Abstract
Immune checkpoint inhibitors (ICIs) have revolutionized the field of cancer immunotherapy. Most commonly, inhibitors of PD-1 and CTLA4 are used having received approval for the treatment of many cancers like melanoma, non-small-cell lung carcinoma, and leukemia. In contrast, to date, clinical studies conducted in patients with CNS malignancies have not demonstrated promising results. However, patients with CNS malignancies have several underlying factors such as treatment with supportive medications like corticosteroids and cancer therapies including radiation and chemotherapy that may negatively impact response to ICIs. Although many clinical trials have been conducted with ICIs, measures that reproducibly and reliably indicate that treatment has evoked an effective immune response have not been fully developed. In this article, we will review the history of ICI therapy and the correlative biology that has been performed in the clinical trials testing these therapies in different cancers. It is our aim to help provide an overview of the assays that may be used to gauge immunologic response. This may be particularly germane for CNS tumors, where there is currently a great need for predictive biomarkers that will allow for the selection of patients with the highest likelihood of responding.
Keywords: biomarkers, clinical correlates, CNS malignancies, immune checkpoint inhibitors
Key Points.
Immune monitoring is critical to interpreting patient response to ICIs.
Predictive biomarkers enrich for GBM patients most likely respond.
Peripheral and intratumoral markers together define immunotherapy response.
One of the hallmarks of cancer is the ability of cancer cells to overcome immunosurveillance.1 Part of the mechanism of circumventing the immune system is the capability of cancer cells to “co-opt” immune checkpoint pathways.2 These findings led to the development of immune checkpoint inhibitors (ICI) to treat cancer. CTLA-4 and PD-1 were among the first to be discovered and are the most commonly targeted checkpoint receptors in several cancers.3 CTLA-4, exclusively expressed on T cells, antagonizes CD28 co-stimulation, thereby inhibiting T cell activation.3 PD-1, on the other hand, is expressed on activated T cells and when engaged by its ligands, PD-L1 or PD-L2 hinders their immune activity.3 Expression of PD-1 ligands occurs both on myeloid cells and tumor cells within the tumor microenvironment, making it a dominant pathway for acquired immune resistance.2
Clinical testing and evaluation of humanized anti-CTLA-4 antibodies, namely ipilimumab and tremelimumab began in the early 2000s in patients with metastatic melanoma.3 Similar studies examining the efficacy of humanized anti-PD-1 antibodies, nivolumab and pembrolizumab, showed success not only in melanoma but also in renal cancer, colon cancer, and lung cancer.3 Anti-PD-1 treatment is less toxic in patients as compared to anti-CTLA-4 and has produced a wide range of responses including tumor regression and increase in long-term overall survival (OS).2 Despite encouraging results, notably improvement in median survival, monotherapies of checkpoint inhibitors have not been as effective as combination regimens in producing complete clinical responses or cures. For example, concurrent treatment of advanced melanoma patients with nivolumab and ipilimumab showed objective responses in over 53% of patients, making it distinctly more efficacious than previous monotherapy regimens.4 The evolving tumor-immune landscape suggests that the use of a combinatorial approach targeting multiple checkpoint pathways may be more effective, although with greater toxicity. Many more recently discovered checkpoint pathways such as Tim-3, Lag3, A2aR, and so on have been shown to work synergistically and/or have an additive effect when targeted simultaneously making them potential targets for cancer immunotherapy.3
Glioblastoma (GBM) is one of the most aggressive CNS malignancies. The discovery of CNS immunosurveillance and a rudimentary lymphatic system, led to interest in using ICI to treat this disease. Checkmate143 was the first large systematic clinical study comparing the therapeutic effectiveness of nivolumab as a single agent to an inhibitor of angiogenesis, bevacizumab.5,6 Importantly, a smaller component of the Checkmate 143 study tested the safety and tolerability of nivolumab with or without ipilimumab in patients with recurrent GBM, enabling consideration of a combination strategy in future trials.7 Unfortunately, although the safety profile of nivolumab in GBM was comparable to other cancers, the primary endpoint of the study remained unmet since nivolumab monotherapy was not more efficacious than bevacizumab alone.6,8 Nevertheless, the durability of response to nivolumab in a subset of GBM patients was encouraging, leaving room for speculation on possible reasons for the failure of this trial. It is important to note that over 40% of patients in each arm of this study were treated with corticosteroids at the time of entry into the study.8,9 Studies conducted by our group and others clearly demonstrate the debilitating effect of steroid treatment on lymphocyte activation and proliferation in the context of checkpoint inhibition.10–12 Additional factors such as the presence of a suppressive tumor micro-environment, impaired lymphocyte trafficking, and reduced tumor mutational burden in these patients may have also contributed to inconsistent responses and thereby collapse of the trial. It is for these reasons that identifying the appropriate patient cohorts most likely to respond to ICI is critical. This underscores the need for predictive biomarkers to enrich clinical trials with patients likely to generate a systemic immune response to immunotherapy, positing that a peripheral blood response is necessary for a tumor response. Although this peripheral blood immune response may not predict tumor response, meaning that it may not be sufficient, it will enable enrichment of the treated patient population who with a systemic immune response, may at least have the possibility of tumor response.
In translational research, a biomarker can serve as a diagnostic, prognostic, or predictive tool. The search for reproducible and accurate markers of response to ICI therapy is an area of active research. A clinically validated and robust biomarker of immune response to checkpoint therapy can potentially revolutionize the field of cancer immunotherapy by identifying populations of patients poised to respond to treatment, especially for GBM.
In this review, we summarize the body of work that has been done to establish correlative markers of immune activity and response in the context of ICI therapy in various cancers, with the aim of providing a better definition of response which may help guide future trials in patients with GBM and other cancers undergoing immunotherapy.
Markers of Peripheral Immune Response to Checkpoint Blockade
Antitumor responses to immunotherapy can most easily be studied by looking at systemic changes in patient peripheral circulation. It also allows for repeat and longitudinal evaluation, the advantage being the ability to compare pre- and post-treatment changes as well as monitor changes occurring during the course of treatment. Often called a “liquid biopsy,” a sampling of patient blood is noninvasive, cost-effective, and provides a snapshot of possible intratumoral immune response to treatment.13 Patient serum allows for evaluation of cytokines and other soluble factors.
Cerebrospinal fluid (CSF) also provides real-time information about the status of the tumor microenviroment.14 Most often, CSF is sampled directly via lumbar puncture, however, in vivo micro dialysis, is yet another method of periodically draining the CSF from the interstitial space in the brain.15,16 This technique has been used successfully to monitor changes in neurotransmitters both in rodents and humans and is currently under investigation for immune monitoring in GBM patients being treated with ICIs.17
Fecal sampling to survey gut microbiota is another mechanism to nonsurgically monitor changes in immune status in patients treated with checkpoint blockade.18 It has been successfully utilized in many cancers, with a growing interest for application in CNS malignancies as well.
Blood Cytology Biomarkers
Systemic inflammation is a seminal feature of cancer.1 Complete blood counts performed routinely as part of clinical care for cancer patients is one of the most fundamental measures of inflammation.19 Clinical studies conducted in patients with metastatic melanoma showed that only patients with a positive change in absolute lymphocyte count (ALC) between baseline to Week 4 after treatment were likely responders to ipilimumab monotherapy.20 Patients with ALC > 1500 cells/μL after 7 weeks of ipilimumab treatment had increased OS as compared to those with ALC < 1500 cells/μL.20 The ratio of absolute neutrophil count to absolute lymphocyte count called the neutrophil-to-lymphocyte ratio (NLR) has also been shown to predict patient ability to respond to checkpoint therapy.21 Patients with metastatic melanoma, treated with ipilimumab, with baseline NLR < 5 displayed both improved progression-free survival (PFS) and as well as increased OS.21 However, in patients with non-small-cell lung carcinoma (NSCLC) undergoing treatment with nivolumab, NLR < 3 alone was not associated with a better prognosis. There was improved OS in patients with NLR < 3 alongside normal levels of lactate dehydrogenase.22
As in many other solid cancers, patients with GBM usually present with pretreatment neutrophilia.23 Studies have shown that a NLR > 4 in GBM patients is associated with poor prognosis.23 However, the effect of checkpoint therapy on neutrophil numbers has yet to be fully evaluated and validated. Although NLR and ALC are prognostically valuable, baseline measurements can be very erratic and vary by race, gender, age, disease stage, and in many cases, changes in total numbers of leukocytes may not reflect response. There could be changes in specific subpopulations or in the activation states of a population of leukocytes (which may be more informative of response), that may not be capitulated by these metrics, thereby limiting the use of these biomarkers to make clinical decisions.
