Abstract
Background
Experimental human immunodeficiency virus (HIV)-1 vaccines frequently elicit antibodies against HIV-1 that may react with commonly used HIV diagnostic tests, a phenomenon known as vaccine-induced seropositivity/seroreactivity (VISP/VISR). We sought to determine, under clinic conditions, whether a patient-controlled HIV test, OraQuick ADVANCE Rapid HIV-1/2 Antibody Test, detected HIV-1 vaccine-induced antibodies.
Methods
Plasma assessment of HIV-1 cross-reactivity was examined in end-of-study samples from 57 healthy, HIV-uninfected participants who received a candidate vaccine that has entered Phase 2B and 3 testing. We also screened 120 healthy, HIV-uninfected, unblinded HIV-1 vaccine participants with VISP/VISR for an assessment using saliva. These participants came from 21 different parent vaccine protocols representing 17 different vaccine regimens, all of which contained an HIV-1 envelope immunogen. OraQuick ADVANCE was compared with results from concurrent blood samples using a series of commercial HIV screening immunoassays.
Results
Fifty-seven unique participant plasma samples were assayed in vitro, and only 1 (1.8%) was reactive by OraQuick ADVANCE. None of the 120 clinic participants (0%; 95% confidence interval, 0% to 3.7%) tested positive by OraQuick ADVANCE, and all were confirmed to be uninfected by HIV-1 viral ribonucleic acid testing. One hundred eighteen of the 120 (98.3%) participants had a reactive HIV test for VISP/VISR: 77 (64%) had at least 1 reactive fourth-generation HIV-1 diagnostic test (P < .0001 vs no reactive OraQuick ADVANCE results), and 41 (34%) only had a reactive test by the less specific third-generation Abbott Prism assay.
Conclusions
These data suggest that this widely available patient-controlled test has limited reactivity to HIV-1 antibodies elicited by these candidate HIV-1 vaccines.
Keywords: HIV diagnostics, HIV vaccine, immunogenicity, vaccine safety
Experimental HIV-1 vaccines frequently elicit antibodies which cross-react with common HIV diagnostic tests. Vaccine-induced seropositivity/seroreactivity (VISP/VISR) can be problematic for clinical trial volunteers. We tested a point-of-care HIV test, OraQuick ADVANCE, to determine if it could detect VISP/VISR.
Control of the human immunodeficiency virus (HIV)-1 pandemic will almost certainly require a safe and effective vaccine [1–3]. To successfully identify an effective HIV-1 vaccine, numerous additional vaccine concepts are currently being tested in an iterative fashion [2, 4, 5]. These experimental vaccines are designed to elicit anti-HIV-1 immune responses, some of which induce anti-HIV-1 antibodies that can be detected by commonly used diagnostic tests used for screening for HIV infection. Vaccine-induced seropositivity (VISP) or vaccine-induced seroreactivity (VISR) is therefore an anticipated likely occurrence with participation in HIV vaccine trials [6] and has been identified as a common reason why potential participants decline participation [7, 8]. Furthermore, VISP/VISR can result in social harms such as misdiagnosis of HIV infection status [9, 10]. During the active phase of an HIV-1 vaccine clinical trial, knowledge of VISP/VISR could result in unblinding of the participant or study staff, and therefore blinding measures need to be undertaken for any on-study HIV tests that are performed. Because memory B cell responses can be long-lived, VISP/VISR may persist well after study participation ends [11], and clinical research sites and sponsors need to mitigate potential harms from the misinterpretation of VISP/VISR results [12]. The HIV Vaccine Trials Network (HVTN) has established a comprehensive program (https://www.hvtn.org/en/participants/visp-hiv-testing.html) to mitigate these risks for participants in their studies. This commitment provides HIV testing to vaccine recipients for as long as necessary to distinguish between vaccine responses and HIV infection, including for vaccinees who have relocated from any clinical trial sites.
For non-HVTN protocols, to meet the ethical obligations of assisting vaccinees poststudy, clinical trial sites may continue to perform all HIV tests [12]. However, poststudy testing for participants who relocate poses considerable logistical challenges including potentially establishing a contract with a phlebotomy service to obtain and ship the sample as well as with a central laboratory that can perform HIV testing to distinguish between VISP/VISR and HIV infection. Alternative methods of rapidly and accurately distinguishing VISP/VISR from actual HIV infection are needed, and the development of such tests is currently being supported [12, 13].
Human immunodeficiency virus testing kits using oral fluid are available and marketed both for rapid testing in emergency rooms, clinicians’ offices, as well as for home self-testing [14–16]. We hypothesized that the antibodies elicited by candidate HIV vaccines would not be detected by one of these patient-controlled testing kits (OraQuick ADVANCE), in contrast to a series of commercial HIV screening immunoassays performed on concurrent blood samples.
