Abstract
Objectives
To examine the relationship between obesity and mortality as a function of polygenic risk for obesity among older U.S. adults.
Method
Using data from the 1994–2014 Health and Retirement Study in conjunction with genome-wide data, we evaluated the risk of mortality as a function of obesity classification, an individual’s polygenic risk score (PGS) for obesity, and their interaction, stratified by sex. We conducted our analyses using cox proportional hazard models.
Results
Among those with an average PGS for obesity (8,143 [68.8%]), obese I (hazard ratio [HR] = 0.79, p = .336) adults show no difference in their risk for mortality and obese II/III (HR = 3.17, p = .000) adults present higher risk of mortality relative to non-obese adults. The interaction of obesity classification and PGS suggests that obese II/III respondents with low PGS in the total sample (HR = 2.71, p = .006) and among women (HR = 3.02, p = .023) are at significantly higher risk of death when compared to obese II/III respondents with average or high PGS.
Discussion
We posit that these findings suggest that the pathway to obesity, in this case, more socio-behavioral rather than genetic, may influence subsequent risk of death in older adults. We suggest that practitioners and population researchers be mindful of these pathways as to better identify and understand mortality risk.
Keywords: Genetics, Health and Retirement Study, Mortality, Obesity
Previous research has shown that obesity increases the risk of mortality (Flegal, Kit, Orpana, & Graubard, 2013) accounting for 16% and 22% of adult deaths among non-Hispanic white men and women, respectively (Masters et al., 2013). These associations are not without controversy, as some have questioned body size classification (Adams et al., 2006), biases involving misclassification due to self-reported measures (Ahima & Lazar, 2013), selection effects due to mortality (Flegal, Kit, & Graubard, 2017), and a limited understanding of the metabolic pathways involved (Read et al., 2017). Though some recent research examines the role genetics plays in the relationship between Body Mass Index (BMI) and chronic disease (Dhana et al., 2018; Gianfrancesco et al., 2017), we propose that some of the inconsistency in the general association between obesity and mortality is because extant research has not differentiated between more socio-behavioral and genetically influenced pathways to obesity.
The reluctance to draw distinctions between putatively environmental pathways like obesogenic neighborhoods (Barrientos-Gutierrez et al., 2017) and more straightforward genetic channels (Locke et al., 2015) reflects a complex, multifactorial etiology. Despite this acknowledged complexity—where genetics and environment not only play independent roles in determining BMI and associated health measures, but also likely interact with one another through epigenetic mechanisms (Rohde et al., 2019)—it may be valuable to consider whether those with relatively more “socio-behaviorally” induced obesity evince systemically different outcomes compared to those whose obesity is more genetically influenced. While we recognize the crude distinction of this classification system, we hypothesize that the association between obesity and mortality will be substantially higher for those with a relatively low genetic propensity for obesity compared to those with a high genetic propensity for obesity. In this study, we use a polygenic risk score (PGS; Dudbridge, 2013) as the measure of genetic propensity, which is a single score reflecting the accumulation of common risk variants across the genome. While all people have some socio-behavioral and genetic risk, we assume that obesity for those with low polygenic risk may have more to do with socio-behavioral risks like general health lifestyle (Cockerham, 2005) when compared to those with a high polygenic risk for obesity. We intuit that these socio-behavioral risk factors (e.g., inactivity, caloric abundance) plausibly carry more severe health consequences for those with a more socio-behavioral disposition to obesity compared to their peers for whom BMI disposition is more largely influenced by polygenic liability. To evaluate this hypothesis, we model the risk of mortality as a function of obesity status, polygenic risk for obesity, and an interaction between the two variables.
