Infection | |||
| Fever | 127 (7.5) | 0 (0.0) | 127 (8.8) |
| lUTI | 90 (5.3) | 0 (0.0) | 90 (6.3) |
| uRTI | 66 (3.9) | 0 (0.0) | 66 (4.6) |
| Neutropenic fever | 33 (2.0) | 0 (0.0) | 32 (2.3) |
| Bone and joint infection | 13 (0.8) | 1 (0.4) | 12 (0.8) |
| Viral infectionc | 12 (0.7) | 0 (0.0) | 12 (0.8) |
| CNS infection | 9 (0.5) | 1 (0.4) | 8 (0.6) |
| Other | 9 (0.5) | 0 (0.0) | 9 (0.6) |
| Other bacterial/parasitic infection | 4 (0.2) | 0 (0.0) | 4 (0.3) |
| No Infection Diagnosis | |||
| Suspected infection | 230 (13.6) | 0 (0.0) | 230 (16.0) |
| Suspected viral infection | 44 (2.6) | 0 (0.0) | 44 (3.1) |
| Inflammatory diseases | 20 (1.2) | 0 (0.0) | 20 (1.4) |
Abbreviations: ABSSSI, acute bacterial skin and skin structure infection; BSI, bloodstream infection; CLABSI, central line-associated BSI; CNS, central nervous system; ICU, intensive care unit; IQR, interquartile range; lRTI, lower respiratory tract infection; lUTI, lower urinary tract infection; LOS, length of stay; SOFA, sequential organ failure assessment; uRTI, upper respiratory tract infection; uUTI, upper urinary tract infection.
NOTE: Numbers are presented as N (%) unless specified.
aCerebrovascular disease included strokes and transient ischemic attack.
bThese were 188 portal catheters and 5 Hickman catheters.
cViral infections were herpes zoster (n = 3), cytomegalovirus (n = 2), Epstein-Barr virus, herpes simplex, enterovirus, measles, rhinovirus, parainfluenza virus, and human immunodeficiency virus (all n = 1).
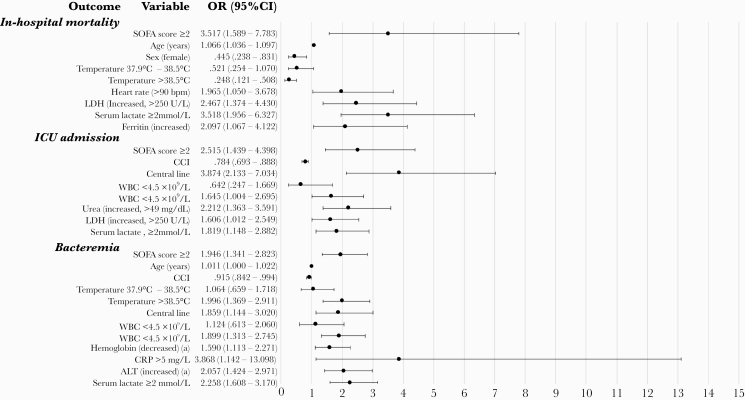
Independent risk factors for in-hospital mortality, ICU admission, and bacteremia are shown in Figures 1 and 2. In model 1 (Figure 1), SOFA score and serum lactate ≥2 mmol/L were independent risk factors for all outcomes. Independent risk factors for in-hospital mortality were older age, male sex, increased heart rate (>90 bpm), increased LDH (>250 U/L), and increased ferritin. High temperature (>38.5°C) at the start of the episode was a negative independent risk factor. For ICU admission, the presence of central lines at admission, leukocytosis (>11 × 109 WBC/L), increased urea (>49 mg/dL), and increased LDH (>250 U/L) were independent risk factors. Bacteremia was associated with older age, temperature >38.5°C, the presence of central lines at admission, leukocytosis (>11 × 109 WBC/L), decreased hemoglobin levels, increased CRP (>5 mg/L), and increased ALT levels. Lower CCI was an independent risk factor for ICU admission and for bacteremia.
Figure 1.
Forest plot of multivariable logistic regression analysis (model 1) of the association of clinical and laboratory parameters with mortality, intensive care unit (ICU) admission, and presence of bacteremia at admission. Model 1: Sequential organ failure assessment (SOFA) score ≥2 and other parameters. aReference levels for abnormal values (decreased or increased) are age and sex dependent. *Odds ratio (OR) and P value for increased hemoglobin were not calculated for this model because of low numbers of patients for that group (data not shown). Two patients with body temperature <35°C were excluded for multivariable analysis because of the low number in that group. ALT, alanine aminotransferase; CCI, Charlson Comorbidity Index; CI, confidence interval; CRP, C-reactive protein; GCS, Glasgow coma scale; LDH, lactate dehydrogenase; MAP, mean arterial pressure; WBC, white blood cell count.