METHODS
Participants and Study Design
A subset of plasma samples from participants who enrolled in the Phase 1/2a HVTN 117/HPX2004 vaccine study [17] were assayed using the OraQuick ADVANCE Rapid HIV-1/2 Antibody Test (OraSure Technologies, Inc., Bethlehem, PA) as per manufacturer’s instructions for blood testing [18]. The OraQuick immunoassay platform uses gp41- and gp36-derived antigens for detection [19]. Participants had been randomized to 2 doses of either adenovirus serotype 26 (Ad26) delivering trivalent or tetravalent mosaic HIV immunogens followed by 2 doses of the same Ad26 in combination with clade C Env gp140 or placebo [17]. These plasma samples had been designated for evaluation of HIV seroreactivity (EOS) and were drawn at a participant’s end-of-study (final) visit, 24 weeks after their final vaccination. These plasma samples comprised all the available EOS samples from US participants in the HVTN 117/HPX2004 study as of May 2018; this subset represents approximately half of US enrollees. Because the parent study was still blinded at the time, samples were recoded with a sequential number so that the HIV results did not inadvertently unblind staff as to which participants received product versus placebo.
The point-of-care OraQuick ADVANCE saliva assessment focused on participants enrolled at the Brigham and Women’s Hospital (BWH) Clinical Research Site who had previously received a candidate HIV-1 vaccine and are followed in 1 of 2 long-term follow-up protocols that assess the persistence of VISP/VISR. The first protocol (HVTN 910) follows participants from HVTN-coordinated preventive HIV vaccine trials [20], whereas the second is a site-specific protocol that provides follow-up testing for participants from HIV-1 vaccine studies conducted at BWH in Boston, Massachusetts. We screened 120 healthy, HIV-uninfected, prior candidate HIV-1 vaccine recipients who were followed at BWH (Table 1).
Table 1.
Oral Fluid Participant Demographics
| Demographic | Number (%) |
|---|---|
| Sex | |
| Female | 69 (58%) |
| Ethnicity | |
| Hispanic | 10 (8%) |
| Race | |
| Black or African American | 11 (9%) |
| Asian | 8 (7%) |
| White or Caucasian | 92 (77%) |
| Mixed or Other | 9 (8%) |
Oral fluid testing was performed in the clinic using the OraQuick ADVANCE by the participant by swabbing the outer gums per manufacturer’s instructions [18]. A blood sample was taken concurrently. Immediately after the oral sample collection, the test device was inserted into the vial of developer solution outside of the participant’s view. After 20–40 minutes of incubation, the device was read and the result of the test was recorded. Standard pretest and posttest HIV counseling was provided to the participants along with the results of their blood tests as per the Centers for Disease Control and Prevention (CDC) guidelines [21].
For the concurrent blood testing as well as for EOS evaluation in HVTN 117/HPX2004 participants, 3 different fourth-generation antibody/antigen-based HIV-1 diagnostic tests were used: Bio-Rad GS HIV Combo Ag/Ab EIA, Abbott Architect HIV Ag/Ab Combo, and Alere Determine HIV-1/2 Ag/Ab Combo. The Bio-Rad GS and Abbott Architect platforms provide a quantitative sample to cutoff (S/CO) ratio for each assay [22]; S:CO ratios between 0.7 and 0.99 were reported as “equivocal.” Several groups have reported that S:CO ratios on the Abbott Architect platform typically exceed 100 in individuals chronically infected with HIV [22, 23]. If reactivity was noted on any of the fourth-generation tests, quantitative HIV-1 viral load testing was done using the Abbott m2000 RealTime PCR HIV-1 platform. If no reactivity was noted on any of the fourth-generation tests, then samples were further assayed with the less specific third-generation Abbott Prism O Plus Anti-HIV-1/2 test.
Patient Consent Statement
This protocol was approved by the BWH institutional review board and written informed consent was obtained from each participant.
Vaccines
The parent protocols (n = 21) in which the oral fluid testing participants had been vaccinated are summarized in Table 2. Most participants had received vaccines delivering gp140 envelope (Env) constructs; gp160 constructs were used in HVTN 065, HVTN 094, HVTN 106 (MVA boost delivered gp150), HVTN 114, and HVTN 205. The Env protein boosts were given in HVTN 117, HVTN 118, and IPCAVD 009 (trimeric gp140) as well as HVTN 114 (monomeric gp120). Participants had been vaccinated between 8 months and 13 years, 11 months (median 34.5 months) before the oral fluid HIV testing.
Table 2.