Circulating Myeloid-Derived Suppressor Cells
Myeloid cells are a heterogenous group of innate immune cells whose activation and mobilization are greatly affected by their environment. Their heightened plasticity makes them efficient at reporting on the evolving status of the tumor microenvironment. Myeloid-derived suppressor cells (MDSCs) are a class of myeloid cells that are stuck in the process of differentiation. These cells express CD33, a common myeloid cell marker, but they lack the expression of HLA-DR, a characteristic of mature myeloid cells. Broadly classified as monocytic or polymorphonuclear,24 these cells can be readily detected by flow cytometry from patient PBMCs. Studies have now identified more diverse subsets of MDSCs based on the expression of surface markers. These include neutrophilic (CD15+ CD33+ HLADR-), monocytic (CD14+ CD33+ HLADR-), and linage-negative (CD15- CD14- CD33+ HLADR-) MDSCs.24 These MDSCs are all notorious for their immunosuppressive properties.25 They inhibit T cell proliferation and antitumor function and also secrete IL-10 and TGF-β that promote development of Tregs.26
A study conducted by Meyer et al showed that peripheral blood of melanoma patients are enriched for Lin-CD14+HLA-DR- monocytic MDSCs in comparison to healthy donors.27 Although these MDSCs did not significantly change from baseline with ipilimumab treatment, there was an increase in the circulating MDSCs in patients with metastatic tumors.27 A separate study in advanced melanoma patients receiving ipilimumab therapy found Lin-CD14-CD11b+CD33+HLA-DRlow/neg polymorphonuclear MDSCs decrease in frequency with the first dose and remained that way throughout the treatment.28 They also found that patients whose monocytic MDSCs diminished within the first 3 weeks of treatment had a significant survival benefit.28 Interestingly, Weber et al. found that in metastatic melanoma, low numbers of monocytic MDSCs significantly correlated with high response rates and improved survival when treated with nivolumab even when the disease progressed following prior treatment with ipilimumab.29
MDSCs are thought to be one of the major causes of therapeutic resistance in many cancers including GBM. High levels of CD33+ HLA-DR- MDSCs have been detected in patients with newly diagnosed GBM with high arginase activity.24 These cells include CD15+ neutrophilic subsets that constitute >60% of the total MDSC population. CD15- CD142- lineage negative cells account for 31% and CD14+ monocytic subsets make up for 6% of the total MDSC population in these patients.24 GBM patients with lower levels of circulating MDSCs in totality survive longer and some studies also show a clustering of clinical response to ICI with micro RNAs that induce MDSC formation.30 The ability of MDSCs to build an immune suppressive secretory landscape coupled with their ability to negatively impact T cell activity and differentiation make them a promising prognostic tool to measure response to immunotherapy. Nevertheless, the reliability of MDSCs as biomarkers for GBM patients receiving ICI therapy has not yet been established.
Peripheral T Lymphocytes
The composition of T cells in peripheral blood is a very important factor in predicting ICI efficacy. CD8 T cells are one of the main antitumor immune cells.25 They get activated by antigen-presenting cells (APCs)25 following which they proliferate, clonally expand, and mature from a naive state. Subsequently, they migrate to the tumor to carry out their cytotoxic function. Some of these cells also develop into tumor-specific memory cells. CD4 T cells are also activated by APCs, via MHC-I and develop into T helper cells or Tregs.25 Tregs can be immunosuppressive.25 Although T cell maturation is greatly dictated by the tumor microenvironment, the T cells traffic from the periphery and their phenotype can differentiate responders from nonresponders.
Cross-sectional prospective analyses of melanoma and NSCLC patients treated with nivolumab showed that a high ratio of Cmemory to Effector T cell ratio (Cm/Eff T) of CD4 and CD8 T cells reflect highly inflamed tumors.31 Within 3 months of nivolumab treatment, patients with a higher baseline Cm/Eff T cell ratio showed further increase in this ratio alongside significant decreases of naive CD4 and CD8 T cells in these patients.31 Further, NSCLC patients with high Cm/Eff T cell ratios also experienced prolonged PFS.31 A separate study in patients with NSCLC treated with anti-PD-1 demonstrated that responders displayed a higher baseline number of CD62low CD4+T cells and a lower CD4+CD25+FoxP3+ Treg count in circulation.32 The CD62lowCD4+T cell numbers also closely correlated with effector CD8 T cell counts and long-term PFS. Decreases in CD62low CD4+ T cells with anti-PD-1 treatment corresponded with acquired treatment resistance.32 On the other hand, in advanced melanoma, patients receiving neoadjuvant ipilimumab experienced a significant increase in circulating Tregs after treatment with nivolumab; a finding associated with increased PFS.33 Additionally, a greater than 3-fold increase in CD3+CD4+IFNγ + T cells in circulation was associated with patients who were progression free at 6 months.33
In a more recent study conducted by Fairfax et al., T cell receptor (TCR) clonality played an integral role in distinguishing response in metastatic melanoma patients treated with a combination of anti-PD-1 and anti-CTLA antibodies.34 They observed that patients who displayed expansion in large clones, defined as clones that constitute over 0.5% of the total T cell repertoire within 3 weeks of treatment, had a significant improvement in long-term response.34 Interestingly, they also found that responders had on average 5.7 or more large clones in comparison to nonresponders. While the clonal diversity closely correlated with central memory T cells, the large clones were mostly associated with CD8 effector memory T cells.34
In the case of GBM, although the peripheral blood immune cells are separated from the brain by the blood–brain barrier (BBB) and/or the blood–CSF barrier (BCSFB), the status of T cells in the periphery may still be predictive of response to treatment, especially with ICI. Since checkpoint antibodies cannot readily cross the BBB, they primarily act on T cells in the periphery which would then need to eventually traffic to the brain tumor for efficacy. For this reason, assaying T cells in the periphery may be helpful in identifying responders among GBM patients on checkpoint therapy. Recent studies have shown that clonal expansion of T cells, expression of specific chemokines receptors, and IFNγ on T cells characterize potential responders to nivolumab treatment.35,36 Another concern with regard to immune treatment of intracranial tumors is the possible sequestration of T cells in the bone marrow as reported by Fecci et al. They observed T cell lymphopenia and shrunken peripheral lymphoid organs in treatment-naive human and mouse subjects with GBM. Tumor secreted factors are suspected to cause this sequestration of lymphocytes by inducing internalization of the S1P1 receptor from the surface of T cells. Studies in murine models have demonstrated that rescuing the T cells from the bone marrow, increases their number in peripheral circulation, resulting in improved response to checkpoint blockade.37 It has also been observed that GBM patients display preferential migration of Tregs, attributable to increased secretion of CCL2 in the tumor microenviroment.38 However, most of these studies were performed on tumor infiltrating lymphocytes (TILs) from patient tumor biopsies and hence must be systematically correlated with T cells from patient peripheral circulation. We believe that characterization of peripheral T cells using the aforementioned metrics especially measuring ratios of CM/Eff T cells and clonal expansion of circulating T cells in response to treatment hold great promise in predicting response to ICI for GBM patients.
Cytokines and Chemokines in Blood
Cytokines and chemokines are soluble factors that play an important role in cell-to-cell communication, cell trafficking, and are critical to shaping the tumor microenvironment with the ability to either enhance or suppress an antitumor immune response. Therefore, quantification of cytokines and their receptors may help to predict immune dysfunction. Cytokines and chemokines can easily be detected in patient plasma/serum as well as CSF using ELISA or by ELISpot assays.
IFNγ is one of the most commonly profiled pro-inflammatory, cytotoxic, type-II interferon in patients treated with ICI, secreted mainly by activated T cells. Recent studies by Tahara et al. in patients with advanced metastatic melanoma treated with nivolumab showed that elevated IFNγ in pretreatment serum of patients corresponded with better objective responses.39 Increased expression of IFNγ also leads to the upregulation of chemokines like CXCL9, CXCL10, and CXCL11 as these play a significant role in mobilizing peripheral T cells to the tumor. Patients with melanoma, NSCLC, and renal cancer treated with anti-PD-L1 antibodies demonstrated a marked increase in CXCL11 early during treatment and elevated baseline levels of CXCL11 corresponded with response.40 In patients with advanced melanoma, increases in CXCL9 and CXCL10 with nivolumab treatment also corresponded to an improved prognosis.39 However, in the case of melanoma patients treated with ipilimumab, elevated baseline expression of CXCL11 correlated with poor survival and was a reliable predictor of response.41
IL-6 and IL-8, pleiotropic cytokines, have been associated with increased mobilization of MDSCs in metastatic melanoma.42 Although these patients treated with ipilimumab showed great variability in baseline levels of serum IL-6 as well as the degree of change in circulating IL-6 levels with treatment, responders displayed a trend of decreasing IL-6 levels from baseline to the fourth cycle of treatment corresponding with their improved outcome.43 High levels of IL-6 after the fourth cycle of ipilimumab treatment was also associated with decreased survival.43 Interestingly, patients with melanoma who responded to nivolumab treatment demonstrated significant increases in IL-6 from baseline and these increases correlated with increases in serum IFNγ and IL-10 expression.39 Patients with NSCLC treated with nivolumab did not show significant differences in baseline serum IL-8 levels between responders and nonresponders.22 Stratifying patients based on the median increase in IL-8 levels within 2 months of initiation of nivolumab treatment demonstrated that increased levels of IL-8 were associated with a significant decrease in OS, but not PFS.22
For GBM immunotherapy, much remains to be studied about the role of cytokines and chemokines as markers of response. Enhanced expression of IFNγ and its targets have traditionally been shown to be associated with better outcomes while the IL-6 axis has been shown to promote tumor growth and M2-like myeloid phenotype.8,44 Despite these promising biomarker results, to date, studies have not stratified patients based on the expression of these cytokines so that the impact of these factors on outcomes with ICI therapy can be evaluated. However, often cytokines and chemokines have varied expression based on tumor location, making it hard to detect these regional differences systemically. In some other instances, the window of cytokine release with successful treatment may be difficult to determine, impacting the ability to truly measure the predictive capability of a particular cytokine. Nevertheless, cytokines remain worth investigating in the context of clinical trials and biomarkers.