Methods
We use a sample of 11,843 non-Hispanic white adults aged 50 years or older from the Health and Retirement Study (HRS) for the years 1994–2014. Genetic data for the HRS are based on samples collected in two phases. First, via buccal swabs in 2006 using the Qiagen Autopure method. Second, via saliva samples collected in 2008 and extracted with Oragene. Genotype calls were then made with the Illumina HumanOmni2.5-4v1 array (National Center for Biotechnology Information, 2012). Using STATA 15, we estimate cox proportional hazard models for the full sample and separately for men and women. All models control for age and sex (in the full model). PGSs were created using results from a large genome-wide association study of BMI in over 300,000 participants of European ancestry (Locke et al., 2015), which provided estimates of the effect size for common genetic variants (i.e., alleles) across the genome on obesity. For each individual in the HRS, a PGS was constructed by adding the number of risk alleles (i.e., those associated with risk for higher BMI) and weighting each risk by the strength of its association with BMI as estimated in Locke and colleagues (2015) (Ware et al., 2018). The resulting PGS has been shown to explain about 20% of the variation in BMI (Locke et al., 2015). Within the HRS, we can explain 6.91% (R2 = 0.0691) of the variation in average BMI across all waves among non-Hispanic whites with the BMI PGS. Further, recent work has shown that high PGS for BMI is associated with a 19% increase in the risk of mortality, suggesting that it is a useful predictor of mortality (Khera et al., 2019). We differentiated between low (referent; PGS ≤ −1σ), average (−1σ < PGS < 1σ), and high (PGS ≥ 1σ) PGS and non-obese1 (referent; 18.5–29.9), obese I (30.0–34.9), and obese II/III (≥35) for BMI. Controls include years of education (0–17+; mean-centered at 13.26 years) and average self-reported functional limitation2 across all waves (0–5; mean = 0.48). Due to potential confounds (i.e., spurious allele associations) that may arise due to systematic differences in allele frequencies across groups with different genetic ancestries (Martin et al., 2017), we limited our sample to non-Hispanic white respondents. Further, we controlled for the top ten principal components derived from the genome-wide data using standard techniques (Patterson, Price, & Reich, 2016). The inclusion of these genetic principal components further rules out the likelihood that our measure of polygenic risk is simply reflecting the observed differences across the genome among those of European ancestry (Price et al., 2006) that are also correlated with obesity but not causal.
Results
Overall, 28.1% of our sample reported an average BMI above 30. We did not observe any sex differences in class I obesity, but there were notable differences in the prevalence of class II/III for women (10.4%) compared to men (7.7%).3 Importantly, there is a clear association between PGS and obesity classification (χ 2 = 462.39, p < .001). Adults with a high PGS for obesity were nearly 2.5 times more likely to be obese (33.5%) compared to those with a low PGS (13.9%). Increasing the BMI PGS from the average level to the high level increased the relative odds of obesity by 102% (odds ratio [OR] = 2.02) and this pattern was consistent for women (OR = 1.96) and men (OR = 2.15).
Table 1 presents the results of cox proportional hazard models for mortality for the total sample and separately by sex and denote the focus of this article. In the total sample, obese I adults (hazard ratio [HR] = 0.79, p = .366) show no difference in their risk for mortality when compared to non-obese adults, and obese II/III adults (HR = 3.17, p = .000) present higher risk of mortality when compared to non-obese adults. Among non-obese adults, low PGS reduced the risk of mortality, suggesting that low PGS may serve a protective effect above and beyond BMI. Similarly, those with high PGS were at higher odds for mortality when compared to low PGS respondents, which substantively suggests that higher PGS increases risk for mortality among normal-weight adults. The direction of the interaction is consistent with our primary hypothesis. The association of obese II/III on mortality increased substantially among those with the low PGS (e.g., the socio-behaviorally obese). We did not find evidence for a reduced effect of high PGS, or genetic obesity, compared to those with an average PGS.
Table 1.
The Interactive Effect of Obesity and Polygenic Risk for Obesity on Increased Risk of Mortality Among Adults in the United States
| Total | Female | Male | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| BMI [Ref. 18.5–29.9] | ||||||
| Obese I [30.0–34.9] | 0.79 | 0.48, 1.31 | 0.61 | 0.25, 1.50 | 0.84 | 0.46, 1.57 |
| Obese II/III [≥35.0] | 3.17 | 1.73, 5.81 | 3.94 | 1.72, 9.04 | 2.63 | 1.07, 6.46 |
| BMI PGS [Ref. Low ≤ −1σ] | ||||||
| Average [−1σ< PGS < 1σ] | 1.18 | 1.02, 1.37 | 1.31 | 1.06, 1.63 | 1.07 | 0.88, 1.31 |
| High [PGS ≥ 1σ] | 1.33 | 1.09, 1.61 | 1.62 | 1.18, 2.09 | 1.10 | 0.84, 1.44 |
| Interaction effects | ||||||
| Obese I × Average | 1.37 | 0.80, 2.32 | 1.79 | 0.71, 4.52 | 1.23 | 0.64, 2.36 |
| Obese II/III × Average | 0.45 | 0.24, 0.85 | 0.39 | 0.16, 0.94 | 0.53 | 0.20, 1.39 |
| Obese I × High | 1.38 | 0.78, 2.46 | 1.73 | 0.65, 4.66 | 1.29 | 0.63, 2.65 |
| Obese II/III × High | 0.37 | 0.18, 0.75 | 0.33 | 0.13, 0.86 | 0.41 | 0.14, 1.17 |
| Years of education | 0.95 | 0.94, 0.97 | 0.94 | 0.92, 0.97 | 0.95 | 0.93, 0.97 |
| Average self-report limitation | 1.42 | 1.32, 1.55 | 1.53 | 1.37, 1.71 | 1.39 | 1.23, 1.57 |
Note: BMI = body mass index; CI = confidence interval; HR = hazard ratio; PGS = polygenic risk score. Data come from the Health and Retirement Study (HRS) for the years 1994–2014. Cell entries are Hazard Ratios derived from Cox Proportional Hazard models among 11,843 respondents (female = 6,739; male = 5,104) of the HRS. All models control for age and the top 10 principal components derived from genome-wide data.