Vaccine Regimens Previously Administered to Clinic Participants Who Underwent Oral Fluid Testing
| Parent Protocol | Number | Prime-Boost Strategy | Participants and Regimens | Env Immunogen | Reference |
|---|---|---|---|---|---|
| HVTN 057 | 1 | DNA + viral vector | DNA + Ad5 | gp140 | NCT00091416 |
| HVTN 065 | 3 | DNA + viral vector | DNA + MVA | gp160 | [24] |
| HVTN 069 | 1 | DNA + viral vector | DNA + Ad5 | gp140 | [25] |
| HVTN 077 | 1 | DNA + viral vector | DNA + Ad35 | gp140 | [26] |
| 1 | Viral vector | Ad35 + Ad5 | |||
| HVTN 082 | 2 | DNA + viral vector | DNA + Ad5 | gp140 | NCT01054872 |
| HVTN 083 | 1 | Viral vector | Ad5 | gp140 | [27] |
| 1 | Viral vector | Ad35 + Ad5 | |||
| HVTN 085 | 2 | Viral vector | Ad5 | gp140 | NCT01479296 |
| HVTN 094 | 5 | DNA + viral vector | DNA + MVA | gp160 | [28] |
| HVTN 106 | 4 | DNA + viral vector | DNA + MVA | gp160 + gp150 | NCT02296541 |
| HVTN 114 | 1 | DNA + viral vector + protein | DNA + MVA + gp120 | gp160 + gp120 | NCT02852005 |
| HVTN 117/ | 28 | Viral vector + protein | Ad26 + gp140 | gp140 | [17] |
| HPX2004 | 1 | Viral vector | Ad5 + Ad26a | ||
| HVTN 118/ HPX2003 | 10 | Viral vector + protein | Ad26 + gp140 | gp140 | NCT02935686 |
| 1 | Viral vector | Ad26b | |||
| HVTN 204 | 3 | DNA + viral vector | DNA + Ad5 | gp140 | [29] |
| HVTN 205 | 1 | DNA + viral vector | DNA + MVA | gp160 | [30] |
| HVTN 505 | 8 | DNA + viral vector | DNA + Ad5 | gp140 | [31] |
| 1 | DNA | DNAc | |||
| IPCAVD 001 | 1 | Viral vector | 1 × 109 Ad26 | gp140 | [32] |
| 2 | Viral vector | 1 × 1010 Ad26 | |||
| IPCAVD 002 | 1 | Viral vector | 1 × 109 Ad5HVR48 | gp140 | [33] |
| 2 | 1 × 1010 Ad5HVR48 | ||||
| 3 | 1 × 1011 Ad5HVR48 | ||||
| IPCAVD 003 | 1 | Viral vector | 5 × 1010 rAd26 | gp140 | [34] |
| IPCAVD 004/ | 3 | Viral vector | Ad26 + Ad35 | gp140 | [35] |
| IAVI B003/ HVTN 091 | 2 | Viral vector | Ad35 + Ad26 | ||
| IPCAVD 006 | 2 | Viral vector | MVA | gp140 | [36] |
| 3 | Viral vector | 1 × 1010 rAd26 + MVA | |||
| 1 | Viral vector | 1 × 1011 rAd26 + MVA | |||
| IPCAVD 009/ HIV-V-A004 | 9 | Viral vector + protein | Ad26 + MVA + gp140d | gp140 | [5] |
| 7 | Ad26 + MVA | ||||
| 3 | Ad26 | ||||
| 4 | Ad26 + gp140 |
Abbreviations: Ad, adenovirus; DNA, deoxyribonucleic acid; Env, envelope; HVTN, HIV Vaccine Trial Network; IAVI, International AIDS Vaccine Initiative; IPCAVD, Integrated Preclinical/Clinical AIDS Vaccine Development; MVA, modified vaccinia Ankara.
aAd5-vectored vaccine received via prior enrollment in HVTN 084 [NCT01159990], not known by site staff until after first vaccination with Ad26-vectored vaccine.
bParticipant missed Ad26 + trimeric Env gp140 boosters.
cParticipant missed Ad5 booster.
dOne participant missed final MVA + gp140 booster.
Statistical Methods
All analyses of the oral fluid data in this post hoc, cross-sectional, cross-protocol study are based on the per-protocol (PP) principle because participants were recruited based on known EOS VISP positivity; analyses of the plasma EOS samples were blinded as to product allocation before study unblinding. Results of the oral fluid and plasma assays are reported as proportions. Differences in proportions were tested with 2-sided McNemar’s test. Two-sided 95% confidence intervals (CIs) for binomial proportions were calculated using the score test method [37]. Tests with a 2-sided P < .05 were considered significant. No adjustment for multiple comparisons was made.