Circulating Tumor DNA
Circulating tumor DNA (ctDNA) may provide a window into the genetic characteristics of a tumor. These are free strands of DNA released from apoptotic or necrotic tumor cells into circulation.45 They are homogenous, reproducible, and can be detected and analyzed both quantitively and qualitatively using droplet digital polymerase chain reaction (PCR) (dd PCR), next-generation sequencing, and bidirectional phosphorolysis activated polymerization PCR (Bi-Pap PCR).46
ctDNA has been most effectively used as a prognostic indicator in NSCLC patients on immunotherapy. A pilot study performed on patients with NSCLC treated either with pembrolizumab or nivolumab showed that changes in ctDNA between baseline to week 8 post-treatment significantly correlated with a decrease in tumor size.46 Clearance of ctDNA corresponded with significant radiological response as well as improved OS.46 Similar results were also observed by Goldberg et al. in patients with metastatic NSCLC on ICI.47 Clinical assessments of patients with advanced melanoma with specific BRAF and NRAF mutations demonstrated a significant association between levels of ctDNA (BRAFmut/NRAFmut) and response to treatment as detected by ddPCR.48 Responders showed a decrease in quantities of ctDNA within 2–4 weeks of nivolumab treatment as compared to the nonresponders.48 Studies examining hotspot mutations such as BRAF, TERT, cKIT, and NRAS in advanced melanoma patients treated with ipilimumab and/or nivolumab49 found that plasma levels of ctDNA in responders, leading to eventual tumor regression, had these hotspot mutations between undetectable to 5.5% of total ctDNA. This decrease in ctDNA over 3 weeks of treatment indicates the utility of longitudinal measurement of ctDNA as a biomarker for ICI treatment.49
The role of ctDNA in predicting response and outcome for GBM patients treated with immunotherapy is a new area of investigation. While isolation of ctDNA from blood has been difficult in patients with GBM, it can more readily be isolated from the CSF of these patients.50 Another source of circulating genetic material from GBMs are extracellular vesicles (EVs), that can be detected both in plasma and CSF.51 EVs are particles made of lipid bilayers enclosing cytosolic material from the cell of their origin.51 Studies show that GBM patients have a larger number of detectable EVs in their plasma compared to healthy controls.52 Significant differences have been detected in EV concentration pre- and post-surgery, indicating their tumor cell origin.52 In part, these EVs were found to contain information on the mutational status of the tumor, the most easily detectable being EGFRvIII mutations along with several others.53 In addition to a genetic payload, EVs also contain proteins that can act as antigens that stimulate the immune system and alert it to the evolving tumor environment.53 The rapid turnover of GBM cells provides large amounts of circulating material including DNA, making it a viable tool as a biomarker.50
Gut Microbiota as a Predictor of Immune Response
The gut microbiome comprises the total population of bacteria, fungi, and viruses that reside in the viscera. They can be detected by performing whole-exome sequencing in combination with 16s microbial analysis on stool samples from a patient.18,54 Otherwise, healthy individuals may display variation in the composition of gut microbiota attributable to lifestyle as well as dietary and medication differences.54 It has been known that antibiotics severely alter the composition of microbes in gut lymphoid tissue and can be inhibitory to commensalic bacteria that enable immune homeostasis.54 Additionally, gut microbes also secrete several metabolites like short-chain fatty acids that lead downstream to the production of cytokines such as IL-6, IL-10, and so on, and can skew T cell development to a Treg phenotype.54 These functions of the gut microbiota make them promising predictors of immune response to therapy.
Clinical studies conducted in a small cohort of melanoma patients treated with ipilimumab, showed that baseline microbiota was unaffected by treatment.55 However, this changed in patients who displayed treatment-induced colitis. Patients whose baseline microbiota was enriched for Faecalibacterium, of the Firmicutes genus had improved OS as compared to those with Bacteriodes.55 Correspondingly, a lower frequency of Tregs was observed in peripheral blood of patients with Faecalibacterium that may have contributed to the clinical benefit that was determined.55 Similarly, studies in melanoma patients treated with anti-PD-1 showed that responders had a unique composition of fecal bacteria compared to nonresponders. Furthermore, significant correlations were also established between these responders and the number of CD8 T cells infiltrating the tumor.18
The recent confirmation of the presence of a lymphatic system in the brain has raised the possibility of a gut-brain axis that is currently under investigation.54 One study in a murine model shows that chronic administration of antibiotics altered the intestinal microbiota, diminished cytotoxic NK cell subsets, and changed the pattern of expression of inflammatory and homeostatic cytokines in microglia, contributing to increased growth of intracranial gliomas.56 Studies are underway where feces from patients with GBM are being transplanted into germ-free mice to compare the composition of the microbiota at baseline to healthy donors as well as pre- and post-ICI therapy. Although this area of investigation is new for CNS malignancies, it offers yet another component that can be non-invasively assessed as part of an immune evaluation in patients.
Markers of Intratumoral Response to Checkpoint Blockade
The tumor microenvironment and the presence of intratumoral immune cell infiltrates is critical to understanding a patient’s response to ICIs. Some intratumoral immune cell infiltrates can be predictive of improved survival57,58 while others can be predict worse survival.59 Evaluation of immune infiltrates by immunohistochemistry (IHC) enables visualization of the location of immune cells within the tumor and allows us to appreciate intratumoral heterogeneity, but it is restricted by the number of markers that can be analyzed at a given time. On the other hand, flowcytometric analysis of TILs is more robust and quantitative, although it too is limited by the inability to differentiate perivascular immune infiltrates from those within the parenchyma. Tumor tissue can also be analyzed with much granularity using single-cell RNA sequencing. Further, genetic profiles for specific immune cells can be deconvoluted using computational algorithms, from RNA sequencing of bulk tumor tissue with fresh, frozen and fixed samples with the same degree of accuracy.60 Nevertheless, these analyses require tumor tissue either through surgical resection or biopsy. Although highly informative, acquisition of tissue specimens for biomarker analysis is far more invasive, expensive, and can be inconvenient for longitudinal evaluations in comparison to markers in peripheral circulation.
Immune Scoring
An immune score is determined by the number of adaptive immune cells that infiltrate the central core of a tumor, the invasive margin, and distant metastasis.61 It is usually computed as a percentile based on the parameters/markers described in a training set and then graded as a bad, intermediate or good prognosis.62 While the density of immune cell infiltrates is often determined via IHC, bioinformatics tools like CIBERSORT (Cell-type Identification By Estimating Relative Subsets Of RNA Transcripts) have been used to calculate the immune score with better resolution using bulk RNA sequencing of the tumor sample.63 It is a meta-gene system that estimates the abundance of member cell types located within mixed cell populations like the tumor microenvironment.63 Unlike other algorithms, CIBERSORT employs linear support vector regression to minimize noise and increase its deconvolution power. Tests performed on synthetic data sets made by combining admixed blood cell lines with colon cancer cells to simulate the tumor tissue showed that CIBERSORT outperformed most other platforms even at less than 50% immune content where most other analyses began to fail.60,64 Additionally, head-to-head comparisons between CIBERSORT’s ability to deconvolute immune subsets from lung tumor biopsies and lymph node biopsies from follicular lymphoma patients with flowcytometric evaluation of the same, demonstrated comparable results between the two measurements.60
ESTIMATE (Estimation of STromal and Immune cells in MAlignant Tumor tissues using Expression data) is yet another bioinformatics tool that uses single-sample gene set-enrichment analysis for the calculation of stromal and immune scores by determining the presence of established markers such as FOXP3, CECR1, and PDGFB.65 In patients diagnosed with breast cancer, the ESTIMATE model showed that increased immune scores correlated with an increase in disease-free survival.66,67 In patients diagnosed with NSCLC, specifically adenocarcinoma, the presence of a greater number of adaptive immune cells correlated with an improved PFS.68 Similar associations have also been made in patients with pancreatic cancer and melanoma69,70 as well as in colorectal cancer. Hence, there is a clear benefit to determining immune scores at baseline and subsequently comparing changes in this metric with treatment to better predict patient outcomes. Unfortunately, there is a paucity of research that specifically explores this in the context of immunotherapy, especially ICI regimens.
At present, there is not a validated immune scoring model for malignant gliomas. Given the immunosuppressive tumor microenvironment37,71 and the immunosuppressive effects of the current standard of care, the ability to score the tumor-immune landscape pre- and post immunotherapy in patients with GBM will allow differentiation of tumor progression from inflammatory “pseudo-progression” that mimic tumor growth on conventional imaging studies. Since positive correlations have been demonstrated between higher immune scores and response to ICI in other cancers, having an effective method of determining immune score in patients with GBM could prove to be valuable.