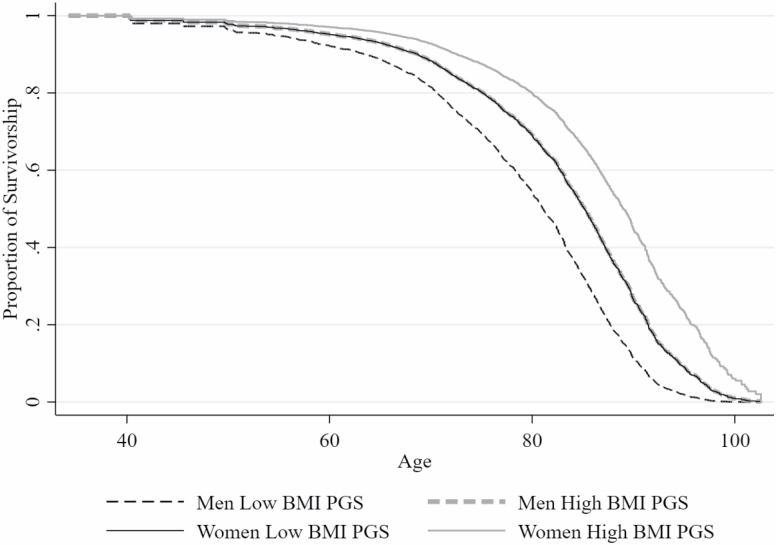
Evaluating these same associations among men and women reveal important differences. First, the association between obesity and mortality is higher among women compared to men. When compared to non-obese adults, neither obese I men (HR = 0.84, p = .593) or women (HR = 0.61, p = .281) show higher risk of death. While both obese II/III men (HR = 2.63, p = .034) and women (HR = 3.94, p = .001) are at significantly greater risk for mortality than the non-obese, the association remains notably higher for women. Second, the association between the interactions describe above (i.e., BMI and PGS) and the risk of mortality are only significant among women. To better illustrate how socio-behaviorally oriented obesity may impact mortality risk, we present survival curves among obese II/III men and women separately as a function of their polygenic risk for obesity (Figure 1). The most important comparison is the two solid lines that differentiate between obese II/III women with a low PGS (the solid black line) and those with a high PGS (the solid gray line). Keeping in mind that the number of men in the obese II/III category is nearly one-half (n = 394) the number of women in this category (n = 703) and while we do not find a significant association among men, it is important to note that a very similar same association is evident among men (the dashed lines).
Figure 1.
Survival curve across age for male and female older adults in the obese II/III category by polygenic risk score
Discussion
We see the expected gradients for obesity on mortality among those with average or high polygenic risk for obesity. We also show that the increased risk of mortality associated with obese II/III depends on cumulative genetic risk for obesity such that a small group of obese older adults with a low polygenetic risk may experience the greatest risk of mortality compared to other groups; socio-behaviorally obese women are at particularly high risk of death. An optimistic interpretation of these results is that these pathways are modifiable. The social and behavioral factors that contribute to obesity can be addressed in order to reduce the risk of obesity-related mortality, especially among women in the US. The increase in obesity prevalence that has been noted by others (Ogden, Carroll, Kit, & Flegal, 2014) cannot be due to changes in the prevalence of risk alleles associated with the morbidity but rather changes in the social and behavioral lifestyles of adults in the United States over the past 30–40 years. Our results echo this, but we note that genotype provides important information about who is at the greatest risk of mortality associated with obesity. By understanding one’s unique genetic risk for elevated BMI, it is possible to characterize obesity as a uniquely risky factor related to mortality.
Limitations
There are several limitations to this study. First, we lack data in early life, which may provide important information about life course exposures (Hayward & Gorman, 2004). Second, this data is limited to the United States and does not provide insight into international trends. Third, these analyses are primarily based on self-report data. Fourth, this initial investigation was limited to non-Hispanic and white respondents for statistical reasons described above. It is important to replicate these analyses among all ethnic and racial groups as PGS based on GWAS that includes larger samples of nonwhites become available to researchers. Finally, the goal of this study was to characterize polygenic risk for obesity without detailing the full contours of socio-behavioral and environmental risks. It is our hope that the results presented here inspire future studies that more rigorously investigate these social and environmental exposures, such as neighborhood walkability (Kowaleski-Jones et al., 2017), in older adulthood and throughout the life course.