RESULTS
Assessment of OraQuick ADVANCE Reactivity Using Plasma
Using routine fourth-generation antigen/antibody (Ag/Ab) tests, blinded HVTN 117/HPX2004 participant EOS plasma samples had a high rate of reactivity. Most (66 of 71 [93%]) samples were concordant across the 3 platforms. Two samples were equivocal by the Bio-Rad GS but nonreactive by the Abbott Architect and Alere Determine. One sample was nonreactive by the Bio-Rad GS but reactive by the Abbott Architect and Alere Determine. Two samples were nonreactive by the Alere Determine but reactive by the Bio-Rad GS and Abbott Architect. Samples that tested nonreactive on all 3 fourth-generation assays were further tested by the Abbott Prism assay.
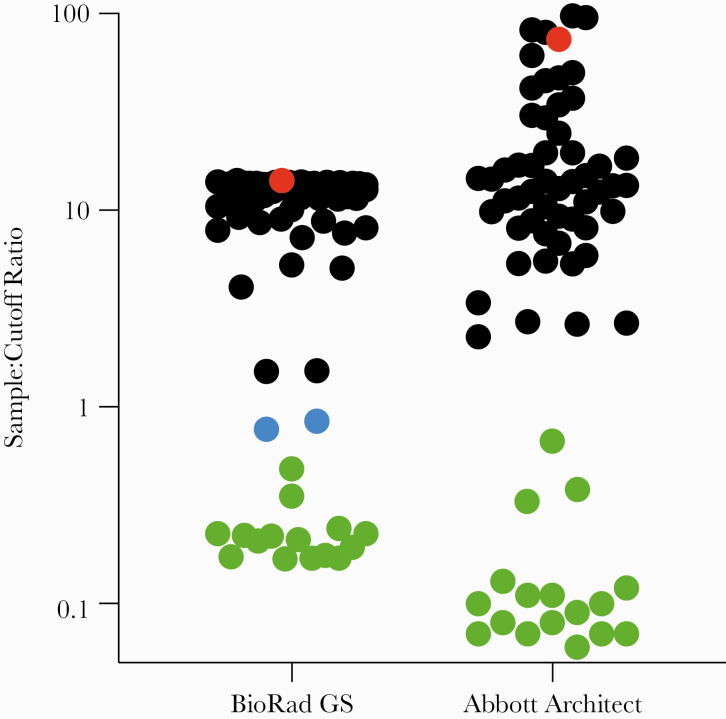
After unblinding of the primary study, we found that the 14 samples that tested nonreactive by all 4 platforms were from placebo recipients. When the 57 plasma samples from active vaccinees were assayed using OraQuick ADVANCE (Table 3), only 1 of 57 (1.8%; 95% CI, 0% to 10.2%) was reactive. The single sample that cross-reacted with OraQuick ADVANCE had a high S/CO ratio on both the Bio-Rad GS and Abbott Architect platforms (Figure 1). All samples that tested positive by any of the immunoassays were found to be negative by HIV-1 ribonucleic acid (RNA) testing.
Table 3.
Analysis of OraQuick ADVANCE Cross-Reactivity With Plasma Samples From HVTN 117/HPX2004
| HIV-1 Diagnostic Test | Participants Testing Reactive n (%) | P Value vs OraQuick ADVANCE |
|---|---|---|
| OraQuick ADVANCE | 1 of 57 (1.8%) | – |
| Bio-Rad GS | 53 of 57 (93%) | <.0001 |
| Abbott Architect | 54 of 57 (95%) | <.0001 |
| Alere Determine | 52 of 57 (91%) | <.0001 |
| Abbott Prisma | 1 of 1 (100%) | – |
Abbreviations: HIV, human immunodeficiency virus; HVTN, HIV Vaccine Trial Network.
aAbbott Prism was only used if samples tested negative on all 3 of the fourth-generation antigen/antibody tests (n = 15).
Figure 1.
Assessment of fourth-generation human immunodeficiency virus (HIV) tests compared with OraQuick ADVANCE. End-of-study plasma samples from participants in the HIV Vaccine Trial Network (HVTN) 117/HPX2004 study were assayed for vaccine-induced seropositivity using 2 routine fourth-generation HIV tests. Sample/cutoff ratios are given: nonreactive samples are green, samples reported as equivocal are blue, reactive samples are black except for the lone sample that was reactive by OraQuick ADVANCE, which is red.