Microsatellite Instability and Mutation-Associated Neoantigens
It is predicted that mutation-associated neoantigens resulting from DNA mismatch repair may be recognized by the immune system and serve as a valuable tumor biomarker.72 Microsatellite instability (MSI) occurs most frequently in colorectal cancer, gastric cancer, and endometrial cancer. The vast majority of these cancers demonstrate a significant level of chromosomal instability leading to aneuploidy and thereafter the activation of oncogenes and loss of tumor suppressors.73 This mechanism of action appears to be through dysfunction in DNA mismatch repair,74 resulting in an overwhelming amount of replication errors, leading to MSI.75
A recent study by Le et al. found that patients treated with pembrolizumab who had colorectal cancers that were mismatch repair deficient had a 78% immune-related PFS as compared to 67% in non-colorectal cancer that was mismatch repair deficient. Further, in patients with mismatch repair proficient colorectal cancer, the immune-related PFS rate was just 11%.76 Kim et al. demonstrated that 85.7% of patients with gastric cancers who demonstrated high microsatellite instability and subsequently high tumor mutational burden, showed a high overall response rate to ICI.77
In CNS malignancies, however, there is not much evidence of the occurrence of MSI. Nonetheless, using whole-exome genome sequencing and neoantigen prediction algorithms, the tumor mutational status of GBM patients have been directly evaluated. These evaluations have led to weak associations being made between tumor mutational burden (TMB) and response to ICIs.78,79 In general, GBMs have a lower TMB in comparison to other cancers. Standard of care for GBM which includes chemo-radiation therapy has often been seen to induce treatment acquired resistance through the DNA mismatch repair pathway (MMR), base excision pathway, and by induction of O6 methylguanine DNA methyl transferase. In rare cases, some patient gliomas have inherent MMR deficiency and hyper-mutational phenotypes where robust responses to ICIs have been reported.80 Studies show that MMR-deficient gliomas have significantly lower T cell infiltration into the tumor and lower rate of response to anti-PD-1 treatment.78 However, these studies did not detect MSIs in MMR-deficient gliomas but revealed the presence of MSIs in treatment-induced hypermutant gliomas.78 While some studies indicate that low-grade gliomas have a higher TMB relative to GBM, others indicate the opposite.81 Studies by Heimberger et al. suggest that although tumor mutational load (TML) does not correlate significantly with tumor grade or age, higher-grade gliomas do harbor a higher TML albeit at reduced frequency. Interestingly, this study also found that tumors from IDH mutated grade 4 gliomas had higher TML when compared with IDH1 wild-type GBM.79 However, there was higher PD-1+ T cell influx in IDH1 wild-type GBMs that was independent of TML compared to IDH1-mutant tumors.79 Currently, clinical studies by our group are underway to determine a correlation between response to treatment with nivolumab and hypermutator phenotype in IDH1- or IDH2-mutant gliomas (NCT03718767). Taken together, it has been seen that either due to de novo defects in DNA repair or those acquired by after initial treatment with chemo-radiation agents, increased TML in GBM may predict sensitivity to ICIs.79
Intratumoral TCR Diversity and Clonality
A fundamental component that drives a patient’s ability to mount an antitumoral response is the activation of T cells that occurs through the TCR. TCRs have unique sequences that are developed in the thymus during T cell maturation. Once T cell activation in the periphery occurs, it undergoes clonal expansion resulting in more T cells harboring the same TCR sequence called the TCR repertoire.78 Research efforts are being focused on finding ways to use these TCR sequences as biomarkers in an effort to monitor immune reactions and predict which patients will likely respond to ICI.82,83
In a study by Wang et al., sequencing of the complementarity determining region 3 of TCRβ chains was performed on patients diagnosed with NSCLC to determine those who were more likely to respond to anti-PD-1 therapy. They found that patients with high PD-1+ CD8+ TCR diversity prior to the initiation of ICI, demonstrated enhanced response to ICI and increased PFS.84 The presence of a diverse population of TCR epitopes derived from several genetic alterations allowed the recognition of tumor neoantigens.
The role and potential benefit of TCR diversity in GBM is currently under investigation. A recent study by Cloughesy et al. found that neoadjuvant anti-PD1 promoted both a survival benefit and immune response in patients with recurrent GBM. The authors note an expansion in the T cell repertoire in the neoadjuvant group with significant correlation between intratumoral and peripheral T cells.35 T cell expansions were also observed in the adjuvant group, although this expansion was not systemic, indicating that anti-PD-1 treatment can result in increased T cell clones irrespective of the timing of administration, but presurgical treatment causes systemic changes making treatment more effective. The authors also detect that baseline elevation in TCR clonality corresponds with decreased survival benefit.35 They postulated that higher baseline T cell clonality augments the effects of pembrolizumab treatment and believe that combinatorial treatment with anti-CTLA 4 may provide more benefit in the neo-adjuvant setting.35 In a study by Jaffee et al., in patients diagnosed with pancreatic ductal adenocarcinoma, there is a distinct survival benefit for patients receiving anti-CTLA4 who had diverse baseline TCR repertoires. Patients with diverse baseline TCR repertoires had a median survival of 8.7 months compared to patients who had clonal repertoires who survived 4.3 months.85 Patients who had increased TCR clonal expansion (more than 100 clones) survived nearly 3 times as long compared to patients who had decreased TCR clonal expansion.85 Specifically, patients with increased TCR clonal expansion had a median survival of 13.2 months compared to patients who had decreased TCR clonal expansion and a median survival of 4.6 months. However, the benefit of TCR diversity is still questioned as studies such as by Li et al., where the opposite effect was observed. In patients diagnosed with GBM receiving a vaccination with heat shock protein-peptide complex-96 (HSPPC-96), it was seen that long-term survivors demonstrated a lower amount of TCR diversity.86 However, TCR clonal expansion was especially important for long-term survival as the study found that four TCR clones were amplified for those in the long-term survival group.86
Role of Supporting Cells in Predicting Tumor-Immune Response
B cells
While the field of immunotherapy has focused mainly on T cells, more recently, the value of B cells in predicting response to ICI is being recognized. A recent study evaluating the response of melanoma patients given neoadjuvant ICI revealed that B cell markers were the most differentially expressed among tumor samples of responders versus non-responders.87 Responding tumors had a larger presence of memory B cells while the nonresponders had more naive B cells.87 Additionally, this study also found more CXCR3+ switched memory B cells in the tumors of the responders.87 This is a critical finding as CXCR3 is involved in the recruitment and activation of CD8+ memory T cells.88 Griss et al. reinforced that depletion of B cells from melanoma tumors causes a significant decrease both in inflammation and infiltration of CD8+T cells.89 For patients with soft tissue sarcoma treated with ICI, the immune landscape can be classified as immune-low, immune-high, or highly vascularized.89 The immune-high tumors were enriched for B cells compared to the immune-low tumors. This richness of immune-high tumors in B cells was a major contributing factor to higher survival and increased response to ICI in these patients.89
The role of B cells in predicting ICI response in CNS malignancies is not well established. Klopfenstein et al. generated transcriptomic profiles for patients diagnosed with GBM to better understand factors in the tumor microenvironment that influence prognosis.90 It was observed that higher infiltrates of B cells correlated with improved outcome. However, the reasons for this B cell benefit remain obscure,90 necessitating more work to better our understanding of the contribution of B cells to prognosis and efficacy of immune therapy in patients with GBM.
Neurons
Recent findings by Venkatesh et al. demonstrated that synapses are formed between glioma cells and neurons within the tumor microenvironment, critical to the discovery that depolarization of glioma/neuron synapses promotes glioma growth.91 However, the effect of the glioma-neuron synapse on the immune landscape remains unknown. Since activated T cells seldom penetrate the brain parenchyma, it is postulated that T cell inhibition typically occurs in the perivascular space.92,93 Neuronal inhibition of T cells can occur in a contact-free manner through neuropeptides and neurotransmitters as shown previously in studies to modulate microglia and subsequent T cell activation.94,95 Neurotransmitters and neuropeptides have not been critically evaluated for their impact on the local immune environment of GBM. However, given that T cells possess dopaminergic and glutamate receptors, understanding the impact of neurotransmitters on T cells may provide additional insights.
In patients with neurodegenerative diseases like Parkinson’s disease, and in animal models of multiple sclerosis, the neuroinflammation is thought to be caused by loss of dopaminergic neurons.96 Blocking this loss of dopamine protects against inflammation.97,98
Thus, dopamine and glutamate may play a significant role in modulating immune responses in GBM. The increased presence of neurotransmitters negatively impacts local T cells.99 Given the role of neurotransmitters in immune modulation in neurodegenerative diseases, we believe that measuring neurotransmitter release may be beneficial in predicting response to ICI treatment in GBM.
Prognostic Biomarkers in CNS Metastatic Disease
While most studies have demonstrated the utility of predictors of immune response for patients with malignancies outside the CNS, few studies explore these predictors in patients with tumors residing inside the brain as highlighted throughout this article. Brain metastases share a common anatomic location with primary CNS malignancies; however, their response to treatment may markedly differ since they are derived from very different primary cancers. Therefore, efforts to develop means to monitor treatment response in these patients may be more rewarding as comparisons of responders with nonresponders is more feasible, given the much higher rate of response in some cancers. Therefore, studies of response markers in these patients may help narrow down viable biomarkers for investigation in primary CNS tumor patients.