Conclusion
To the best of our knowledge, this represents the first empirical analysis of the interactive relationship between polygenic risk for BMI, reported BMI, and mortality. It is commonplace for both physicians and researchers to identify family history and socio-behavioral risk factors to better understand the risk of obesity and associated mortality. Thus, the distinction between obesity as a result of family factors, which include genetics, or obesity as a result of behaviors and overall health lifestyle (Cockerham, 2005) unique to a specific individual is nothing new. To date, however, understanding this distinction with respect to identifying the mechanisms responsible for an individual’s selection into obesity, has not been part of the standard toolkit of gerontologists, demographers, or epidemiologists. We believe that the identification and consideration of individually oriented polygenic risk, alongside these familial and socio-behavioral factors, maybe a central component to understanding the risk and prevalence of obesity. We encourage other researchers to extend this same approach to other important morbidities especially those with a strong behavioral component. We believe that this understanding is very much in line with Fundamental Cause Theory (Link and Phelan, 1995), which argues that the proximate determinants of death have changed considerably over time from infectious to chronic disease, and yet those at the lowest rung of the economic ladder are still more likely to die earlier compared to those at the top of the ladder. As such, socioeconomic status remains a fundamental determinant of health. We believe that by characterizing the composition of the obese population as primarily (but not exclusively) social and genetic paths of entry, our work demonstrates that the link between obesity and mortality may be primarily due to social rather than genetic pathways. Thus, while some have been critical of the use of genetics in social epidemiologic research (Shostak, 2003) because of the overly inward focus, we hope to have made the case that the use of individual polygenic risk for a specific morbidity can change the focus to an outward orientation which stresses and reinforces the perspective that the social environment remains a fundamental feature of individual and population health.
Funding
This work was supported, in part, by grants from the National Institute on Aging (T32AG052371) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (P2CHD066613). The Health and Retirement Study is supported by the Division of Behavioral and Social Research of the National Institute on Aging of the National Institutes of Health (U01 AG009740, RC2AG036495, and RC4AG039029) and is conducted by the University of Michigan.
Author Contributions
J. Vinneau was lead author and responsible for data preparation and analysis. All authors were involved in conceptualizing the study and contributed to draft writing and revisions.
Conflict of Interest
None reported.
Supplementary Material
Footnotes
“Non-obese” excludes underweight respondents (n = 85; 0.71% of the genotyped non-Hispanic white sample). Models including the underweight sample show the same trends but with less precision.
This measure is an average score of “instrumental activities of daily living” limitations that are self-reported at each wave for five activities: using a telephone, taking medication, handling money, shopping, and preparing meals. Respondents can report between 0 and 5 limitations in each wave of the HRS.
Please see Supplementary Tables 1 and 2 for full descriptive and bivariate statistics.
References
- Adams K. F., Schatzkin A., Harris T. B., Kipnis V., Mouw T., Ballard-Barbash R.,…Leitzmann M. F (2006). Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. The New England Journal Of Medicine, 355, 763–778. doi:10.1056/NEJMoa055643 [DOI] [PubMed] [Google Scholar]
- Ahima R. S., & Lazar M. A (2013). Physiology. The health risk of obesity–better metrics imperative. Science, 341, 856–858. doi:10.1126/science.1241244 [DOI] [PubMed] [Google Scholar]
- Barrientos-Gutierrez T., Moore K. A. B., Auchincloss A. H., Mujahid M. S., August C., Sanchez B. N., & Diez Roux A. V (2017). Neighborhood physical environment and changes in body mass index: Results from the Multi-Ethnic Study of Atherosclerosis. American Journal of Epidemiology, 186, 1237–1245. doi:10.1093/aje/kwx186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockerham W. C. (2005). Health lifestyle theory and the convergence of agency and structure. Journal of Health and Social Behavior, 46, 51–67. doi:10.1177/002214650504600105 [DOI] [PubMed] [Google Scholar]