Assessment of OraQuick ADVANCE Reactivity Using Oral Fluid
The 120 subjects that participated in the saliva study were derived from 21 different candidate HIV-1 vaccine protocols. Of the 120 participants, 76 received an Ad26-vectored HIV-1 vaccine (Table 2), which included the following: 8 received Ad26 alone, 11 received Ad26 prime with a modified vaccinia Ankara (MVA) boost, 42 received Ad26 with a protein boost, 9 received Ad26 with both MVA and protein boost, and 5 received Ad26 and Ad35 in a heterologous prime/boost study; 1 participant received Ad5 before Ad26 because of inadvertent prior enrollment. In addition, 27 received other adenovirus vectors: 3 received Ad5, 15 received deoxyribonucleic acid (DNA) priming with an Ad5 boost, 2 received Ad35 and Ad5 heterologous prime-boosting, 1 received a DNA prime and an Ad35 boost, and 6 received an Ad5HVR48 chimeric vector. The remaining 17 received a DNA prime with an MVA boost: 14 received DNA and MVA, 2 received MVA alone, and 1 received DNA alone. All participants had received vaccines that included an Env immunogen.
None of the 120 participants tested reactive (0%; 95% CI, 0% to 3.7%) by OraQuick ADVANCE, and all were negative by HIV-1 RNA testing. When comparing the participants’ plasma test results (Table 4), 77 (64%) tested reactive by 1 or more of the fourth-generation antibody/antigen-based HIV-1 diagnostic assays; 65 (54%) were reactive by Bio-Rad GS HIV Combo Ag/Ab EIA, 76 (63%) were reactive by Abbott Architect HIV Ag/Ab Combo, and 58 (48%) were reactive by Alere Determine HIV-1/2 Ag/Ab Combo. Fifty-four participants were reactive by all 3 standard assays; 14 participants were reactive by 2 of the assays (either Bio-Rad GS and Abbott Architect or Alere Determine and Abbott Architect); and 9 participants were reactive by 1 of the assays (8 on the Abbott Architect and 1 who was only reactive by Bio-Rad GS). The remaining 41 (34%) participants were only reactive by the Abbott Prism assay. Two participants did not test reactive by any blood diagnostic test.
Table 4.
Analysis of OraQuick ADVANCE Saliva Cross-Reactivity With Blood Tests
| HIV-1 Diagnostic Test | Participants Testing Reactive n (%) | P Value vs OraQuick ADVANCE |
|---|---|---|
| OraQuick ADVANCE | 0 of 120 (0%) | – |
| Bio-Rad GS | 65 of 120 (54%) | <.0001 |
| Abbott Architect | 76 of 120 (63%) | <.0001 |
| Alere Determine | 58 of 120 (48%) | <.0001 |
| Abbott Prisma | 41 of 43 (95%) | <.0001 |
Abbreviations: HIV, human immunodeficiency virus.
aAbbott Prism was only used if samples tested negative on all 3 of the fourth-generation antigen/antibody tests (n = 41).
Of note, after unblinding, the lone plasma sample that tested positive by OraQuick ADVANCE was found to have come from a participant at the BWH site who had received the tetravalent Ad26 and clade C gp140 Env regimen. This participant’s oral fluid tested negative by OraQuick ADVANCE, although the 2 assays were performed 14 months apart.
DISCUSSION
Persistence of HIV-1 vaccine-elicited antibodies can lead to diagnostic challenges as well as adverse social impacts in participants who received candidate HIV-1 vaccines [10, 12]. Our data suggest that the OraQuick ADVANCE oral point-of-care test infrequently detects HIV-1 antibodies elicited by the different candidate HIV-1 vaccines evaluated in these trials. Given the low rates of reactivity with plasma and oral fluid, it is possible that the HIV-1-derived antigens used in this point-of-care assay have limited epitope overlap with the immunogens in some of these candidate vaccines. Alternatively, there could be differences in epitope presentation or immunogenicity between vaccination and infection. However, because oral fluid levels of immunoglobulins are lower than in serum, our results may also reflect this assay’s relative insensitivity for low antibody titers.
There are several limitations to our study. For in-home testing, the OraQuick In-Home HIV Test, available for over-the-counter purchase, would be used, and per the package insert, the test missed identifying 8 of 96 individuals with HIV infection (1 in 12), and it is to be used no less than 3 months after a potential exposure [38]. The test has insufficient sensitivity for detecting HIV infection during this time period [39]. There may also be delays in the appearance of HIV-1-specific antibodies associated with the use of antiretrovirals for pre-exposure prophylaxis [40]. Our study is further limited to the specific HIV-1 vaccine vectors and immunogens that were used in the parent vaccine studies. This is particularly true for the in vitro plasma testing, which assessed only a single vaccine regimen. Other candidate HIV-1 vaccines under development could be assessed for cross-reactivity by this (or other) detection systems to determine potential utility during the clinical trial to ensure minimization of the risk for possible unblinding. Furthermore, our data from plasma samples suggest that there may be a risk of reactivity with this point-of-care test at high antibody titers, as might occur at peak time points after vaccination. Because blood and tissue donation programs may use less specific tests such as the third-generation Abbott Prism [41], former vaccine recipients should be counseled that nonreactivity by this point-of-care testing may not exclude blood test reactivity by all testing platforms.