Berghoff et al. explored the correlation between TIL density and OS for patients with brain metastases (BM). They analyzed 116 BM specimens by IHC for CD3, CD8, CD45RO, FOXP3, PD-1, and PD-L1 markers and calculated immune scores as well. They were able to detect TILs in 115 (99%) of these samples and immune scores correlated positively with median OS, indicating a possible prognostic role for TIL densities in these tumors.100 Additionally, the authors also observed that the highest TIL density was seen in BM for patients with melanoma, followed by renal cell cancer and lung cancer, a finding that has been confirmed by studies conducted by other groups as well.101 Interestingly, CD8+ TIL density also correlated positively with peritumoral edema seen on preoperative MRI.
The study by Zakaria et al. further supports the prognostic potential of TIL density in BM through their observations in patients with lung, breast, colon, skin (melanoma), or renal cancer, where TIL density impacted patient survival.102 After studying multiple biomarkers, they found that only increased density of CD3+ TILs present within the region of the brain that had reduced fractional anisotropy (due to more white matter tract disruption) was predictive of longer OS102 in various types of cancer that had metastasized to the brain, suggesting that this phenomenon is disease agnostic.
In another study, Berghoff et al. determined that the density of TILs and PD-L1 expression in NSCLC BM and matched primary tumors were different from each other. A dense infiltration of CD3+, CD8+, CD45RO+, and PD1+ TILs was more often observed in the systemic tumors, whereas an increased expression of PD-L1 was observed mainly in BM as compared to primary tumors.103 This highlights a potential differential response to treatment depending on tumor type, and it may be interesting to explore this in future clinical trials as it may be predictive of prognosis for BM patients receiving ICI but not necessarily for the primary systemic cancer. Additionally, studies conducted by Zhou et al. found that there were reduced numbers of CD8+ TILs in BM as compared to primary tumors for patients with NSCLC.104 Lung cancer patients have also been observed to have lower numbers of TILs in BM as compared to metastases from extra-cranial sites.105 This difference in TIL density affected patient survival. The reduced levels of stromal CD8+ TILs in BM was associated with a significant reduction in OS as compared to patients who demonstrated a high number of stromal CD8+ TILs.104 These studies support the concept that the density of TILs within tumors of the brain is capable of serving as a valuable biomarker regardless of cancer type.
Taken together, these data suggest that evaluating intratumoral T cells is a potential biomarker of response, albeit the significance of this determination in tracking response to ICI still remains to be clinically validated both in metastatic disease as well as in primary brain tumors.
Combining Radiologic Responses With Laboratory-Based Immune Monitoring to Predict Response in GBM Patients
Patient tumor imaging is critical for response assessment and cancer therapy. It is especially important for the evaluation of CNS cancers because of difficulties with accessing tissue. MRI has become the most widely used tool to guide therapy. T2/FLAIR and post-contrast-enhanced T1 are most commonly used to determine therapeutic response. Unfortunately, changes induced by therapy can look similar to disease progression, and terms such as “pseudo-progression” are often used to highlight the paradoxical difference of robust treatment response that emulates tumor progression by imaging.106 Concerns that imaging changes may not reflect tumor response or progression led to the publication of the Immune Response Assessment in Neuro-oncology (iRANO) criteria in 2015 with a proposed confirmation of progression on follow-up imaging 3 months after initial radiographic progression if a defined set of clinical and therapeutic criteria are met.107 However, practical application of these criteria can be challenging since tumor progression, and inflammatory changes can look the same using MRI.108 The ability of functional imaging studies such as functional MRI, blood-oxygenation level-dependent MRI, and diffusion tensor imaging to track specific molecules and cells, can potentially allow for the study of therapeutic agents and immune cell migration into the CNS also enhancing our understanding of immune cell interactions with brain tumor cells.109–111
Positron emission tomography (PET) has emerged as an alternative to conventional structural MRI since it uses fluorodeoxyglucose (FDG) or amino acids to assess cell metabolism or proliferation, or antibodies targeting specific molecules and provides complementary information to structural imaging. However, several of the PET compounds, such as FDG are likely to yield some false positives as immune cells have glucose requirements that are comparable to tumor cells. Despite these caveats, preliminary data from immunotherapy clinical trials suggest that this technology with a more specific substrate for either tumor or immune cells may be very important, although validation is essential before adoption in routine clinical practice.112–114
Radiomics is a developing technique that may circumvent difficulties with interpretation of imaging data from CT, MRI, and PET.115 It involves extrapolation of qualitative and quantitative information from morphological and functional clinical images of tumor growth and metastasis that indicate the underlying pathophysiology of a tumor.115 A given set of clinical images for a patient are segmented or broken down into their individual components from which multiple parameters such as semantics and changes in shape, structure, and spatial orientation of the tumor are analyzed using trained computer algorithms. Data from these algorithms help build models which predict response to treatment and disease progression. Although very promising, radiomics is still an evolving field of research and its accuracy is currently limited by dependance on quality of image acquisition as well as post-acquisition processing and segmentation (which can be done manually, semi-automated or completely automated).115
Over the last few years, radiomics has begun to be successfully applied to GBMs. This method has been shown to potentially be capable of distinguishing between low-grade gliomas that will progress to GBM compared to those that will not progress to GBM. This technique has made this distinction with a high degree of accuracy.116 Preoperative T1-weighted contrast material-enhanced and T2-weighted fluid-attenuation inversion recovery MRIs analyzed from a total of 79 GBM patients by Cui et al., using semiautomated tumor delineation and fully automated tumor segmentation led to the discovery of at least 5 prognostic imaging biomarkers based on surface area and intensity distributions of the tumor and its various subregions.117 Applying machine learning to make radiomic assessments has been shown to predict survival in patients with GBM. Using a deep-learning based OS prediction method, Tang et al. used a convolutional neural network to derive the genotypic features of patient tumors from preoperative multimodal MRI images, and then used these results to predict OS with comparable success and accuracy to any other state-of-the-art prediction tool.118 Thus, radiomics coupled with machine learning possess the capability to make predictions of patients response to treatment potentially enabling prediction of response to a treatment regimen and providing a non-invasive tool to enrich for patients more likely respond to a specific treatment. However, the utility of radiomics to predict response of GBM patients to ICI remains to be evaluated.
Conclusion
Immunotherapy has proven to be revolutionary in a wide spectrum of cancers. With rare exceptions, this efficacy has not been demonstrated in CNS cancers. An extensive list of contributing factors has been established ranging from tumor-associated factors to the tumor microenvironment, to patient-related and iatrogenic causes. However, despite these impediments to treatment, a subgroup of patients does demonstrate an effective immune-related tumor response. In order to build on these findings, robust predictors of response are required. In this review, we consider the various peripheral and intratumoral biomarkers that have been proposed as predictors of response to ICI for many solid tumors. Peripheral blood biomarkers which are easily obtained and suitable for longitudinal evaluation include total lymphocyte count, now enhanced by more specific evidence such as T cell expansion, presence of specific types of myeloid cells, cytokine and chemokine secretions, as well as ctDNA. In contrast, given the requirements for an invasive procedure, there are fewer clinically validated intratumoral biomarkers than those for the peripheral blood. While MSI and DNA MMR have proven to be invaluable markers for developing effective treatment protocols for patients with colorectal cancer as well as many others, these are not uniformly predictive of response. Furthermore, MSI is not a common mechanism in CNS cancers, therefore, evaluation of mutational burden requires more extensive genomic testing, typically whole-exome sequencing of tumor with comparison to germline sequencing. Mutational burden is further enhanced by RNA sequencing with better prediction of neoantigen expression. This assessment is enhanced by assessing TCR diversity, an intratumoral biomarker that when increased is predictive of response to ICI in patients with NSCLC. This has been hypothesized to correlate with increased recognition of tumor neoantigens.
Peripheral biomarkers can be detected using cost effective and relatively noninvasive liquid biopsies (blood samples, CSF, and stools) and provide a quick, yet mostly reliable snapshot of the immune environment within a tumor. However, peripheral tissue findings may not always be representative of what is occurring within the confines of the tumor. This makes it imperative to validate the findings from liquid biopsies with data from surgical biopsies of the tumor, although this process is far more complex and invasive for patients. While immune scoring has historically been helpful for predicting response to ICI in multiple cancers, the current models have limitations because they cannot track T cell densities over time as they require tissue samples from the patient which would require repeated invasive procedures. In many instances, components of the immune responses are transient, in which case infrequently procured tumor tissue will not capture the dynamic changes thereby affecting clinical decisions.