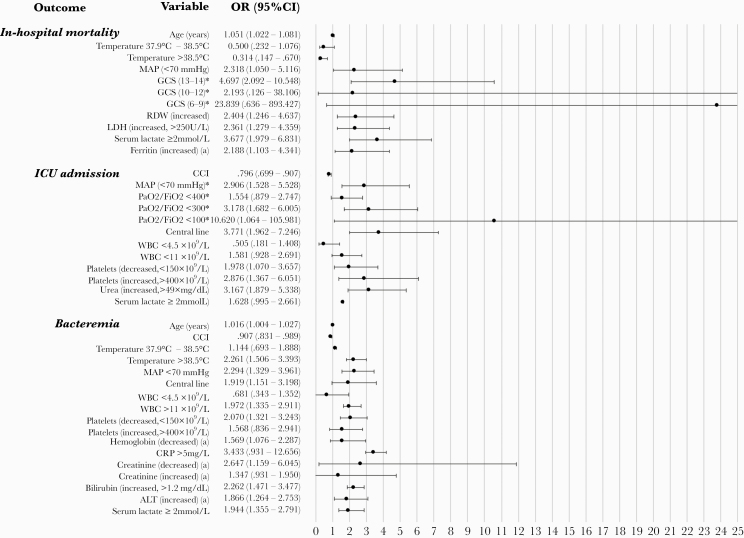
Figure 2.
Forest plot of multivariable logistic regression analysis (model 2) of the association of clinical and laboratory parameters with mortality, intensive care unit (ICU) admission, and presence of bacteremia at admission. Model 2: Separate variables of sequential organ failure assessment (SOFA) score and other parameters. aReference levels for abnormal values (decreased or increased) are age and sex dependent. *Odds ratio (OR) and P value for decreased red blood cell distribution width (RDW), increased hemoglobin, a Glasgow coma scale (GCS) <6, and a PaO2/FiO2 <200 were not calculated for this model because of low numbers of patients for that group (data not shown). Two patients with body temperature <35°C were excluded for multivariable analysis because of the low number in that group. ALT, alanine aminotransferase; CCI, Charlson Comorbidity Index; CI, confidence interval; LDH, lactate dehydrogenase; MAP, mean arterial pressure; WBC, white blood cell count.
In model 2 (Figure 2), patients with MAP <70 mmHg had a 2-fold risk for all outcomes. Temperature >38.5°C was negatively related to in-hospital mortality, but older age, decreased GCS (13–14 and <9), increased RDW, increased LDH (>250 U/L), serum lactate ≥2 mmol/L, and increased ferritin were independent risk factors. For ICU admission, serum lactate (≥2 mmol/L) and LDH (>250 U/L) were eliminated in model 2. The SOFA variables associated with ICU admission were decreased PaO2/FiO2 ratio (<300 and <100) and abnormal platelet counts (<150 × 109/L and >400 × 109/L). Regarding bacteremia, increased CRP (>5 mg/L) was not an independent risk factor in model 2. Sequential organ failure assessment variables decreased platelet count (<150 × 109/L), decreased creatinine levels, and increased bilirubin levels (>1.2 mg/dL) were independent risk factors for bacteremia.
For all models, performance characteristics to predict the respective outcome at admission are shown in Supplementary Table 4, and receiver operating curves are shown in Supplementary Figure 1. Model 1 was able to predict mortality, ICU admission, and bacteremia with a sensitivity and specificity of 7.9% and 99.5%, 5.4% and 99.9%, and 4.2% and 99.1%, respectively. Model 2 was able to predict mortality, ICU admission, and bacteremia with a sensitivity and specificity of 18.0% and 99.7%, 10.0% and 99.9%, and 16.2% and 98.1%, respectively.