The rigorous HIV diagnostic algorithm established by the HVTN has dual purposes: (1) to identify HIV infection, and (2) to fully inform vaccinees of the likelihood that they may test HIV antibody positive in tests commonly used in circumstances such as blood donation, medical exams, during pregnancy and delivery, for life insurance applications, presurgical consultations, military service, international travel, and certain employment. Without the knowledge of their likelihood to test antibody positive in these circumstances, vaccinees may experience issues such as denial of life insurance, permanent deferral from blood or tissue donation, postponement of elective surgical procedures, and, in the case of perinatal care, the newborn may be unnecessarily placed on antiretroviral therapy [12].
CONCLUSIONS
Taken together, our data suggest that this point-of-care test may be an alternative, participant-controlled, HIV screening modality for individuals at low risk for HIV infection who previously participated in certain HIV-1 vaccine studies, developed VISP/VISR by routine blood tests, and have been counseled in the limitations of the test. This point-of-care test has the potential to provide an option to such vaccinees who have not had a potential exposure during the preceding 3 months, do not need to know their VISP status for circumstances for HIV testing in the community (eg, medical exam), prefer the convenience of in-home testing, and have the resources to purchase the test (US ~$45).
Acknowledgments
We thank National Institute of Allergy and Infectious Diseas (NIAID)/National Institutes of Health (NIH) staff (Mary Marovich and Michael Pensiero) and our collaborators at International AIDS Vaccine Initiative (Dagna Laufer and Harriet Park) and the US Military HIV Research Program (Merlin Robb and Nelson Michael). We also thank our volunteers for their generous participation in this study as well as the dedicated staff at the Harvard HIV Vaccine Clinical Research Site (Jennifer A. Johnson, Jane A. Kleinjan, Seo Yim Shin, Naina Rao, Jane Higley, Hannah Jin, Xhoi Mitre, Jessica Cauley, Bruce Bausk, and Monica Feeley), the HIV Vaccine Trials Network (Ann Duerr, Jessica Andriesen, and Ashley Clayton), and the affiliated laboratories (University of Washington Retrovirology Laboratory: Glenda Daza, Socorro Harb, Carol Gallardo, Michael Corey Scherrer, Jose Pax Ortega, and Yuan-Jun Deng) who made this study possible. Finally, we thank Fatima Laher, Kathryn Mngadi, and Stephaun Wallace for their thoughtful review of the manuscript.
Financial support. This work was funded by National Institute of Allergy and Infectious Diseases and the National Center for Advancing Translational Sciences (US Public Health Service Grants UM1 AI068614 [Leadership and Operations Center: HIV Vaccine Trials Network], UM1 AI068618 [Laboratory Center: HIV Vaccine Trials Network], UM1 AI069412 [Harvard/Boston/Providence Clinical Trials Unit] and UL1 RR025758 [to Harvard University], and P30 AI027757 and UM1 AI106701 [to University of Washington]).
Potential conflicts of interest. S. R. W. has received clinical trial support from Janssen Vaccines; L. L. is a consultant at Janssen Vaccines & Prevention; and F. T. is an employee of Janssen Pharmaceutical Research and Development.