In the case of CNS malignancies like GBM, the location of the tumor and its invasion of the normal brain parenchyma possess unique challenges. The brain is immune separated from the periphery, and most treatment antibodies fail to cross the BBB and/or the BCSFB. These tumors also have extensive intratumoral heterogeneity thereby limiting the ability to obtain a comprehensive assessment of the tumor immune-related microenvironment for the entire cancer. Therefore, a combination of biomarkers both from the periphery and the tumor will likely be needed for the optimal predictive panel. For example, in a recent study conducted by Cloughesy et al. on patients with recurrent and surgically resectable GBM, patients who started treatment before surgery (neoadjuvant) with an anti-PD-1 ICI demonstrated longer survival compared to those treated after surgery. They observed an increase in intratumoral expression of IFNγ, T cell clonal expansion, as well as increased CD8 tumor infiltration in the neoadjuvant group.35 These intratumoral changes with treatment in the neoadjuvant treated patients were accompanied by decreased expression of PD-1 on peripheral T cells, an increase in clonally expanded T cells in circulation, and a decrease in circulating monocytes.35
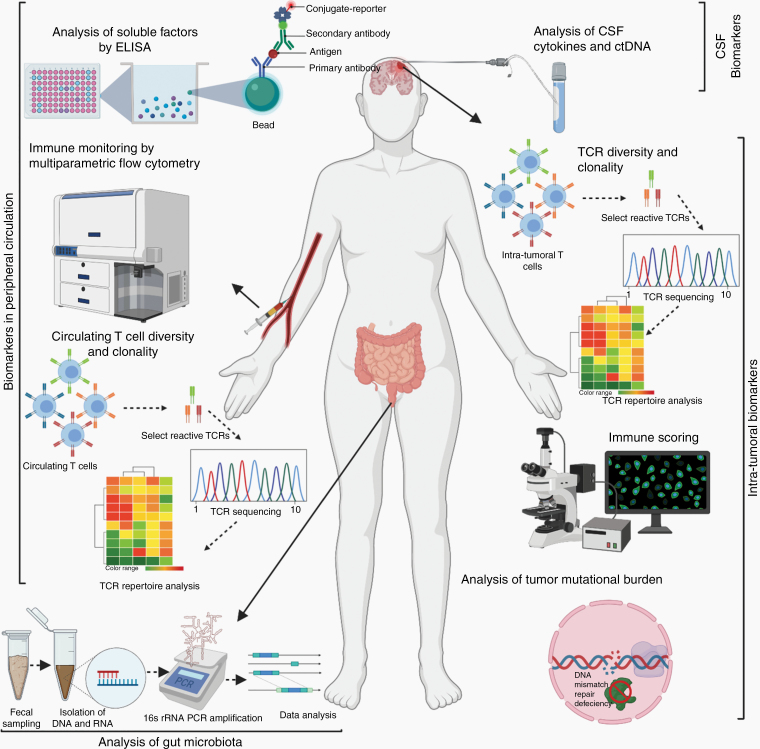
The successful implementation of immunotherapy, particularly ICI, for patients with CNS cancers will require the development of robust markers that predict whether a patient is capable of generating an immune response to the treatment regimen and whether the tumor is likely to respond to treatment as well. We propose that a combination of local (tumor based) and systemic (blood/CSF/Stool-based) markers listed in Table 1 and illustrated in Figure 1 may help to develop these predictors and measures of response, enabling selection of patients most likely to benefit from treatment.
Table 1.
List of All the Biomarkers Reviewed in This Study and the Tissue Required for Determining the Metrics
| Biomarker | Tissue Type | References |
|---|---|---|
| Blood cytology (CBC): ALR, NLR | Peripheral blood | 20–23 |
| Circulating myeloid-derived suppressor cells | Peripheral blood | 24–30 |
| Peripheral T lymphocytes: Cm/Eff T cell ratio, Eff Memory cell counts | Peripheral blood | 31,34 |
| Peripheral T lymphocytes: Treg counts | Peripheral blood | 32,33,38 |
| Peripheral T lymphocytes: Activated, IFNγ expressing T cell counts | Peripheral blood | 35,36 |
| Peripheral T lymphocytes: Clonal expansion if T cells, number of T cell large clones | Peripheral blood | 34–36 |
| Cytokines and chemokines in blood: IFNγ, CXCL9, CXCL10, CXCL11, IL-6, and IL-8 | Peripheral blood | 8,22,39–44 |
| Circulating tumor DNA: Changes in total ctDNA, ctDNA with hotspot mutations, Extracellular vesicles | Peripheral blood/ CSF for brain tumors | 45–53 |
| Gut microbiota | Feces | 18,54–56 |
| Immune scoring: Type and location of intratumoral T lymphocytes -CD3, CD4, CD8, FOXP3, CD68, CD163, CECR1, and PDGFB in the tumor core, invasive margin and distant metastasis | Intratumoral | 63–71 |
| DNA mismatch repair dysfunction: Mutation-associated neoantigens and MSI | Intratumoral | 72–79 |
| TCR diversity and clonality | Intratumoral | 35,78,82–86 |
| Role of supporting cells in predicting immune response B cells and neurons | Intratumoral | 87–99 |
MSI, microsatellite instability; NLR, neutrophil-to-lymphocyte ratio; TCR, T cell receptor.
Figure 1.
Schematic representing select biomarkers both peripheral and intratumoral, proposed for assessment of immune responses of glioblastoma patients treated with immune checkpoint inhibitors.
Funding
This research was supported in part by the Intramural Research Program of the National Institutes of Health/National Cancer Institute (NIH/NCI).
Authorship Statement. N.M.R. and S.C.F. collaborated to review and write this article and are equal contributors to this manuscript. J.A.G. provided his expertise on the clinical aspects. M.R.G. conceptualized and helped to write the manuscript.
Conflict of interest statement. The authors declare that they have no competing interests.
References
- 1. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. [DOI] [PubMed] [Google Scholar]
- 2. Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27(4):450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Callahan MK, Horak CE, Curran MA, et al. Peripheral blood and tumor biomarkers in patients with advanced melanoma treated with combination nivolumab (anti-PD-1, BMS-936558, ONO-4538) and ipilimumab. J Immunother Cancer. 2013;1(S1):O6. [Google Scholar]
- 5. Reardon DA, Brandes AA, Omuro A, et al. Effect of Nivolumab vs Bevacizumab in patients with recurrent glioblastoma: the checkmate 143 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(7):1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reardon DA, Omuro A, Brandes AA, et al. OS10.3 Randomized phase 3 study evaluating the efficacy and safety of nivolumab vs bevacizumab in patients with recurrent glioblastoma:CheckMate 143. Neuro Oncol. 2017;19(Suppl 3):iii21. [Google Scholar]
- 7. Omuro A, Vlahovic G, Lim M, et al. Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: results from exploratory phase I cohorts of CheckMate 143. Neuro Oncol. 2018;20(5):674–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buerki RA, Chheda ZS, Okada H. Immunotherapy of primary brain tumors: facts and hopes. Clin Cancer Res. 2018;24(21):5198–5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Anna C. Filley MHMD. Recurrent glioma clinical trial, CheckMate-143: the game is not over yet. Oncotarget. 2008;8(53):91779–91794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Giles AJ, Hutchinson MND, Sonnemann HM, et al. Dexamethasone-induced immunosuppression: mechanisms and implications for immunotherapy. J Immunother Cancer. 2018;6(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gustafson MP, Lin Y, New KC, et al. Systemic immune suppression in glioblastoma: the interplay between CD14+HLA-DRlo/neg monocytes, tumor factors, and dexamethasone. Neuro Oncol. 2010;12(7):631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jackson CM, Choi J, Lim M. Mechanisms of immunotherapy resistance: lessons from glioblastoma. Nat Immunol. 2019;20(9):1100–1109. [DOI] [PubMed] [Google Scholar]
- 13. Selleck MJ, Senthil M, Wall NR. Making meaningful clinical use of biomarkers. Biomark Insights. 2017;12:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lynes JP, Nwankwo AK, Sur HP, et al. Biomarkers for immunotherapy for treatment of glioblastoma. J Immunother Cancer. 2020;8(1):e000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xiao F, Lv S, Zong Z, et al. Cerebrospinal fluid biomarkers for brain tumor detection: clinical roles and current progress. Am J Transl Res. 2020;12(4):1379–1396. [PMC free article] [PubMed] [Google Scholar]
- 16. Darvesh AS, Carroll RT, Geldenhuys WJ, et al. In vivo brain microdialysis: advances in neuropsychopharmacology and drug discovery. Expert Opin Drug Discov. 2011;6(2):109–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lynes J, Jackson S, Sanchez V, et al. Cytokine microdialysis for real-time immune monitoring in glioblastoma patients undergoing checkpoint blockade. Neurosurgery. 2019;84(4):945–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gong J, Chehrazi-Raffle A, Placencio-Hickok V, Guan M, Hendifar A, Salgia R. The gut microbiome and response to immune checkpoint inhibitors: preclinical and clinical strategies. Clin Transl Med. 2019;8(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Howard R, Kanetsky PA, Egan KM. Exploring the prognostic value of the neutrophil-to-lymphocyte ratio in cancer. Sci Rep. 2019;9(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Di Giacomo AM, Danielli R, Calabrò L, et al. Ipilimumab experience in heavily pretreated patients with melanoma in an expanded access program at the University Hospital of Siena (Italy). Cancer Immunol Immunother. 2011;60(4):467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ferrucci PF, Gandini S, Battaglia A, et al. Baseline neutrophil-to-lymphocyte ratio is associated with outcome of ipilimumab-treated metastatic melanoma patients. Br J Cancer. 2015;112(12):1904–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Agulló-Ortuño MT, Gómez-Martín Ó, Ponce S, et al. Blood predictive biomarkers for patients with non–small-cell lung cancer associated with clinical response to Nivolumab. Clin Lung Cancer. 2020;21(1):75–85. [DOI] [PubMed] [Google Scholar]
- 23. Massara M, Persico P, Bonavita O, et al. Neutrophils in gliomas. Front Immunol. 2017;8(OCT):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Raychaudhuri B, Rayman P, Ireland J, et al. Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neuro Oncol. 2011;13(6):591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hernandez C, Arasanz H, Chocarro L, et al. Systemic blood immune cell populations as biomarkers for the outcome of immune checkpoint inhibitor therapies. Int J Mol Sci. 2020;21(7):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Park SM, Youn JI. Role of myeloid-derived suppressor cells in immune checkpoint inhibitor therapy in cancer. Arch Pharm Res. 2019;42(7):560–566. [DOI] [PubMed] [Google Scholar]