DISCUSSION
This study shows several independent risk factors for in-hospital mortality, ICU admission, and bacteremia in a population presenting at the ED with suspected sepsis. Most importantly, SOFA score of ≥2 and serum lactate of ≥2 mmol/L were associated with all outcomes. Mean arterial pressure <70 mmHg was associated with an increased risk for all outcomes in one model. Age and CCI were important confounders. This is in accordance with previous research, in which CCI was higher in patients with Staphylococcus aureus bacteremia for more than 1 day [11] and was found to be an independent predictor for mortality in bloodstream infection due to Enterobacterales [12]. Independent risk factors for mortality were clinical (temperature ≤38.5°C, decreased GCS) and laboratory parameters (leukocytosis, increased RDW, LDH, and ferritin). It is interesting to note that, consistent with other studies in the ED [13], higher temperature at admission was negatively associated with mortality. Reasons can be late recognition of infection, and consequently a later start of antibiotics in the absence of fever, or a poor immune and fever response [13]. Hypothermia is associated with an increased mortality risk [13], which could not be confirmed due to the small number of patients with hypothermia in our cohort. Intensive care unit admission was associated with decreased PaO2/FiO2, the presence of central lines at admission, leukocytosis, and increased levels of urea and LDH. In addition, lower CCI was an independent risk factor for ICU admission, although this can reflect patient management in our hospital. Risk factors for bacteremia were temperature >38.5°C, the presence of central lines at admission, and laboratory parameters (leukocytosis, increased levels of CRP and ALT, and decreased levels of platelets, hemoglobin, and creatinine). All measurements can be easily and rapidly determined at admission.
According to the sepsis-3 guidelines, SOFA score is useful for defining sepsis, and a score of ≥2 is associated with a 10% mortality risk. Furthermore, hyperlactatemia is associated with a 25.7% mortality risk [2], and significant increases in OR of in-hospital mortality were reported with each increase in serum lactate values [14]. In our population, the association of mortality with SOFA score and serum lactate was confirmed. In addition, both factors were associated with ICU admission and bacteremia. Furthermore, when SOFA score was replaced by its individual variables, most independent risk factors that were identified in model 1 remained similar. Mean arterial pressure <70 mmHg was an independent risk factor at admission for all outcomes, confirming the earlier findings of one retrospective study [15]. Moreover, fever, hypotension, and leukocytosis were identified as independent risk factors for bacteremia. These parameters are part of the SIRS criteria in the sepsis-1 and -2 guidelines [16]. Although studies show better performance of SOFA score in describing prognosis [17], these factors could still be useful in risk stratification, and our findings suggest the use of a combination of both assessment scores.
All models were able to predict all outcomes with high specificity but with low sensitivity. Therefore, these risk factors could be used in the ED as a screening tool for “ruling in” patients most at risk for a severe disease course, but they should not be used for ruling these patients out. Sensitivity increased in model 2 compared to model 1. This indicates that the use of separate independent risk factors can add value in screening patients at the ED, compared with using SOFA score as a composite score.
The prospective inclusion of patients and collection of data is a major strength of this study. This resulted in a well defined and uniform cohort and a limited amount of missing data; for all variables, more than 90% of values were available (data not shown). Furthermore, the complete study period covered 1 year, which accounted for a representation of different infectious diseases in all seasons and a large sample size of approximately 1700 episodes. The last patient included was on February 29, 2020 before the start of the coronavirus disease 2019 pandemic in Belgium. Some limitations exist. First, our population is a subset of patients that present to the ED with severe symptoms of infection. Therefore, the extrapolation of these results to all patients with suspected infection at admission is impossible. Second, this is a single-center setting with a low level of antimicrobial resistance, and the external validity could be limited. However, patient demography, diagnoses of infection, and isolated pathogens from blood are comparable to other similar settings in a high-income country [18, 19]. Third, all parameters were assessed at the start of a new suspected sepsis episode. We did not follow patients during hospitalization, and later events could have affected mortality and ICU admission.
CONCLUSIONS
In conclusion, several independent risk factors for in-hospital mortality, ICU admission, and bacteremia were identified in a population of patients with suspected sepsis at admission. The outcomes are representative of a severe disease course, and these factors can help to identify patients most at risk and guide management and empirical antibiotic therapy. The SOFA score and hyperlactatemia, as defined in the sepsis-3 guidelines, and MAP <70 mmHg are important risk factors for all outcomes. These findings can be of high value in early risk stratification, and they could be used to direct diagnostics and close patient monitoring, leading to earlier therapies in high-risk patients.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank Dr. Liesbeth Bruckers (Interuniversity institute for Biostatistics and Statistical Bioinformatics, Hasselt University) for providing statistical advice and reviewing the statistical methodology of this study. This study is part of the Limburg Clinical Research Center UHasselt-ZOL-Jessa, supported by the foundation Limburg Sterk Merk, Hasselt University, Ziekenhuis Oost-Limburg and Jessa Hospital.
Financial support. This work was funded by the European Union’s Horizon 2020 Research and Innovation Program under Grant Agreement 634137 and is part of the Fast Assay for Pathogen Identification and Characterisation project.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Presented in part: ECCMID 2020.