References
- 1. Fauci AS, Folkers GK, Marston HD. Ending the global HIV/AIDS pandemic: the critical role of an HIV vaccine. Clin Infect Dis 2014; 59 Suppl 2:S80–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gray GE, Laher F, Lazarus E, et al. Approaches to preventative and therapeutic HIV vaccines. Curr Opin Virol 2016; 17:104–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pitisuttithum P, Marovich MA. Prophylactic HIV vaccine: vaccine regimens in clinical trials and potential challenges. Expert Rev Vaccines 2020; 19:133–42. [DOI] [PubMed] [Google Scholar]
- 4. Russell ND, Marovich MA. Pox-protein public private partnership program and upcoming HIV vaccine efficacy trials. Curr Opin HIV AIDS 2016; 11:614–9. [DOI] [PubMed] [Google Scholar]
- 5. Barouch DH, Tomaka FL, Wegmann F, et al. Evaluation of a mosaic HIV-1 vaccine in a multicentre, randomised, double-blind, placebo-controlled, phase 1/2a clinical trial (APPROACH) and in rhesus monkeys (NHP 13-19). Lancet 2018; 392:232–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cooper CJ, Metch B, Dragavon J, et al. ; NIAID HIV Vaccine Trials Network (HVTN) Vaccine-Induced Seropositivity (VISP) Task Force. Vaccine-induced HIV seropositivity/reactivity in noninfected HIV vaccine recipients. JAMA 2010; 304:275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Newman PA, Rubincam C, Slack C, et al. Towards a science of community stakeholder engagement in biomedical HIV prevention trials: an embedded four-country case study. PLoS One 2015; 10:e0135937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Richardson S, Seekaew P, Koblin B, et al. Barriers and facilitators of HIV vaccine and prevention study participation among young black MSM and transwomen in New York City. PLoS One 2017; 12:e0181702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schmidt C, Jaoko W, Omosa-Manyonyi G, et al. Long-term follow-up of study participants from prophylactic HIV vaccine clinical trials in Africa. Hum Vaccin Immunother 2014; 10:714–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Couderc M. Being “False Positive”: An “Inconvenience”? Debates and questions regarding the notion of vaccine-induced seropositivity (VISP) in the recruitment of healthy volunteers for a preventive anti-HIV vaccine trial. J Acquir Immune Defic Syndr 2018; 79 Suppl 1:20–9. [DOI] [PubMed] [Google Scholar]
- 11. Durier C, Desaint C, Lelièvre JD, et al. ; ANRS/VRI COHVAC Study Group. Long-term safety and vaccine-induced seropositivity in healthy volunteers from HIV vaccine trials. AIDS 2019; 33:2061–71. [DOI] [PubMed] [Google Scholar]
- 12. Voronin Y, Zinszner H, Karg C, et al. ; VISR Working Group of the Global HIV Vaccine Enterprise. HIV vaccine-induced sero-reactivity: a challenge for trial participants, researchers, and physicians. Vaccine 2015; 33:1243–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Penezina O, Krueger NX, Rodriguez-Chavez IR, et al. ; HIV Selectest Study Group. Performance of a redesigned HIV Selectest enzyme-linked immunosorbent assay optimized to minimize vaccine-induced seropositivity in HIV vaccine trial participants. Clin Vaccine Immunol 2014; 21:391–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Centers for Disease Control and Prevention. Approval of a new rapid test for HIV antibody. MMWR Morb Mortal Wkly Rep 2002; 51:1051–2. [PubMed] [Google Scholar]
- 15. Nkenfou CN, Kembou JE, Temgoua ES, et al. Evaluation of OraQuick® HIV-1/2 as oral rapid test. Afr J Infect Dis 2013; 7:27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stekler JD, O’Neal JD, Lane A, et al. Relative accuracy of serum, whole blood, and oral fluid HIV tests among Seattle men who have sex with men. J Clin Virol 2013; 58 Suppl 1:e119–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baden LR, Stieh DJ, Sarnecki M, et al. ; Traverse/HVTN 117/HPX2004 Study Team. Safety and immunogenicity of two heterologous HIV vaccine regimens in healthy, HIV-uninfected adults (TRAVERSE): a randomised, parallel-group, placebo-controlled, double-blind, phase 1/2a study. Lancet HIV 2020; 7:e688–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. OraQuick ADVANCE HIV -1/2 rapid antibody test [package insert]. Available at: https://www.fda.gov/media/73607/download. Accessed 01 December 2020.
- 19. Branson BM. Update on HIV diagnostic technologies. In: 2012 National Summit on HIV and Viral Hepatitis Diagnosis, Prevention, and Access to Care. Washington, DC: Available at: https://forumresearch.org/projects/69-general/754-2012-summit-breakout-sessions Accessed 01 December 2020. [Google Scholar]
- 20. Hural J, Metch B, Grunenberg N, et al. HIV vaccine trials network (HVTN) 910: long-term follow-up of HIV vaccine-induced seropositivity (VISP) among HIV vaccinees. AIDS Res Hum Retroviruses 2016; 32:OA22.06. [Google Scholar]
- 21. Centers for Disease Control and Prevention. HIV testing in clinical settings. Available at: https://www.cdc.gov/hiv/testing/clinical/index.html. Accessed 01 December 2020.