- 27. Meyer C, Cagnon L, Costa-Nunes CM, et al. Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab. Cancer Immunol Immunother. 2014;63(3):247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Coaña YP, Wolodarski M, Poschke I, et al. Ipilimumab treatment decreases monocytic MDSCs and increases CD8 effector memory T cells in long-term survivors with advanced melanoma. Oncotarget. 2017;8(13):21539–21553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weber J, Gibney G, Kudchadkar R, et al. Phase I/II study of metastatic melanoma patients treated with nivolumab who had progressed after ipilimumab. Cancer Immunol Res. 2016;4(4):345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ding AS, Routkevitch D, Jackson C, Lim M. Targeting myeloid cells in combination treatments for glioma and other tumors. Front Immunol. 2019;10:1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Manjarrez-Orduño N, Menard LC, Kansal S, et al. Circulating T cell subpopulations correlate with immune responses at the tumor site and clinical response to PD1 inhibition in non-small cell lung cancer. Front Immunol. 2018;9:1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kagamu H, Kitano S, Yamaguchi O, et al. CD4+ T-cell immunity in the peripheral blood correlates with response to anti-PD-1 therapy. Cancer Immunol Res. 2020;8(3):334–344. [DOI] [PubMed] [Google Scholar]
- 33. Tarhini AA, Edington H, Butterfield LH, et al. Immune monitoring of the circulation and the tumor microenvironment in patients with regionally advanced melanoma receiving neoadjuvant ipilimumab. PLoS One. 2014;9(2):e87705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fairfax BP, Taylor CA, Watson RA, et al. Peripheral CD8+ T cell characteristics associated with durable responses to immune checkpoint blockade in patients with metastatic melanoma. Nat Med. 2020;26(2):193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cloughesy TF, Mochizuki AY, Orpilla JR, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. 2019;25(3):477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schalper KA, Rodriguez-Ruiz ME, Diez-Valle R, et al. Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nat Med. 2019;25(3):470–476. [DOI] [PubMed] [Google Scholar]
- 37. Chongsathidkiet P, Jackson C, Koyama S, et al. Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat Med. 2018;24(9):1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jordan JT, Sun W, Hussain SF, DeAngulo G, Prabhu SS, Heimberger AB. Preferential migration of regulatory T cells mediated by glioma-secreted chemokines can be blocked with chemotherapy. Cancer Immunol Immunother. 2008;57(1):123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yamazaki N, Kiyohara Y, Uhara H, et al. Cytokine biomarkers to predict antitumor responses to nivolumab suggested in a phase 2 study for advanced melanoma. Cancer Sci. 2017;108(5):1022–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Koguchi Y, Hoen HM, Bambina SA, et al. Serum immunoregulatory proteins as predictors of overall survival of metastatic melanoma patients treated with ipilimumab. Cancer Res. 2015;75(23):5084–5092. [DOI] [PubMed] [Google Scholar]
- 42. Tobin RP, Jordan KR, Kapoor P, et al. IL-6 and IL-8 are linked with myeloid-derived suppressor cell accumulation and correlate with poor clinical outcomes in melanoma patients. Front Oncol. 2019;9(November):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bjoern J, Juul Nitschke N, Zeeberg Iversen T, Schmidt H, Fode K, Svane IM. Immunological correlates of treatment and response in stage IV malignant melanoma patients treated with Ipilimumab. Oncoimmunology. 2016;5(4):e1100788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sampson JH, Gunn MD, Fecci PE, Ashley DM. Brain immunology and immunotherapy in brain tumours. Nat Rev Cancer. 2020;20(1):12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li L, Zhang J, Jiang X, Li Q. Promising clinical application of ctDNA in evaluating immunotherapy efficacy. Am J Cancer Res. 2018;8(10):1947–1956. [PMC free article] [PubMed] [Google Scholar]
- 46. Cabel L, Riva F, Servois V, et al. Circulating tumor DNA changes for early monitoring of anti-PD1 immunotherapy: a proof-of-concept study. Ann Oncol. 2017;28(8):1996–2001. [DOI] [PubMed] [Google Scholar]
- 47. Goldberg SB, Patel AA. Monitoring immunotherapy outcomes with circulating tumor DNA. Immunotherapy. 2018;10(12):1023–1025. [DOI] [PubMed] [Google Scholar]
- 48. Ashida A, Sakaizawa K, Uhara H, Okuyama R. Circulating tumour DNA for monitoring treatment response to anti-PD-1 immunotherapy in melanoma patients. Acta Derm Venereol. 2017;97(10):1212–1218. [DOI] [PubMed] [Google Scholar]
- 49. Lipson EJ, Velculescu VE, Pritchard TS, et al. Circulating tumor DNA analysis as a real-time method for monitoring tumor burden in melanoma patients undergoing treatment with immune checkpoint blockade. J Immunother Cancer. 2014;2(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Saenz-Antoñanzas A, Auzmendi-Iriarte J, Carrasco-Garcia E, et al. Liquid biopsy in glioblastoma: opportunities, applications and challenges. Cancers (Basel). 2019;11(7):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9(8):581–593. [DOI] [PubMed] [Google Scholar]
- 52. Osti D, Del Bene M, Rappa G, et al. Clinical significance of extracellular vesicles in plasma from glioblastoma patients. Clin Cancer Res. 2019;25(1):266–276. [DOI] [PubMed] [Google Scholar]
- 53. Yekula A, Yekula A, Muralidharan K, Kang K, Carter BS, Balaj L. Extracellular vesicles in glioblastoma tumor microenvironment. Front Immunol. 2020;10(January):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mehrian-Shai R, Reichardt JKV, Harris CC, Toren A. The gut-brain axis, paving the way to brain cancer. Trends Cancer. 2019;5(4):200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chaput N, Lepage P, Coutzac C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol. 2017;28(6):1368–1379. [DOI] [PubMed] [Google Scholar]
- 56. D’Alessandro G, Antonangeli F, Marrocco F, et al. Gut microbiota alterations affect glioma growth and innate immune cells involved in tumor immunosurveillance in mice. Eur J Immunol. 2020;50(5):705–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dobrzanski MJ. Expanding roles for CD4 T cells and their subpopulations in tumor immunity and therapy. Front Oncol. 2013;3(March):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Protti MP, De Monte L. Immune infiltrates as predictive markers of survival in pancreatic cancer patients. Front Physiol. 2013;4(August):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fortis SP, Sofopoulos M, Sotiriadou NN, et al. Differential intratumoral distributions of CD8 and CD163 immune cells as prognostic biomarkers in breast cancer. J Immunother Cancer. 2017;5(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Newman AM, Liu CL, Green MR, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12(5):453–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kwak Y, Koh J, Kim DW, Kang SB, Kim WH, Lee HS. Immunoscore encompassing CD3+ and CD8+ T cell densities in distant metastasis is a robust prognostic marker for advanced colorectal cancer. Oncotarget. 2016;7(49):81778–81790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hermitte F. Biomarkers immune monitoring technology primer: Immunoscore® Colon. J Immunother Cancer. 2016;4:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yang X, Shi Y, Li M, et al. Identification and validation of an immune cell infiltrating score predicting survival in patients with lung adenocarcinoma. J Transl Med. 2019;17(1):217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chen B, Khodadoust MS, Liu CL, Newman AM, Alizadeh AA.. Profiling Tumor Infiltrating Immune Cells with CIBERSORT BT - Cancer Systems Biology: Methods and Protocols. In: von Stechow L, ed. New York, NY: Springer New York; 2018:243–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wang H, Wu X, Chen Y. Stromal-immune score-based gene signature: a prognosis stratification tool in gastric cancer. Front Oncol. 2019;9(November):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wang J, Li Y, Fu W, et al. Prognostic nomogram based on immune scores for breast cancer patients. Cancer Med. 2019;8(11):5214–5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yoshihara K, Shahmoradgoli M, Martínez E, et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat Commun. 2013;4:2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Öjlert ÅK, Halvorsen AR, Nebdal D, et al. The immune microenvironment in non-small cell lung cancer is predictive of prognosis after surgery. Mol Oncol. 2019;13(5):1166–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tahkola K, Leppänen J, Ahtiainen M, et al. Immune cell score in pancreatic cancer-comparison of hotspot and whole-section techniques. Virchows Arch. 2019;474(6):691–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gartrell RD, Marks DK, Rizk EM, et al. Validation of melanoma immune profile (MIP), a prognostic immune gene prediction score for stage II–III melanoma. Clin Cancer Res. 2019;25(8):2494–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lehtipuro S, Nykter M, Granberg KJ. Modes of immunosuppression in glioblastoma microenvironment. Oncotarget. 2019;10(9):920–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lengauer C, Vogelstein KW. Genetic instabilities in human cancers. Nature. 1998. 396(6712):643–649. [DOI] [PubMed] [Google Scholar]