References
- 1. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 2017; 43:304–77. [DOI] [PubMed] [Google Scholar]
- 2. Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016; 315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Innocenti F, Bianchi S, Guerrini E, et al. Prognostic scores for early stratification of septic patients admitted to an emergency department-high dependency unit. Eur J Emerg Med 2014; 21:254–9. [DOI] [PubMed] [Google Scholar]
- 4. Tusgul S, Carron PN, Yersin B, et al. Low sensitivity of qSOFA, SIRS criteria and sepsis definition to identify infected patients at risk of complication in the prehospital setting and at the emergency department triage. Scand J Trauma Resusc Emerg Med 2017; 25:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Churpek MM, Snyder A, Han X, et al. Quick sepsis-related organ failure assessment, systemic inflammatory response syndrome, and early warning scores for detecting clinical deterioration in infected patients outside the intensive care unit. Am J Respir Crit Care Med 2017; 195:906–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eliakim-Raz N, Bates DW, Leibovici L. Predicting bacteraemia in validated models–a systematic review. Clin Microbiol Infect 2015; 21:295–301. [DOI] [PubMed] [Google Scholar]
- 7. Kyriazopoulou E, Leventogiannis K, Norrby-Teglund A, et al. ; Hellenic Sepsis Study Group. Macrophage activation-like syndrome: an immunological entity associated with rapid progression to death in sepsis. BMC Med 2017; 15:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention. Collecting cultures: a clinician guide. Available at: https://www.cdc.gov/antibiotic-use/core-elements/collecting-cultures.html. Accessed 29 June 2020.
- 9. Wiersinga WJ, Bonten MJ, Boersma WG, et al. Management of community-acquired pneumonia in adults: 2016 guideline update from the Dutch Working Party on Antibiotic Policy (SWAB) and Dutch Association of Chest Physicians (NVALT). Neth J Med 2018; 76:4–13. [PubMed] [Google Scholar]
- 10. U.S. Department of Health and Human Services. Food and Drug Administration. Center for Drug Evaluation and Research (CDER). Complicated Urinary Tract Infections: Developing Drugs for Treatment. Guidance for Industry. Revision 1. Silver Spring, MD: Center for Drug Evaluation and Research; 2018. [Google Scholar]
- 11. Kuehl R, Morata L, Boeing C, et al. ; International Staphylococcus aureus Collaboration Study Group and the ESCMID Study Group for Bloodstream Infections, Endocarditis and Sepsis. Defining persistent Staphylococcus aureus bacteraemia: secondary analysis of a prospective cohort study. Lancet Infect Dis 2020; 20:1409–17. [DOI] [PubMed] [Google Scholar]
- 12. Russo A, Falcone M, Gutiérrez-Gutiérrez B, et al. ; REIPI/ESGBIS/INCREMENT investigators. Predictors of outcome in patients with severe sepsis or septic shock due to extended-spectrum β-lactamase-producing Enterobacteriaceae. Int J Antimicrob Agents 2018; 52:577–85. [DOI] [PubMed] [Google Scholar]
- 13. Yamamoto S, Yamazaki S, Shimizu T, et al. Body Temperature at the emergency department as a predictor of mortality in patients with bacterial infection. Medicine (Baltimore) 2016; 95:e3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shetty AL, Thompson K, Byth K, et al. Serum lactate cut-offs as a risk stratification tool for in-hospital adverse outcomes in emergency department patients screened for suspected sepsis. BMJ Open 2018; 8:e015492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coeckelenbergh S, Van Nuffelen M, Mélot C. Sepsis is frequent in initially non-critical hypotensive emergency department patients and is associated with increased mortality. Am J Emerg Med 2019; 37:2242–5. [DOI] [PubMed] [Google Scholar]
- 16. Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992; 101:1644–55. [DOI] [PubMed] [Google Scholar]
- 17. Freund Y, Lemachatti N, Krastinova E, et al. ; French Society of Emergency Medicine Collaborators Group. Prognostic accuracy of sepsis-3 criteria for in-hospital mortality among patients with suspected infection presenting to the emergency department. JAMA 2017; 317:301–8. [DOI] [PubMed] [Google Scholar]
- 18. Latten G, Hensgens K, de Bont EGPM, et al. How well are sepsis and a sense of urgency documented throughout the acute care chain in the Netherlands? A prospective, observational study. BMJ Open 2020; 10:e036276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rothe K, Wantia N, Spinner CD, et al. Antimicrobial resistance of bacteraemia in the emergency department of a German university hospital (2013-2018): potential carbapenem-sparing empiric treatment options in light of the new EUCAST recommendations. BMC Infect Dis 2019; 19:1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.