- 22. Jensen TO, Robertson P, Whybin R, et al. A signal-to-cutoff ratio in the Abbott architect HIV Ag/Ab Combo assay that predicts subsequent confirmation of HIV-1 infection in a low-prevalence setting. J Clin Microbiol 2015; 53:1709–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Adhikari EH, Macias D, Gaffney D, et al. Diagnostic accuracy of fourth-generation ARCHITECT HIV Ag/Ab Combo assay and utility of signal-to-cutoff ratio to predict false-positive HIV tests in pregnancy. Am J Obstet Gynecol 2018; 219:408 e1–9. [DOI] [PubMed] [Google Scholar]
- 24. Goepfert PA, Elizaga ML, Sato A, et al. ; National Institute of Allergy and Infectious Diseases HIV Vaccine Trials Network. Phase 1 safety and immunogenicity testing of DNA and recombinant modified vaccinia Ankara vaccines expressing HIV-1 virus-like particles. J Infect Dis 2011; 203:610–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koblin BA, Casapia M, Morgan C, et al. ; NIAID HIV Vaccine Trials Network. Safety and immunogenicity of an HIV adenoviral vector boost after DNA plasmid vaccine prime by route of administration: a randomized clinical trial. PLoS One 2011; 6:e24517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fuchs JD, Bart PA, Frahm N, et al. Safety and immunogenicity of a recombinant adenovirus serotype 35-vectored HIV-1 vaccine in adenovirus serotype 5 seronegative and seropositive individuals. J AIDS Clin Res 2015; 6:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Walsh SR, Moodie Z, Fiore-Gartland AJ, et al. ; HVTN 083 Study Group and the NIAID HVTN. Vaccination with heterologous HIV-1 envelope sequences and heterologous adenovirus vectors increases T-cell responses to conserved regions: HVTN 083. J Infect Dis 2016; 213:541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Buchbinder SP, Grunenberg NA, Sanchez BJ, et al. ; HIV Vaccine Trials Network (HVTN) 094 Study Group. Immunogenicity of a novel Clade B HIV-1 vaccine combination: results of phase 1 randomized placebo controlled trial of an HIV-1 GM-CSF-expressing DNA prime with a modified vaccinia Ankara vaccine boost in healthy HIV-1 uninfected adults. PLoS One 2017; 12:e0179597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Churchyard GJ, Morgan C, Adams E, et al. ; NIAID HIV Vaccine Trials Network. A phase IIA randomized clinical trial of a multiclade HIV-1 DNA prime followed by a multiclade rAd5 HIV-1 vaccine boost in healthy adults (HVTN204). PLoS One 2011; 6:e21225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Goepfert PA, Elizaga ML, Seaton K, et al. ; HVTN 205 Study Group; National Institutes of Allergy and Infectious Diseases HIV Vaccines Trials Network. Specificity and 6-month durability of immune responses induced by DNA and recombinant modified vaccinia Ankara vaccines expressing HIV-1 virus-like particles. J Infect Dis 2014; 210:99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hammer SM, Sobieszczyk ME, Janes H, et al. ; HVTN 505 Study Team. Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. N Engl J Med 2013; 369:2083–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Baden LR, Walsh SR, Seaman MS, et al. First-in-human evaluation of the safety and immunogenicity of a recombinant adenovirus serotype 26 HIV-1 Env vaccine (IPCAVD 001). J Infect Dis 2013; 207:240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baden LR, Walsh SR, Seaman MS, et al. First-in-human evaluation of a hexon chimeric adenovirus vector expressing HIV-1 Env (IPCAVD 002). J Infect Dis 2014; 210:1052–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Baden LR, Liu J, Li H, et al. Induction of HIV-1-specific mucosal immune responses following intramuscular recombinant adenovirus serotype 26 HIV-1 vaccination of humans. J Infect Dis 2015; 211:518–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baden LR, Karita E, Mutua G, et al. ; B003-IPCAVD004-HVTN091 Study Group. Assessment of the safety and immunogenicity of 2 novel vaccine platforms for HIV-1 prevention: a randomized trial. Ann Intern Med 2016; 164:313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Baden LR, Walsh SR, Seaman MS, et al. ; IPCAVD006/RV380/HIV-V-A002 Study Group. First-in-human randomized, controlled trial of mosaic HIV-1 immunogens delivered via a modified vaccinia Ankara vector. J Infect Dis 2018; 218:633–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Agresti A, Coull B. Approximate is better than “exact” for interval estimation of binomial proportions. Am Stat 1998; 52:119–26. [Google Scholar]
- 38. OraQuick in-home HIV test [package insert]. Available at: https://www.fda.gov/media/83607/download. Accessed 01 December 2020.
- 39. Stekler JD, Tapia K, Maenza J, et al. No time to delay! Fiebig stages and referral in acute HIV infection: seattle primary infection program experience. AIDS Res Hum Retroviruses 2018; 34:657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Curlin ME, Gvetadze R, Leelawiwat W, et al. ; OraQuick Study Group. Analysis of false-negative human immunodeficiency virus rapid tests performed on oral fluid in 3 international clinical research studies. Clin Infect Dis 2017; 64:1663–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kiely P, Catton M, Brown D, et al. The potential complexity and need for caution when interpreting atypical human immunodeficiency virus reactivity in blood donors. Blood Transfus 2015; 13:669–71. [DOI] [PMC free article] [PubMed] [Google Scholar]