- Dhana K., Braun K. V. E., Nano J., Voortman T., Demerath E. W., Guan W.,…Dehghan A (2018). An epigenome-wide association study of obesity-related traits. American Journal of Epidemiology, 187, 1662–1669. doi:10.1093/aje/kwy025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudbridge F. (2013). Power and predictive accuracy of polygenic risk scores. PLoS Genetics, 9, e1003348. doi:10.1371/journal.pgen.1003348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal K. M., Kit B. K., & Graubard B. I (2017). Bias in hazard ratios arising from misclassification according to self-reported weight and height in observational studies of body mass index and mortality. American Journal of Epidemiology, 187, 125–134. doi:10.1093/aje/kwx193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal K. M., Kit B. K., Orpana H., & Graubard B. I (2013). Association of all-cause mortality with overweight and obesity using standard body mass index categories: A systematic review and meta-analysis. JAMA, 309, 71–82. doi:10.1001/jama.2012.113905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianfrancesco M. A., Glymour M. M., Walter S., Rhead B., Shao X., Shen L.,…Barcellos L. F (2017). Causal effect of genetic variants associated with body mass index on multiple sclerosis susceptibility. American Journal of Epidemiology, 185, 162–171. doi:10.1093/aje/kww120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward M. D., & Gorman B. K (2004). The long arm of childhood: The influence of early-life social conditions on men’s mortality. Demography, 41, 87–107. doi:10.1353/dem.2004.0005 [DOI] [PubMed] [Google Scholar]
- Khera A. V., Chaffin M., Wade K. H., Zahid S., Brancale J., Xia R.,…Kathiresan S (2019). Polygenic prediction of weight and obesity trajectories from birth to adulthood. Cell, 177, 587–596.e9. doi:10.1016/j.cell.2019.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowaleski-Jones L., Grown B. B., Fan J. X., Hanson H. A., Smith K. R., & Zick C. D (2017). The joint effects of family risk of obesity and neighborhood environment on obesity among women. Social Science & Medicine, 195, 17–25. doi:10.1016/j.socscimed.2017.10.018 [DOI] [PubMed] [Google Scholar]
- Link B. G., & Phelan J (1995). Social conditions as fundamental causes of disease. Journal of Health and Social Behavior, Spec No, 80–94. doi:10.2307/2626958 [PubMed] [Google Scholar]
- Locke A. E., Kahali B., Berndt S. I., & Speliotes E. K (2015). Genetic studies of body mass index yield new insights for obesity biology. Nature, 518, 197–206. doi:10.1038/nature14177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. R., Gignoux C. R., Walters R. K., Wojcik G. L., Neale B. M., Gravel S.,…Kenny E. E (2017). Human demographic history impacts genetic risk prediction across diverse populations. American Journal of Human Genetics, 100, 635–649. doi:10.1016/j.ajhg.2017.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters R. K., Reither E. N., Powers D. A., Yang Y. C., Burger A. E., & Link B. G (2013). The impact of obesity on US mortality levels: The importance of age and cohort factors in population estimates. American Journal of Public Health, 103, 1895–1901. doi:10.2105/AJPH.2013.301379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Biotechnology Information dbGaP (2012). Health and Retirement Study Bethesda, MD: National Center for Biotechnology Information; Retrieved from www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000428.v1.p1 [Google Scholar]
- Ogden C. L., Carroll M. D., Kit B. K., & Flegal K. M (2014). Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA, 311, 806–814. doi:10.1001/jama.2014.732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson N., Price A. L., & Reich D (2016). Population structure and eigenanalysis. PLoS Genetics, 2, e190. doi:10.1371/journal.pgen.0020190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price A. L., Patterson N. J., Plenge R. M., Weinblatt M. E., Shadick N. A., & Reich D (2006). Principal components analysis corrects for stratification in genome-wide association studies. Nature genetics, 38, 904–909. doi:10.1038/ng1847 [DOI] [PubMed] [Google Scholar]
- Read S. H., Lewis S. C., Halbesma N., & Wild S. H (2017). Measuring the association between body mass index and all-cause mortality in the presence of missing data: Analyses from the Scottish National Diabetes Register. American Journal of Epidemiology, 185, 641–649. doi:10.1093/aje/kww162 [DOI] [PubMed] [Google Scholar]
- Rohde K., Keller M., la Cour Poulsen L., Blüher M., Kovacs P., & Böttcher Y (2019). Genetics and epigenetics in obesity. Metabolism: Clinical and Experimental, 92, 37–50. doi:10.1016/j.metabol.2018.10.007 [DOI] [PubMed] [Google Scholar]
- Shostak S. (2003). Locating gene-environment interaction: At the intersections of genetics and public health. Social Science & Medicine (1982), 56, 2327–2342. doi:10.1016/s0277-9536(02)00231-9 [DOI] [PubMed] [Google Scholar]
- Ware E. B., Schmitz L. L., Gard A. M., & Faul J (2018). HRS polygenic scores – release 2. Ann Arbor, MI: Survey Research Center, Institute for Social Research, University of Michigan. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.