- 73. Grady WM, Carethers JM. Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology. 2008;135(4):1079–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Altonen LA, Salovaara S, Kristo P, et al. Incidence of hereditary nonpolyposis colorectal cancer and the feasibility of molecular screening for the disease. N Engl J Med. 1998;338(21):1481–1487. [DOI] [PubMed] [Google Scholar]
- 75. Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993;260(5109):816–819. [DOI] [PubMed] [Google Scholar]
- 76. Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kim ST, Cristescu R, Bass AJ, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24(9):1449–1458. [DOI] [PubMed] [Google Scholar]
- 78. Touat M, Li YY, Boynton AN, et al. Mechanisms and therapeutic implications of hypermutation in gliomas. Nature. 2020;580(7804):517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hodges TR, Ott M, Xiu J, et al. Mutational burden, immune checkpoint expression, and mismatch repair in glioma: implications for immune checkpoint immunotherapy. Neuro Oncol. 2017;19(8):1047–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bouffet E, Larouche V, Campbell BB, et al. Immune checkpoint inhibition for hypermutant glioblastoma multiforme resulting from germline biallelic mismatch repair deficiency. J Clin Oncol. 2016;34(19):2206–2211. [DOI] [PubMed] [Google Scholar]
- 81. Wang L, Ge J, Lan Y, et al. Tumor mutational burden is associated with poor outcomes in diffuse glioma. BMC Cancer. 2020;20(1):213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Cui JH, Lin KR, Yuan SH, et al. TCR repertoire as a novel indicator for immune monitoring and prognosis assessment of patients with cervical cancer. Front Immunol. 2018;9(NOV):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Charles J, Mouret S, Challende I, et al. T-cell receptor diversity as a prognostic biomarker in melanoma patients. Pigment Cell Melanoma Res. 2020;33(4):612–624. [DOI] [PubMed] [Google Scholar]
- 84. Han J, Duan J, Bai H, et al. TCR repertoire diversity of peripheral PD-1+CD8+ T cells predicts clinical outcomes after immunotherapy in patients with non-small cell lung cancer. Cancer Immunol Res. 2020;8(1):146–154. [DOI] [PubMed] [Google Scholar]
- 85. Hopkins AC, Yarchoan M, Durham JN, et al. T cell receptor repertoire features associated with survival in immunotherapy-treated pancreatic ductal adenocarcinoma. JCI Insight. 2018;3(13):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zhang Y, Mudgal P, Wang L, et al. T cell receptor repertoire as a prognosis marker for heat shock protein peptide complex-96 vaccine trial against newly diagnosed glioblastoma. Oncoimmunology. 2020;9(1):1749476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Helmink BA, Reddy SM, Gao J, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020;577(7791):549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Maurice NJ, McElrath MJ, Andersen-Nissen E, Frahm N, Prlic M. CXCR3 enables recruitment and site-specific bystander activation of memory CD8+ T cells. Nat Commun. 2019;10(1):4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Griss J, Bauer W, Wagner C, et al. B cells sustain inflammation and predict response to immune checkpoint blockade in human melanoma. Nat Commun. 2019;10(1):4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Klopfenstein Q, Truntzer C, Vincent J, Ghiringhelli F. Cell lines and immune classification of glioblastoma define patient’s prognosis. Br J Cancer. 2019;120(8):806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Venkatesh HS, Morishita W, Geraghty AC, et al. Electrical and synaptic integration of glioma into neural circuits. Nature. 2019;573(7775):539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Tian L, Rauvala H, Gahmberg CG. Neuronal regulation of immune responses in the central nervous system. Trends Immunol. 2009;30(2):91–99. [DOI] [PubMed] [Google Scholar]
- 93. Smolders J, Heutinck KM, Fransen NL, et al. Tissue-resident memory T cells populate the human brain. Nat Commun. 2018;9(1):4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Biber K, Neumann H, Inoue K, Boddeke HW. Neuronal ‘On’ and ‘Off’ signals control microglia. Trends Neurosci. 2007;30(11):596–602. [DOI] [PubMed] [Google Scholar]
- 95. Ganea D, Gonzalez-Rey E, Delgado M. A novel mechanism for immunosuppression: from neuropeptides to regulatory T cells. J Neuroimmune Pharmacol. 2006;1(4):400–409. [DOI] [PubMed] [Google Scholar]
- 96. Stojkovska I, Wagner BM, Morrison BE. Parkinson’s disease and enhanced inflammatory response. Exp Biol Med (Maywood). 2015;240(11):1387–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Geed M, Garabadu D, Ahmad A, Krishnamurthy S. Silibinin pretreatment attenuates biochemical and behavioral changes induced by intrastriatal MPP+ injection in rats. Pharmacol Biochem Behav. 2014;117:92–103. [DOI] [PubMed] [Google Scholar]
- 98. Hansen AM, Caspi RR. Glutamate joins the ranks of immunomodulators. Nat Med. 2010;16(8):856–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ganor Y, Levite M. The neurotransmitter glutamate and human T cells: glutamate receptors and glutamate-induced direct and potent effects on normal human T cells, cancerous human leukemia and lymphoma T cells, and autoimmune human T cells. J Neural Transm (Vienna). 2014;121(8):983–1006. [DOI] [PubMed] [Google Scholar]
- 100. Berghoff AS, Fuchs E, Ricken G, et al. Density of tumor-infiltrating lymphocytes correlates with extent of brain edema and overall survival time in patients with brain metastases. Oncoimmunology. 2016;5(1):e1057388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Madden K, Kasler MK. Immune checkpoint inhibitors in lung cancer and melanoma. Semin Oncol Nurs. 2019;35(5):150932. [DOI] [PubMed] [Google Scholar]
- 102. Zakaria R, Platt-Higgins A, Rathi N, et al. T-cell densities in brain metastases are associated with patient survival times and diffusion tensor MRI changes. Cancer Res. 2018;78(3):610–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Berghoff AS, Ricken G, Wilhelm D, et al. Tumor infiltrating lymphocytes and PD-L1 expression in brain metastases of small cell lung cancer (SCLC). J Neurooncol. 2016;130(1):19–29. [DOI] [PubMed] [Google Scholar]
- 104. Zhou J, Gong Z, Jia Q, Wu Y, Yang ZZ, Zhu B. Programmed death ligand 1 expression and CD8+ tumor-infiltrating lymphocyte density differences between paired primary and brain metastatic lesions in non-small cell lung cancer. Biochem Biophys Res Commun. 2018;498(4):751–757. [DOI] [PubMed] [Google Scholar]
- 105. Lu BY, Gupta R, Ribeiro M, et al. PD-L1 expression and tumor-infiltrating lymphocytes in lung cancer brain metastases. J Clin Oncol. 2018;36(15_suppl):e24116–e24116. [Google Scholar]
- 106. Dietrich J, Winter SF, Klein JP. Neuroimaging of brain tumors: pseudoprogression, pseudoresponse, and delayed effects of chemotherapy and radiation. Semin Neurol. 2017;37(5):589–596. [DOI] [PubMed] [Google Scholar]
- 107. Okada H, Weller M, Huang R, et al. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol. 2015;16(15):e534–e542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ranjan S, Quezado M, Garren N, et al. Clinical decision making in the era of immunotherapy for high grade-glioma: report of four cases. BMC Cancer. 2018;18(1):239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wei W, Jiang D, Ehlerding EB, Luo Q, Cai W. Noninvasive PET imaging of T cells. Trends Cancer. 2018;4(5):359–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. De Belder FE, Oot AR, Van Hecke W, et al. Diffusion tensor imaging provides an insight into the microstructure of meningiomas, high-grade gliomas, and peritumoral edema. J Comput Assist Tomogr. 2012;36(5):577–582. [DOI] [PubMed] [Google Scholar]
- 111. Secretariat MA. Functional brain imaging: an evidence-based analysis. Ont Health Technol Assess Ser. 2006;6(22):1–79. [PMC free article] [PubMed] [Google Scholar]
- 112. Chiba Y, Kinoshita M, Okita Y, et al. Use of (11)C-methionine PET parametric response map for monitoring WT1 immunotherapy response in recurrent malignant glioma. J Neurosurg. 2012;116(4):835–842. [DOI] [PubMed] [Google Scholar]
- 113. Rashidian M, Ingram JR, Dougan M, et al. Predicting the response to CTLA-4 blockade by longitudinal noninvasive monitoring of CD8 T cells. J Exp Med. 2017;214(8):2243–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Vrabec M, Van Cauter S, Himmelreich U, et al. MR perfusion and diffusion imaging in the follow-up of recurrent glioblastoma treated with dendritic cell immunotherapy: a pilot study. Neuroradiology. 2011;53(10):721–731. [DOI] [PubMed] [Google Scholar]
- 115. Rizzo S, Botta F, Raimondi S, et al. Radiomics: the facts and the challenges of image analysis. Eur Radiol Exp. 2018;2(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Narang S, Lehrer M, Yang D, Lee J, Rao A. Radiomics in glioblastoma: current status, challenges and potential opportunities. Transl Cancer Res. 2016;5(4):383–397 [Google Scholar]
- 117. Cui Y, Tha KK, Terasaka S, et al. Prognostic imaging biomarkers in glioblastoma: development and independent validation on the basis of multiregion and quantitative analysis of MR images. Radiology. 2016;278(2):546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Tang Z, Xu Y, Jin L, et al. Deep learning of imaging phenotype and genotype for predicting overall survival time of glioblastoma patients. IEEE Trans Med Imaging. 2020;39(6):2100–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]